Abstract
The development of tasks measuring behaviors specific to the three major symptom categories for autism makes it possible to differentiate mouse models of autism spectrum disorders (ASD) in terms of changes in these specific categories. Prior studies indicate that BTBR T+tf/J mice, the strain that has been evaluated most extensively, show autism-relevant changes in all three symptom categories; reciprocal social interactions; communication; and repetitive, ritualized behaviors. This report reviews the behaviors of oxytocin receptor (Oxtr) and Mecp2308/Y wild-type (WT) and knockout (KO) mice, in a number of tests specifically designed to provide information on behaviors that may show functional parallels to the core symptoms of ASD. Oxtr KO mice show robust decreases in reciprocal social interactions, and reduced levels of communication, but no changes in repetitive, ritualized behaviors; whereas Mecp2308/Y KO mice show a slight but consistent enhancement of social behavior and communication, and no changes in repetitive, ritualized behaviors. This data base, although small, strongly indicates that mouse models can sort the diagnostic symptoms of autism, and suggests that biological and physiological analyses of these strains may be capable of providing differential information on the brain systems involved in particular symptoms of this disorder. Profiles of behavioral changes in other mouse models of ASD should provide additional specificity in the search for biomarkers associated with particular ASD symptoms and symptom clusters.
Keywords: Autism, Oxytocin Receptor, Mecp2, Social Behavior, Mouse Models
1. Introduction
Autism spectrum disorders (ASD) are a group of highly prevalent and severe neurodevelopmental conditions defined by occurrence of three main symptom clusters [1,2]. Abnormal reciprocal social interactions include low levels of social approach, and other impairments such as deficits in non-verbal expression (e.g. deficits in eye-to-eye gaze) and failure to develop peer relationships [3,4]. Deficits in communication include delayed development of speech and poor expressive language [5,6]. Stereotyped, repetitive, and ritualized behaviors, resistance to change a learned response, obsessions, and other persistent behavioral patterns are components of the third diagnostic indicator of ASD [7]. Although there is considerable evidence for a strong genetic component in the etiology of ASD [8,9], this seems to be polygenic, with hundreds of contributing loci [10,11]. A range of environmental factors, including fetal exposure to teratogenic compounds or viral infections, also appear to influence rates of ASD [12–14], and the predominant current view of the disorder is that genetic/environmental interactions are implicated in its occurrence [15,16].
The primary diagnostic indices of ASD are abnormal behaviors, as no consistent biochemical or physiological markers have yet been identified [17–19]. Therefore, suitable animal models for ASD require a strong relationship to the types of social deficits that are considered the core symptoms of this group of disorders, including impairments in social interactions and deficiencies in other functional domains [20–22]. Mice are especially useful for analysis of genetic disorders, given the many genetic resources available for this species, and the high level of homology between the mouse and human genomes [23–25]. There are two basic approaches in genetics for ASD mouse model research. Reverse genetic approaches involve elucidation of the behavioral phenotypes of specific mouse strains with targeted mutations of candidate genes for ASD (see for instance 26–29). Forward genetic approaches identify inbred mouse strains with phenotypes relevant to ASD, and explore mechanisms responsible for these phenotypes (see for instance 30–32). A wealth of studies using the latter approaches have identified several inbred mouse strains displaying low levels of sociability, an impairment that is analogous to the first diagnostic symptom of ASD [33–35]. Of particular interest is BTBR T+tf/J (BTBR), an inbred strain that consistently displays behaviors relevant to all three core symptom domains of ASD. Compared to the commonly used C57BL/6J (B6) strain, BTBR mice exhibit lower reciprocal social interactions, reduced social approach and impaired juvenile play [36–38], emit fewer ultrasonic vocalizations in several social settings [39,40], and display higher levels of repetitive self-grooming throughout developmental stages [37,38,41]. Normal scores on measures of general health, motor functions, and sensory abilities [31,37] corroborate an interpretation of remarkably specific autism-relevant abnormalities in BTBR.
Recently, much attention has been given to systematic behavioral analyses of mouse models with targeted mutations of various candidate genes potentially implicated in ASD [42,43]. Prior evidence suggests that the complex set of genes associated with ASD can be categorized according to their involvement in shaping the development and function of regions and pathways affected in such disorders [44]. Synaptic development genes implicated in ASD include neurexins, neuroligins, shanks, reelin, integrins, cadherins and contactins [45–49]. Signaling, transcription, methylation, and neurotrophic genes are mainly represented by methyl-CpG-binding protein 2 (MECP2), engrailed 2 (EN2), fragile X mental retardation 1 (FMR1), tuberous sclerosis 2 (TSC2), and brain-derived neurotrophic factor (BDNF) [44,50–53]. Neurotransmission genes include oxytocin (OXTR) and vasopressin (AVPR) receptor, serotonin transporter (5-HTT), and GABA receptor genes [54–57]. This report reviews the behaviors of Oxtr and Mecp2308/Y wild-type (WT) and knockout (KO) mice, in comparison to the extensively described behavioral changes of BTBR mice [58], in a number of tests specifically designed to provide information on behaviors that may show functional parallels to the core symptoms of ASD. These candidate genes were selected taking into account their prior implications in ASD, neurodevelopmental diseases comorbid with ASD, or by having a known role in the regulation of rodent social behaviors. The assays used were developed to reveal subtle and overt distinctions in sociality, communication, and repetitive or stereotypical behavioral patterns, and have proven to be relevant variables for mouse models of neurodevelopmental disorders [31,59–63].
2. General Procedures
2.1. Subjects
WT and KO mice from Oxtr and Mecp2308/Y strains were bred from a B6-backcrossed stock obtained from Dr. Scott Young’s Laboratory (B6.129SJL-Oxtrtm1.1Wsy/J; Bethesda, MD) and The Jackson Laboratory (B6.129S-Mecp2tm1Hzo/J; Bar Harbor, ME), respectively. Mutant mouse genotype was determined according to PCR parameters obtained from Dr. Scott Young and The Jackson laboratories with purified DNA collected from tail biopsy after weaning at PND 25. Whole body Oxtr KO mice (Oxtr−/−) were generated from crosses of heterozygous progenies with one Oxtr allele inactivated (Oxtr+/−), as previously described in [64]. Mecp2308/Y is an X-linked gene, and therefore only WT (y/+) and KO (y/−) males were obtained and compared in behavioral testing. Stimulus mice used for social behavior tests were male adult CD-1 mice bred in-house from stock obtained from Charles River Labs (Wilmington, MA), and B6 mice bred from stock obtained from The Jackson Laboratory. All procedures were conducted in accordance with protocols approved by the University of Hawaii Laboratory Animal Service Institutional Animal Care and Use Committee.
2.2. Group size
Except as noted, each study involved WT and KO mice from Oxtr and Mecp2308/Y strains in groups of 9 or more subjects per genotype. All subjects were male adults, with a minimum age of 70 days.
2.3. Behavior ratings
All procedures were videotaped and later rated by experimenters who had been trained to a minimum 90% agreement on each test to be scored. Experimenters and scorers were blind to genotype conditions.
2.4. Behavioral test schedule
Groups of mice were subjected to a series of behavioral tests in a determined sequence: Visible Burrow System, Three-chambered Social Approach Test, Self-Grooming, Social Proximity, Repetitive Novel Object Contact Task, and Urinary Scent Marking. Interval between tests was of 24 h, except for the Scent Marking analysis for which mice were individually housed for at least 7 days before testing. Unless otherwise noted, behavioral tests were performed under ambient fluorescent lighting during the light phase of the light/dark cycle from 9:00 am to 5:00 pm. Temperature (22±1°C) and humidity (50–70%) were controlled in the experimental room.
3. Studies of social behaviors in Mecp2308/Y and Oxtr KO mice [65,66]
3.1. Visible Burrow System
3.1.1. Apparatus and procedures
Visible burrow systems (VBSs), constructed as previously described [59], are bins containing about 0.4 m2 of floor space with an open area to which three chambers are connected by tunnels. Mice maintained in these habitats tend to sleep in the chambers during an inactive period (lights on) and to utilize both the chambers and the open area, showing a range of active social, consummatory, and self-grooming behaviors during the active period (lights off). Groups comprising 3 age-matched adult male mice of the same genotype, either WT or KO, from Oxtr and Mecp2308/Y strains, all naïve, were placed in each VBS. Subjects in each group were unfamiliar to each other prior to group formation. A total of 24 h of video-recordings, 4 h each on days 1, 2 and 3 in the dark period and days 2, 3 and 4 in the light period, were made and behaviors were analyzed by time sampling, with a 30-s sample being taken every 10 min for each mouse. Individual mice had been marked with crème-based hair bleach prior to colony formation, and behaviors were entered for each animal separately. Behaviors analyzed included: huddle: lying in contact with another animal for more than 10 s of the 30-s time sample; allo-grooming: lick or rub with paws, another animal; self-grooming: lick or rub self; approach to the front or to the back of another animal was defined in terms of a line bisecting the approached mouse, perpendicular to the long axis of its body; flight: rapid locomotion away from an approaching animal; and chase/follow: rapid locomotion toward another animal, or a slow approach toward an animal that was moving away. VBS data displayed in Figs. 1 and 2 represent an average of 24 h collected over the four days of VBS testing, from both dark and light periods. Behavioral data obtained in the VBS were analyzed by two-way analyses of variance (ANOVA), with strain (Oxtr or Mecp2308/Y) as between-subjects factor, and genotype (WT or KO) as within group factor. Post hoc comparisons were made using the Newman-Keuls test.
Fig. 1.
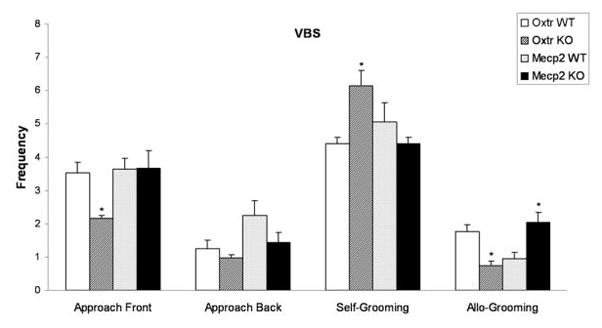
Frequencies (mean ± S.E.M.) of approaches to the front, approaches to the back, self-grooming, and allo-grooming behaviors in the VBS, for WT and KO mice from Oxtr (n=12 for each group) and Mecp2308/Y (n=9 for each group) strains; *p<0.05 compared to WT by Newman-Keuls test.
Fig. 2.
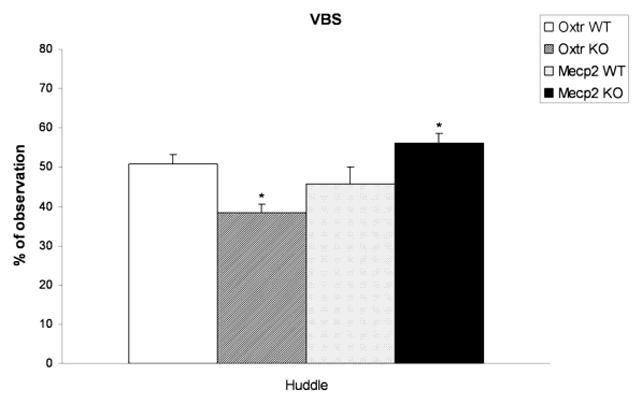
Frequencies (mean ± S.E.M.) of huddling behavior in the VBS, for WT and KO mice from Oxtr (n=12 for each group) and Mecp2308/Y (n=9 for each group) strains; *p<0.05 compared to WT by Newman-Keuls test.
3.1.2. Results
Table 1 provides results of statistical tests for measures of the VBS analysis, by strain, genotype, along with interaction effects.
Table 1.
Summary of the statistic results obtained in the VBS, Social Proximity, and Scent-Marking analyses
| ANOVA results
|
|||
|---|---|---|---|
| Main effect
| |||
| Strain | Genotype | Strain x Genotype | |
| VBS | |||
| Approach front | F(1,38)= 6.27; p<0.05* | F(1,38)= 4.26; p<0.05* | F(1,38)= 4.79; p<0.05* |
| Approach back | F(1,38)= 6.65; p<0.05* | F(1,38)= 3.58; p=0.065 n.s. | F(1,38)= 0.83; p=0.36 n.s. |
| Self- grooming | F(1,38)= 1.92; p=0.17 n.s. | F(1,38)= 1.96; p=0.16 n.s. | F(1,38)= 9.42; p<0.01** |
| Allo-grooming | F(1,38)= 1.25; p=0.26 n.s. | F(1,38)= 0.01; p=0.90 n.s. | F(1,38)= 23.71; p<0.01** |
| Huddle | F(1,38)= 5.04; p<0.05* | F(1,38)= 0.14; p=0.71 n.s. | F(1,38)= 16.54; p<0.01** |
| Social Proximity | |||
| Nose to Nose | F(1,43)= 10.50; p<0.01** | F(1,43)= 3.97; p=0.052 n.s. | F(1,43)= 4.74; p<0.05* |
| Nose to Face | F(1,43)= 0.63; p=0.42 n.s. | F(1,43)= 2.76; p=0.10 n.s. | F(1,43)= 7.00; p<0.05* |
| Nose to Anus | F(1,43)= 24.35; p<0.01** | F(1,43)= 2.45; p=0.12 n.s. | F(1,43)= 3.22; p=0.07 n.s. |
| Crawl Over | F(1,43)= 19.01; p<0.01** | F(1,43)= 4.63; p<0.05* | F(1,43)= 5.57; p<0.05* |
| Crawl Under | F(1,43)= 18.56; p<0.01** | F(1,43)= 0.69; p=0.40 n.s. | F(1,43)= 3.45; p=0.06 n.s. |
| Upright | F(1,43)= 14.07; p<0.01** | F(1,43)= 0.36; p=0.54 n.s. | F(1,43)= 0.36; p=0.54 n.s. |
| Scent-Marking | |||
| Baseline | F(1,43)= 0.28; p=0.59 n.s. | F(1,43)= 0.91; p=0.34 n.s. | F(1,43)= 6.97; p<0.05* |
| CD-1 | F(1,43)= 4.96; p<0.05* | F(1,43)= 0.31; p=0.57 n.s. | F(1,43)= 5.84; p<0.05* |
p<0.05;
p<0.01
3.1.2.1. Front and Back Approach, Flight, and Chase/Follow
Fig. 1 presents frontal and back approach measures for WT and KO mice from Oxtr and Mecp2308/Y strains. The analysis revealed a significant effect of genotype and strain for Approach Front; frequencies of this behavior were significantly reduced in Oxtr KO mice, when compared to their WT counterparts. No significant differences were found in the frequencies of Approach Back for Oxtr KO mice, while Mecp2308/Y KO mice showed reduced rates of this measure, as compared to WT. Flight and Chase/Follow (not shown) were both significantly reduced in Oxtr KO mice, as compared to WT; Mecp2308/Y KO mice did not differ from their WT littermates in the rates of these behaviors. BTBR mice showed significant reductions in the frequencies of approach (front and back), flight and chase/follow behaviors in the VBS, as compared to B6 [63]. Oxtr KO mice displayed reduced rates in three of the latter four measures, when compared to WT, suggesting a similar behavioral pattern to that revealed for BTBR mice.
3.1.2.2. Self- and allo-grooming
Fig. 1 displays the frequencies of self-grooming and allo-grooming behaviors for WT and KO mice from Oxtr and Mecp2308/Y strains. Self-grooming was significantly increased, and allo-grooming significantly reduced, in Oxtr KO mice, as compared to WT. In addition, Mecp2308/Y KO mice displayed increased frequencies of allo-grooming behavior, as compared to their WT counterparts. BTBR mice displayed enhanced frequencies of self-grooming, and decreased frequencies of allo-grooming [63], when compared to B6, which is consonant with the results described for Oxtr KO mice.
3.1.2.3. Huddle
The mean frequencies of huddling behavior for WT and KO mice from Oxtr and Mecp2308/Y strains are displayed in Fig. 2. The main effect of strain, and the interaction between strain and genotype were statistically significant for huddling; frequencies of this behavior were respectively reduced in Oxtr KO mice, and increased in Mecp2308/Y KO mice, as compared to WT. BTBR mice displayed a significant reduction in the percentage of observations of huddling behavior, as compared to B6 [63].
3.1.2.4. Discussion
The VBS is a semi-natural environment that was designed to provide many of the features of natural habitats of rodents, including a substantial area ensuring spacing and avoidance, multiple tunnels and burrows in addition to an open surface area, a consistent social group, and extended periods of observation of social interactions [67,68]. Rodent groups maintained in this context display considerable consistency in terms of time and activity budgets, even though there are few external constraints on their behavior aside from a standard light/dark cycle [59,69]. Interestingly, the behavioral profile of Oxtr KO mice in the VBS is similar to that described for BTBR mice [63]; both strains showed consistent reductions in interactive behaviors: frontal approach, huddling, allo-grooming, and flight, with more time spent alone, and in self-grooming. In contrast, Mecp2308/Y KO mice preferentially approach one another from the front rather than the back; a preference that seems to be associated with pro-social motivation [59]. Also, Mecp2308/Y KO mice displayed increased frequencies of huddling, and allo-grooming behaviors in the VBS, as compared to WT. These results for three mouse models potentially related to ASD indicate that VBS measures can provide ethologically valid indices of a range of social activities, potentially including some (e.g. frontal approach) that may enable differentiation of pro-social motivations as opposed to other motives (e.g. sexual or aggressive) for social interactions.
3.2. Three-chambered social approach test
3.2.1. Apparatus and procedures
The three-chambered social approach test has been extensively used in studies of sociality in a variety of mouse strains [30–32,70]. WT and KO mice from Oxtr and Mecp2308/Y strains were run individually in a three-chambered arena, constructed as previously described [30]. Briefly, the subject mouse was placed into the middle chamber of the divided apparatus, which contained two empty, inverted wire cups placed in each of the two outer compartments. For the habituation phase, the subject mouse was given free access to the three chambers during a 10 min session. Following habituation, the mouse was placed back into the center, and an unfamiliar male CD-1 mouse was placed into one of the two cups, with the subject mouse permitted to explore the entire apparatus for 10 min. Time spent in each compartment during both sessions was collected in real-time with two stopwatches by a single observer who was blind to the genotype of the subject. Data from the three-chambered test were analyzed using within-group repeated measures ANOVA for comparison of time spent in the empty cup side with time spent in the CD-1 mouse side.
3.2.2. Results and discussion
Fig. 3 displays the duration of time that WT and KO mice from Oxtr and Mecp2308/Y strains spent in both side compartments of the three-chambered apparatus. The analysis revealed that WT mice from both Oxtr and Mecp2308/Y strains, and Mecp2308/Y KO mice showed a significant preference for spending time in the side of the test box containing the unfamiliar CD-1 mouse used as stimulus vs. the opposite side. Unlike these latter groups, Oxtr KO mice did not show a significant preference for one side over the other.
Fig. 3.

Time spent in each side (mean ± S.E.M.) of the three-chambered apparatus, for Oxtr WT (n=13), Oxtr KO (n=16), Mecp2308/Y WT (n=9) and Mecp2308/Y KO (n=9) mice; *p<0.05, within-group repeated measures ANOVA comparison.
These results obtained in the three-chambered social approach test are in agreement with the previous VBS data (section 3.1.2). The finding of reduced sociability for Oxtr KO mice is similar to the behavioral pattern displayed by BTBR mice in this same situation [37,63,71]. In addition, Oxtr WT, Mecp2308/Y WT and KO mice showed a significant preference for spending time in the side of the three-chambered apparatus containing the stimulus mouse, a pattern that is similar to the one described for B6, a high social standard control strain [31].
3.3. Social proximity test
Although the VBS and Three-Chamber tests produced a congruent set of results, both of these tests utilized relatively large enclosures configured to allow effective avoidance of social stimuli. Because avoidance was readily available, neither of these tests provided satisfactory measures of potential behavioral differences for these mutant strains when in close contact with other mice. The social proximity test was developed specifically to ensure such close contact, enabling a clearer analysis of specific components of potential investigatory, orientation, and avoidance behaviors.
3.3.1. Apparatus and procedures
Social proximity testing was conducted in a clear rectangular Plexiglas chamber (14 x 7 x 30 cm H) constructed according to previously reported parameters [61]. For testing, the subject mouse and an unfamiliar male B6 mouse were placed simultaneously into the apparatus during a single 10 min test. Dimensions of the chamber allowed both mice of a pair to stand normally on the substrate but ensured a substantial degree of contact as they moved. Mice were marked for individual identification, and measures taken reflected specific types of contact by each mouse to the other. The following behaviors were manually quantified by an observer blind to the subject’s genotype: nose to nose: subject’s nose tip and/or vibrissae contact the nose tip and/or vibrissae of the other mouse; nose to face: subject’s nose tip contacts the head of the stimulus mouse; nose to anogenital: subject’s nose or vibrissae contacts the base of the tail or anogenital region of the other mouse; crawl over: subject’s forelimbs cross the midline of the dorsal surface of the other mouse; crawl under: subject’s head goes under the ventral surface of the other mouse to a depth of at least the ears of the subject animal crossing the midline of the other mouse’s body; and upright: subject displays a reared posture oriented towards the other mouse with head and/or vibrissae contact. Data from the social proximity test were analyzed by two-way analyses of variance (ANOVA), with strain (Oxtr or Mecp2308/Y) as between-subjects factor, and genotype (WT or KO) as within group factor. Post hoc comparisons were made using the Newman-Keuls test.
3.3.2. Results and discussion
Results of statistical tests for measures of the social proximity test, by strain, genotype, along with interaction effects are shown in Table 1. Fig. 4 displays effects on social behaviors elicited by confinement with an unfamiliar B6 mouse in the social proximity chamber for Oxtr and Mecp2308/Y WT and KO mice. Oxtr KO mice showed a significant reduction in the frequencies of nose to nose, and nose to anogenital behaviors oriented to the B6 mouse, as compared to WT. Mecp2308/Y KO mice displayed elevated frequencies and/or durations of crawl over, crawl under behaviors, and nose to face investigation, when compared to their WT littermates in the social proximity test.
Fig. 4.
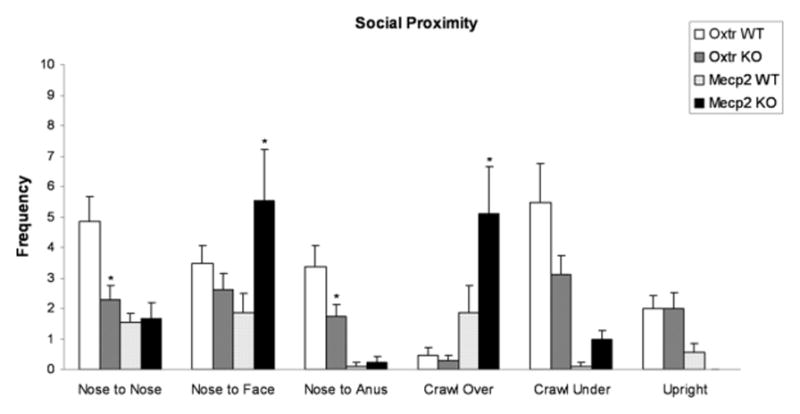
Frequency (mean ± S.E.M.) of social behaviors obtained when Oxtr WT (n=13), Oxtr KO (n=16), Mecp2308/Y WT (n=9) and Mecp2308/Y KO (n=9) mice were confined with an unfamiliar B6 mouse in the social proximity chamber; *p<0.05 compared to WT by Newman-Keuls test.
Reduced frequencies of nose to nose behaviors in Oxtr KO mice confirm an interpretation of impaired sociality in these mutants; this was also described in BTBR mice confined either with a partner of the same strain or a B6 mouse in the social proximity chamber [61]. However, when compared to their B6 partners, BTBR mice also displayed enhanced frequencies of nose to anogenital contacts in the social proximity test [61], in contrast to the reduced rates seen in Oxtr KO mice. One possibility is that social contact for BTBR mice may be anxiogenic, with enhanced nose to anogenital contacts reflecting an increase in risk assessment behaviors. This interpretation of increased social anxiety for BTBR mice is consonant with diazepam-linked changes in other social behaviors for this strain [61,71]. In this context, reduced frequencies of nose to anogenital contacts described for Oxtr KO mice might reflect their normal scores on measures of emotional-like reactivity [64,72], in combination with reductions in social motivations.
The significant differences of Mecp2308/Y KO mice, in comparison to their WT, were increased nose to face, crawl under and crawl over behaviors. The most straightforward interpretation of these changes is that they are consonant with the increased huddling and allo-grooming of Mecp2308/Y KO in the VBS. While it is interesting that BTBR mice confined with a B6 partner also showed enhanced frequencies of crawl under/over behaviors in the social proximity test, administration of diazepam significantly reduced these behaviors [61]. This was interpreted in terms of enhanced BTBR social anxiety, with crawl under behaviors enabling escape from visual contact with the partner mouse, and crawl over as a passive result of having the BTBR pair mate crawl under the focal animal. However, evidence for social anxiety in the Mecp2308/Y mouse is both less direct, and mixed: constitutive mutants of the gene encoding the Mecp2 protein tend to show decreased anxiety (see 73 for a review), although Shabazian et al. [74] did note increased anxiety-like behaviors for Mecp2308/Y as part of a later-developing neurological spectrum. Recent data from our lab did not show elevated anxiety for Mecp2308/Y mice to nonanimate threat stimuli, although mutant mice displayed sharply increased defensive responses to predator stimuli (Pearson et al., unpublished). Thus, although an interpretation of increased crawl under behavior as escape from visual confrontation with a social stimulus based on enhanced anxiety to animate stimuli remains possible for the Mecp2308/Y mouse social proximity data, it is currently more speculative than is an interpretation of these behaviors in terms of social affiliation.
4. Tests of motor and cognitive stereotypies in Mecp2308/Y and Oxtr KO mice [65,66]
4.1. Self-grooming analyses
4.1.1. Apparatus and procedures
Subjects were individually assessed for grooming microstructure in the same chamber that was used in the social proximity test (section 3.3.1), as previously described [62]. Videotapes of spontaneous self-grooming episodes were scored for the frequency and duration of paw licking, head washing, body grooming, leg licking, and tail/genital grooming. Mice typically display a clear sequential pattern of grooming, following the order of body contact listed above [75,76], and transitions from one body part to another that did not follow this sequence (“incorrect transitions”) were measured, as well as “interrupted bouts” - grooming bouts that were interrupted, but for less than 6 s; interruptions of more than 6 s resulted in the designation of a new bout. Following a previously validated syntactical grooming analysis [77], we calculated the number of bouts, the number of interrupted bouts, and the proportion of interrupted bouts, as well as the number of transitions between grooming stages, the number of incorrect transitions, and the proportion of incorrect transitions. Unpaired t tests were performed to compare average frequencies and durations of each behavior category in the self-grooming analyses within each strain, with genotype (WT or KO) as the grouping variable.
4.1.2. Results and discussion
Analyses of the data revealed slight alterations in body site-specific patterns of grooming in Mecp2308/Y KO mice: this group showed increased frequency of tail/genital grooming, and increased duration of paw licking, as compared to WT (not shown). No differences were found between Oxtr WT and KO genotypes for any of the grooming subtypes. Similarly, no significant differences were revealed between WT and KO genotypes from either Oxtr or Mecp2308/Y strains for any of the syntactical parameters of self-grooming (number of bouts, number and proportion of interrupted bouts, number of transitions between grooming stages, number and proportion of incorrect transitions).
The pattern displayed by BTBR mice in this same experimental situation [62] differs from that found in Oxtr and Mecp2308/Y mutants. BTBR mice showed elevated frequencies of all grooming subtypes, increased proportion of interrupted bouts, but proportionately fewer incorrect transitions between grooming stages, as compared to B6. These results indicate that while BTBR mice display more self-grooming, they actually show proportionately less deviation than B6 mice from a typical mouse grooming sequence, suggesting enhanced stereotypy in addition to a higher level of repetitive behaviors. Taken together, this set of data suggests that while BTBR mice display a consistent pattern of repetitive and stereotyped behaviors, no major changes in such behaviors are revealed for mice lacking Oxtr and Mecp2 genes.
4.2. Repetitive Novel Object Contact Task
4.2.1. Apparatus and procedures
Subjects were assessed for the frequency of repetitive contacts with novel objects in a previously habituated polypropylene cage, 26.5 cm x 17 cm x 11.5 (H) cm, as previously reported [62]. The cage contained four small novel objects (children’s toys, 1.5–4 cm in length) made of high density plastic and located approximately 3 cm from each of the four corners in a fixed pattern. Recorded DVDs were scored for the occurrence of investigation of the four toys, defined as clear facial or vibrissae contact with or burying of each object. Occurrences of identical patterns of sequential contact with three, or with four toys were counted, as well as the frequencies of contact with each of the four toys. In order to determine if there was a genotype effect on the tendency to display preferences for particular toys, frequencies of contact with each object were ranked in decreasing order from maximum to minimum preference values for each subject, averaged by genotype and compared. Scoring of the number of transitions between quadrants in the habituation session was used for assessment of any possible differences in motor activation or exploratory tendencies between genotypes. Unpaired t tests were performed to compare measures obtained in the repetitive novel object contact task within each strain, with genotype (WT or KO) as the grouping variable.
4.2.2. Results and discussion
During the habituation phase, no significant differences were revealed between WT and KO genotypes from Oxtr and Mecp2308/Y strains in the number of transitions between quadrants. In addition, WT and KO mice from Oxtr and Mecp2308/Y strains investigated novel objects at a similar rate. Similarly, when proportional preferences for each toy were ranked and averaged for each genotype, no significant difference in object preferences was found. Finally, when the number of identical three- and four-object sequences was compared between genotypes of Oxtr and Mecp2308/Y strains, no differences were observed.
In the repetitive novel object contact task, BTBR mice showed stronger preferences for specific objects, and a stronger preference differentiation among the four objects, than did B6 mice. Also, BTBR mice displayed a significantly higher number of visits that included a repetitive sequence of three or four objects [62]. These findings indicate that stereotyped patterns of BTBR mice are not confined to motor behaviors, but encompass a tendency to prefer some objects over others, as well as a more repetitive pattern of sequential approaches to these objects. On the other hand, WT and KO mice from Oxtr and Mecp2308/Y strains did not differ in their cognitive, object investigation-based measures of stereotypy or restricted interest.
5. Analyses of communication levels in Mecp2308/Y and Oxtr KO mice [65,66]
5.1. Urinary scent marking
5.1.1. Apparatus and procedures
Subjects were assessed for baseline scent marking after being individually housed for at least seven days, as previously described [60]. The scent marking arena was an inverted rat cage with a steel mesh divider wall installed to bisect the arena. This apparatus was placed on top of a 30 x 45 cm section of drawing paper, and the subject mouse was placed on one side for a 20 min baseline session. Twenty-four hours later, the mouse was tested for urinary scent marking to an unfamiliar male CD-1 mouse that had just been placed in the opposite half of the arena, on the other side of the steel mesh divider. The drawing paper’s orientation in the test compartment was marked to ensure that only subject markings were counted, and the paper was allowed to dry overnight. It was then fixed and stained with a 6% solution of ninhydrin (Fisher) in methanol to label urinary scent marks, and dried. To quantify the amount of urinary scent marking, a 1×1 cm printed transparency grid was placed over the paper and the number of squares containing a stained mark was counted manually by an assistant blind to the genotype of the subject. Data from the scent marking analysis were compared by two-way analyses of variance (ANOVA), with strain (Oxtr or Mecp2308/Y) as between-subjects factor, and genotype (WT or KO) as within group factor. Post hoc comparisons were made using the Newman-Keuls test.
5.1.2. Results and discussion
Table 1 provides results of statistical tests for measures of the scent marking analysis, by strain, genotype, along with interaction effects. The mean number of squares containing scent marks of WT and KO mice from Oxtr and Mecp2308/Y strains in the baseline and social conditions is displayed in Fig. 5. The number of scent marks was reduced in Oxtr KO mice, and increased in Mecp2308/Y KO mice, when compared to their WT littermates, in both social and non-social contexts; it is worth noting however that such changes in scent marking did not reach statistical significance. Interestingly, BTBR mice showed a significant increase in the number of scent marks in non-social contexts, as compared to B6 (unpublished results). This set of results indicates that while Oxtr KO mice display an overall decrease in communication levels, Mecp2308/Y KO mice exhibit an enhanced deposition of scent marks across social and non-social contexts, as compared to WT.
Fig. 5.

Number (mean ± S.E.M.) of squares with scent marks of Oxtr WT (n=13), Oxtr KO (n=16), Mecp2308/Y WT (n=9) and Mecp2308/Y KO (n=8) mice assessed in two experimental conditions.
6. Overall summary and discussion
The studies reviewed in this report compared WT and KO genotypes from Oxtr and Mecp2308/Y strains with the extensively described behavioral changes of BTBR mice in a variety of situations designed to provide parallels to the three major symptom clusters of ASD. These mouse models were selected on the basis of previous implications in ASD, neurodevelopmental diseases comorbid with ASD, or by having a known role in the regulation of rodent social behaviors. A wealth of prior evidence supports a role for oxytocin as a central mediator of complex social behaviors [78,79], and mice lacking the ability to synthesize Oxtr display impaired social recognition and deficits in other behavioral domains relevant for ASD [64,80]. In addition, previous evidence indicates that loss of function mutations in the X-linked gene responsible for encoding the Mecp2 protein underlie the majority of cases of Rett syndrome, an ASD with a known genetic cause [81,82]; Mecp2308/Y KO mice, expressing a truncated form of the Mecp2 protein, recapitulate many of the features of the Rett syndrome [74].
Lack of social approach and disruption of reciprocal social interactions, indicators of the first major symptom of ASD, were assessed in these mouse models in three specific situations: VBS, three-chambered and social proximity tests. The behavioral changes observed in these mutant mouse strains were consistent and highly significant. In the VBS, while Oxtr KO mice displayed consistent reductions in interactive behaviors (frontal approach, huddling, allo-grooming, and flight), with more time spent alone and in self-grooming, Mecp2308/Y KO mice showed behavioral changes suggesting enhanced affiliative social behavior (increased rates of allo-grooming and huddling behaviors), as compared to WT. These results were corroborated in the three-chambered test: unlike Oxtr WT, Mecp2308/Y WT and KO mice, Oxtr KO mice failed to spend more time in the side of the test box containing the unfamiliar CD-1 mouse. A recently published study revealed similar impairments in social approach for Oxtr KO mice evaluated in the three-chambered test [72]. Alterations in sociability levels were also described for Mecp2308/Y KO mice: although there were no differences in aggression or exploration of novel inanimate stimuli, these mutants took less initiative and were less decisive approaching unfamiliar males in various social interaction assays [83]. It is worth noting that Mecp2308/Y KO mice from the latter study were on a pure 129/SvEv genetic background, while those used by Pearson et al. [65] were on a C57BL/6J. The behavioral results described when Oxtr and Mecp2308/Y KO mice were confined with an unfamiliar B6 mouse in the social proximity chamber support and extend the data obtained in the VBS and three-chamber tests, with Oxtr KO mice displaying a significant reduction in the frequencies of social investigatory behaviors oriented to the B6 mouse, and Mecp2308/Y KO mice showing enhanced rates of crawl over/under behaviors, and nose to face investigation, as compared to their WT counterparts.
Existing mouse models of ASD commonly exhibit patterns of restricted repetitive behaviors: in a hole-board task, BTBR mice displayed inflexibility in exploratory behavior and failed to shift their preference towards an appetitive olfactory stimulus, as compared to B6, BALB/cByJ and FVB/NJ strains [84]. BTBR mice showed a significant increase in the frequency of all subtypes of self-grooming, and a stronger preference for specific objects as well as a significantly higher number of visits that include a repetitive sequence of 3 or 4 objects [62]. In contrast to these data, except for slight changes in body site-specific patterns of grooming revealed in Mecp2308/Y KO mice, no reliable differences were found between WT and KO genotypes from either Oxtr or Mecp2308/Y strains in repetitive or stereotypical behavioral patterns assessed in two specific situations. These results indicate that unlike BTBR mice which display a robust pattern of restricted and repetitive behaviors, no major alterations in such behaviors were described for mice with mutations in Oxtr or Mecp2 genes.
Deposition of urinary scent marks toward conspecifics and emission of ultrasonic vocalizations are two major models of mouse communication [85,86]. Scattoni et al. [87] reported that when compared to B6, FVB/NJ, and 129X1/SvJ mouse pups, BTBR pups produced an unusual repertoire of ultrasonic vocalizations in response to separation from their mothers and siblings, suggesting a link to communication deficits. Prior studies revealed that Oxtr and Mecp2308/Y KO pups emitted fewer ultrasonic vocalizations calls than their WT counterparts upon separation from their mothers [80,88]. Adult male BTBR mice showed lower scent marking and minimal ultrasonic vocalizations to female urinary pheromones, as compared to B6 [40]. Analyses of the results reviewed in this report revealed that when compared to their WT littermates, adult Oxtr KO mice displayed a non-significant reduction in number of urinary scent marks across social and non-social contexts, while Mecp2308/Y KO mice showed a non-significant increase in deposition of these urinary traces in both contexts.
In summary, the data reviewed in this report revealed that while Oxtr KO mice displayed consistent social deficits, and reduced levels of communication, a profile that is similar to the pattern found in BTBR mice [40,58], Mecp2308/Y KO mice showed a slight but consistent enhancement of social behavior and communication; no changes in repetitive, ritualized behaviors were described for Oxtr and Mecp2308/Y KO mice. It should be noted that there are a number of KO strains engineered for each gene potentially implicated in ASD (e.g. for the Mecp2 gene see 89). Ideally, comparisons involving each of these KO strains would be necessary for evaluating the roles of the genes involved in behaviors relevant to autism. However, although the current data base is small, it indicates that mouse models can sort the diagnostic symptoms of ASD, and suggests that biological and physiological analyses of these strains may be capable of providing differential information on the brain systems involved in particular symptoms of this group of disorders. Moreover, considering that the primary diagnostic indices of ASD are abnormal behaviors, the current analysis illustrates the importance of a detailed description of social and stereotyped behavioral patterns in inbred mouse strains carrying mutations in genes potentially associated with ASD.
Highlights.
Oxtr KO mice display consistent social deficits, and reduced levels of communication.
Mecp2308/Y KO mice show a slight but consistent enhancement of social behavior and communication.
No changes in repetitive, ritualized behaviors were described for Oxtr and Mecp2308/Y KO mice.
This data base, although small, strongly indicates that mouse models can sort the diagnostic symptoms of autism.
Acknowledgments
This research was supported by the NIH grant MH081845 to RJB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.A.P.A. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 2.Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. 2009;65:591–8. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- 3.Kanner L. Autistic disturbances of affective contact. Acta Paedopsychiatr. 1968;35:100–36. [PubMed] [Google Scholar]
- 4.Clifford S, Young R, Williamson P. Assessing the early characteristics of autistic disorder using video analysis. J Autism Dev Disord. 2007;37:301–13. doi: 10.1007/s10803-006-0160-8. [DOI] [PubMed] [Google Scholar]
- 5.Groen WB, Zwiers MP, van der Gaag RJ, Buitelaar JK. The phenotype and neural correlates of language in autism: an integrative review. Neurosci Biobehav Rev. 2008;32:1416–25. doi: 10.1016/j.neubiorev.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- 7.Szatmari P, Georgiades S, Bryson S, Zwaigenbaum L, Roberts W, Mahoney W, et al. Investigating the structure of the restricted, repetitive behaviours and interests domain of autism. J Child Psychol Psychiatry. 2006;47:582–90. doi: 10.1111/j.1469-7610.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- 8.Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2:943–55. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- 9.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–55. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betancur C. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 11.Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn Sci. 2011;15:409–16. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dufour-Rainfray D, Vourc’h P, Tourlet S, Guilloteau D, Chalon S, Andres CR. Fetal exposure to teratogens: evidence of genes involved in autism. Neurosci Biobehav Rev. 2011;35:1254–65. doi: 10.1016/j.neubiorev.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Landrigan PJ. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 2010;22:219–25. doi: 10.1097/MOP.0b013e328336eb9a. [DOI] [PubMed] [Google Scholar]
- 14.Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17:389–94. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persico AM, Bourgeron T. Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci. 2006;29:349–58. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–9. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Happe F, Ronald A. The ‘fractionable autism triad’: a review of evidence from behavioural, genetic, cognitive and neural research. Neuropsychol Rev. 2008;18:287–304. doi: 10.1007/s11065-008-9076-8. [DOI] [PubMed] [Google Scholar]
- 18.Rapin I, Tuchman RF. Autism: definition, neurobiology, screening, diagnosis. Pediatr Clin North Am. 2008;55:1129–46. doi: 10.1016/j.pcl.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Volkmar FR, State M, Klin A. Autism and autism spectrum disorders: diagnostic issues for the coming decade. J Child Psychol Psychiatry. 2009;50:108–15. doi: 10.1111/j.1469-7610.2008.02010.x. [DOI] [PubMed] [Google Scholar]
- 20.Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–59. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moy SS, Nadler JJ. Advances in behavioral genetics: mouse models of autism. Mol Psychiatry. 2008;13:4–26. doi: 10.1038/sj.mp.4002082. [DOI] [PubMed] [Google Scholar]
- 22.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bucan M, Abel T. The mouse: genetics meets behaviour. Nat Rev Genet. 2002;3:114–23. doi: 10.1038/nrg728. [DOI] [PubMed] [Google Scholar]
- 24.Flint J, Mott R. Applying mouse complex-trait resources to behavioural genetics. Nature. 2008;456:724–7. doi: 10.1038/nature07630. [DOI] [PubMed] [Google Scholar]
- 25.Seong E, Seasholtz AF, Burmeister M. Mouse models for psychiatric disorders. Trends Genet. 2002;18:643–50. doi: 10.1016/s0168-9525(02)02807-x. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton SM, Spencer CM, Harrison WR, Yuva-Paylor LA, Graham DF, Daza RA, et al. Multiple autism-like behaviors in a novel transgenic mouse model. Behav Brain Res. 2011;218:29–41. doi: 10.1016/j.bbr.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scearce-Levie K, Roberson ED, Gerstein H, Cholfin JA, Mandiyan VS, Shah NM, et al. Abnormal social behaviors in mice lacking Fgf17. Genes Brain Behav. 2008;7:344–54. doi: 10.1111/j.1601-183X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 28.Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, et al. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–37. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva-Paylor LA, et al. Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic-like responses. Autism Res. 2011;4:40–56. doi: 10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 31.Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, et al. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118–29. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 34.Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, et al. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8:129–42. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS. Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain. Behav Brain Res. 2010;208:178–88. doi: 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–6. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+ tf/J mice. Genes Brain Behav. 2008;7:152–263. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 38.Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007;25:515–21. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wöhr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, et al. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007;1:1–9. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ey E, Leblond CS, Bourgeron T. Behavioral profiles of mouse models for autism spectrum disorders. Autism Res. 2011;4:5–16. doi: 10.1002/aur.175. [DOI] [PubMed] [Google Scholar]
- 43.Patterson PH. Modeling autistic features in animals. Pediatr Res. 2011;69:34R–40R. doi: 10.1203/PDR.0b013e318212b80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abrahams BS, Geschwind DH. Connecting genes to brain in the autism spectrum disorders. Arch Neurol. 2010;67:395–9. doi: 10.1001/archneurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–4. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–11. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Kelleher RJ, Bear MF. The autistic neuron: troubled translation? Cell. 2008;135:401–6. doi: 10.1016/j.cell.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 48.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–11. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye H, Liu J, Wu JY. Cell adhesion molecules and their involvement in autism spectrum disorder. Neurosignals. 2010;18:62–71. doi: 10.1159/000322543. [DOI] [PubMed] [Google Scholar]
- 50.Bassel GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–14. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benayed R, Choi J, Matteson PG, Gharani N, Kamdar S, Brzustowicz LM, et al. Autism associated haplotype affects the regulation of the homebox gene, ENGRAILED 2. Biol Psychiatry. 2009;66:911–7. doi: 10.1016/j.biopsych.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levitt P, Campbell DB. The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J Clin Invest. 2009;119:747–54. doi: 10.1172/JCI37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishimura K, Nakamura K, Anitha A, Yamada K, Tsujii M, Iwayama Y, et al. Genetic analyses of the brain-derived neurotrophic factor (BDNF) gene in autism. Biochem Biophys Res Commun. 2007;356:200–6. doi: 10.1016/j.bbrc.2007.02.135. [DOI] [PubMed] [Google Scholar]
- 54.Campbell DB, Datta D, Jones ST, Batey Lee E, Sutcliffe JS, Hammock EA, et al. Association of oxytocin receptor (OXTR) gene variants with multiple phenotype domains of autism spectrum disorder. J Neurodev Disord. 2011;3:101–12. doi: 10.1007/s11689-010-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kistner-Griffin E, Brune CW, Davis LK, Sutcliffe JS, Cox NJ, Cook EH., Jr Parent-of-origin effects of the serotonin transporter gene associated with autism. Am J Med Genet B Neuropsychiatr Genet. 2011;156:139–44. doi: 10.1002/ajmg.b.31146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prasad HC, Steiner JA, Sutcliffe JS, Blakely RD. Enhanced activity of human serotonin transporter variants associated with autism. Phil Trans R Soc Lond. 2009;364:163–73. doi: 10.1098/rstb.2008.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tansey KE, Hill MJ, Cochrane LE, Gill M, Anney RJ, Gallagher L. Functionality of promoter microsatellites of arginine vasopressin receptor 1A (AVPR1A): implications for autism. Mol Autism. 2011;2:3. doi: 10.1186/2040-2392-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blanchard DC, Defensor EB, Meyza KZ, Pobbe RL, Pearson BL, Bolivar VJ, et al. BTBR T+tf/J mice: Autism-relevant behaviors and reduced fractone-associated heparin sulfate. Neurosci Biobehav Rev. 2012;36:285–96. doi: 10.1016/j.neubiorev.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arakawa H, Blanchard DC, Blanchard RJ. Colony formation of C57BL/6J mice in visible burrow system: identification of eusocial behaviors in a background strain for genetic models of autism. Behav Brain Res. 2007;176:27–39. doi: 10.1016/j.bbr.2006.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Social features of scent-donor mice modulate scent marking of C57BL/6J recipient males. Behav Brain Res. 2009;205:138–45. doi: 10.1016/j.bbr.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Defensor EB, Pearson BL, Pobbe RL, Bolivar VJ, Blanchard DC, Blanchard RJ. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+tf/J mice. Behav Brain Res. 2011;217:302–8. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pearson BL, Pobbe RL, Defensor EB, Oasay L, Bolivar VJ, Blanchard DC, et al. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:228–35. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214:443–9. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., III A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–63. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pearson BL, Defensor EB, Pobbe RL, Yamamoto LH, Bolivar VJ, Blanchard DC, et al. Mecp2 truncation in male mice promotes affiliative social behavior. Behav Genet. 2012;42:299–312. doi: 10.1007/s10519-011-9501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Young WS, III, Lee HJ, et al. Oxytocin receptor knockout mice display deficits in the expression of autism-related behaviors. Horm Behav. doi: 10.1016/j.yhbeh.2011.10.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol. 1989;103:70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- 68.Bouchard PR, Lynch CB. Burrowing behavior in wild house mice: variation within and between populations. Behav Genet. 1989;19:447–56. doi: 10.1007/BF01066170. [DOI] [PubMed] [Google Scholar]
- 69.Blanchard RJ, Dulloog L, Markham C, Nishimura O, Compton JN, Jun A, et al. Sexual and aggressive interactions in a visible burrow system with provisioned burrows. Physiol Behav. 2001;72:245–54. doi: 10.1016/s0031-9384(00)00403-0. [DOI] [PubMed] [Google Scholar]
- 70.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–14. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 71.Pobbe RL, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T+tf/J mouse strain. Behav Brain Res. 2011;216:446–51. doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–82. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 73.Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–37. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–54. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 75.Fentress JC, Stilwell FP. Grammar of a movement sequence in inbred mice. Nature. 1973;244:52–3. doi: 10.1038/244052a0. [DOI] [PubMed] [Google Scholar]
- 76.Berridge KC. Comparative fine structure of action: rules of form and sequence in the grooming patterns of six rodent species. Behaviour. 1990;113:21–56. [Google Scholar]
- 77.Kalueff AV, Aldridge JW, LaPorte JL, Murphy DL, Tuohimaa P. Analyzing grooming microstructure in neurobehavioral experiments. Nat Protoc. 2007;2:2538–44. doi: 10.1038/nprot.2007.367. [DOI] [PubMed] [Google Scholar]
- 78.Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–79. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–47. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA. 2005;102:16096–101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–8. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 82.Schaevitz LR, Moriuchi JM, Nag N, Mellot TJ, Berger-Sweeney J. Cognitive and social functions and growth factors in a mouse model of Rett syndrome. Physiol Behav. 2010;100:255–63. doi: 10.1016/j.physbeh.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 83.Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–20. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- 84.Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, et al. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res. 2008;188:178–94. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ. Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev. 2008;32:1236–48. doi: 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33:508–15. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Filippis B, Ricceri L, Laviola G. Early postnatal behavioral changes in the Mecp2-308 truncation mouse model of Rett syndrome. Genes Brain Behav. 2010;9:213–23. doi: 10.1111/j.1601-183X.2009.00551.x. [DOI] [PubMed] [Google Scholar]
- 89.Ricceri L, De Filippis B, Laviola G. Mouse models of Rett syndrome: from behavioural phenotyping to preclinical evaluation of new therapeutic approaches. Behav Pharmacol. 2008;19:501–17. doi: 10.1097/FBP.0b013e32830c3645. [DOI] [PubMed] [Google Scholar]


