Abstract
ZnT2 (zinc transporter-2) expression is restricted to tissues with unique zinc requirements such as mammary and prostate glands. We previously determined that ZnT2 plays a major role in zinc export from mammary glands, as women with a mutation in the gene encoding ZnT2 (SLC30A2) had an ~75% reduction in milk zinc concentration. Two distinct human ZnT2 isoforms (~42 and 35 kDa) are predicted to result from alternative splicing of SLC30A2. We examined the localization and function of each ZnT2 isoform, in cells generated to express ZnT2–HA (haemagglutinin) fusion proteins. The 42 kDa isoform was localized primarily to the endosomal/secretory compartment and overexpression resulted in increased zinc vesicularization. In contrast, the 35 kDa isoform is associated with the plasma membrane. Importantly, zinc transport was higher in cells over-expressing each isoform, indicating that both proteins are functional. Endogenous expression of the secretory vesicle-associated ZnT2 isoform predominates in mammary cells and expression is higher in secreting cells, whereas the smaller isoform plays a minor role in zinc export, directly reflecting the secretory function of the mammary gland. Together our data shed further light on the complex integration of cellular zinc transport mechanisms, which may be facilitated by multiple isoforms of specific zinc transporters with unique cellular functions.
Keywords: exocytotic vesicles, lactation, mammary gland, plasma membrane, zinc transporters, ZnT2 variants
INTRODUCTION
Zinc is required for numerous proteins involved in DNA and protein synthesis, mitosis and cell division, serving both a structural and catalytic role, thus strict regulation of cellular Zn transport mechanisms (import, export and intracellular partitioning) is critical for cell survival. Cellular Zn homoeostasis is co-ordinated through the functional activities of numerous Znspecific transporters which are members of two distinct gene families. Members of the SLC39A gene family [Zip1-14 (ZRTL-like import proteins1–14)] are responsible for Zn import into the cytoplasm, either across the plasma membrane or out of intracellular organelles [1]. In contrast, members of the SLC30A gene family [ZnT1-10 (zinc transporter-1–10)] export Zn from the cytoplasm, either across the plasma membrane into the extracellular space or into intracellular organelles [2]. Few studies have focused on ZnT2 and its role in Zn transport, which may reflect the restricted distribution of mammalian ZnT2 to tissues with unique Zn requirements such as mammary gland, prostate, retina and pancreas. Previously, we identified a mis-sense mutation in human SLC30A2 (ZnT2) which substitutes an arginine for a conserved histidine residue in the N-terminal domain. This substitution results in reduced Zn export from the mammary gland into milk during lactation [3], documenting an important role for ZnT2 in the mammary gland; however, the mechanisms through which ZnT2 facilitates Zn export are not understood. Limited characterization suggests that ZnT2 transports Zn into ‘acidic vesicles’ in BHK (baby hamster kidney) cells [4] and into an unknown intracellular compartment(s) in mouse pancreatic cells, small intestine enterocytes [5] and rat mammary epithelial cells [6]. Intracellular localization and a positive correlation between Zn exposure and ZnT2 abundance has led to the suggestion that ZnT2 participates in vesicular Zn sequestration and perhaps export or secretion from these tissues [5]. However, mechanistic information regarding ZnT2 localization and function is lacking.
In silico analysis suggests two distinct human ZnT2 isoforms may be translated; a long isoform (~42 kDa; accession number NM_032513) and a short isoform (~35 kDa; accession number NM_001004434) resulting from alternative splicing of exon 3. We speculate that the resultant ZnT2 isoforms represent distinct protein variants with physiologically relevant Zn transporting functions, as we previously detected two ZnT2 proteins (~52 and ~45 kDa) in rat mammary gland [6]. Concurrent immunohistology demonstrated that ZnT2 is localized to an intracellular compartment within the mammary epithelial cell, proximal to the apical membrane. In contrast, when expressed in rat small intestine, ZnT2 was reported to have a lower molecular mass (~28 kDa) and be localized to the apical membrane [7]. We postulate that tissue-specific expression of ZnT2 isoforms may be responsible for the reported differences in molecular mass and localization. Results from several studies suggest that multiple isoforms of other ZnT proteins may be expressed [6,8,9]. In fact, a recent study by Jackson et al. [10] elegantly illustrated that two ZnT5 isoforms are translated as a result of alternative splicing of the SLC30A5 gene in response to Zn and these proteins are differentially localized.
Our interest in the role of ZnT2 in mammary gland Zn metabolism has led us to characterize the function of ZnT2 and to pose the questions: does endogenous expression of multiple ZnT2 isoforms exist and if so, what is their physiological relevance with respect to mammary gland Zn metabolism and secretion? In the present manuscript, we determined that both the 42 and 35 kDa human ZnT2 isoforms are functional Zn-transporting proteins. Importantly, they are localized to specific sub-cellular compartments; the larger 42 kDa isoform is localized to the secretory compartment, whereas the smaller 35 kDa isoform is associated with the plasma membrane. Both isoforms are endogenously expressed in normal mammary epithelial cells; however, expression of the 35 kDa isoform is very low, whereas, in stark contrast, expression of the 42 kDa isoform is quite abundant. Importantly, treatment of mammary cells with lactogenic hormones increases the abundance of the larger isoform in the secretory compartment without affecting the smaller isoform at the plasma membrane, clearly reflecting the secretory function of the mammary gland.
EXPERIMENTAL
Cell culture
HC11 cells were a gift from Dr Jeffrey Rosen (Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX, U.S.A.) and used with permission of Dr Bernd Groner (Institute for Biomedical Research, Frankfurt, Germany). Cells were routinely maintained in a non-secretory phenotype by culturing in growth medium [RPMI 1640 supplemented with 10% fetal bovine serum, insulin (5 μg/ml; Sigma–Aldrich), EGF (10 ng/ml; Sigma–Aldrich) and gentamycin (50 mg/l; Sigma–Aldrich)]. Cells were differentiated to a secreting phenotype following stimulation with lactogenic hormones (prolactin, 1 μg/ml and cortisol, 1 μM; Sigma–Aldrich) in secretion medium consisting of serum-free RPMI 1640, supplemented with insulin for up to 48 h where indicated.
Generation of plasmid DNA constructs and expression of ZnT2–HA (haemagglutinin) fusion proteins
The long and short isoforms of human SLC30A2 cDNA were obtained from cDNA clones (long isoform: accession number CR592804, Invitrogen; short isoform: accession number BC006251, Open Biosystems) and C-terminally tagged with HA using methods previously described [3] to produce pcDNA3.1-longHA or pcDNA3.1-shortHA. The orientation and fidelity of the insert and incorporation of the HA-tag were confirmed by directed sequencing (University of California Davis, Division of Biological Sciences Automated DNA Sequencing Facility and The Nucleic Acid Facility at Pennsylvania State University, PA, U.S.A.). Large scale plasmid purification was carried out using the Plasmid Midi Kit (Qiagen).
Cells were plated in antibiotic-free Opti-MEM medium (Invitrogen) in 6-well plates (2.5 × 106 cells/well) for cell surface biotinylation or in 24-well plates (6 × 105 cells/well) for Zn export and sub-cellular localization (on glass coverslips) experiments and cultured overnight until ~95% confluent. Cells were transiently transfected with 0.8 μg (24-well plates) or 4 μg (6-well plates) of pcDNA3.1, pcDNA3.1-longHA or pcDNA3.1-shortHA in antibiotic-free Opti-MEM medium using Lipofectamine 2000 (Invitrogen) at a transfection reagent/DNA ratio of 1:2.5 according to manufacturer's specifications for up to 24 h prior to experiments.
Immunoblotting
Cells were washed in PBS, scraped into lysis buffer containing protease inhibitors as previously described [11] and sonicated for 20 s on ice. Cellular debris and nuclei were pelleted by centrifugation at 500 g for 5 min and protein concentration of the post-nuclear supernatant was determined using the Bradford assay. To isolate the crude membrane fraction, the post-nuclear supernatant was centrifuged at 150000 g for 20 min at 4°C. Protein (50–100 μg) was diluted in Laemmli sample buffer containing 100 mM DTT (dithiothrietol) and incubated at 95°C for 5 min. Proteins were separated by electrophoresis, transferred to nitrocellulose for 60 min at 350 mA then immunoblotted with anti-rabbit HA (0.5 μg/ml; Invitrogen) or mouse anti-β-actin (1:10000; Sigma–Aldrich) and detected with horseradish peroxidase-conjugated IgG as previously described [3]. Proteins were visualized by chemiluminescence after exposure to autoradiography film, and relative band density and molecular mass relative to standard molecular mass markers (Amersham Pharmacia) was assessed using the Chemi-doc Gel Quantification System (Bio-Rad).
Sub-cellular localization of HA-tagged and endogenous ZnT2
To determine the sub-cellular localization of HA-tagged ZnT2 isoforms, transfected cells, plated on to glass coverslips, were fixed in phosphate buffered-paraformaldehyde (4%; w/v), pH 7.4, for 10 min, washed in PBS, and permeabilized with Triton X-100 (0.2% in PBS) for 10 min. Non-specific binding was blocked with 5% goat serum/1% bovine serum albumin in PBS for 20 min followed by detection with Alexa Fluor® 488-conjugated anti-rabbit HA (1 μg/ml, Invitrogen) for 45 min at room temperature (25°C), shielded from light. Cells were washed extensively in PBS, coverslips were drained, mounted in ProLong Gold (Invitrogen) and sealed with nail polish. To determine the sub-cellular localization of endogenous ZnT2, cells were plated on to glass coverslips and cultured overnight until ~50–80% confluent in the presence of lactogenic hormones. Cells were fixed and blocked as described above followed by incubation with affinity purified ZnT2 antibody. After extensive washing with PBS, ZnT2 antibody was detected with Alexa Fluor® 488-conjugated anti-rabbit IgG (1 μg/ml; Invitrogen) for 45 min at room temperature shielded from light. Cells were washed, mounted and coverslips were sealed. Sub-cellular co-localization markers were as follows: plasma membrane (mouse anti-mouse pan-cadherin, 1 μg/ml; Abcam) endoplasmic reticulum (mouse anti-human protein disulphide isomerase, 1:100; Abcam); endosomes [mouse anti-human M6PR (mannose 6-phosphate receptor), 5 μg/ml; Abcam]; and exocytotic vesicles (goat anti-human vesicle-associated membrane protein-8, 1:100; Santa Cruz Biotechnology). Co-localization antibodies were detected with Alexa Fluor® 568-conjugated anti-mouse or anti-goat IgG (Invitrogen). Immunofluorescent imaging was performed using an Olympus BX50WI, with UPlanApo 100 × oil lens NA (numerical aperture) 1.35 and digital images were captured sequentially (LaserSharp2000, version 4.1; BioRad) to eliminate potential interference between fluorochromes, and images were saved as .jpg files to maintain image quality. Co-localization analysis was performed with ImageJ Software (version 1.29X, National Institutes of Health, Bethesda, MD, http://rsb.info.gov./ij/) and co-localized pixels were pseudocoloured yellow.
FluoZin-3 fluorimetry assay
To illustrate specific vesicularization and detection of labile Zn pools by FluoZin-3, cells were cultured on glass coverslips and loaded with FluoZin-3 AM (acetoxymethyl ester) (1 μM, Invitrogen) in Opti-MEM containing 0.2% pluoronic acid 127 for 60 min, as recommended by the manufacturer. Cells were rinsed twice with PBS, pH 7.4, and incubated in PBS for 1 h at 25°C with constant shaking. Immunofluorescent imaging was performed using an Olympus BX50WI, with UPlanApo 100 × oil lens, NA 1.35, and digital images were captured sequentially (LaserSharp2000, version 4.1; BioRad). To verify that the ZnT2 long-HA isoform facilitates the accumulation of Zn into vesicles containing labile Zn, cells were plated in antibiotic-free growth medium in 96-well optical bottom plates and cultured until 90–95% confluent. Cells were transiently transfected with 0.2 μg of pcDNA3.1 or pcDNA3.1 ZnT2 long-HA for 24 h prior to experiments. Cells were rinsed with 1 × PBS, pH 7.4, and loaded with FluoZin-3 AM in Opti-MEM containing 0.2% pluoronic acid 127, as recommended by the manufacturer. Cells were rinsed twice with PBS, pH 7.4, and incubated for 1 h at 25°C with constant shaking. Fluorescence of FluoZin-3 (emission, 495 nm; excitation, 516 nm) was measured at 25°C using a FLUOstar OPTIMA plate reader (BMG Labtech) spectrofluorimeter with FLUOstar OPTIMA software version 1.32R2. Cellular protein concentration was determined using the Bradford assay and fluorescence measurements were normalized to total protein concentration.
Cellular zinc retention and secretion
To verify that both ZnT2 isoforms were able to transport Zn, HC11 cells were generated to express either the long- or short-ZnT2-HA-tagged fusion proteins as described above. In some experiments, cells were differentiated to a secretory phenotype as described above where indicated. Cells were loaded with [65Zn] (Oakridge National Laboratory, Oakridge, TN, U.S.A.) for 3 h at 37°C, then washed briefly with cold PBS containing 1 mM EDTA to remove exofacial-bound Zn. Cells were cultured in serum-free medium for up to 210 min as indicated and the cellular retention or cellular export of [65Zn] into the culture medium was quantified in a gamma counter.
Detection of ZnT2 variants by cell surface biotinylation
Sulfo-NHSS (N-hydroxy-sulfosuccinimide) biotin (Pierce) was used to label cell surface proteins to detect HA-tagged ZnT2 isoforms or endogenous ZnT2 variants at the plasma membrane. Cells were cultured until confluent and biotinylated with Sulfo-NHSS biotin (0.5 mg/ml) at room temperature for 30 min. Cells were washed twice with 50 mM glycine, pH 5, followed by three washes with ice-cold PBS, scraped into ice-cold lysis buffer (50 mM Tris/HCl, pH 7.4, 2 mM EDTA and 2 mM EGTA, plus protease inhibitors) and sonicated for 30 s on ice. The crude membrane fraction was pelleted by ultracentrifugation at 63000 rev./min in a Thermosorvall S12DAT2 for 30 min at 4°C and resuspended in lysis buffer containing 0.1 M NaCl, and membranes were solubilized with SDS (final concentration, 0.2%) at 60°C for 5 min and Triton X-100 (final concentration, 1%), then briefly sonicated on ice. Insoluble material was pelleted by ultracentrifugation at 53000 rev./min in a Thermosorvall S12DAT2 for 20 min at 4°C and the supernatant was incubated with 75 μl of a 1:1 slurry of Ultralink-neutravidin beads (Pierce) while rocking at room temperature for 1 h. Beads were pelleted by centrifugation at 1000 g for 1 min and washed four times with PBS plus 1% Triton X-100. Biotinylated proteins were eluted by heating to 95°C in Lammelli buffer containing DTT (100 mM) and immunoblotted with anti-HA or anti-ZnT2 antibodies as described below.
Affinity purification and validation of ZnT2 antibody
The antibody against ZnT2 was generated in rabbits immunized (1 mg peptide/rabbit) against a peptide sequence in the predicted C-terminus of human and mouse ZnT2 (GKFNFHTMTIQIESYSEDMKSCQECQGPSE; Genemed Synthesis, South San Francisco, CA, U.S.A.). Peptides were synthesized with an additional cysteine residue for conjugation to KLH (keyhole limpet haemocyanin) at the C-terminal end. Sequences were verified by amino acid analysis and MS. ZnT2 antibody was affinity purified using the Sulfolink Kit (Pierce) following the manufacturer's recommendations. To validate antibody specificity, immunoblotting was conducted with pre-immunized rabbit serum, then stripped and re-incubated with affinity purified ZnT2 antibody as described below. Additionally, immunospecificity was confirmed by immunoblot of isolated crude membranes following transient siRNA (small interfering RNA)-mediated ZnT2 suppression.
siRNA-mediated gene attenuation
To verify the specificity of our ZnT2 antibody, cells were plated in antibiotic-free Opti-MEM medium in 6-well plates (4 × 105 cells/well) and cultured overnight until 50% confluent. Cells were transfected with 100 pmol of either SLC30A2-specific (sense: 5′-CCGAGCUGCCUUCAUUCAUGUGAUU-3′, antisense: 5′-AAUCACAUGAAUGAAGGCAGCUCGG-3′) or mismatch control siRNA (sense: 5′-CCGCGUCCUUCCUUAUGUAGGAAUU-3′, antisense: 5′-AAUUCCUACAUAAGGAAGGA CGCGG-3′) (Invitrogen Stealth siRNA) in antibiotic-free Opti-MEM medium using LipofectamineTM 2000 at an oligonuceotide/transfection reagent ratio of 25:1 for 16 h prior to experiments.
Deglycosylation by tunicamycin and PGNase (peptide: N-glycosidase)
To determine if ZnT2 contains N-linked mannose and complex oligosaccharides, cells were pre-treated with tunicamycin or crude membrane proteins were treated with PGNase. Briefly, cells were treated overnight in serum-free growth medium containing tunicamycin (5 μg/ml). Cells were washed briefly with PBS and scraped with lysis buffer as previously described in [11]. Crude membrane fraction was prepared following centrifugation at 100000 g for 30 min at 4°C and resuspended in lysis buffer. Crude membrane proteins (50 μg) were diluted in Laemmli sample buffer containing DTT (100 mM) and incubated at 95°C. Proteins were resolved by SDS/PAGE and immunoblotted for ZnT2 as described above. Immunoblotting for the glycosylated protein prolactin receptor (Affinity Bioproducts, 1 μg/ml) was used as a positive control. Alternatively, crude membrane proteins were isolated as described above and treated with PGNase following the manufacturer's instructions (New England Biolabs). Crude membrane proteins (40 μg) were diluted in Laemmli sample buffer containing DTT (100 mM) and incubated at 95°C. Proteins were resolved by SDS/PAGE and immunoblotted for ZnT2 as described above.
Statistical analysis
Results are presented as means ± S.D. from triplicate samples from three independent experiments. Statistical comparisons were performed using the Student's t test on Prism Graph Pad and a significant difference was demonstrated at P < 0.05.
RESULTS
Characterization and functional relevance of human ZnT2 isoforms
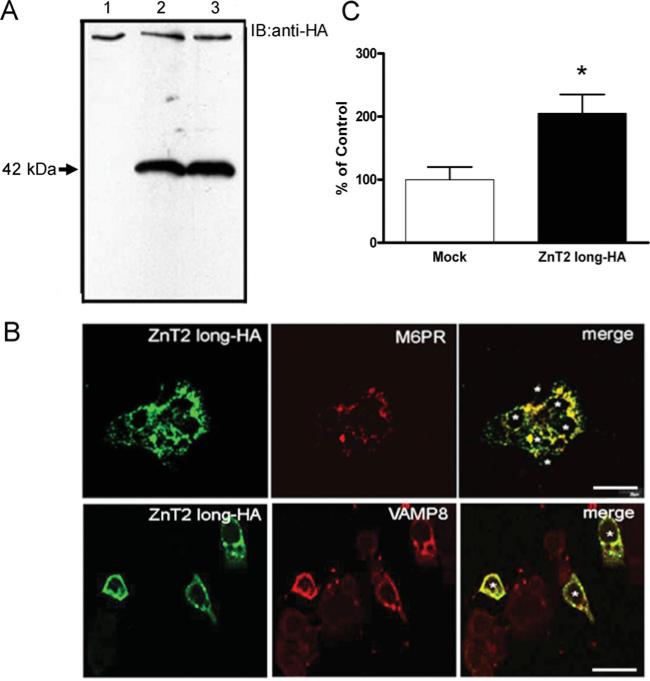
We previously determined that ZnT2 plays a major role in Zn export from the mammary gland in lactating women [3]. Two human ZnT2 isoforms (accession numbers NM_001004434 and NM_032513) resulting from alternative splicing have been putatively identified (Supplementary Figure S1 at http://www.BiochemJ.org/bj/422/bj4220043add.htm). To characterize the function of these two ZnT2 isoforms and determine the role they play in Zn export from the mammary gland, we first constructed ZnT2–HA fusion proteins and determined their subcellular localization in mammary cells by confocal microscopy (Figures 1–3). Previous studies have detected ZnT2 in lysosomes or an unknown vesicular compartment in transfected cell models [4], or at the plasma membrane in the small intestine [7]. Our results illustrated visually that the ZnT2 long–HA isoform was detected in distinct vesicular compartments which specifically co-localized with the M6PR (a endosomal vesicle marker) and VAMP8 (vesicle-associated membrane protein 8), an exocytotic vesicle marker (Figure 1B). Based on these data, we postulated that the ZnT2 long isoform facilitates Zn accumulation into a vesicular compartment destined for secretion from the cell. To examine this possibility, we used the fluorescent dye FluoZin-3, which fluoresces upon binding to labile Zn within intracellular vesicles [12]. Our data clearly demonstrated that cells over-expressing the ZnT2 long-HA isoform had significantly greater fluorescence (~2-fold) and thus enhanced Zn accumulation into a labile Zn pool (Figure 1C).
Figure 1. The ZnT2 long isoform is localized to the secretory compartment in mammary epithelial cells.
(A) Representative immunoblot of crude membrane proteins (100 μg protein/lane) from HC11 cells transfected with pcDNA3.1 (lane 1, mock-transfected control) or cells expressing the 42 kDa ZnT2 HA-fusion protein (lanes 2–3) detected with HA antibody. (B) Confocal micrographs of cells transfected with pcDNA3.1-longHA to express an HA-tagged 42 kDa ZnT2 fusion protein (ZnT2 long–HA) detected with HA antibody and visualized with Alexa Fluor® 488-conjugated anti-mouse IgG. Confocal micrographs illustrate that the long ZnT2 isoform (green) resides in distinct vesicular compartments and co-localizes (merge, yellow) with M6PR (red, endosome marker) and VAMP8 (red, exocytotic vesicle marker). Asterisks represent individual transfected cells (n = 3 and 6 cells expressing ZnT2 long-HA fusion protein). Bar, 20 μm. (C) Accumulation of Zn into vesicles was assessed using FluoZin-3 fluorescence in cells transfected to over-express the long ZnT2 isoform (ZnT2 long–HA) and compared with mock-transfected cells. Data represent mean percentage fluorescence relative to mock-transfected cells ±S.D. (n = 6–8 samples/group). *P < 0.05, a significant effect of over-expressing ZnT2 isoform on Zn vesicularization.
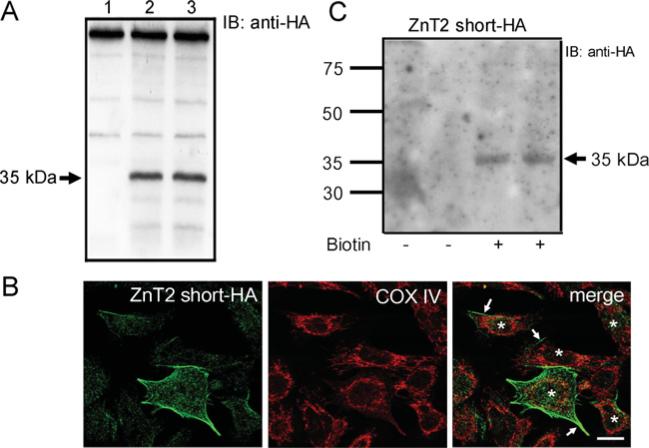
Figure 3. The short ZnT2 isoform is localized to the plasma membrane in mammary epithelial cells.
(A) Representative immunoblot (IB) of crude membrane proteins (100 μg protein/lane) from HC11 cells transfected with pcDNA3.1 (lane 1, mock-transfected control) or cells expressing the 35 kDa ZnT2 short–HA (lanes 2–3) detected with HA antibody. (B) Confocal micrographs of cells transfected with pcDNA3.1-shortHA, to express ZnT2 short–HA, detected with HA antibody and visualized with Alexa Fluor® 488-conjugated anti-mouse IgG. Confocal micrographs illustrate detection of the ZnT2 short-HA at the cell periphery (arrows); COX IV (cytochrome c oxidase subunit IV; red) was utilized for spatial orientation. Asterisks represent individual transfected cells (n = 5 cells expressing ZnT2 short-HA). Bar, 10 μm. (C) Plasma membrane association was verified by immunoblot of cell surface proteins isolated from cells expressing the 35 kDa ZnT2 short-HA, captured with Ultralink Neutravidin, detected with HA antibody. Non-specific binding to avidinated beads was assessed in proteins captured from cells not exposed to biotin (—) and compared with biotinylated cell surface proteins.
Having identified the secretory compartment as the subcellular compartment within which the ZnT2 long isoform is localized, we next aimed to verify its participation in Zn export from mammary cells. To do so, we measured the ability of ZnT2-overexpressing cells to efflux [65Zn] into the culture medium after being pre-loaded with [65Zn] for 3 h. Over-expression of the ZnT2 long-HA isoform in non-secreting cells resulted in a modest but significant (11%) increase in Zn efflux (results not shown). In order to detect a robust effect on Zn efflux resulting from over-expression of the ZnT2 long-HA isoform, the cells were differentiated to a secretory phenotype through hormonal manipulation with the lactogenic hormone prolactin. Activation of secretory mechanisms clearly resulted in the co-localization of the ZnT2 long-HA isoform with the plasma membrane marker pan-cadherin (Figure 2A) and a significant increase in Zn secretion (~25%, P < 0.001) from mammary cells (Figure 2B). Importantly, there was no effect of the HA tag on the Zn-transporting function of ZnT2. Together, these data provide direct evidence that the long ZnT2 isoform resides within the secretory pathway in mammary epithelial cells and facilitates Zn export from secreting mammary cells.
Figure 2. ZnT2 localized to vesicles traffics to the plasma membrane and facilitates Zn secretion.
(A) Confocal micrographs documented co-localization of the long ZnT2 isoform (ZnT2 long-HA; green) with pan-cadherin (red) illustrating localization (yellow) at the plasma membrane in secreting cells. Bar, 20 μm. (B) Expression of ZnT2 long-HA (ZnT2-HA) resulted in significantly higher Zn secretion from mammary cells relative to mock-transfected cells. There was no effect of the HA-tag (ZnT2-no HA) on Zn secretion observed. *P < 0.05, a significant effect of over-expressing the long ZnT2 isoform on Zn secretion.
In contrast, we clearly detected the ZnT2 short-HA isoform at the cell periphery (Figure 3B) in cells generated to express the ZnT2 short-HA isoform (Figure 3A), which was confirmed by immunoblotting biotinylated cell membrane proteins captured on avidinated beads with anti-HA antibody (Figure 3C). To determine if the ZnT2 short isoform functionally transports Zn, [65Zn] export from cells pre-loaded for 3 h was measured over 60 min. Our data indicated that cells expressing the ZnT2 short-HA isoform indeed exported ~21% more Zn (0.54 ± 0.01 pmol Zn/μg of protein) compared with cells transfected with the empty vector (0.43 ± 0.03 pmol Zn/μg of protein, P < 0.0001). As observed with the ZnT2 long-HA isoform, there was no effect of the HA tag on Zn transporting function (results not shown). Taken together, these data clearly indicate that both the long and short ZnT2 isoforms are functionally capable of exporting Zn from the cell and do so through discrete sub-cellular compartments; the long isoform through intracellular vesicular movement, followed by secretion, and the short isoform directly across the cell membrane. It is interesting to note that, in contrast with mammary cells generated to over-express the ZnT2 long isoform, cells transfected to over-express the ZnT2 short isoform were sensitive to over-expression and importantly were not viable for more than 24 h after transfection. We speculate that this reflects the ~4-fold increase in the rate of Zn export from cells over-expressing the ZnT2 short-HA isoform (0.009 pmol Zn/μg protein per min) compared with cells over-expressing the ZnT2 long-HA isoform (0.002 pmol Zn/μg protein per min). This effect seems reasonable given that the ZnT2 short isoform is localized at the plasma membrane, whereas ZnT2 long must traffic to the plasma membrane. Total cell protein was significantly lower (P < 0.0001) in cells over-expressing the short ZnT2 isoform after 24 h (5.3 ± 0.6 μg protein/well) compared with mock-transfected cells (41.8 ± 1.3 μg protein/well). We interpret this data to suggest that over-expression of the short plasma membrane-associated ZnT2 isoform results in hyper-elimination of Zn from mammary cells and decreased cell viability.
Functional relevance of endogenous ZnT2 in mammary cells
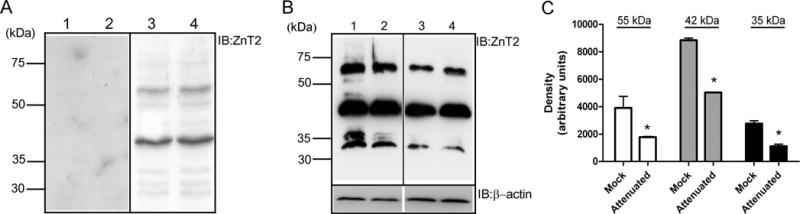
We next aimed to explore the functional relevance of both ZnT2 isoforms on endogenous Zn export in cultured mammary cells. We first confirmed that both ZnT2 isoforms are endogenously expressed in mouse mammary epithelial cells by immunoblotting (Figure 4). The major isoform in the mammary gland was the long 42 kDa isoform which was consistently more abundant (~50-fold greater) than the smaller 35 kDa isoform (often negligible or difficult to detect). Additionally, a third immunoreactive protein with a molecular mass of ~55 kDa was observed which was routinely ~ 10-fold less abundant than the major isoform. The specificity of our ZnT2 antibody with respect to each ZnT2 isoform was verified by immunoblot of crude membrane proteins using pre-immunized rabbit serum in place of ZnT2 antibody (Figure 4A). These immunoblots were then stripped and re-probed with ZnT2 antibody. The fact that all three proteins were ZnT2 gene products was further confirmed by immunoblotting of crude membrane proteins isolated from cells transfected with SLC30A2 siRNA to suppress ZnT2 expression (Figure 4B). The degree of attenuation reflected the relative abundance of each ZnT2 isoform and resulted in an ~60% (55 kDa isoform), ~45% (42 kDa isoform) and ~60% (35 kDa isoform) decrease in abundance, indicating that all three proteins are indeed ZnT2 isoforms. We previously determined that two different ZnT2 proteins are expressed in rat mammary gland tissue (~52 and ~45 kDa) and the largest ZnT2 isoform was a product of glycosylation. To determine if the largest isoform identified in our mammary cell model was also a product of glycosylation, we undertook two different approaches. We pre-treated cells with either tunicamycin or treated cell extracts with PGNase. Neither treatment was effective in robustly altering the molecular mass of the larger 55 kDa ZnT2 protein in isolated mammary cells (Supplementary Figure S2 at http://www.BiochemJ.org/bj/422/bj4220043add.htm), suggesting that glycosylation may be a minimal determinant of the larger molecular mass we observed.
Figure 4. Three specific ZnT2 endogenous isoforms are detected in mammary epithelial cells.
(A) Representative immunoblots (IB) of crude membrane fractions (50 μg protein/lane) isolated by ultracentrifugation from non-secreting mammary epithelial cells. Crude membrane proteins were separated by electrophoresis and ZnT2 was detected by immunoblotting with pre-immune rabbit serum (lanes 1–2) or ZnT2 antibody (lanes 3–4). (B) To verify antibody specificity to each isoform, crude membrane proteins isolated from cells transfected with mismatched control siRNA (lanes 1–2) and cells transfected with SLC30A2-specific siRNA (lanes 3–4) were immunoblotted with ZnT2 antibody. Both methods illustrate specific detection of three ZnT2 proteins at ~55, ~42 and ~35 kDa. Equal loading was verified by immunoblotting for β-actin. (C) Densitometric analysis of immunoblot illustrating a significant effect of siRNA-mediated SLC30A2 attenuation on all three ZnT3 isoforms. * P < 0.05, a significant effect of SLC30A2 attenuation on isoform abundance.
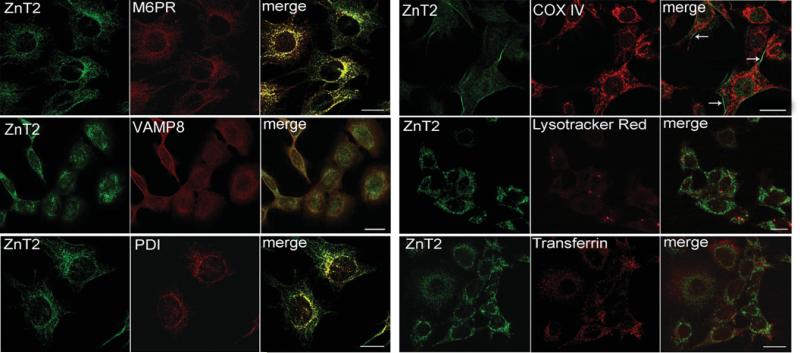
Given the dependence upon ZnT2 for Zn export from the mammary gland during lactation [3], we next aimed to verify the subcellular localization of endogenous ZnT2 in cultured mammary epithelial cells differentiated to a secreting phenotype. Double immunofluorescence confocal imaging confirmed that endogenously expressed ZnT2 was associated to a minimal extent with PDI (protein disulphide isomerase; an endoplasmic reticulum marker) (Figure 5), which is reasonable given that ZnT2 is a multipass transmembrane protein and thus is translated in the endoplasmic reticulum. Importantly, endogenously expressed ZnT2 was co-localized with secretory compartment markers such as M6PR and VAMP8. Together, our results indicate that this compartmentalization reflects the localization of the long ZnT2 isoform. A minimal amount of ZnT2 was occasionally detected at the plasma membrane surface by confocal microscopy. No co-localization between ZnT2 and Lysotracker Red (a lysozome marker) or transferrin (a recycling endosome marker) was observed in secreting mammary epithelial cells. Cell surface biotinylation demonstrated that the endogenously expressed ZnT2 short isoform was clearly associated with the plasma membrane (Figure 6). Similar to what we observed in cells transfected to express the ZnT2 long-HA fusion protein, we detected a minimal amount of the endogenously expressed ZnT2 long isoform but a distinct absence of the 55 kDa ZnT2 isoform at the plasma membrane by cell surface biotinylation (Figure 6). However, as illustrated in Figure 6, detection of the long ZnT2 isoform at the plasma membrane was spurious and not discrete. We interpret this to reflect the transient association of ZnT2-associated secretory vesicles with the plasma membrane.
Figure 5. Confocal microscopy identified specific sub-cellular compartments associated with endogenous ZnT2 isoforms in secreting mammary epithelial cells.
HC11 cells were treated with prolactin and cortisol for 24 h to cause differentiation to a secreting phenotype. Double-immunofluorescence imaging of ZnT2 (green) and sub-cellular markers (red) in secreting mammary epithelial cells illustrating co-localization (merge, yellow) with M6PR (late endosome/secretory vesicle marker), VAMP8 (exocytotic vesicle marker) and to a lesser extent with PDI (endoplasmic reticulum marker). Minimal localization at the cell periphery was occasionally observed (arrows). No co-localization between ZnT2 isoforms and lysozomes (Lysotracker Red) or recycling endosomes (Transferrin) was observed in secreting mammary epithelial cells. Bars, 10 μm.
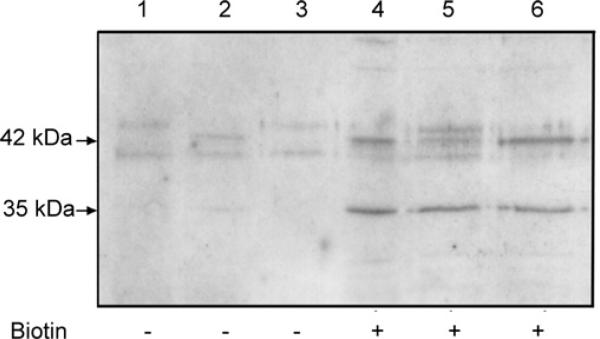
Figure 6. Cell surface biotinylation detects the expression of the endogenous 35 kDa ZnT2 isoform and a minimal amount of the 42 kDa ZnT2 isoform at the plasma membrane in secreting mammary epithelial cells.
Representative immunoblot of plasma membrane-associated proteins captured with Ultralink Neutravidin detected with ZnT2 antibody. Non-specific binding to avidinated beads was assessed in proteins captured from cells not exposed to biotin (—; lanes 1–3) and compared with biotinylated cell surface proteins (+; lanes 4–6). Distinct localization of the 35 kDa isoform at the plasma membrane was routinely detected, whereas spurious and variable abundance of the 42 kDa isoform was observed.
We previously determined that attenuation of endogenous ZnT2 abundance (~75% reduction) in mammary epithelial cells reduced Zn export 2-fold [3]. An interesting question relates to the functional relevance of the expression of multiple ZnT2 isoforms on Zn transport and metabolism in the mammary gland. To investigate the functional relevance of endogenous expression of these ZnT2 isoforms on Zn export from mammary epithelial cells, we first examined the effects of differentiation to a secretory phenotype on abundance of each ZnT2 isoform. Our results illustrated that expression of the short ZnT2 isoform was always very low and often difficult to detect and abundance of the short 35 kDa and largest 55 kDa ZnT2 isoforms did not appear to be phenotype-dependent. In contrast, the long ZnT2 isoform was more abundant in secreting mammary epithelial cells compared with non-secreting cells (Figure 7A). Taken together, our results indicate that abundance of the long ZnT2 isoform is dependent upon a secretory phenotype and further suggest that if the short ZnT2 isoform plays a role in Zn export from mammary cells, it is probably minimal. To explore effects of the increased abundance of the long ZnT2 isoform on vesicular Zn accumulation and Zn export, labile Zn pools were detected with FluoZin-3 in non-secreting and secreting mammary epithelial cells. As illustrated in Figure 7(B), cells differentiated to a secretory phenotype resulted in increased vesicularized Zn pools by confocal imaging and a 2-fold increase in FluoZin-3 fluorescence (P < 0.05). To verify a role in Zn secretion, cells were also loaded with [65Zn] and Zn export over 120 min was measured. Our data clearly demonstrated that Zn export from mammary epithelial cells differentiated to a secretory phenotype was significantly higher (~30%, P < 0.01) compared with non-secreting cells (Figure 7C). Additionally, differentiation to a secretory phenotype resulted in an enhanced rate of Zn secretion (0.042 ± 0.003 pmol Zn/min) compared with non-secreting cells (0.029 ± 0.003 pmol Zn/min, P < 0.05). We speculate that this reflects−increased expression of ZnT2 in combination with an increased rate of the secretory process in general [13]. Collectively, these results confirm that the long 42 kDa ZnT2 isoform resides in secretory vesicles and plays the primary role in Zn secretion from the mammary gland and as such is dependent upon activation to a secretory phenotype in mammary epithelial cells.
Figure 7. Enhanced Zn accumulation and secretion is associated with higher abundance of the vesicular ZnT2 isoform in secreting mammary epithelial cells.
(A) Representative immunoblot of ZnT2 associated with the crude membrane fraction isolated from mammary epithelial cells with a non-secreting and secretory phenotype (100 μg protein/lane; n = 3 samples/phenotype). Immunoblotting illustrated that a secretory phenotype was associated with greater abundance of the vesicular ZnT2 isoform, whereas abundance of the plasma membrane-associated ZnT2 isoform was negligible and not related to phenotype. Relative sample loading was visualized by immunoblotting for β-actin. (B) Zinc accumulation into vesicular pools was detected by confocal microscopy and quantified by fluorimetry in non-secreting and secreting mammary cells. Data represent mean fluorescence/μg of protein ± S.D. *P < 0.05, a significant effect of phenotype on Zn accumulation. (C) Zinc secretion over 120 min was measured in secreting and non-secreting mammary cells pre-loaded with [65Zn] (n = 6 samples/phenotype per time). High-resolution confocal micrographs permit the visualization of increased vesicluarized Zn pools in secreting cells. *P < 0.05, a significant effect of phenotype on Zn secretion.
DISCUSSION
ZnT2 expression is particularly abundant in unique tissues with high Zn requirements such as mammary and prostate glands. We previously identified a mis-sense mutation in human SLC30A2 which resulted in phenotypically low Zn secretion into milk during lactation [3], indicating that ZnT2 plays a major role in Zn export from the mammary gland. The aim of this study was to further characterize the function of ZnT2 in mammary gland Zn metabolism. Our current documentation of two discrete human ZnT2 isoforms which export Zn through two distinct mechanisms in mammary epithelial cells sheds further light on this process. Importantly, our data indicate that Zn secretion from mammary cells is primarily facilitated by a ZnT2 isoform localized primarily within a vesicular compartment, which in this cell-type includes the endosomal/secretory compartment, whereas a minor ZnT2 isoform may export Zn across the plasma membrane. Regulation of secretory vesicle trafficking is mediated by SNARE (soluble-N-ethylamleimide-sensitive factor attachment protein receptor) proteins, which help facilitate membrane fusion [14]. VAMP8 is a vesicular SNARE protein and has been shown to be expressed in secretory tissues such as the lactating mammary gland and prostate [15], thus the co-localization of the long ZnT2 isoform and VAMP8 indicates that the long 42 kDa ZnT2 isoform is associated with the secretory compartment. A key question is, do these ZnT2-associated vesicles contain labile Zn? FluoZin-3 has been shown to be a good indicator of vesicularized, labile Zn pools [12]. Our results clearly revealed that over-expression of the long ZnT2 isoform directly results in the accumulation of labile Zn into a vesicular compartment which is then ultimately destined for secretion. In light of our previous detection of ZnT2 proximal to the apical membrane in lactating mammary glands in vivo, data from this study provides mechanistic evidence that these secretory vesicles traffic to the apical membrane to export Zn into milk. Importantly, Zn secretion through this mechanism is enhanced by stimulation of the secretory process, a key consideration due to the transient activation of the secretory process in this cell type. Evidence from others suggest that abundance of the long ZnT2 isoform is also increased by Zn in BHK cells [4], and pancreatic acinar cells [5], which is likely to be a sequestration mechanism to protect cells from the consequences of excessive Zn exposure and/or toxicity.
In contrast with the abundant expression of the long ZnT2 isoform, abundance of the short ZnT2 isoform is very low in mammary cells, similar to our observations in rat lactating mammary gland [6,16]. This suggests that the short ZnT2 isoform plays a minor role in Zn export from the mammary gland during lactation, as we have determined that this protein is indeed functional. As abundance of this short ZnT2 isoform is low and does not appear to be affected by hormonal differentiation to a secretory phenotype, we presume that the 35 kDa ZnT2 isoform facilitates minimal, constitutive Zn efflux across the plasma membrane [6]. Similarly, Luizzi et al. [7] detected only the smaller ZnT2 variant (~28 kDa) at the apical membrane of rat small intestine, indicating that robust expression of the short ZnT2 isoform may be tissue-specific. The relevance of this observation may reflect the need to quickly export Zn back into the lumen of the small intestine to limit Zn absorption in this non-secretory tissue. It is interesting to note the ZnT2 in the small intestine and mammary gland is associated with or proximal to the apical membrane. This begs the question of the physiological relevance of multiple ZnT2 isoforms which export Zn from mammary cells. Mammary epithelial cells utilize both secretory (facilitated via vesicular trafficking) and efflux (facilitated directly across the plasma membrane) mechanisms to transport milk components in a vectorial manner into the lumen of the mammary gland alveoli [17]. The mammary gland is unusual in that it must export an extraordinary amount of Zn across mammary epithelial cells during lactation (~1–2 mg Zn/day), thus a hormonally-regulated secretory system would permit this large amount of Zn transfer. Results from this study indicate mammary epithelial cells utilize primarily vesicular secretory mechanisms to accumulate Zn into vesicles, allowing for the secretion of copious amounts of Zn into milk. The necessity to express the smaller ZnT2 isoform is curious, and the extremely low abundance may reflect its coincidental expression or a yet unknown facet of Zn metabolism regulation. Expression of a second minor ZnT2 protein of ~55 kDa was endogenously detected by immunoblotting. Our previous data in lactating rat mammary gland similarly identified a ZnT2 protein of ~52 kDa and suggested that this ZnT2 protein is a glycosylated product of the 42 kDa isoform [6]. We were able to recapitulate these observations in our murine mammary epithelial cell model to a limited extent, suggesting that the larger ZnT2 protein may be glycosylated as well. Alternatively, the larger molecular mass of this Zn exporter may result from other post-translational modifications such as polyubiquitination [18], as has been observed for the Zn importer Zip4, or ZnT2 may exist in complex with other proteins, similar to the interaction between ZnT3 and AP3 [19]. Further studies are clearly needed to characterize this protein.
An important question is, how are these ZnT2 isoforms targeted to different sub-cellular compartments? Trafficking of proteins to the plasma membrane is the default pathway for proteins with multiple transmembrane domains. This suggests that the motif that targets the 42 kDa ZnT2 isoform to the secretory pathway probably resides within a cytoplasmic sequence unique to this isoform. The most common motifs are either tyrosine-based signals of the forms NPXY or YXXΦ (where X = any residue, and Φ = large hydrophobic residues) and dileucine-based motifs (LL). In fact, examination of the amino acid sequence of ZnT2 reveals the presence of two dileucine motifs, one at Leu9 and the other at Leu293. Alternative splicing may result in eliminating one or both of these two LL motifs from the cytoplasmic domain and thus direct its targeting away from the secretory compartment. The contribution of these dileucine motifs to sub-cellular trafficking of ZnT2 is currently being evaluated.
Our characterization of two discrete ZnT2 isoforms may help to explain the significantly reduced (75% of normal), but not completely abolished, milk Zn concentration in women with an amino acid substitution (H54R) in ZnT2. For example, the H54R mutation may only significantly affect the major ZnT2 isoform that resides in the secretory compartment allowing for residual Zn secretion via the smaller, plasma membrane-associated ZnT2 isoform. The H54R substitution resides in the N-terminal region and importantly is predicted to be within a cytoplasmic domain of the 42 kDa ZnT2 isoform [3]. Sequence motifs found in the cytoplasmic terminal regions of membrane proteins generally controls their sub-cellular localization and trafficking. Thus the H54R substitution may affect protein localization and/or function of the larger, vesicular ZnT2 isoform, ultimately targeting it for aggresomal degradation [3]. Alternatively, as we have shown that expression of the long ZnT2 isoform is hormonally-dependent, the mutation may result in a major reduction in hormonally-stimulated Zn secretion. On the other hand, the 35 kDa isoform is predicted to result from alternative splicing of exon 3. This splicing event is predicted to eliminate a 49 amino acid region, including the second transmembrane domain and an adjacent cytoplasmic region (TopPred, http://mobyle.pasteur.fr/cgi-bin/portal.py?form=toppred). Thus the topology of the smaller 35 kDa ZnT2 isoform is certainly inherently different from that of the longer 42 kDa isoform, and thus the H54R substitution may not necessarily affect the localization or function of the short ZnT2 isoform or target this short isoform for degradation. Although expression of the short isoform is very low, it is localized to the plasma membrane, thus one would predict that limited Zn efflux directly across the plasma membrane might be maintained. Of course, the contribution of other Zn transporting proteins to milk Zn secretion such as ZnT4 [20] must also be considered.
In summary, results from the present study provide direct and functional evidence that detection of multiple ZnT2 isoforms represent expression of distinct molecular variants. Two of these ZnT2 isoforms probably result from alternative splicing of the SLC30A gene. Our data indicate that the primary isoform in the mammary gland is the long, 42 kDa isoform which we have clearly determined transports Zn into the secretory compartment for eventual efflux from the mammary cell in a hormonally dependent manner. Current studies are underway to determine the molecular regulation of ZnT2 by the lactogenic hormone prolactin to further our understanding of this essential mammalian process.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge the excellent technical expertise of Dr Winyoo Chowanadisai, Jose Zambra, Eskil Eskilsson, Nick McCormick and Young Ah Seo. Moreover, we are grateful to Dr Bo Lönnerdal for his insight and guidance and Dr Colin Duckett, Dr Dennis Winge and Dr David Eide for their critical evaluation and gracious input.
FUNDING
This work was supported by intramural grants from the University of California Davis, Pennsylvania State University and the NIH (National Institues for Health) [grant number HD058614 (to S. L. K.)].
Abbreviations used
- AM
acetoxymethyl ester
- BHK cell
baby hamster kidney cell
- DTT
dithiothrietol
- HA
haemagglutinin
- M6PR
mannose 6-phosphate receptor
- NA
numerical aperture
- NHSS
N-hydroxy-sulfosuccinimide
- PDI
protein disulphide isomerase
- PGNase
peptide: N-glycosidase
- siRNA
small interfering RNA
- SNARE
soluble-N-ethylamleimide-sensitive factor attachment protein receptor
- VAMP8
vesicle-associated membrane protein 8
- Zip
ZRTL-like import protein
- ZnT
zinc transporter
Footnotes
AUTHOR CONTRIBUTION
Veronica Lopez and Shannon Kelleher were equally responsible for experimental design, data collection and interpretation.
REFERENCES
- 1.Eide DJ. The SLC39 family of metal ion transporters. Pflugers Arch. 2004;447:796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- 2.Palmiter RD, Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. 2004;447:744–751. doi: 10.1007/s00424-003-1070-7. [DOI] [PubMed] [Google Scholar]
- 3.Chowanadisai W, Lönnerdal B, Kelleher SL. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J. Biol. Chem. 2006;281:39699–39707. doi: 10.1074/jbc.M605821200. [DOI] [PubMed] [Google Scholar]
- 4.Palmiter RD, Cole TB, Findley SD. ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration. EMBO J. 1996;15:1784–1791. [PMC free article] [PubMed] [Google Scholar]
- 5.Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc. Natl. Acad. Sci. U.S.A. 2004;101:14355–14360. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelleher SL, Lönnerdal B. Zn transporter levels and localization change throughout lactation in rat mammary gland and are regulated by Zn in mammary cells. J. Nutr. 2003;133:3378–3385. doi: 10.1093/jn/133.11.3378. [DOI] [PubMed] [Google Scholar]
- 7.Liuzzi JP, Bobo JA, Cui L, McMahon RJ, Cousins RJ. Zinc transporters 1, 2 and 4 are differentially expressed and localized in rats during pregnancy and lactation. J. Nutr. 2003;133:342–351. doi: 10.1093/jn/133.2.342. [DOI] [PubMed] [Google Scholar]
- 8.Cragg RA, Christie GR, Phillips SR, Russi RM, Kury S, Mathers JC, Taylor PM, Ford D. A novel zinc-regulated human zinc transporter, hZTL1, is localized to the enterocyte apical membrane. J. Biol. Chem. 2002;277:22789–22797. doi: 10.1074/jbc.M200577200. [DOI] [PubMed] [Google Scholar]
- 9.McMahon RJ, Cousins RJ. Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4841–4846. doi: 10.1073/pnas.95.9.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson KA, Helston RM, McKay JA, O'Neill ED, Mathers JC, Ford D. Splice variants of the human zinc transporter ZnT5 (SLC30A5) are differentially localized and regulated by zinc through transcription and mRNA stability. J. Biol. Chem. 2007;282:10423–10431. doi: 10.1074/jbc.M610535200. [DOI] [PubMed] [Google Scholar]
- 11.Kelleher SL, Lönnerdal B. Zip3 plays a major role in zinc uptake into mammary epithelial cells and is regulated by prolactin. Am. J. Physiol. Cell Physiol. 2005;288:C1042–C1047. doi: 10.1152/ajpcell.00471.2004. [DOI] [PubMed] [Google Scholar]
- 12.Muylle FA, Adriaensen D, De Coen W, Timmermans JP, Blust R. Tracing of labile zinc in live fish hepatocytes using FluoZin-3. Biometals. 2006;19:437–450. doi: 10.1007/s10534-005-4576-y. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Chiu CT, Nakamura T, Walker AM, Petridou B, Trousdale MD, Hamm-Alvarez SF, Schechter JE, Mircheff AK. Elevated prolactin redirects secretory vesicle traffic in rabbit lacrimal acinar cells. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1122–E1134. doi: 10.1152/ajpendo.00381.2006. [DOI] [PubMed] [Google Scholar]
- 14.Wong SH, Zhang T, Xu Y, Subramaniam VN, Griffiths G, Hong W. Endobrevin, a novel synaptobrevin/VAMP-like protein preferentially associated with the early endosome. Mol. Biol. Cell. 1998;9:1549–1563. doi: 10.1091/mbc.9.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang CC, Shi H, Guo K, Ng CP, Li J, Gan BQ, Chien Liew H, Leinonen J, Rajaniemi H, Zhou ZH, et al. VAMP8/endobrevin as a general vesicular SNARE for regulated exocytosis of the exocrine system. Mol. Biol. Cell. 2007;18:1056–1063. doi: 10.1091/mbc.E06-10-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelleher SL, Lönnerdal B. Zinc transporters in the rat mammary gland respond to marginal zinc and vitamin A intakes during lactation. J. Nutr. 2002;132:3280–3285. doi: 10.1093/jn/132.11.3280. [DOI] [PubMed] [Google Scholar]
- 17.McManaman JL, Reyland ME, Thrower EC. Secretion and fluid transport mechanisms in the mammary gland: comparisons with the exocrine pancreas and the salivary gland. J. Mammary Gland Biol. Neoplasia. 2006;11:249–268. doi: 10.1007/s10911-006-9031-3. [DOI] [PubMed] [Google Scholar]
- 18.Mao X, Kim BE, Wang F, Eide DJ, Petris MJ. A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J. Biol. Chem. 2007;282:6992–7000. doi: 10.1074/jbc.M610552200. [DOI] [PubMed] [Google Scholar]
- 19.Salazar G, Love R, Werner E, Doucette MM, Cheng S, Levey A, Faundez V. The zinc transporter ZnT3 interacts with AP-3 and it is preferentially targeted to a distinct synaptic vesicle subpopulation. Mol. Biol. Cell. 2004;15:575–587. doi: 10.1091/mbc.E03-06-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L, Gitschier J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat. Genet. 1997;17:292–297. doi: 10.1038/ng1197-292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.