Abstract
Background
We previously demonstrated upregulation of c-myc, survivin, and cyclin D1 in CD34+ bone marrow mononuclear cells (BMMNCs) of patients with trisomy 8 and monosomy 7 myelodysplastic syndromes (MDS). “Knockdown” of cyclin D1 by RNA interference decreased trisomy 8 cell growth, suggesting that this might be a therapeutic target in MDS.
Experimental Design
We performed preclinical studies using BMMNCs from patients with MDS and AML to examine the effects of the styryl sulfone ON 01910.Na on cyclin D1 accumulation, aneuploidy, and CD34+ blast percentage. We next treated twelve patients with higher risk MDS and two trisomy 8 AML patients with ON01910.Na on a phase I clinical protocol (NCT00533416).
Results
ON 01910.Na inhibited cyclin D1 expression, and was selectively toxic to trisomy 8 cells in vitro. Flow cytometry studies demonstrated increased mature CD15+ myeloid cells and decreased CD34+ blasts. Three patients treated with ON01910.Na on a clinical had decreased bone marrow blasts by ≥50%, and three patients had hematologic improvements, one of which was sustained for 33 months. Patients with hematologic responses to ON 01910.Na had decreased cyclin D1 expression in their CD34+ cells.
Conclusions
The preclinical results and responses of patients on a clinical trial warrant further investigation of ON 01910.Na as a potential novel targeted therapy for higher risk MDS patients.
Keywords: MDS, Treatment, ON 01910.Na, Cyclin D1
Introduction
The myelodysplastic syndromes (MDS) are a group of bone marrow diseases with wide variation in clinical presentation and disease severity [1]. Typically patients are older and suffer co-morbidities [2]. MDS is characterized by cytopenias due to ineffective hematopoiesis and a variable risk of leukemic progression. Treatment of high risk MDS is particularly difficult. Chemotherapy has a limited role in MDS management [3], and stem cell transplantation is poorly tolerated in most older individuals. 5-azacytidine provides lasting hematological responses and improved survival in some patients [4], and lenalidomide improves blood counts in patients with 5q- syndrome [5,6]. However, some patients fail to respond to, or relapse after these therapies. Therefore, additional therapies with good safety profiles are needed.
Monosomy 7 and trisomy 8 are among the most common cytogenetic abnormalities seen in patients with MDS [7]. In de novo MDS patients with monosomy 7 and trisomy 8, and in those with trisomy 8 AML, there is up-regulation of cyclin D1 and Wilms Tumor 1 (WT1) gene expression, which allows for the expansion of aneuploid cells despite a vigorous immune response against them[8,9]. Cyclin D1 is over-expressed in trisomy 8 and monosomy 7 cells when examined by microarray [8], realtime PCR [10] and immunoblot [10]. Cyclin D1 is also increased in high risk MDS bone marrows [11–13]. Up-regulation of cyclin D1 increases proliferation of leukemia and lymphoma cells, and may upregulate survivin, an anti-apoptotic protein [10, 12, 14]. Survivin blocks apoptosis upstream of caspase 8, and may be responsible for MDS clones failing to complete apoptosis [15]. “Knockdown” of either c-myc or surviving diminishes the trisomy 8 clone in MDS without affecting normal hematopoiesis [10]. Collectively, these findings raise the possibility that MDS and trisomy 8 AML bone marrows may be susceptible to pharmacologic inhibition of cell cycle control proteins such as cyclin D1.
ON 01910.Na is a styryl sulfonyl which is a potent inhibitor of molecules that influence cell cycle progression, including polo-like kinase 1, cyclin D1, and c-myc pathways [16]. It shows significant in vitro activity against mantle cell lymphoma lines which over-express cyclin D1, decreases c-myc and cyclin D1 levels, and stimulates apoptosis through release of caspases and modulation of Bcl-2. Animal studies using ON 01910.Na show little toxicity, and this compound is well-tolerated in phase I studies of patients solid tumors [17,18]. We examined the effects of ON 01910.Na on MDS cells in vitro, and report the results of a phase I clinical trial treating patients with high risk MDS and trisomy 8 AML.
Patients, Methods
Cell preparation and culture
BMMNCs were aspirated from the posterior iliac crest into syringes containing media supplemented 1:10 with heparin and prepared by density gradient centrifugation using lymphocyte separation medium (Organon). Aliquots of BMMNC were placed in Myelocult (Stem Cell Technologies) with growth factors as described previously [19]. For colony forming assays, cells were plated in methylcellulose with growth factors for fourteen days and then counted. To assess the effect of ON 01910.Na on aneuploidy, BMMNC were cultured in liquid media with growth factors at varying concentrations of ON 01910.Na. Cells were counted, viability was assessed, and FISH was performed after fourteen days.
Fluorescence in situ hybridization
Cells were treated with hypotonic buffer comprising KCl, HEPES (N-2-hydroxyethypiperazine-N'-2-ethanesulfonic acid), EGTA (ethylene-glycotetra-acetic acid), and NaOH, then fixed onto slides using methanol/acetic acid (3:1). FISH was performed with probes for chromosomes 5q, 7, 11 and 8 (Vysis Inc.) as described previously [15].
Quantitative real-time polymerase chain reaction (PCR)
RNA was isolated from a minimum of 106 PBMCs using RNeasy mini-kits (QIAGEN). cDNA was synthesized using the Advantage RT-for-PCR kit (Clontech). ABL expression was used as the cDNA quantity control [20]; its expression was measured using 300 nM primers and 200 nM probe [21]. Expression of Cyclin D1 was measured using TaqMan Assays-on-demand probe-and-primer reagents (Applied Biosystems) for Cyclin D1, Hs00277039_m1, and expressed as a ratio of Cyclin D1/ABL. All reactions by quantitative reverse-transcription (RT)–PCR using the ABI PRISM 7900 sequence detection system (Applied Biosystems) were performed in triplicate in 10 µL volume using standard conditions with 40 cycles of amplification.
Monoclonal antibodies
The following commercially available fluorochrome-conjugated monoclonal antibodies (mAbs) were used: CD33-PECy5 (BD Pharmingen), CD33-PECy5 (Ebioscience), CD34-PECy7 (BD Pharmingen), CD13-APCCy7 (Biolegend), CD15-Pacific Blue (Invitrogen), Cyclin-D1-FITC, CD184-APC, and CD49d PE (BD Pharmingen).
Flow cytometry
To assess intracellular cyclin-D staining by flow cytometry, PBMCs were isolated by density gradient centrifugation as described previously [22]. Prior to staining, 106 PBMCs were suspended in IMDM (Gibco Invitrogen) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific), 2 mM L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin (Invitrogen), and 8 units/mL RNase-free recombinant DNase-I (Roche) (I-10), and incubated at 37°C 5% CO2. PBMCs were stained with live-dead discrimination dye BiViD (Invitrogen), and then stained with mABs specific for surface markers. For cyclin D1 studies, PBMCs were fixed and permeabilized with fix/perm buffer (Ebioscience), washed, and blocked with mouse serum (Caltag Laboratories). Cells were stained intracellularly with cyclin-D mABs (BD Pharmingen) and analyzed using a LSR-II or LSR Fortessa flow cytometer (BD). At least 200,000 events were acquired per tube. Data analysis was performed using FlowJo software (Tree Star, Inc).
Phase I clinical trial description
All patients signed informed consent according to the protocol 07-H-0225 approved by the Institutional Review Board of the NHLBI, and were treated at the Mark O. Hatfield Clinical Research Center at the NIH in Bethesda, MD, USA. The study was a non-randomized, dose escalating, open label, Phase I study of ON 01910.Na. This trial was registered at clinicaltrials.gov as NCT00533416.
Eligiblity criteria
Eligibility criteria included MDS patients having a World Health Organization classification [23] of RAEB-1 or 2, or AML with trisomy 8, and cytopenia(s) defined as one of the following: anemia requiring transfusion of > 1 unit PRBC /mo, or a hemoglobin <9g/dL or a reticulocyte count <60,000 cells/uL; and/or thrombocytopenia (platelet count less than 50,000 cells/uL), and/or neutropenia (absolute neutrophil count less than 500/ul). The trial was open to patients with any cytogenetic abnormality and >5% blasts, based on the fact that we previously observed up-regulation of cyclin D1 in monosomy 7 [8], and others have demonstrated increased cyclin D1 expression in MDS patients with various karyotypes [12]. Patients had an ECOG performance status of 0, 1, or 2.
Treatment plan
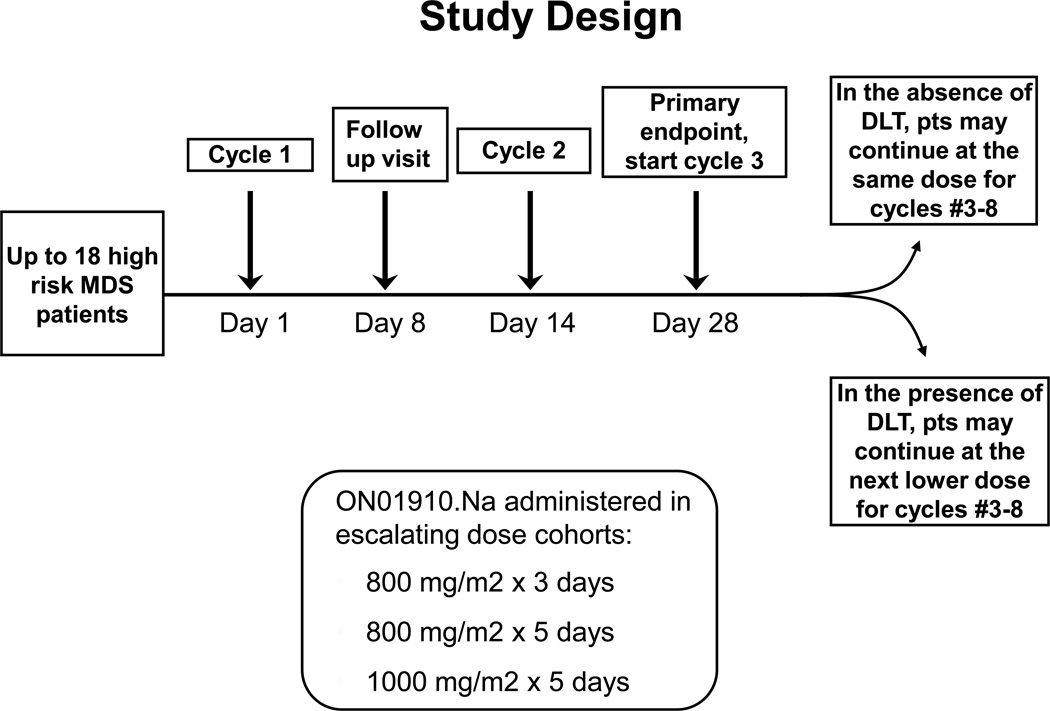
A standard phase I trial design was utilized [24] (trial scheme depicted in Fig 2), with three cohorts receiving ON 01910.Na with increases in drug exposure (either by dose or duration) with each successive cohort. We completed our planned accrual to this trial. Three dose levels of ON 01910.Na (800mg/m2 / day × 3 days, 800mg/m2/ day × 5 days, or 1000mg/m2 /day × 5 days) were considered with three to six evaluable patients at each dose level to enroll up to 18 patients in the event of dose-limiting toxicities. In the absence of dose limiting toxicity, a cohort received a continuous infusion repeated every 2 weeks with dose escalation in each subsequent cohort until the maximum tolerated dose was identified. A cycle was defined as the duration of treatment (3 or 5 days) plus the subsequent days thereafter for a total of 14 days. Any death considered to be possibly, probably, or definitely related to ON 01910.Na was considered for early stopping of the corresponding dose cohort and the cohorts with higher dose levels.
Figure 2.
Clinical trial design.
Endpoints
The primary endpoint was toxicity as measured by Common Terminology Criteria for Adverse Events Version 3.0. Secondary endpoints measured after completion of two cycles of ON 01910.Na included hematologic improvements, transfusion independence, and cytogenetic responses, as defined according to the International Working Group (IWG) criteria [25], and decreases in bone marrow blasts. Metaphase cytogenetics were performed in a certified clinical laboratory (Quest Diagnostics, Madison, NJ), and assessment of bone marrow aspirate blast percentages were performed retrospectively by a single hematopathologist (IM) in a blinded manner. Pharmacokinetics were performed at the first two dose levels for the first two cycles of treatment as described previously [26].
Statistical methods
Laboratory parameters were expressed as mean ± SEM. Paired t-tests were used to compare the laboratory parameters before and after ON 01910.Na treatment. The linear mixed models were used to examine the change in the proportions of CD34+ and CD15+ BMMNCs at varying concentrations of ON 01910.Na. A positive or negative slope indicates the increase or reduction in the mean percent cell counts with increasing concentrations of drug. The statistical significance was set at p < 0.05. Data analysis was performed using the Prism software (GraphPad, La Jolla, CA) and the SAS 9.2 software (SAS Institute Inc., Cary, NC, USA)
Results
ON 01910.Na inhibits cyclin D1 expression in MDS bone marrow cells in vitro
We examined the effects of ON 01910.Na on cyclin D1 accumulation on cultured BMMNCs from patients with high risk MDS with trisomy 8. Cells were incubated with 50–100nM ON 01910.Na or vehicle control for 14 days, and cyclin D1 expression was determined by quantitative real time PCR. As shown in supplemental figure 1, ON 01910. Na reduced cyclin D1 transcript levels (measured by real time PCR), in a concentration dependent manner.
ON 01910.Na reduces bone marrow blasts and trisomy 8 aneuploidy in vitro
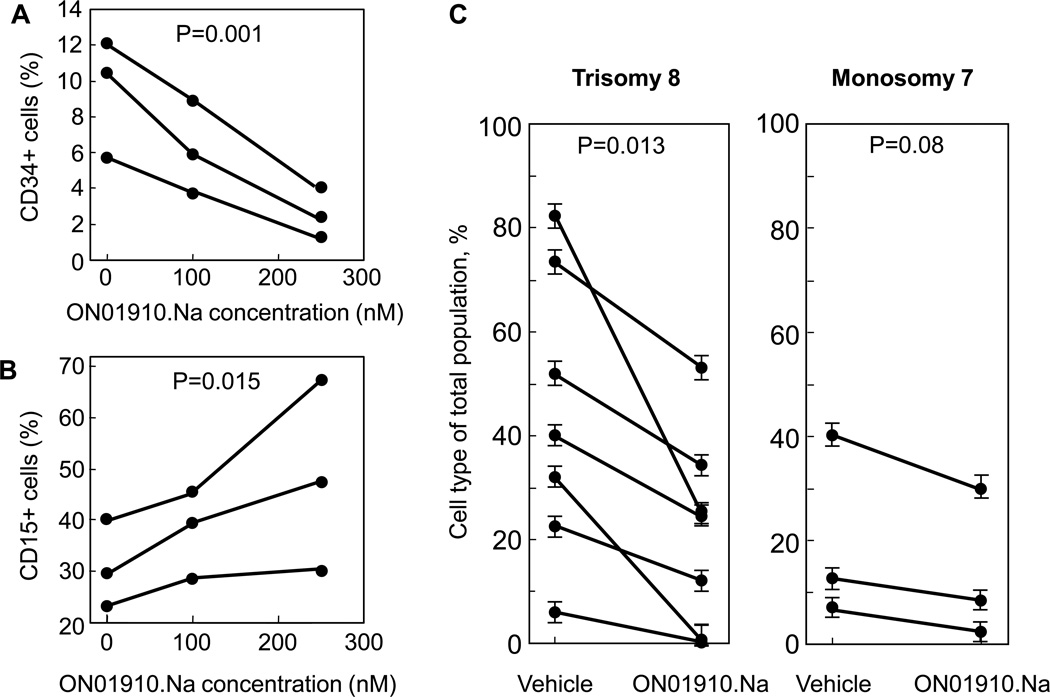
To assess the effects of ON 01910.Na on hematopoiesis, short- term colony culture assays were performed at varying concentrations of drug. BMMNCs from 5 healthy controls were placed in culture with growth factors in the presence of vehicle or increasing concentrations of drug. There were no differences in erythroid or myeloid colony formation in either the treated or untreated normal cells (data not shown). Because of the ease of identifying the MDS clone in trisomy 8 patients, and because cyclin D1 is over-expressed in this population10, we evaluated the effects of ON 01910.Na on blasts in BMMNCs cultured from patients with high risk trisomy 8 MDS in the presence of vehicle or increasing concentrations of ON 01910.Na in vitro. Cells treated with ON 01910.Na had decreased numbers of blasts when examined morphologically (not shown), as well as decreased percentages of CD34+ bone marrow precursors when evaluated by flow cytometry (fig 1A), with a corresponding increase in mature CD15+ myeloid cells (fig 1B). In order to assess the effect of ON 01910.Na on trisomy 8 aneuploidy in patients with high risk MDS, we quantitated trisomy 8 clone size by FISH in bone marrow mononuclear cells treated in vitro with ON 01910.Na or vehicle control. As shown in the left panel on figure 1C, trisomy 8 clone size decreased upon treatment with ON 01910.Na 250nM (p=0.013), whereas percentage of diploid bone marrow mononuclear cells correspondingly increased (not shown). The mean percentage of monosomy 7 cells was also decreased, but not significantly, perhaps related to the small sample size (n=3), after treatment with ON 01910.Na (Fig. 1C, right panel).
Figure 1.
Effects of ON 01910.Na on CD34+ blasts, CD15+ myeloid cells, and aneuploidy in MDS cells in vitro. BMMNCs from patients with RAEB-1, RAEB-2 were grown in the presence of vehicle or increasing concentrations of ON 01910.Na. The percentage of CD34+ blasts (panel A), CD15+ myeloid cells (panel B) were determined by flow cytometry, and percentages of aneuploid and diploid cells were determined by FISH expressed as a percentage of the total cell population (panel C) as described in Materials and Methods. Individual lines represent independent patient samples. Each data point in panel C represents a mean +/− SEM from 3 replicate determinations.
Phase I clinical trial: patient characteristics
Based on the results described above, we conducted a phase I protocol examining the safety and tolerability of ON 01910.Na in patients with higher risk MDS or trisomy 8 AML. Twelve higher risk MDS patients with >5% blasts and two leukemic patients were enrolled, which completed our planned accrual for this trial. Patients were treated in escalating dosing cohorts with ON 01910.Na at 800 mg/m2 for three days (n=3), 800 mg/m2 for five days (n=8), and 1000 mg/m2 for five days (n=3), with each cycle repeated every two weeks for a maximum of eight cycles (Fig. 2). The demographics, World Health Organization Prognostic Scoring Scale (WPSS) risk category [27], baseline bone marrow blast percentage, and cytogenetics for the fourteen patients treated with ON 01910.Na are depicted in Table 1. Median age of the patients was 73 years (range 56–86 years), and all MDS patients had a WPSS score of high or very high.
Table 1.
Patient Characteristics and Responses to ON 01910.Na.
| Patient characteristics | Pre-ON1910.Na | Post-ON1910.Na | Current Status | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN | Age | ECOG score |
Sex | Race | WPSS | Dose Level (mg/m2) |
Cytogenetics | FISH | Blasts | Cellularity | Cytogenetics | FISH | Blasts | IWG response |
Follow up (months) |
Outcome |
| 1 | 65 | 0 | M | W | Very high |
800 ×3d | 46XY,+8,−7,5q-, 12p- 16/20 | 68% | 13% | hyper | 46XY,+8,−7,5q-, 12p- 20/20 | 41% | 4% | HI-2, BM |
4 | Deceased |
| 2 | 68 | 1 | M | W | Very high |
800 ×3d | 45XY,−7 20/20 | 92% | 10% | hyper | 45XY,−7 20/20 | 72% | 5% | HI-1, BM |
42 | Alive |
| 3 | 78 | 0 | M | W | Very high |
800 ×3d | 46XY,+8 (6/20),−7(12/20) | 11% | 6% | hyper | 47XY,+8 1/20 | 2% | 5% | NR | 7 | Deceased |
| 4 | 76 | 1 | M | W | High | 800 × 5d | 47XY, +8 20/20 | 58% | 11% | hyper | 47XY, +8 1/20 | 15% | 11% | NR | 6 | Deceased |
| 5 | 71 | 1 | M | W | Very high |
800 × 5d | hyperploidy in 19/20 metaphases |
44% | 15% | hyper | hyperploidy in 19/20 metaphases |
ND | 10% | NR | 8 | Deceased |
| 6 | 68 | 1 | F | W | AML | 800 × 5d | 47XX,+8 10/20, XXXX 3/20 | 57% | 93% | hypo | 47XX +8 4/20 | 15% | 35% | BM | 10 | Deceased |
| 7 | 67 | 1 | F | H | High | 800 × 5d | 46, XX, t(3;21)(q26.2;q22) 19/20 |
ND | 7% | hypo | 46, XX, t(3;21)(q26.2;q22) 19/20 |
ND | 6% | NR | 19 | Deceased |
| 8 | 75 | 1 | M | B | AML | 800 × 5d | 47XY,+8 ,20/20 | 28% | 94% | hyper | 47, XX +8 18/20 | ND | ND | NR | 1 | Deceased |
| 9 | 56 | 1 | M | W | Very high |
800 × 5d | 47XY,+11, 19/20 | 78% | 11% | hyper | 47XY,+11, 20/20 | 76% | 43% | NR | 11 | Deceased |
| 10 | 77 | 1 | M | W | High | 800 × 5d | 46XY | ND | 8% | hyper | 46XY | ND | 6% | HI-1 | 21 | Alive |
| 11 | 86 | 1 | M | B | High | 800 × 5d | 47XY,+8 20/20 | 92% | 5% | hyper | 46 XY | 22% | 4% | NR | 20 | Alive |
| 12 | 63 | 1 | F | W | Very high |
1000 × 5d | 45XX,−7, 20/20 | 90% | 13% | hyper | 46 XY,−7 (2/15) | 25% | 10% | NR | 7 | Deceased |
| 13 | 77 | 1 | M | W | High | 1000 × 5d | 47 XY, t(3;9)+8 72% | 52% | 9% | hyper | 47 XY, t(3/9), +8, 85% | 40% | 10% | NR | 4 | Alive |
| 14 | 77 | 1 | M | B | Very high |
1000 × 5d | 43 XY, complex with −7q, - 5, −8, +10, −15, −18, −20 and 11 others ; 7/20 |
ND | 7% | hyper | 43 XY, complex with −7q, - 5, −8, +10, −15, −18, −20 and 11 others ; 19/20 |
ND | 6% | NR | 2 | Alive |
Abbreviations: AML, acute myeloid leukemia; B, black; BM, bone marrow response; ECOG, Eastern Cooperative Oncology Group; F, female; FISH, florescence in situ hybridization; H, Hispanic; HI, hematologic improvement; Hyper, hypercellular marrow; hypo, hypocellular arrow; Int-2, intermediate-2; IWG, International Working Group; M, male; ND, not done; UPN, nique patient number; W, white; WPSS, World Health Organization Prognostic Scoring Scale
Clinical toxicity of ON 01910.Na
ON 01910.Na infusion was well tolerated. All serious adverse events (SAEs) and all non-hematologic adverse events (AEs) of grade 2 and higher that were possibly, probably, or definitely attributed to treatment are listed in Table 2. There were no grade 4 AEs. Two patients with histories of atrial fibrillation had episodes of this arrhythmia while on study, both of which were considered unlikely related to treatment, and one patient had hypotension after an erythrocyte transfusion. One patient with trisomy 8 AML and neutropenia died of Clostridium difficile colitis and vancomycin resistant enterococcus bacteremia after receiving two cycles of ON 1910.Na (dose limiting toxicity (DLT) at the 800 mg/m2 for 5 days dose level). One patient developed pharyngitis, and as cultures for streptococcus, herpesvirus, and fungi were negative the cause was assumed to be viral. One patient had a grade 3 skin inflammation in an area previously irradiated for nasopharyngeal carcinoma, and another patient had two episodes of gross hematuria (DLT at the 1000 mg/m2 for 5 days dose level) . A second patient developed bilateral conjunctivitis, as well as inflammation of her distal left fourth and fifth fingers after two cycles of treatment (DLT at the 1000 mg/m2 for 5 days dose level). She was taken off study and her symptoms fully resolved after a short course of prednisone. Grade 2 toxicities included upper respiratory infection, chest pain, irritation at catheter insertion site, and rash. The maximum tolerated dose was therefore defined as 800 mg/m2/day infusion of ON 01910.Na for 5 consecutive days every other week.
Table 2.
Serious Adverse Events, and Grade 2 or Higher Non-hematologic Adverse Events.
| Severe adverse events | |||
|---|---|---|---|
| Category | Event | N | Dose level |
| Cardiovascular | Atrial fibrillation/flutter Hypotension |
2 1 |
800 mg/m2 × 3 days 800 mg/m2 × 3 days |
| Infection | VRE bacteremia, C. diff colitis Pharyngitis |
1 1 |
800 mg/m2 × 5 days 1000 mg/m2 × 5 days |
| Ocular visual/Dermatologic | Conjunctivitis, inflammation of left 4th, 5th digits |
1 | 1000 mg/m2 × 5 days |
| Renal/Genitourinary | Gross hematuria |
2 | 1000 mg/m2 × 5 days |
| Non-hematologic AEs | Grade 2 | Grade 3 | Grade 4 |
|
Cardiovascular Chest pain |
1 |
- |
- |
|
Dermatology/skin Rash Dry skin Radiation recall |
1 1 - |
- - 1 |
- - - |
|
Hemorrhage Epistaxis Gum bleeding |
1 1 |
- - |
- - |
|
Infection/febrile neutropenia Upper respiratory tract Febrile neutropenia Urinary tract Prostatitis Oral thrush |
4 - 1 1 1 |
- 1 - - - |
- - - - - |
|
Metabolic Decreased calcium Elevated LDH Increased bilirubin |
- - - |
1 1 1 |
- - - |
|
Pain Nose |
1 |
- |
- |
|
Vascular IVC site inflammation |
2 |
- |
- |
AE, adverse event;C. diff., clostridium difficile; IVC, intravenous catheter; LDH, lactate dehydrogenase; SAE, serious adverse event; URI, upper respiratory tract infection; VRE, vancomycin resistant entercoccus
MDS patients treated with ON 01910.Na exhibit decreased cyclin D1 expression, reductions in trisomy 8 aneuploidy and blast counts, and improvements in blood counts
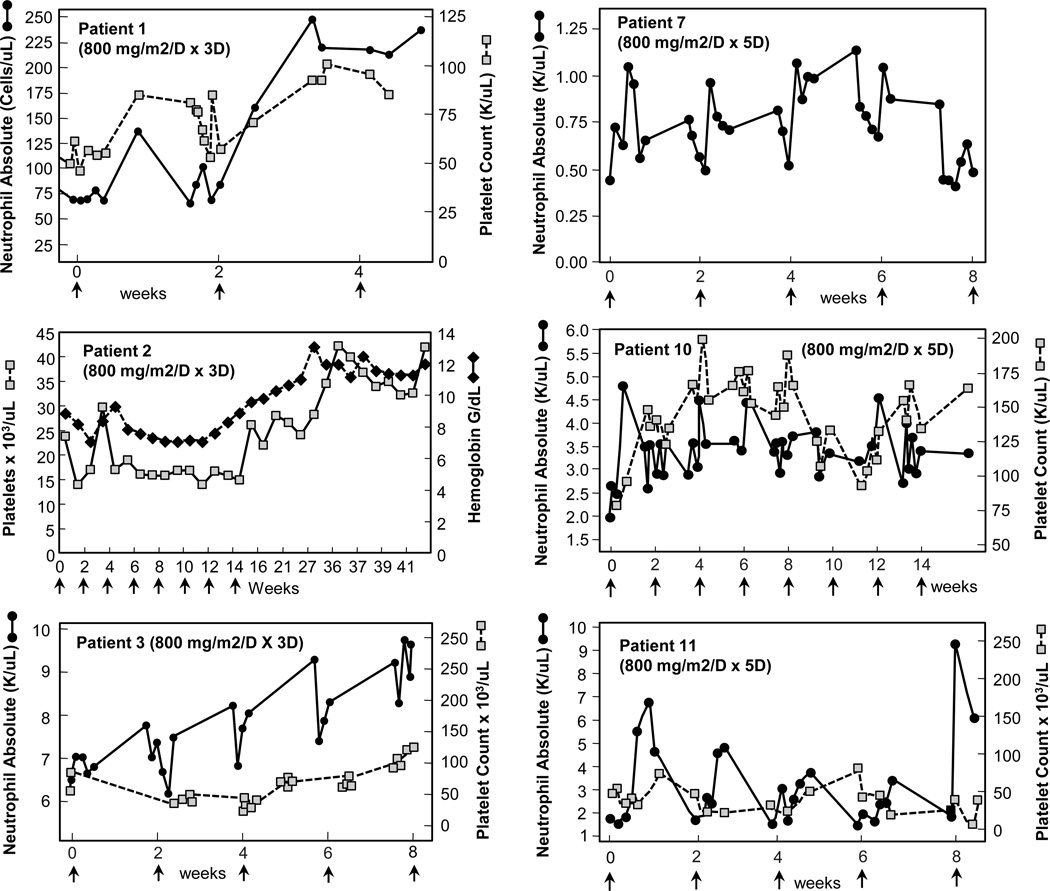
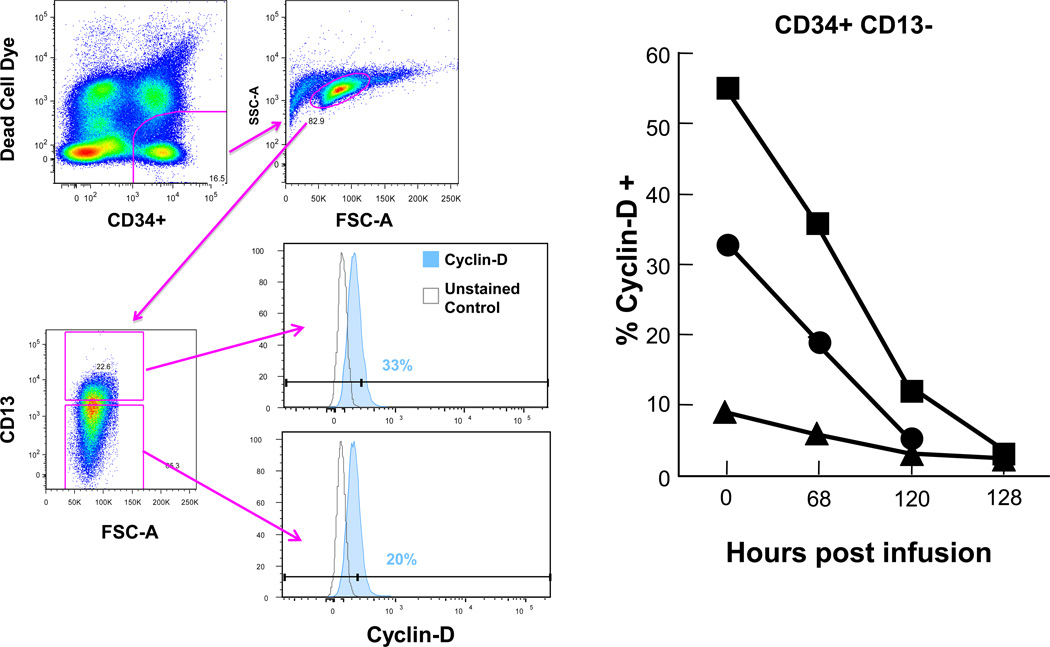
The hematologic, bone marrow, and cytogenetic responses are depicted in Table 1. Three patients treated with ON 01910.Na had ≥ 50% reductions in bone marrow blast percentages (Table 1). MDS aneuploidy was quantitated by FISH as well as metaphase cytogenetics. Four of six patients bearing trisomy 8 clones had reductions in their clone size, including one patient with complex cytogenetics that included trisomy 8 (Table 1). Six patients treated with ON 01910.Na improved their neutrophil and platelet counts relative to pre-treatment baselines (Fig 3), and three patients achieved hematologic improvements by IWG criteria [25] (Table 1). One patient with monosomy 7 who was transfusion-dependent for packed red blood cells completed ON 01910.Na therapy and was transfusion independent for 33 months after treatment. We quantitated cyclin D1 levels in CD34+ peripheral blood cells to evaluate whether ON 01910.Na inhibits cyclin D1 in patients treated systemically. As shown in Fig 4, ON 01910.Na decreased cyclin D1 expression in three of three patients with hematologic improvements tested. Patients who did not achieve hematologic improvements had no changes in cyclin D1 levels (not shown). These results indicate that short- term treatment with ON 01910.Na selectively improves bone marrow blast percentages, hematologic parameters and trisomy 8 clone size in MDS patients.
Figure 3.
Relationship of ON 01910.Na administration and peripheral blood counts. Peripheral blood counts of patients receiving ON 1910.Na who demonstrated increased blood counts coincident with administration of drug. Start of each cycle of infusion indicated by arrows. All counts depicted are without growth factor or transfusion support.
Figure 4.
Decreasing levels of cyclin D1 in peripheral blood CD34+ cells following administration of ON 1910.Na Cyclin D1 was measured by flow cytometry as described in Materials and Methods in peripheral bood CD34+ cells of patients receiving ON1910. Gating strategy is seen at far left: live CD33+ cells were discriminated from dead cells by a CD33 versus BiViD gate. Mononuclear cells were identified by a forward scatter-area (FSC-A) versus side scatter-area (SSC-A) plot. Viable CD33+ cells were then dichotomized on the basis of maturation by a CD13+ versus CD34+ bivariate plot. Cyclin-D1 positives were then identified by a CD33 versus cyclin-D plot, with positive events for cyclin-D1 defined on the basis of a fluorescence-minus-one (FMO) control. Percentages of CD34+ cells expressing cyclin D1 from three patients are seen in the right panel.
Survival and hematologic response duration after ON1910.Na
Five patients remain alive after ON 01910.Na therapy. The median survival time is 10 months (range 1–42 months, supplemental fig 2). Two patients relapsed at 4 and 33 months after treatment, and both remain alive with transfusion support. Three non-responders are alive with stable disease. One patient relapsed two months after treatment and subsequently died of disease progression. Five patients without hematologic responses died after progression to AML, one died after conditioning for HSCT, and two died after undergoing HSCT.
Pharmacokinetic studies
Pharmacokinetic studies performed on patients receiving 800mg/m2 ON 01910.Na for three days (n=3) and five days (n=2) are shown in supplemental figure 3. The steady-state plasma levels of ON 01910.Na were maintained at 3070 ± 675 ng/mL during the course of the infusion. The half-life of the drug was 1.3 ± 0.5 hours, with a clearance of 11.9 ± 2.4 L/hr/m2and an apparent volume of distribution of 20 L/m2. Plasma levels of ON 01910.Na plateaued rapidly when the drug was administered by continuous IV infusion, and remained at steady state throughout the infusion.
Discussion
Styryl sulfonyl compounds such as ON 01910.Na inhibit cyclin D1by negative effects on the PI-3K/Akt/mTOR/eIF4E-BP signaling pathway. They also promote apoptosis through a cytochrome c-dependent process [16], and they suppress translation of c-myc, a gene located on chromosome 8 [13]. Both c-myc and Cyclin D1 expression are increased in trisomy 8 and monosomy 7 cells [7,8] as well as in MDS bone marrows undergoing transformation to AML [11,12]. Up regulation of cyclin D1 increases proliferation of these cells, and may up-regulate survivin, an anti-apoptotic protein [9] “Knock down” of survivin expression by siRNA leads to apoptosis of trisomy 8 MDS cells without affecting normal hematopoiesis [10]. In this study, we applied these observations towards the development of a targeted therapy for patients with high risk MDS and trisomy 8 AML.
Cyclin D1 transcripts were decreased in cells cultured with ON 01910.Na in vitro, and cyclin D1 levels were decreased in patients receiving the drug systemically. We observed decreased numbers of trisomy 8 cells concurrently with decreased blasts when bone marrow was cultured with ON 1910.Na in vitro, and three patients had improvements in bone marrow blast percentages after two cycles of treatment. Six patients had improved blood counts after receiving ON 01910.Na, and three patients achieved hematologic improvements by IWG criteria despite the relatively brief treatment period on this translational study. In one patient, the hematologic improvement was associated with PRBC transfusion independence that was sustained for 33 months. Clinical responses were observed in patients with trisomy 8 or monosomy 7, and occurred even when these chromosomal abnormalities were part of a complex karyotype.
ON 01910.Na showed no deleterious effect on normal hematopoiesis; indeed it improved blood counts in patients with trisomy 8 and monosomy 7. The mechanism(s) for improved hematopoiesis is unclear. Defective hematopoiesis is frequently observed in patients with MDS and leukemia, and may occur with low disease burden as estimated by bone marrow morphology. MDS cells may express neoantigens that elicit an immune response with killing of normal cells via local release of TNFα and IFNγ by activated lymphocytes [28]. Elimination of these cells could potentially decrease migration of activated autoreactive lymphocytes to the marrow, resulting in improved hematopoiesis. One novel mechanism for suppression of normal hematopoiesis in chronic myelogenous leukemia involves the destruction of GCSF and GCSFR by elastase elaborated by the malignant cells [29]. Leukemic cells may also impair hematopoiesis by disrupting progenitor cell bone marrow niches [30]. In a mouse model, neutralization of stem cell factor (SCF) secreted by leukemic cells inhibited CD34+ cell migration into malignant niches resulting in restoration of normal CD34+ cell numbers in leukemic mice [30]. The hematologic improvements after treatment with ON 01910.Na warrant further study for its use in cytopenic MDS patients who cannot withstand standard chemotherapy.
Continuous intravenous (CIV) dosing of ON 01910.Na was well tolerated in MDS patients, with no grade 4 adverse events. Two patients experienced idiopathic inflammatory reactions. One patient developed severe inflammation in areas of previous irradiation for carcinoma of the nasal pharynx, and another had conjunctivitis and inflammation of two distal fingers that required termination of therapy. No infectious etiology was implicated for either condition. The mechanism for these inflammatory reactions is unclear, but may relate to CIV dosing, as such reactions were not observed in a phase I trial using ON 01910.Na in solid tumor patients dosed in a bolus manner [18]. In contrast, patients dosed by CIV did not report grade 2 or higher gastrointestinal side effects or fatigue as was observed with bolus dosing [18].
In a recently published Phase I/II clinical trial [31], 13 higher risk MDS patients unresponsive to hypomethylating therapy were treated with ON01910.Na. Four patients had marrow complete responses among eight with stable disease, associated with good drug tolerance. In a subset of patients, AKT2 phosphorylation was decreased in CD34+ marrow cells from patients responding to therapy but not in those who progressed on therapy. Overall median survival was similar in this study (10 months).
In summary, we show both in vitro and in patients receiving drug that ON 01910.Na inhibits cyclin D1 accumulation, decreases trisomy 8 and monosomy 7 aneuploidy, decreases bone marrow blast counts, and improves hematopoiesis in some patients with MDS. Further studies are needed to define the therapeutic benefit of inhibiting cell cycle control proteins in higher risk MDS patients.
Supplementary Material
ON 01910.Na inhibits cyclin D1 transcript levels in MDS cells in vitro. BMMNCs were grown in the presence of vehicle or increasing concentrations of ON01910.Na, and cyclin D1 expression was measured by quantitative real time PCR as described in Materials and Methods. Results are means +/− SEMs from a representative patient sample performed in triplicate (n=3 independent experiments) expressed as a ratio of cyclin D1/ABL ratio.
Kaplan Meier survival curve is shown for all MDS and AML patients treated with ON 01910.Na.
Pharmacokinetics of ON1910.Na dosed by continuous infusion. Pharmacokinetic studies performed on patients receiving 800mg/m2 for three days (n=3, dark circles) and five days (n=2, grey squares).
Acknowledgments
Funding sources: This research was supported by the Intramural Research Program of the NIH, National Heart, Lung and Blood Institute. Onconova Therapeutics provided ON1910.Na and research funding to Dr. Sloand for correlative laboratory experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at American Society of Hematology Meeting, Abstract 120, December 6, 2009.
Authors’ contributions: M.J.O. designed the research, performed research, analyzed data, wrote the paper. A.S. designed the research, performed the research, analyzed data. B.W. performed research. L.P. performed research, analyzed data. K.L. performed research, analyzed data. Z.T. performed research, analyzed data. X.T. performed statistical analysis. M.K. performed statistical analysis. F.W. analyzed data. A.Y. performed research, analyzed data. I.M. performed research. M.M. performed research, analyzed data. P.S. performed research, analyzed data. J.G. performed research, analyzed data. N.S.Y. obtained funding, analyzed data, edited the paper. E.M.S. designed research, performed research, analyzed data, wrote the paper.
Conflict of interest: Onconova Therapeutics provided ON1910.Na and research funding to Dr. Sloand for correlative laboratory experiments. Francois Wilhelm is Chief Medical Officer and Senior Vice President at Onconova Therapeutics Inc.
References
- 1.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 2.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109:1536–1542. doi: 10.1002/cncr.22570. [DOI] [PubMed] [Google Scholar]
- 3.Nimer SD. Myelodysplastic syndromes. Blood. 2008;111:4841–4851. doi: 10.1182/blood-2007-08-078139. [DOI] [PubMed] [Google Scholar]
- 4.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 6.Ades L, Boehrer S, Prebet T, et al. Efficacy and safety of lenalidomide in intermediate-2 or high-risk myelodysplastic syndromes with 5q deletion: results of a phase 2 study. Blood. 2009;113:3947–3952. doi: 10.1182/blood-2008-08-175778. [DOI] [PubMed] [Google Scholar]
- 7.Haase D, Germing U, Schanz J, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110:4385–4395. doi: 10.1182/blood-2007-03-082404. [DOI] [PubMed] [Google Scholar]
- 8.Schoch C, Kohlmann A, Dugas M, et al. Genomic gains and losses influence expression levels of genes located within the affected regions: a study on acute myeloid leukemias with trisomy 8, 11, or 13, monosomy 7, or deletion 5q. Leukemia. 2005;19:1224–1228. doi: 10.1038/sj.leu.2403810. [DOI] [PubMed] [Google Scholar]
- 9.Chen G, Zeng W, Miyazato A, et al. Distinctive gene expression profiles of CD34 cells from patients with myelodysplastic syndrome characterized by specific chromosomal abnormalities. Blood. 2004;104:4210–4218. doi: 10.1182/blood-2004-01-0103. [DOI] [PubMed] [Google Scholar]
- 10.Sloand EM, Pfannes L, Chen G, et al. CD34 cells from patients with trisomy 8 myelodysplastic syndrome (MDS) express early apoptotic markers but avoid programmed cell death by up-regulation of antiapoptotic proteins. Blood. 2007;109:2399–2405. doi: 10.1182/blood-2006-01-030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saberwal G, Broderick E, Janssen I, et al. Involvement of cyclin D1 and E2F1 in intramedullary apoptosis in myelodysplastic syndromes. J Hematother Stem Cell Res. 2003;12:443–450. doi: 10.1089/152581603322286079. [DOI] [PubMed] [Google Scholar]
- 12.Jaroslav P, Martina H, Jiri S, et al. Expression of cyclins D1, D2, and D3 and Ki-67 in Leukemia. Leuk Lymphoma. 2005;46:1605–1612. doi: 10.1080/10428190500215100. [DOI] [PubMed] [Google Scholar]
- 13.Economopoulou C, Pappa V, Papageorgiou S, et al. Cell cycle and apoptosis regulatory gene expression in the bone marrow of patients with de novo myelodysplastic syndromes (MDS) Ann Hematol. 2010;89:349–358. doi: 10.1007/s00277-009-0835-2. [DOI] [PubMed] [Google Scholar]
- 14.Bortul R, Tazzari PL, Cappellini A, et al. Constitutively active Akt1 protects HL60 leukemia cells from TRAIL-induced apoptosis through a mechanism involving NF-kappaB activation and cFLIP(L) up-regulation. Leukemia. 2003;17:379–389. doi: 10.1038/sj.leu.2402793. [DOI] [PubMed] [Google Scholar]
- 15.Sloand EM, Mainwaring L, Fuhrer M, et al. Preferential suppression of trisomy 8 compared with normal hematopoietic cell growth by autologous lymphocytes in patients with trisomy 8 myelodysplastic syndrome. Blood. 2005;106:841–851. doi: 10.1182/blood-2004-05-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad A, Park IW, Allen H, et al. Styryl sulfonyl compounds inhibit translation of cyclin D1 in mantle cell lymphoma cells. Oncogene. 2009;28:1518–1528. doi: 10.1038/onc.2008.502. [DOI] [PubMed] [Google Scholar]
- 17.Chun A, Cosenza S, Taft D, Maniar M. Preclinical pharmacokinetics and in vitro activity of ON 01910.Na, a novel anti-cancer agent. Cancer Chemother Pharmacol. 2010;9:1022–1029. doi: 10.1007/s00280-009-1022-9. [DOI] [PubMed] [Google Scholar]
- 18.Jimeno A, Li J, Messersmith WA, et al. Phase I study of ON 01910.Na, a novel modulator of the Polo-like kinase 1 pathway, in adult patients with solid tumors. J Clin Oncol. 2008;26:5504–5510. doi: 10.1200/JCO.2008.17.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sloand EM, Yong AS, Ramkissoon S, et al. Granulocyte colony-stimulating factor preferentially stimulates proliferation of monosomy 7 cells bearing the isoform IV receptor. Proc Natl Acad Sci U S A. 2006;103:14483–14488. doi: 10.1073/pnas.0605245103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beillard E, Pallisgaard N, van der Velden VH, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using 'real-time' quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia. 2003;17:2474–2486. doi: 10.1038/sj.leu.2403136. [DOI] [PubMed] [Google Scholar]
- 21.Yong AS, Szydlo RM, Goldman JM, Apperley JF, Melo JV. Molecular profiling of CD34+ cells identifies low expression of CD7, along with high expression of proteinase 3 or elastase, as predictors of longer survival in patients with CML. Blood. 2006;107:205–212. doi: 10.1182/blood-2005-05-2155. [DOI] [PubMed] [Google Scholar]
- 22.Sloand EM, Fuhrer M, Keyvanfar K, et al. Cytogenetic abnormalities in paroxysmal nocturnal haemoglobinuria usually occur in haematopoietic cells that are glycosylphosphatidylinositol-anchored protein (GPI-AP) positive. Br J Haematol. 2003;123:173–176. doi: 10.1046/j.1365-2141.2003.04562.x. [DOI] [PubMed] [Google Scholar]
- 23.Swerdlow SH, editor. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Fourth Edition. Lyon: IARC; 2010. Myelodysplastic Syndromes. [Google Scholar]
- 24.Storer BE. Design and analysis of phase I clinical trials. Biometrics. 1989;45:925–937. [PubMed] [Google Scholar]
- 25.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Zhao M, Jimeno A, et al. Validation and implementation of a liquid chromatography/tandem mass spectrometry assay to quantitate ON 01910.Na, a mitotic progression modulator, in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856:198–204. doi: 10.1016/j.jchromb.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503–3510. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 28.Olnes MJ, Sloand EM. Targeting immune dysregulation in myelodysplastic syndromes. JAMA. 2011;305:814–819. doi: 10.1001/jama.2011.194. [DOI] [PubMed] [Google Scholar]
- 29.El-Ouriaghli F, Sloand E, Mainwaring L, et al. Clonal dominance of chronic myelogenous leukemia is associated with diminished sensitivity to the antiproliferative effects of neutrophil elastase. Blood. 2003;102:3786–3792. doi: 10.1182/blood-2003-03-0861. [DOI] [PubMed] [Google Scholar]
- 30.Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 31.Seetharam M, Fan AC, Tran M, et al. Treatment of Higher Risk Myelodysplastic Syndrome Patients Unresponsive to Hypomethylating Agents with ON 01910.Na. Leukemia Research. 2012;36:98–103. doi: 10.1016/j.leukres.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ON 01910.Na inhibits cyclin D1 transcript levels in MDS cells in vitro. BMMNCs were grown in the presence of vehicle or increasing concentrations of ON01910.Na, and cyclin D1 expression was measured by quantitative real time PCR as described in Materials and Methods. Results are means +/− SEMs from a representative patient sample performed in triplicate (n=3 independent experiments) expressed as a ratio of cyclin D1/ABL ratio.
Kaplan Meier survival curve is shown for all MDS and AML patients treated with ON 01910.Na.
Pharmacokinetics of ON1910.Na dosed by continuous infusion. Pharmacokinetic studies performed on patients receiving 800mg/m2 for three days (n=3, dark circles) and five days (n=2, grey squares).