Abstract
Human immunodeficiency virus type 1 (HIV-1) transactivator of transcription (Tat) protein possesses a unique membrane-transduction property. Interestingly, Tat transduction could be dramatically increased 1000-fold based on LTR-transactivation assay when complexed with cationic liposomes (lipo-Tat), compared with Tat alone. Therefore, underlining mechanisms were explored further. Microscopy and flow cytometry showed that this effect was associated with enhanced membrane binding, large particle formation (1–2 μm) and increased intracellular uptake of Tat fluorescent proteins. Using pharmacological assays and immune colocalizations, it was found that lipid raft-dependent endocytosis and macropinocytosis were major pathways involved in lipo-Tat uptake, and actin-filaments played a major role in intracellular trafficking of lipo-Tat to the nucleus. Furthermore, we found that the Tat hydrophobic domain (aa 36–47) mediated formation of two positively charged molecules into lipo-Tat complexes via hydrophobic bonds, based on LTR-transactivation inhibition assay. Thus, the hydrophobic domain may play an important role in Tat protein uptake and be useful for intracellular delivery of biomacromolecules if coupled together with Tat basic peptide, a cell-penetrating peptide.—Li, G.-H., Li, W., Mumper, R. J., Nath, A. Molecular mechanisms in the dramatic enhancement of HIV-1 Tat transduction by cationic liposomes.
Keywords: transactivation, uptake, endocytosis, intracellular delivery, hydrophobic peptide
Naturally, human immunodeficiency virus type 1 (HIV-1) transactivator of transcription (Tat) is a protein of 101 aa encoded from two exons, expressed early in infection and mainly localized in the nucleus (1–3). An 86-aa Tat has only been found in a few laboratory viral strains (e.g., HXB2; refs. 1, 4). Tat is essential for viral replication. In the absence of Tat, transcription terminates prematurely and short RNA transcripts of 60–80 nt are produced due to inefficient elongation by RNA polymerase II (5, 6). The first exon encodes aa 1-72, containing necessary, functional regions (e.g., Cys-rich region, core region, and basic region) for transactivation response (TAR) RNA binding and transcriptional activation (1, 7, 8). Although the integrity of the Cys-rich region (aa acids 22–37) is required for transactivation, the “core” region (amino acids 37–48), and Arg-rich basic region (aa 49–57) are directly involved in binding and recognition of TAR RNA (1, 3, 7, 9). The basic RKKRRQRRR motif also confers the nuclear/nucleolar targeting property of Tat protein (3, 9, 10).
Tat can be secreted from infected or transfected cells, including lymphocytes (11, 12), monocytes (13, 14), and astrocytes (15), by a leaderless, unconventional pathway (16–18). However, Tat protein can be taken up by cells from extracellular environment (12, 19), which is initiated by the Arg-rich domain binding to anionic cell membrane (20). Studies have shown that Tat can bind to different receptors (including CD26, LRP, and CXCR4; ref. 21). However, heparan sulfate proteoglycan (HSPG), a major source of macromolecular polyanions at the cell membrane (20, 22), is broadly recognized as a receptor for the internalization of Tat protein. This condition is based on the fact that Tat uptake can be inhibited by competing with soluble heparin or specifically removing heparan sulfate (HS) chains from the cell surface using glycosaminoglycan lyases and that no detectable Tat appears in HSPG-deficient mutant cells (23). Nevertheless, this is not true for the Arg-rich peptide derived from Tat, the uptake of which is unaffected in HSPG-deficient cells or by removing HS chains (24). It is possible that other anionic structures on the cell surface (e.g., anionic amino acids of membraneous proteins, anionic heads of phospholipids) can be utilized by the short basic peptide (20). Surprisingly, a branched-chain arginine peptide as well as the corresponding linear polymer can also be taken up efficiently (25). More efficient uptake can be observed in the corresponding d-isomer and retroinverse isomers of Arg-rich Tat49–57 peptide (26). Apparently, no particular spatial structure is required for the transduction of the short basic peptides except for exclusive involvement of cationic charges (20). Since HSPG also mediates transduction of a variety of other ligands (polybasic peptides, polycation-nucleic acid complexes, polyamines, etc.; ref. 22), it may not be a traditional, specific receptor.
Tat Arg-rich peptide, as well as other transmembrane basic peptides found in Drosophila Antennapedia protein, herpes simplex virus protein VP22 and other proteins, are called protein transduction domains (PTDs) or cell-penetrating peptides (CPPs) and have been widely exploited as vehicles for intracellular delivery of macromolecules, including oligonucleotides, peptides or proteins, low-molecular-mass drugs, nanoparticles, and liposomes (22, 27). Transduction of Tat protein or Tat-fusion proteins reportedly involves caveolar endocytosis (28, 29), clathrin-dependent endocytosis (30, 31), and macropinocytosis (31, 32), although the uptake process is actually inefficient (33). However, in addition to these endocytic pathways (34, 35), an energy-independent, spontaneous translocation is also involved in the transduction of short Tat basic peptides (24, 36, 37).
Recent novel therapeutic approaches for eradicating HIV involve activating the virus in latent reservoirs of patients adequately treated with antiretroviral drugs. These viral reservoirs can potentially be activated by delivery of Tat protein. Conversely, a dominant negative Tat protein can be used to block replication of HIV in the productively infected cells. In addition, Tat protein is a candidate for a therapeutic vaccine in HIV-infected patients (38). Thus, approaches for enhancing Tat delivery to cells may have important therapeutic implications.
We observed that the delivery of Tat proteins to the nucleus could be dramatically enhanced by a cationic liposome reagent and thus focused on how this phenomenon could result from combining two cationic macromolecules. Interestingly, we found that the enhancement not only benefited from modulating Tat-transducing pathways but also from the detoured Tat intracellular trafficking. Liposomes facilitated Tat transversion through the membrane lipid bilayer primarily via lipid-dependent endocytosis and also promoted the release from endosomes/lysosomes to the cytosol thereby reducing Tat degradation. Furthermore, a hydrophobic domain, mainly located in the core region of Tat, was identified that mediated formation of liposome-Tat (lipo-Tat) complexes via hydrophobic bonds.
MATERIALS AND METHODS
Cells and culture
The HeLa-derivative cell lines, HL3T1 and TZM-bl, were obtained from the U.S. National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID). HL3T1 was generated by the cotransfection of pL3CATt and pSV2neo and selected in geneticin (G418), containing the stably integrated, silent gene of chloramphenicol acetyl transferase (CAT) linked to the promoter of HIV-1 long terminal repeat (LTR) (39, 40). TZM-bl was generated from the parent cell line, JC.53, by introducing a lentiviral vector reconstructed with luciferase and β-galactosidase (β-gal) genes under the control of HIV-1 LTR. This cell line also stably expressed large amounts of CD4 and CCR5 (41–43). SVGA-LTR-CAT cells were selected from SVGA cells, a human astrocyte cell line, transfected with pLTR-CAT (15, 44). These cell lines were passaged twice a week in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic-animistic liquid (Invitrogen, Carlsbad, CA, USA).
Tat plasmids and Tat proteins
pET16b-Tat72 was constructed from vector pET16b (EMD Biosciences, San Diego, CA, USA) by inserting Tat72 cDNA that was amplified from pTat72 (45). Similarly, Tat101 DNA was amplified from pTat101 (45) and inserted into vector pFastBac1 (Invitrogen) generating pFastBac1/Tat101. This plasmid was further inserted with a mutant of green fluorescence protein gene, Venus, to construct a Tat fusion plasmid, pFastBac1-Tat101v. The fused Tat101-venus DNA was finally introduced into pET16b generating pET16b-Tat101v. The plasmids pET16b-Tat72 and pET16b-Tat101v were transformed into BL21-Gold competent cells (Stratagene, La Jolla, CA, USA). After overnight culture, expression of Tat72 or Tat101v was induced by 3 mM isopropyl-β-d-thiogalactoside for 4 h. Recombinant proteins were purified through a 1-ml IMAC cartridge on the Profinia Protein Purification System (Bio-Rad, Hercules, CA, USA), lyophilized, and stored at −20°C. Recombinant Tat86 was provided by the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID (46). Tat-FITC was purchased from Autogen Bioclear UK Ltd. (Wilts, UK).
Preparation of lipo-Tat complexes and LTR transactivation
Lipofectamine2000 (Lipo2000), Lipofectamine RNAiMAX, and DMRIE-C were purchased from Invitrogen. Tat proteins were dissolved in Opti-MEM I reduced-serum medium (Invitrogen), portioned into aliquots, and frozen at −80°C. Cationic liposome-Tat complexes were initially prepared in Opti-MEM I according to the manufacturer's protocols except for substituting Tat proteins for DNA, incubated for 20–30 min at room temperature, and then transferred to the cell cultures in a final ratio of <1:250 (v/v) of lipo-Tat to culture medium. The LTR-transactivation assays were performed in HL3T1, TZM-bl, or SVGA-LTR-CAT cells with 60–80% confluence (96-well plate); cells were lysed 24–48 h postincubation with Tat protein or the lipo-Tat complexes. LTR transactivation levels in the cell lysates were measured by CAT or β-gal ELISA kit (Roche Applied Science, Indianapolis, IN, USA).
Flow cytometry
To investigate the attachment and uptake of Tat-FITC in HL3T1 and TZM-bl cells, exponentially growing cells were dissociated with nonenzymatic cell dissociation medium (Sigma-Aldrich, St. Louis, MO, USA), centrifuged, and resuspended in Opti-MEM. Cells (1×106) were treated with 10 μg/ml of Tat-FITC with or without Lipo2000 coupling in a total of 150 μl Opti-MEM in each well of a 48-well plate. For analysis of the attachment, cells were incubated for 20 min at 37°C and then washed with precooled Dulbecco's phosphate-buffered saline (DPBS; Invitrogen) and put on ice until acquisition by flow cytometer (BD FACSCalibur; BD Biosciences, San Jose, CA, USA). However, an extra step of trypsinization was performed to analyze intracellular uptake (47). Briefly, the cells were treated as described above for 2 h, digested in trypsin solution (Invitrogen) for 15 min at 37°C, washed with precooled DPBS, and then put on ice until acquisition.
Immunostaining and colocalization
HL3T1 or TZM-bl cells were seeded in 48-well plates (BD Biosciences) placed with polylysine precoated coverslips (Electron Microscopy Sciences, Hatfield, PA, USA) at 1 d prior to the experiment. The cells with 80% confluence were treated with Tat101v alone or the lipo-Tat101v complexes in 100 μl of Opti-MEM, together with or without Alexa Fluor 568-conjugated transferrin or Alexa Fluor 594-conjugated cholera toxin B (Invitrogen) for 15 min to 1 h, washed with precooled DPBS, and then fixed in 4% (w/v) paraformaldehyde for 20 min at room temperature. Monoclonal anti-clathrin heavy chain and rabbit anti-caveolin1 antibodies were purchased from Sigma-Aldrich. Goat anti-mouse Alexa Fluor 594 and goat anti-rabbit Alexa Fluor 594 were purchased from Invitrogen. The fixed cells were incubated with primary antibody (1:200 dilution for mouse anti-clathrin and 1:3000 for rabbit anti-caveolin1) for 2 h at room temperature, washed 3 times, incubated with secondary antibody (1:500 dilution) for 1 h at room temperature, and then washed 3 times. Coverslips were mounted on slides with Gel-Mount fluid (Electron Microscopy Sciences) and examined under a Zeiss 510-Meta confocal microscope (Carl Zeiss, Oberkochen, Germany). Photomicrographs were taken in the Z-stack mode.
Chemicals and inhibition of Tat uptake
All pharmacological compounds were purchased from Sigma-Aldrich, including chloroquine diphosphate (ChQ), chlorpromazine hydrochloride (CPZ), filipin III, methyl-β-cyclodextrin (MβCD), genistein, amiloride hydrochloride hydrate, 5-(N-ethyl-N-isopropyl) amiloride (EIPA), nocodazole, paclitaxel, cytochalasin D, latrunculin A, bafilomycin A1, brefeldin A, heparin sodium, dextran sulfate sodium, diethylaminoethyl cellulose (DEAE)-dextran hydrochloride, hexadimethrine bromide (polybrene), poly-l-lysine hydrobromide, and spermine tetrahydrochloride. The cultures were pretreated with pharmacological compounds for 30–60 min; the Tat-LTR transactivation assays were then performed as described above. Each compound was tested in the specified cell lines for toxicity before carrying out the Tat uptake experiments, all of which were performed below the concentration showing minimal toxicity of the compounds.
Hydrophobic peptides and inhibition assay of the Tat-LTR transactivation
A 15-mer Tat peptide complete set was obtained from the NIH AIDS Research and Reference Reagent Program. A Tat hydrophobic peptide (VCFITKALGISYG) and a scrambled (SC) hydrophobic peptide (SVGLPISMYFWGT) were synthesized from Chi Scientific (Maynard, MA, USA) and purified by high-pressure liquid chromatography with a purity of ≥95%. Lipo2000 was first complexed with specific concentrations of the peptides for 30 min, then mixed with Tat86 protein for another 30 min, and finally transferred to TZM-bl or HL3T1 cultures. The cells were cultivated for another day, and levels of β-gal were quantified in the cell lysates.
Inhibition assay of HIV-1 replication
TZM-bl cells were preseeded in 96-well plates. Cells with 60–80% confluence were pretreated with the serially diluted peptides complexed to Lipo2000 for 1 h at 37°C and then infected with HIV-1 JRCSF at 2000 TCID50/ml in a final 100 μl volume of culture medium. Levels of β-gal and HIV-1 p24 were quantified in the cell lysates and the supernatants, respectively.
Statistical analysis
Mean values and sd were calculated for experiments performed in duplicates or triplicates and repeated ≥3 times. Comparisons were made using Student's t test, as shown in the figure legends.
RESULTS
Dramatic increase in transduction of Tat protein to the nucleus by cationic liposomes
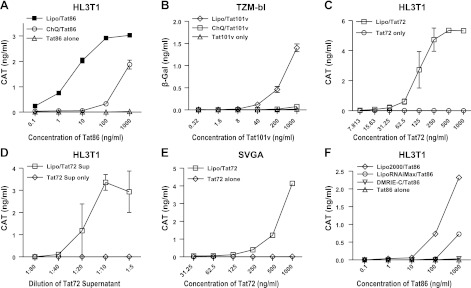
To determine the mechanism by which Tat protein enters cells and results in LTR transactivation, we initially incubated cells that were stably transfected with the LTR-CAT gene or LTR-β-galactosidase with recombinant Tat proteins. LTR transactivation was detected only using high concentrations of the recombinant Tat proteins (Tat86 or Tat101: >5 μg/ml; Tat72: >10 μg/ml) in these cells. A lysosomotropic agent, ChQ, was previously used to enhance Tat trafficking from cytoplasm to nucleus. ChQ is a weak base that neutralizes the acidic compartments of lysosomes and prevents Tat degradation, thereby enhancing its nuclear delivery (19). Consistent with this observation, we found that ChQ enhanced Tat uptake (Fig. 1A, B). However, we found that the delivery of Tat proteins to the nucleus could be dramatically enhanced via a cationic liposome reagent, Lipo2000, using each of the recombinant Tat proteins (Fig. 1A–C) as well as the supernatants from Hela cells transfected with pcDNA-Tat72 (Fig. 1D). This enhancement was dose dependent, as determined by the concentration of Tat proteins complexed with the liposomes and then serially diluted. LTR transactivation could be detected with concentrations of Tat86 or Tat101v at <10 ng/ml when complexed to the cationic liposomes. Thus, the effects of Tat were enhanced ≥1000-fold for Tat86 and >500-fold for Tat101v. For most of the subsequent experiments, we used Tat86 due to the ease of availability from the NIH AIDS reference reagent program.
Figure 1.
Enhancement of Tat uptake by complexing Tat proteins with cationic liposomes. Tat uptake was monitored in cell lines stably transfected with HIV-LTR conjugated to a reporter gene, CAT or β-galactosidase (β-gal). A) LTR transactivation of Tat86 was significantly enhanced by Lipo2000 (1:500) or ChQ (60 μM) in HL3T1 cells. B) LTR transactivation of Tat101v was enhanced by Lipo2000 (1:500) or ChQ (60 μM) in TZM-bl cells. C) LTR transactivation of Tat72 was significantly enhanced by Lipo2000 (1:250) in HL3T1 cells. D) LTR transactivation was enhanced using pcDNA-Tat72-transfected supernatant (Tat72 Sup) in the presence of Lipo2000 (1:500). E) Enhancement of Tat72-LTR transactivation with Lipo2000 (1:250) was observed in SVGA-LTR-CAT cells. F) LTR transactivation of Tat86 was enhanced by different cationic liposome reagents (1:500) in HL3T1 cells. Results are representative of ≥3 experiments for each condition.
This enhancement was consistently demonstrated in several cell lines stably transfected with LTR-CAT or LTR-β-gal using the lipo-Tat complexes. These included HL3T1 (Fig. 1A, C) and TZM-bl cells (Fig. 1B) derived from Hela cells and SVGA cells (Fig. 1E) derived from human astrocytes. Thus, it appears that Tat uptake mediated by lipo-Tat complexes was independent of cell type, although the HL3T1 cells were more sensitive compared to the TZM-bl and SVGA cells.
Different cationic liposome reagents were further tested for their ability to modulate Tat protein uptake. Lipo2000 was most effective in enhancing Tat-mediated LTR transactivation, followed by Lipofectamine RNAiMAX, while DMRIE-C had only minimal activity (Fig. 1F). The variability of liposome reagents to facilitate the uptake of Tat protein may be due to several factors, including lipid structure, binding affinity of Tat to the cationic lipid, and/or ability of the cationic lipid to facilitate Tat-mediated effects.
Increased membrane binding and intracellular uptake of Tat proteins by cationic liposomes
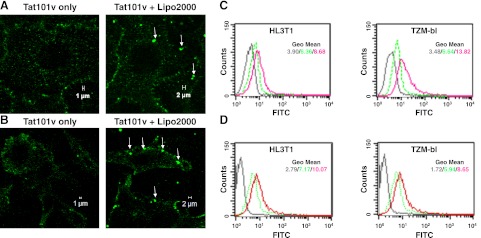
Tat membrane binding and subcellular localization were investigated using fluorescently labeled Tat (Tat101v or Tat-FITC). In the cells treated with the lipo-Tat101v, enhanced binding and 1- to 2-μm fluorescent particles were observed on the cell membrane (Fig. 2A, B). However, only a few particles <1 μm were observed on the cells treated with Tat101v alone. This suggests that these large particles were associated with the facilitation of Tat transduction by cationic liposomes.
Figure 2.
Morphological characterization of fluorescent Tat proteins complexed to cationic liposomes. A) Binding of Tat101v without or with Lipo2000 (1:500) in HL3T1 cells after 20 min incubation. Lipo-Tat complexes are seen on the cell membrane (arrows). B) Binding of Tat101v without or with Lipo2000 (1:500) in TZM-bl cells after 20 min incubation. C) Membrane attachment of Tat-FITC complexed with Lipo2000 (1:200) or uncomplexed on HL3T1 and TZM-bl cells after 20 min incubation. D) Intracellular uptake of Tat-FITC with or without Lipo2000 (1:250) in HL3T1 and TZM-bl cells after incubation for 2 h and trysinization for 15 min. Colors in graphs of flow cytometry: dark gray, untreated cells; green, Tat-FITC only; red, Tat-FITC complexed with Lipo2000. View in images: ×630.
Analysis by flow cytometry confirmed that more attachment of lipo-Tat-FITC was detected in both HL3T1 [41.69% or mean channel fluorescence (mcf) of 8.68] and TZM-bl cells (48.53% or mcf of 13.82) when compared with cells treated with Tat-FITC alone (13.04% or mcf 6.36 in HL3T1 cells, and 5.12% or mcf of 6.64 in TZM-bl cells; Fig. 2C). Moreover, when cells were treated with lipo-Tat-FITC or Tat-FITC alone for 2 h, then digested with trypsin to remove the membrane-bound Tat protein and finally analyzed by FACS, the results still showed considerably more intracellular Tat-FITC taken up in the lipo-Tat-FITC-treated cells, compared with treatment without the lipo-Tat complexes (43.62% or mcf of 10.07 vs. 17.11% or mcf of 7.17 in HL3T1 cells; 47.28% or mcf of 8.65 vs. 19.10% or mcf of 5.94 in TZM-bl cells; Fig. 2D). This finding indicates that the liposomes increased Tat attachment and uptake in both cell types nearly 2- to 3-fold.
Uptake of lipo-Tat complexes may occur via lipid raft-mediated endocytosis and macropinocytosis.
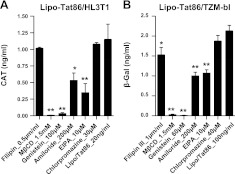
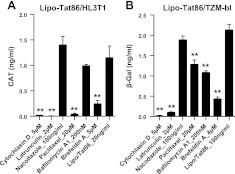
To further characterize the mechanism of Tat uptake by these cells, we used a panel of pharmacological compounds that target various endocytic pathways. We initially treated HL3T1 and TZM-bl with cholesterol-sequestering or depleting agents, filipin III and MβCD, and then exposed them to the lipo-Tat complexes. Although filipin III failed to influence the uptake of lipo-Tat very significantly, MβCD almost completely inhibited the uptake of lipo-Tat (Fig. 3), suggesting that lipo-Tat uptake required an intact cholesterol-rich membrane and may be involved in lipid raft-mediated endocytosis. Similarly, the cholesterol-independent tyrosine kinase inhibitor, genistein, almost completely abolished LTR transactivation of Tat86 in both cell lines (Fig. 3), suggesting that lipo-Tat uptake was tyrosine kinase dependent. Blockers of macropinocytosis, amiloride and EIPA partially inhibited the transduction of lipo-Tat in both HL3T1 and TZM-bl cells (Fig. 3), indicating that macropinocytosis played a partial role in lipo-Tat uptake. This finding was consistent with the observation that the larger green particles were present in the cells treated with lipo-Tat101v (Fig. 2A, B). However, the clathrin-mediated endocytosis inhibitor, CPZ, had no significant effect on lipo-Tat uptake in either HL3T1 or TZM-bl cells (Fig. 3).
Figure 3.
Effect of endocytic blockers on Tat-LTR transactivation in the presence of Lipo2000. A) LTR transactivation was blocked partially or completely by endocytic inhibitors in HL3T1 cells treated with the lipo-Tat86 complexes (Tat86=20 ng/ml). B) LTR transactivation was blocked partially or completely by endocytic inhibitors in TZM-bl cells treated with the lipo-Tat86 complexes (Tat86=100 ng/ml). Results represent ≥3 experiments. *P < 0.05, **P < 0.01; Student's t test.
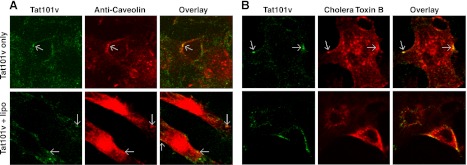
The mechanism of Tat uptake was also explored by immunostaining. We observed a small amount of colocalization of Tat101v with anti-clathrin antibody (Fig. 4A) or transferrin (classic indicator of clathrin-mediated endocytosis; Fig. 4B) in H3T1 cells, but no colocalization for lipo-Tat101v. This finding suggested that clathrin-mediated endocytosis was partially involved in Tat uptake alone but was abolished by Tat complexing to cationic liposomes (Figs. 3A and 4A). In contrast, colocalization was noted for both Tat101v and lipo-Tat101v with anti-caveolin antibody (Fig. 5A) or cholera toxin B (a marker of caveolae-dependent endocytosis; Fig. 5B) in TZM-bl cells, confirming the role of caveolae-mediated endocytosis in Tat or lipo-Tat uptake. Together, lipo-Tat uptake is largely restricted by the lipid bilayer of cell membranes involved in lipid raft-mediated endocytosis, and occurs in part by macropinocytosis. These effects are summarized in Table 1.
Figure 4.
Colocalization of Tat with anti-clathrin heavy chain (HC) or transferrin. A) Tat101v (green) colocalizes with anti-clathrin HC (red) in HL3T1 cells (arrows), but not in the presence of Lipo2000. B) Similar results are also found for Tat101v (green) and Alexa Fluor 568 transferrin (red) in the presence or absence of Lipo2000 in HL3T1 cells. View: ×630.
Figure 5.
Colocalization of Tat with anti-caveolin or cholera toxin B. A) Focal areas of colocalizations (arrows) are shown for Tat101v (green) and anti-caveolin (red) in both the presence and absence of Lipo2000 in TZM-bl cells. B) Tat101v (green) colocalized with Alexa Fluor 594 cholera toxin B (red) in both the presence and absence of Lipo2000 in TZM-bl cells. Magnification: ×630.
Table 1.
Effects of endocytic and trafficking blockers on Tat uptake and intracellular delivery
| Blocker | Target and mechanism | Lipo/Tat-LTR transactivation |
|---|---|---|
| Macropinocytosis | ||
| Amiloride | Selective T-type calcium channel blocker and blocker of epithelial sodium channel | ↓↓ |
| EIPA | Selective blocker of Na+/H+ antiport | ↓↓ |
| Clathrin-dependent endocytosis | ||
| Chlorpromazine | A cationic amphiphilic agent that inhibits formation of clathrin-coated pits | No |
| Caveolae/lipid raft endocytosis | ||
| Filipin III | Sequestering lipid from membrane structure and inducing a profound distortion of the structure and functions of the cholesterol-rich membrane domain | ↓ |
| MβCD | Extraction of cholesterol | ↓↓↓↓ |
| Genistein | Cholesterol-independent tyrosine kinase inhibitor | ↓↓↓↓ |
| Trafficking | ||
| Nocodazole | Disrupting polymerized microtubules | No |
| Paclitaxel | Binding to the N-terminal region of β-tubulin and promoting formation of highly stable microtubules that resist depolymerization | ↓↓↓ |
| Cytochalasin D | Shortening actin filaments by blocking monomer addition at the fast end of polymer | ↓↓↓↓ |
| Latrunculin A | Impairing the integrity of actin-filament by sequestering actin monomers; affecting endosomal trafficking | ↓↓↓↓ |
| Bafilomycin A | Inhibitor of vacuolar proton ATPase, leading increase of endosomal pH | ↓↓ |
| Brefeldin A | Causing early endosomes to form a tubular network and preventing early-to-late-endosome transition; disrupting movement of material from ER to Golgi apparatus | ↓↓↓ |
Intracellular trafficking of the lipo-Tat complexes is mediated via actin filaments
Protein transport in cytoplasm may be mediated by actin filaments and microtubules. To determine their role in Tat trafficking to the nucleus, cells were pretreated with compounds known to alter their function and then exposed to Tat. Disruption of actin filaments using cytochalasin D or latrunculin A, led to a significant decrease in lipo-Tat transport in either HL3T1 (Fig. 6A) or TZM-bl (Fig. 6B). However, impairment of polymerized microtubules with nocodazole had no effect, while enhancing microtubule polymerization with paclitaxel inhibited Tat transport (Fig. 6), although the magnitude of effect differed between the two cell lines. In addition, inhibition of vacuolar-type H(+)-ATPase by bafilomycin A1, which leads to deacidification of cellular organelles, had a minor effect while inhibition of intracellular protein transport by brefeldin A (Fig. 6) significantly prevented lipo-Tat transport. Collectively, these results support the major role that actin-filaments play in the intracellular transport of lipo-Tat complexes (Table 1).
Figure 6.
Effect of trafficking blockers on the functional nuclear delivery of Tat86 in the presence of Lipo2000. A) LTR transactivation was completely or partially blocked by cytochalasin D, latrunculin A, paclitaxel and brefeldin A, but not by nacodazole and bafilomycin A1 in HL3T1 cells treated with the lipo-Tat86 complexes (20 ng/ml). B) LTR transactivation was completely or partially blocked by cytochalasin D, latrunculin A, paclitaxel, bafilomycin A1 and brefeldin A, but not by nacodazole in TZM-bl cells treated with the lipo-Tat86 complexes (100 ng/ml). Results represent ≥3 experiments. *P < 0.05, **P < 0.01; Student's t test.
Cellular uptake of the lipo-Tat complexes is charge dependent
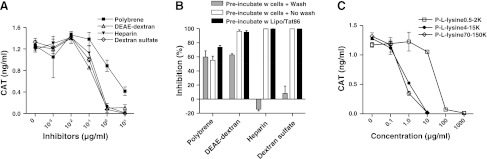
Previous studies defined the role of negatively charged HSPGs on the cell membrane both in interacting with Tat and in its uptake (20, 23). Therefore, we determined whether the interactions of the lipo-Tat complexes with the cell membrane were also charge dependent. Both negatively (−) and positively (+) charged chemicals were tested for their ability to influence lipo-Tat uptake. LTR transactivation assay was used to indicate uptake of lipo-Tat. Interestingly, both negative (heparin and dextran sulfate) and positive (DEAE-dextran and polybrene) macromolecules dose-dependently inhibited lipo-Tat uptake (Fig. 7A). When HL3T1 cells were pretreated with the macromolecules, followed by a washing step before incubating with the lipo-Tat complexes, the inhibitory effect of the negatively charged macromolecules was no longer seen, while the washing step had no significant effect on inhibiting lipo-Tat uptake by the positively charged macromolecules. These data were compared with experiments in which the washing step was eliminated or the macromolecules were preincubated with the lipo-Tat complexes and then added to the cells (Fig. 7B). This finding suggests that the macromolecules may interfere with Tat uptake by applying their negative charges to the lipo-Tat complexes or their positive charges to the cell membrane.
Figure 7.
Inhibition of lipo-Tat uptake by macromolecules carrying positive or negative charges. A) Lipo-Tat uptake was inhibited by polybrene carrying positive charges and polysaccharides carrying positive charges (DEAE-dextran) or negative charges (heparin and dextran sulfate; Tat86=20 ng/ml). B) Inhibition of negative macromolecules on Tat uptake was abolished by pretreating the cells for 30 min, followed by a washing step, and then adding lipo-Tat86 complexes (50 ng/ml); however, only slight or no influence was found on the inhibition using the same protocol for positive macromolecules or following preincubation of negative/positive macromolecules with lipo-Tat complexes. C) Uptake of the lipo-Tat complexes was inhibited by poly-l-lysine hydrobromide (P-l-lysine) with different molecular weights (carrying positive charges; Tat72=400 ng/ml). Results represent 3 experiments.
To determine whether the mass of macromolecules played a role in lipo-Tat uptake, poly-l-lysine bromides of different molecular masses (500 to >15,000) were used to perform the inhibition assay. The molecules with higher molecular mass showed more potent inhibition (Fig. 7C). Another positively charged molecule with a small molecular mass (spermine tetrahydrochloride) also showed inhibitory activity, but the potency was weaker (data not shown). Therefore, lipo-Tat uptake is not only charge dependent, but also influenced by steric hindrance, which is related to the molecular mass of poly-l-lysine.
Hydrophobic bonds play a key role in the formation of the lipo-Tat complexes
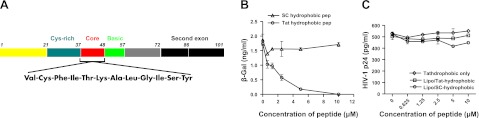
Cationic liposome reagents are used widely to deliver negatively charged DNA or siRNA into cells. Since Tat, which is positively charged, could also be delivered similarly, we determined whether the effect was specific for Tat by substituting Tat with recombinant GFP protein. However, no translocation of GFP was noted (data not shown). We next determined whether a hydrophobic domain located in Tat 36–47 played a role in these interactions. A 13-mer peptide covering the hydrophobic domain of Tat was synthesized (Fig. 8A) and an SC hydrophobic peptide based mainly on the composition of Tat hydrophobic domain was used as a control. The peptides were preincubated with Lipo2000, followed by incubation with Tat protein. Dose-dependent inhibition of Tat-LTR transactivation was observed with Tat hydrophobic peptide (Fig. 8B), suggesting that the hydrophobic domain of Tat may play a critical role in the formation of lipo-Tat complexes, which is very likely mediated by hydrophobic bonds between Tat protein and cationic liposomes.
Figure 8.
Role of Tat hydrophobic domain on the uptake of the lipo-Tat complexes. A) Diagram of HIV-1 Tat protein and sequence of its hydrophobic domain. B) Tat86 uptake and its LTR-transactivation was inhibited by preincubating Lipo2000 with Tat hydrophobic peptide for 30 min before complexing Tat86 (200 ng/ml), but no significant inhibition was found if it was replaced by an SC hydrophobic peptide. Results represent 3 experiments. C) No significant inhibition of HIV-1 JRCSF replication was observed when TZM-bl cells were pretreated with Tat or SC hydrophobic peptides complexed to Lipo2000 or uncomplexed.
To exclude the possibility that the hydrophobic peptide might directly enter into the cells and inhibit LTR transactivation, TZM-bl cells were pretreated with the serially diluted peptides and then infected with HIV-1 JRCSF. Both HIV-1 p24 and β-gal ELISA were performed in the supernatants and cell lysates, respectively. No significant inhibition was noted with any of the peptides (Fig. 8C and data not shown).
DISCUSSION
The membrane-transduction property of Tat protein was first observed soon after the discovery of the protein itself (19, 48). Based on this property, Tat peptides (residues 1–72 or 37–72) have been chemically linked to macromolecules such as β-gal and horseradish peroxidase; these Tat chimeras are shown to be taken up by the cells in vitro and in vivo following intravenous injection (49, 50). Tat-penetrating peptide derived from the basic region has proven to be an effective vehicle for delivering diverse cargoes of macromolecules into different cells (22, 27). In addition, the basic Tat peptide has been linked to negatively charged DNA or liposomes to increase DNA transfection efficiency (23, 36). Surprisingly, we found that commercially available cationic liposomes used for DNA transfection could significantly increase Tat uptake in the cells; Lipofectamine2000 was most efficient among the tested reagents.
Endocytosis is considered the major mechanism involved in Tat uptake (51). It is shown that a lysosomotropic agent, ChQ, can facilitate Tat transportation to the nucleus and significantly increase LTR transactivation (19). In the absence of ChQ treatment, the majority of Tat is trapped in cytoplasmic vesicles, with only a trace amount of nuclear delivery (31). The functional nuclear delivery of Tat depends on its efficient intracellular trafficking or escaping from degradation in lysosomes (31, 32). Lysosomotropic agents are frequently employed to increase Tat release from endosomal/lysosomal lumina and provide access to the nucleus (52). However, the associated toxicity at the effective concentration prevents lysosomotropic agents from being used therapeutically (32). Hence, other strategies are used for endosomal/lysosomal escape of cargoes (53). For example, the N-terminal region of influenza virus hemagglutinin protein, HA2, which can destabilize lipid membranes at low pH, is linked to the basic region of Tat; this fusogenic peptide markedly enhances Tat escape from macropinosomes (32, 53). A biotinylated, pH-responsive polymer (propylacrylic acid) is incorporated in Tat-streptavidin complexes, and its nuclear localization is enhanced (31). A previous study showed ∼20% increased uptake of Tat using Lipofectamine (54). However, we found that Lipo2000 could enhance the functionally nuclear delivery of Tat proteins by 1000-fold without apparent toxicity. Another study also showed that Lipofectin could significantly increase the functional delivery of Tat to the nucleus (55). Nevertheless, no further approach was performed for the mechanism in which Tat uptake is facilitated by cationic liposomes. Therefore, we explored the underlying mechanisms of the cationic liposome-mediated uptake of Tat proteins.
Cationic liposomes enhanced Tat uptake via several steps. The enhanced binding of the lipo-Tat complexes to the cell membrane was observed by microscopy and confirmed by flow cytometry, which was followed by increased Tat intracellular uptake. Our data further showed that this uptake may be mediated largely via lipid raft-dependent endocytosis and macropinocytosis. Recently, it was clarified that clathrin-independent, lipid raft-dependent endocytosis at least includes caveolar and noncaveolar endocytosis (56–61), and lipid raft-dependent, noncaveolar endocytosis can be dynamin dependent or independent (61). Studies also show macropinocytosis is possibly lipid raft dependent, but dynamin independent (32, 59, 61). Hence, the endocytic mechanism involved in the lipo-Tat uptake is consistent with our studies.
The marked enhancement of LTR transactivation by the lipo-Tat complexes cannot be explained only by the intensified attachment and modulation of endocytic pathways. Cationic liposomes might also play a crucial role in the intracellular trafficking of Tat proteins. Although the translocation of Tat protein from endosomes to cytosol may occur by inserting a single Trp on Tat 11 into the endosomal membrane (62), this process is not efficient (33), which was further confirmed by our data. Studies indicate that cationic liposomes containing dioleoyl phosphatidylethanolamine (DOPE) may destabilize the endosomal membrane postendocytosis and release macromolecules (e.g., DNA and proteins) into the cytosol (63, 64); however, little is known about how the destabilization and the concomitant release of macromolecules proceed via cationic lipids (65). The cationic liposomes not only facilitated Tat release from endosomes/lysosomes, but also modified its trafficking pathways to the nucleus postendosomal release (28). We observed that microfilaments were critical to the nuclear delivery of Tat and the stabilization of polymerized microtubules impaired this delivery because of Tat's binding affinity to microtubules (66). This is not surprising since microfilaments are concentrated beneath the cell membrane, and microtubules radiate throughout cytoplasm and are usually required for the transport of most cellular cargoes (67). In addition, blocking the trafficking between endoplasmic reticulum and Golgi apparatus partially impaired Tat transport, indicating that these organelles might also be involved in the intracellular delivery of Tat protein.
We also explored the mechanism by which liposomes complexed with Tat and mediated its attachment to the cell membrane particularly because both liposomes and Tat are positively charged macromolecules. We confirmed that the interaction between the positive charges of the lipo-Tat complexes and the anionic structures on cell membranes (mainly heparan sulfate proteoglycans) were still essential for the subsequent transduction of Tat as previously reported (20, 22, 23, 68). Notably, we found that the hydrophobic domain of Tat played a critical role in mediating the formation of complexes between Tat and cationic liposomes. Tat basic peptides are complexed with DNA and/or liposomes to mediate DNA transfection or the delivery of liposomes (69–71); hence, it is likely that the additional incorporation of the hydrophobic domain of Tat might increase its efficiency. In a previous study, we showed that the cysteine-rich peptide (aa 28–42) could significantly enhance Tat protein uptake (33), which likely benefits from the enhanced hydrophobicity of Tat due to formation of disulfide bonds between the cysteine-rich peptide and Tat protein (72). Tat basic peptide-based methods have been extensively explored to deliver different macromolecules into cells in vitro and in vivo because of its potential ability to deliver diverse cargoes into all types of organs and cells (73). Using the hydrophobic and basic domains together is worthy of further exploration for such deliveries.
In summary, the facilitation of cationic liposomes on Tat delivery is a multiple-step process, which includes formation of lipo-Tat complexes by hydrophobic bonds, enhancement of attachment, modulation of endocytic pathways, increased release from endosomes/lysosomes, and modification of intracellular trafficking. All of these events result in dramatically increasing the nuclear delivery of Tat proteins. These findings implicate an important role for both the Arg-rich region and the hydrophobic domain in the uptake of Tat protein, and provide multiple targets for optimizing Tat peptide-mediated delivery of macromolecules and for using Tat protein as a therapeutic vaccine.
Acknowledgments
This study was supported by U.S. National Insitutes of Health grants R01NS039253 (A.N.) and R01AI058842 (R.J.M.).
The authors thank Yan Huang (A.N. laboratory) and Michael Delannoy (Johns Hopkins Medical InstitutionsMicroscope Facility, Baltimore, MD, USA) for technical assistance.
Footnotes
- β-gal
- β-galactosidase
- CAT
- chloramphenicol acetyl transferase
- ChQ
- chloroquine diphosphate
- CPP
- cell-penetrating peptide
- CPZ
- chlorpromazine hydrochloride
- DEAE
- diethylaminoethyl cellulose
- DPBS
- Dulbecco's phosphate-buffered saline
- EIPA
- 5-(N-ethyl-N-isopropyl) amiloride
- FBS
- fetal bovine serum
- HIV-1
- human immunodeficiency virus type 1
- HS
- heparan sulfate
- HSPG
- heparan sulfate proteoglycan
- lipo-Tat
- liposome-Tat
- Lipo2000
- Lipofectamine2000
- LTR
- long terminal repeat
- MβCD
- methyl-β-cyclodextrin
- mcf
- mean channel fluorescence
- PTD
- protein transduction domain
- SC
- scrambled
- TAR
- transactivation response
- Tat
- transactivator of transcription
- Tat101v
- Tat101venus
REFERENCES
- 1. Rana T. M., Jeang K. T. (1999) Biochemical and functional interactions between HIV-1 Tat protein and TAR RNA. Arch. Biochem. Biophys. 365, 175–185 [DOI] [PubMed] [Google Scholar]
- 2. Stauber R. H., Pavlakis G. N. (1998) Intracellular trafficking and interactions of the HIV-1 Tat protein. Virology 252, 126–136 [DOI] [PubMed] [Google Scholar]
- 3. Jones K. A., Peterlin B. M. (1994) Control of RNA initiation and elongation at the HIV-1 promoter. Ann. Rev. Biochem. 63, 717–743 [DOI] [PubMed] [Google Scholar]
- 4. Bilodeau P. S., Domsic J. K., Stoltzfus C. M. (1999) Splicing regulatory elements within tat exon 2 of human immunodeficiency virus type 1 (HIV-1) are characteristic of group M but not group O HIV-1 strains. J. Virol. 73, 9764–9772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosen C. A., Terwilliger E., Dayton A., Sodroski J. G., Haseltine W. A. (1988) Intragenic cis-acting art gene-responsive sequences of the human immunodeficiency virus. Proc. Natl. Acad. Sci. U. S. A. 85, 2071–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Y., Wang Z., Rana T. M. (1996) Visualizing a specific contact in the HIV-1 Tat protein fragment and trans-activation responsive region RNA complex by photocross-linking. J. Biol. Chem. 271, 10391–10396 [DOI] [PubMed] [Google Scholar]
- 7. Ruben S., Perkins A., Purcell R., Joung K., Sia R., Burghoff R., Haseltine W. A., Rosen C. A. (1989) Structural and functional characterization of human immunodeficiency virus Tat protein. J. Virol. 63, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuppuswamy M., Subramanian T., Srinivasan A., Chinnadurai G. (1989) Multiple functional domains of Tat, the trans-activator of HIV-1, defined by mutational analysis. Nucl. Acids Res. 17, 3551–3561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jeang K. T., Xiao H., Rich E. A. (1999) Multifaceted activities of the HIV-1 transactivator of transcription, Tat. J. Biol. Chem. 274, 28837–28840 [DOI] [PubMed] [Google Scholar]
- 10. Subramanian T., Kuppuswamy M., Venkatesh L., Srinivasan A., Chinnadurai G. (1990) Functional substitution of the basic domain of the HIV-1 trans-activator, Tat, with the basic domain of the functionally heterologous Rev. Virology 176, 178–183 [DOI] [PubMed] [Google Scholar]
- 11. Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. (1990) Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature 345, 84–86 [DOI] [PubMed] [Google Scholar]
- 12. Ensoli B., Buonaguro L., Barillari G., Fiorelli V., Gendelman R., Morgan R. A., Wingfield P., Gallo R. C. (1993) Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 67, 277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turchan J., Anderson C., Hauser K. F., Sun Q., Zhang J., Liu Y., Wise P. M., Kruman I., Maragos W., Mattson M. P., Booze R., Nath A. (2001) Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci. 2, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnston J. B., Zhang K., Silva C., Shalinsky D. R., Conant K., Ni W., Corbett D., Yong V. W., Power C. (2001) HIV-1 Tat neurotoxicity is prevented by matrix metalloproteinase inhibitors. Ann. Neurol. 49, 230–241 [DOI] [PubMed] [Google Scholar]
- 15. Chauhan A., Turchan J., Pocernich C., Bruce-Keller A., Roth S., Butterfield D. A., Major E. O., Nath A. (2003) Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J. Biol. Chem. 278, 13512–13519 [DOI] [PubMed] [Google Scholar]
- 16. Chang H. C., Samaniego F., Nair B. C., Buonaguro L., Ensoli B. (1997) HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 11, 1421–1431 [DOI] [PubMed] [Google Scholar]
- 17. Rayne F., Debaisieux S., Yezid H., Lin Y. L., Mettling C., Konate K., Chazal N., Arold S. T., Pugniere M., Sanchez F., Bonhoure A., Briant L., Loret E., Roy C., Beaumelle B. (2010) Phosphatidylinositol-(4,5)-bisphosphate enables efficient secretion of HIV-1 Tat by infected T-cells. EMBO J. 29, 1348–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rayne F., Debaisieux S., Bonhoure A., Beaumelle B. (2010) HIV-1 Tat is unconventionally secreted through the plasma membrane. Cell Biol. Internat. 34, 409–413 [DOI] [PubMed] [Google Scholar]
- 19. Frankel A. D., Pabo C. O. (1988) Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55, 1189–1193 [DOI] [PubMed] [Google Scholar]
- 20. Vives E. (2003) Cellular uptake [correction of utake] of the Tat peptide: an endocytosis mechanism following ionic interactions. J. Mol. Recognit. 16, 265–271 [DOI] [PubMed] [Google Scholar]
- 21. Debaisieux S., Rayne F., Yezid H., Beaumelle B. (2012) The ins and outs of HIV-1 Tat. Traffic 13, 355–363 [DOI] [PubMed] [Google Scholar]
- 22. Belting M. (2003) Heparan sulfate proteoglycan as a plasma membrane carrier. Trends Biochem. Sci. 28, 145–151 [DOI] [PubMed] [Google Scholar]
- 23. Tyagi M., Rusnati M., Presta M., Giacca M. (2001) Internalization of HIV-1 Tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 276, 3254–3261 [DOI] [PubMed] [Google Scholar]
- 24. Silhol M., Tyagi M., Giacca M., Lebleu B., Vives E. (2002) Different mechanisms for cellular internalization of the HIV-1 Tat-derived cell penetrating peptide and recombinant proteins fused to Tat. Eur. J. Biochem. FEBS 269, 494–501 [DOI] [PubMed] [Google Scholar]
- 25. Futaki S., Nakase I., Suzuki T., Youjun Z., Sugiura Y. (2002) Translocation of branched-chain arginine peptides through cell membranes: flexibility in the spatial disposition of positive charges in membrane-permeable peptides. Biochemistry 41, 7925–7930 [DOI] [PubMed] [Google Scholar]
- 26. Wender P. A., Mitchell D. J., Pattabiraman K., Pelkey E. T., Steinman L., Rothbard J. B. (2000) The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc. Natl. Acad. Sci. U. S. A. 97, 13003–13008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Howl J., Nicholl I. D., Jones S. (2007) The many futures for cell-penetrating peptides: how soon is now? Biochem. Soc. Trans. 35, 767–769 [DOI] [PubMed] [Google Scholar]
- 28. Fittipaldi A., Ferrari A., Zoppe M., Arcangeli C., Pellegrini V., Beltram F., Giacca M. (2003) Cell membrane lipid rafts mediate caveolar endocytosis of HIV-1 Tat fusion proteins. J. Biol. Chem. 278, 34141–34149 [DOI] [PubMed] [Google Scholar]
- 29. Ferrari A., Pellegrini V., Arcangeli C., Fittipaldi A., Giacca M., Beltram F. (2003) Caveolae-mediated internalization of extracellular HIV-1 Tat fusion proteins visualized in real time. Mol. Ther. 8, 284–294 [DOI] [PubMed] [Google Scholar]
- 30. Vendeville A., Rayne F., Bonhoure A., Bettache N., Montcourrier P., Beaumelle B. (2004) HIV-1 Tat enters T cells using coated pits before translocating from acidified endosomes and eliciting biological responses. Mol. Biol. Cell 15, 2347–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rinne J., Albarran B., Jylhava J., Ihalainen T. O., Kankaanpaa P., Hytonen V. P., Stayton P. S., Kulomaa M. S., Vihinen-Ranta M. (2007) Internalization of novel non-viral vector TAT-streptavidin into human cells. BMC Bio/Tech. 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wadia J. S., Stan R. V., Dowdy S. F. (2004) Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 10, 310–315 [DOI] [PubMed] [Google Scholar]
- 33. Ma M., Nath A. (1997) Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J. Virol. 71, 2495–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duchardt F., Fotin-Mleczek M., Schwarz H., Fischer R., Brock R. (2007) A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic 8, 848–866 [DOI] [PubMed] [Google Scholar]
- 35. Jones A. T. (2008) Gateways and tools for drug delivery: endocytic pathways and the cellular dynamics of cell penetrating peptides. Int. J. Pharm. 354, 34–38 [DOI] [PubMed] [Google Scholar]
- 36. Ziegler A., Nervi P., Durrenberger M., Seelig J. (2005) The cationic cell-penetrating peptide CPP(TAT) derived from the HIV-1 protein TAT is rapidly transported into living fibroblasts: optical, biophysical, and metabolic evidence. Biochemistry 44, 138–148 [DOI] [PubMed] [Google Scholar]
- 37. Herce H. D., Garcia A. E. (2007) Molecular dynamics simulations suggest a mechanism for translocation of the HIV-1 TAT peptide across lipid membranes. Proc. Natl. Acad. Sci. U. S. A. 104, 20805–20810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bellino S., Francavilla V., Longo O., Tripiciano A., Paniccia G., Arancio A., Fiorelli V., Scoglio A., Collacchi B., Campagna M., Lazzarin A., Tambussi G., Din C. T., Visintini R., Narciso P., Antinori A., D'Offizi G., Giulianelli M., Carta M., Di Carlo A., Palamara G., Giuliani M., Laguardia M. E., Monini P., Magnani M., Ensoli F., Ensoli B. (2009) Parallel conduction of the phase I preventive and therapeutic trials based on the Tat vaccine candidate. Rev. Rec. Clin. Trials 4, 195–204 [DOI] [PubMed] [Google Scholar]
- 39. Wright C. M., Felber B. K., Paskalis H., Pavlakis G. N. (1986) Expression and characterization of the trans-activator of HTLV-III/LAV virus. Science 234, 988–992 [DOI] [PubMed] [Google Scholar]
- 40. Felber B. K., Pavlakis G. N. (1988) A quantitative bioassay for HIV-1 based on trans-activation. Science 239, 184–187 [DOI] [PubMed] [Google Scholar]
- 41. Wei X., Decker J. M., Liu H., Zhang Z., Arani R. B., Kilby J. M., Saag M. S., Wu X., Shaw G. M., Kappes J. C. (2002) Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46, 1896–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Derdeyn C. A., Decker J. M., Sfakianos J. N., Wu X., O'Brien W. A., Ratner L., Kappes J. C., Shaw G. M., Hunter E. (2000) Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 74, 8358–8367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Platt E. J., Wehrly K., Kuhmann S. E., Chesebro B., Kabat D. (1998) Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72, 2855–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Major E. O., Miller A. E., Mourrain P., Traub R. G., de Widt E., Sever J. (1985) Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc. Natl. Acad. Sci. U. S. A. 82, 1257–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li W., Huang Y., Reid R., Steiner J., Malpica-Llanos T., Darden T. A., Shankar S. K., Mahadevan A., Satishchandra P., Nath A. (2008) NMDA receptor activation by HIV-Tat protein is clade dependent. J. Neurosci. 28, 12190–12198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bohan C. A., Kashanchi F., Ensoli B., Buonaguro L., Boris-Lawrie K. A., Brady J. N. (1992) Analysis of Tat transactivation of human immunodeficiency virus transcription in vitro. Gene Exp. 2, 391–407 [PMC free article] [PubMed] [Google Scholar]
- 47. Richard J. P., Melikov K., Vives E., Ramos C., Verbeure B., Gait M. J., Chernomordik L. V., Lebleu B. (2003) Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 278, 585–590 [DOI] [PubMed] [Google Scholar]
- 48. Green M., Loewenstein P. M. (1988) Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 55, 1179–1188 [DOI] [PubMed] [Google Scholar]
- 49. Fawell S., Seery J., Daikh Y., Moore C., Chen L. L., Pepinsky B., Barsoum J. (1994) Tat-mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. U. S. A. 91, 664–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schwarze S. R., Ho A., Vocero-Akbani A., Dowdy S. F. (1999) In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285, 1569–1572 [DOI] [PubMed] [Google Scholar]
- 51. Mann D. A., Frankel A. D. (1991) Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 10, 1733–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Caron N. J., Quenneville S. P., Tremblay J. P. (2004) Endosome disruption enhances the functional nuclear delivery of Tat-fusion proteins. Biochem. Biophys. Res. Commun. 319, 12–20 [DOI] [PubMed] [Google Scholar]
- 53. Pujals S., Fernandez-Carneado J., Lopez-Iglesias C., Kogan M. J., Giralt E. (2006) Mechanistic aspects of CPP-mediated intracellular drug delivery: relevance of CPP self-assembly. Biochim. Biophys. Acta 1758, 264–279 [DOI] [PubMed] [Google Scholar]
- 54. Huang L., Farhood H., Serbina N., Teepe A. G., Barsoum J. (1995) Endosomolytic activity of cationic liposomes enhances the delivery of human immunodeficiency virus-1 trans-activator protein (TAT) to mammalian cells. Biochem. Biophys. Res. Commun. 217, 761–768 [DOI] [PubMed] [Google Scholar]
- 55. Fong S. E., Smanik P., Smith M. C., Jaskunas S. R. (1997) Cationic liposome-mediated uptake of human immunodeficiency virus type 1 Tat protein into cells. J. Virol. Methods 66, 149–157 [DOI] [PubMed] [Google Scholar]
- 56. Nabi I. R., Le P. U. (2003) Caveolae/raft-dependent endocytosis. J. Cell Biol. 161, 673–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nichols B. (2003) Caveosomes and endocytosis of lipid rafts. J. Cell Sci. 116, 4707–4714 [DOI] [PubMed] [Google Scholar]
- 58. Parton R. G., Richards A. A. (2003) Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic 4, 724–738 [DOI] [PubMed] [Google Scholar]
- 59. Kirkham M., Parton R. G. (2005) Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim. Biophys. Acta 1746, 349–363 [DOI] [PubMed] [Google Scholar]
- 60. Lajoie P., Nabi I. R. (2007) Regulation of raft-dependent endocytosis. J. Cell. Mol. Med. 11, 644–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mayor S., Pagano R. E. (2007) Pathways of clathrin-independent endocytosis. Nat. Rev. 8, 603–612 [DOI] [PubMed] [Google Scholar]
- 62. Yezid H., Konate K., Debaisieux S., Bonhoure A., Beaumelle B. (2009) Mechanism for HIV-1 Tat insertion into the endosome membrane. J. Biol. Chem. 284, 22736–22746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhou X., Huang L. (1994) DNA transfection mediated by cationic liposomes containing lipopolylysine: characterization and mechanism of action. Biochim. Biophys. Acta 1189, 195–203 [DOI] [PubMed] [Google Scholar]
- 64. Farhood H., Serbina N., Huang L. (1995) The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim. Biophys. Acta 1235, 289–295 [DOI] [PubMed] [Google Scholar]
- 65. Hoekstra D., Rejman J., Wasungu L., Shi F., Zuhorn I. (2007) Gene delivery by cationic lipids: in and out of an endosome. Biochem. Soc. Trans. 35, 68–71 [DOI] [PubMed] [Google Scholar]
- 66. Chen D., Wang M., Zhou S., Zhou Q. (2002) HIV-1 Tat targets microtubules to induce apoptosis, a process promoted by the pro-apoptotic Bcl-2 relative Bim. EMBO J. 21, 6801–6810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ross J. L., Ali M. Y., Warshaw D. M. (2008) Cargo transport: molecular motors navigate a complex cytoskeleton. Curr. Opin. Cell Biol. 20, 41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rusnati M., Tulipano G., Spillmann D., Tanghetti E., Oreste P., Zoppetti G., Giacca M., Presta M. (1999) Multiple interactions of HIV-I Tat protein with size-defined heparin oligosaccharides. J. Biol. Chem. 274, 28198–28205 [DOI] [PubMed] [Google Scholar]
- 69. Torchilin V. P., Rammohan R., Weissig V., Levchenko T. S. (2001) TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl. Acad. Sci. U. S. A. 98, 8786–8791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Torchilin V. P., Levchenko T. S., Rammohan R., Volodina N., Papahadjopoulos-Sternberg B., D'Souza G. G. (2003) Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc. Natl. Acad. Sci. U. S. A. 100, 1972–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pappalardo J. S., Quattrocchi V., Langellotti C., Di Giacomo S., Gnazzo V., Olivera V., Calamante G., Zamorano P. I., Levchenko T. S., Torchilin V. P. (2009) Improved transfection of spleen-derived antigen-presenting cells in culture using TATp-liposomes. J. Control. Release 134, 41–46 [DOI] [PubMed] [Google Scholar]
- 72. Bhattacharyya R., Pal D., Chakrabarti P. (2004) Disulfide bonds, their stereospecific environment and conservation in protein structures. Protein Eng. Des. Sel. 17, 795–808 [DOI] [PubMed] [Google Scholar]
- 73. Rapoport M., Lorberboum-Galski H. (2009) TAT-based drug delivery system–new directions in protein delivery for new hopes? Expert Opin. Drug Deliv. 6, 453–463 [DOI] [PubMed] [Google Scholar]