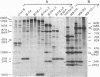
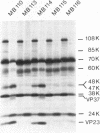
Abstract
A series of specific deletion mutants derived from a full-length cDNA clone of cowpea mosaic virus (CPMV) B RNA was constructed with the aim to study the role of viral proteins in the proteolytic processing of the primary translation products. For the same purpose cDNA clones were constructed having sequences derived from both M and B RNA of CPMV. In vitro transcripts prepared from these clones with T7 RNA polymerase, were efficiently translated in rabbit reticulocyte lysates. The translation products obtained were processed in the lysate by specific proteolytic cleavages into smaller products, which made it possible to study subsequently the effect of the various mutations on this process. The results obtained indicate that the B RNA-encoded 24K polypeptide represents a protease responsible for all cleavages in the polyproteins produced by both CPMV B and M RNA. For efficient cleavage of the glutamine-methionine site in the M RNA encoded polyprotein the presence of a second B RNA encoded protein, the 32K polypeptide, is essential, although the 32K polypeptide itself does not have proteolytic activity. A number of cleavage-site mutants were constructed in which the coding sequence for the glutamine-glycine cleavage site between the two capsid proteins was changed. Subsequent in vitro transcription and translation of these cleavage site mutants show that a correct dipeptide sequence is a prerequisite for efficient cleavage but that the folding of the polypeptide chain also plays an important role in the formation of a cleavage site.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubert S. D., Bruening G., Najarian R. C. Protein bound to the genome RNAs of cowpea mosaic virus. Eur J Biochem. 1978 Dec 1;92(1):45–51. doi: 10.1111/j.1432-1033.1978.tb12721.x. [DOI] [PubMed] [Google Scholar]
- Franssen H., Goldbach R., Broekhuijsen M., Moerman M., van Kammen A. Expression of Middle-Component RNA of Cowpea Mosaic Virus: In Vitro Generation of a Precursor to Both Capsid Proteins by a Bottom-Component RNA-Encoded Protease from Infected Cells. J Virol. 1982 Jan;41(1):8–17. doi: 10.1128/jvi.41.1.8-17.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H., Moerman M., Rezelman G., Goldbach R. Evidence That the 32,000-Dalton Protein Encoded by Bottom-Component RNA of Cowpea Mosaic Virus is a Proteolytic Processing Enzyme. J Virol. 1984 Apr;50(1):183–190. doi: 10.1128/jvi.50.1.183-190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach R. W., Schilthuis J. G., Rezelman G. Comparison of in vivo and in vitro translation of cowpea mosaic virus RNAs. Biochem Biophys Res Commun. 1981 Mar 16;99(1):89–94. doi: 10.1016/0006-291x(81)91716-2. [DOI] [PubMed] [Google Scholar]
- Goldbach R., Rezelman G. Orientation of the cleavage map of the 200-kilodalton polypeptide encoded by the bottom-component RNA of cowpea mosaic virus. J Virol. 1983 May;46(2):614–619. doi: 10.1128/jvi.46.2.614-619.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonossoff G. P., Shanks M. The nucleotide sequence of cowpea mosaic virus B RNA. EMBO J. 1983;2(12):2253–2258. doi: 10.1002/j.1460-2075.1983.tb01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Rezelman G., Goldbach R., Van Kammen A. Expression of bottom component RNA of cowpea mosaic virus in cowpea protoplasts. J Virol. 1980 Nov;36(2):366–373. doi: 10.1128/jvi.36.2.366-373.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Rottier P., Davies J. W., Zabel P., Van Kammen A. A protein linked to the 5' termini of both RNA components of the cowpea mosaic virus genome. Nucleic Acids Res. 1978 Dec;5(12):4505–4522. doi: 10.1093/nar/5.12.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Renz M. An optimized freeze-squeeze method for the recovery of DNA fragments from agarose gels. Anal Biochem. 1983 Jul 1;132(1):14–19. doi: 10.1016/0003-2697(83)90419-0. [DOI] [PubMed] [Google Scholar]
- Verver J., Goldbach R., Garcia J. A., Vos P. In vitro expression of a full-length DNA copy of cowpea mosaic virus B RNA: identification of the B RNA encoded 24-kd protein as a viral protease. EMBO J. 1987 Mar;6(3):549–554. doi: 10.1002/j.1460-2075.1987.tb04789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos P., Verver J., van Wezenbeek P., van Kammen A., Goldbach R. Study of the genetic organisation of a plant viral RNA genome by in vitro expression of a full-length DNA copy. EMBO J. 1984 Dec 20;3(13):3049–3053. doi: 10.1002/j.1460-2075.1984.tb02256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellink J., Rezelman G., Goldbach R., Beyreuther K. Determination of the proteolytic processing sites in the polyprotein encoded by the bottom-component RNA of cowpea mosaic virus. J Virol. 1986 Jul;59(1):50–58. doi: 10.1128/jvi.59.1.50-58.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Manna M. M., Bruening G. Polyadenylate sequences in the ribonucleic acids of cowpea mosaic virus. Virology. 1973 Nov;56(1):198–206. doi: 10.1016/0042-6822(73)90299-7. [DOI] [PubMed] [Google Scholar]
- van Wezenbeek P., Verver J., Harmsen J., Vos P., van Kammen A. Primary structure and gene organization of the middle-component RNA of cowpea mosaic virus. EMBO J. 1983;2(6):941–946. doi: 10.1002/j.1460-2075.1983.tb01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]