Abstract
Three forms of serpinin peptides, serpinin (Ala26Leu), pyroglutaminated (pGlu)-serpinin (pGlu23Leu), and serpinin-Arg-Arg-Gly (Ala29Gly), are derived from cleavage at pairs of basic residues in the highly conserved C terminus of chromogranin A (CgA). Serpinin induces PN-1 expression in neuroendocrine cells to up-regulate granule biogenesis via a cAMP-protein kinase A-Sp1 pathway, while pGlu-serpinin inhibits cell death. The aim of this study was to test the hypothesis that serpinin peptides are produced in the heart and act as novel β-adrenergic-like cardiac modulators. We detected serpinin peptides in the rat heart by HPLC and ELISA methods. The peptides included predominantly Ala29Gly and pGlu-serpinin and a small amount of serpinin. Using the Langendorff perfused rat heart to evaluate the hemodynamic changes, we found that serpinin and pGlu-serpinin exert dose-dependent positive inotropic and lusitropic effects at 11–165 nM, within the first 5 min after administration. The pGlu-serpinin-induced contractility is more potent than that of serpinin, starting from 1 nM. Using the isolated rat papillary muscle preparation to measure contractility in terms of tension development and muscle length, we further corroborated the pGlu-serpinin-induced positive inotropism. Ala29Gly was unable to affect myocardial performance. Both pGlu-serpinin and serpinin act through a β1-adrenergic receptor/adenylate cyclase/cAMP/PKA pathway, indicating that, contrary to the β-blocking profile of the other CgA-derived cardiosuppressive peptides, vasostatin-1 and catestatin, these two C-terminal peptides act as β-adrenergic-like agonists. In cardiac tissue extracts, pGlu-serpinin increased intracellular cAMP levels and phosphorylation of phospholamban (PLN)Ser16, ERK1/2, and GSK-3β. Serpinin and pGlu-serpinin peptides emerge as novel β-adrenergic inotropic and lusitropic modulators, suggesting that CgA and the other derived cardioactive peptides can play a key role in how the myocardium orchestrates its complex response to sympathochromaffin stimulation.—Tota, B., Gentile, S., Pasqua, T., Bassino, E., Koshimizu, H., Cawley, N. X., Cerra, M. C., Loh, Y. P., Angelone, T. The novel chromogranin A-derived serpinin and pyroglutaminated serpinin peptides are positive cardiac β-adrenergic-like inotropes.
Keywords: heart, adenylate cyclase, PKA
Chromogranin A (human: CHGA; rodent: Chga) is a 48- to 52-kDa acidic secretory prohormone that can give rise to several peptides of biological importance (1, 2), including the dysglycemic hormone pancreastatin (PST; ref. 3), the N-terminally derived vasodilator, cardiosuppressive vasostatin 1 (VS-1; ref. 4), and the antiadrenergic, antihypertensive, cardioinhibitory catestatin (CST; refs. 5, 6). The chromogranin A (CgA) protein, highly conserved in the vertebrate secretory granules of the diffuse neuroendocrine system (2), is also present in the heart, where it is costored and cosecreted with catecholamines (CAs) and natriuretic peptides (7, 8, 9). The level of plasma CgA, previously used for clinical applications as a biomarker of neuroendocrine tumors (10, 11), has emerged as a marker of cardiovascular dysfunctions, such as essential hypertension, hypertrophic/dilatative cardiomyopathy, and heart failure (12). More recently, Jansson et al. (13) and Røsjø et al. (14) showed in acute coronary syndromes that circulating levels of CgA provide prognostic information independently from conventional risk markers, predicting long-term mortality and heart failure hospitalizations during follow-up. It is, therefore, of pathophysiological interest that among the bioactive, proteolytically generated peptides, both VS-1 and CST exert striking cardiosuppression at nanomolar concentrations through an antiadrenergic-nitric oxide (NO)-cGMP mediated mechanism (15). The involvement of CgA in cardiac biology is also supported by the observation that genetic ablation of Chga in mice results in high blood pressure, which can be rescued by either pretreatment with CST or the introduction of the human CgA gene in the Chga−/− background (5). These data support the idea that VS-1 and CST can function as cardiac counterregulators in “zero steady-state error” homeostasis, particularly under intense CA-induced myocardial stress, a hypothesis that is gaining support, particularly among cardiologists (16, 17).
This area of research is now enriched by the recent discovery that CgA is intracellularly processed at its highly conserved C terminus to yield a 2.9-kDa peptide named serpinin (Ala26Leu) that can be modified at its N terminus to form a pyroglutamate residue (pGlu23Leu) (18, 19). Both forms of serpinin are found in the pituitary cell line, AtT20, and are secreted into the medium in an activity-dependent manner. Both these peptides inhibit pituitary and neuronal cell death induced by oxidative stress (19). They also promote secretory granule biogenesis in endocrine cells by up-regulating the expression of the protease inhibitor, protease nexin-1 (PN-1), which prevents granule protein degradation in the Golgi apparatus. This, in turn, elevates the level of granule proteins within the Golgi, leading to an increase in secretory granule biogenesis. It is noteworthy that in AtT-20 pituitary cells, serpinin up-regulates PN-1 mRNA transcription by binding to a cognate receptor to increase cAMP levels and protein kinase A (PKA) activity (18). The finding that this novel CgA-derived peptide signals through an adenylate cyclase (AD)-cAMP-PKA dependent excitatory mechanism suggests it may counterbalance the VS-1- and CST-induced antiadrenergic cardioinhibitory effects. To test this hypothesis, and in the absence of information on its putative cardiovascular influence, we investigated whether serpinin peptides are present in the heart and how they might affect myocardial performance and coronary vasoactivity. To this end, we identified several serpinin peptides in rat heart tissue extracts, and, using synthetic peptides in both isolated Langendorff perfused rat heart and papillary muscle, we found that pGlu-serpinin and serpinin exerted dose-dependent positive contractile (inotropic) and relaxing (lusitropic) myocardial effects. As shown by the Langendorff perfused rat heart analysis, these actions were accompanied by a slight, although nonsignificant, coronary dilatation. A synthetic C-terminally extended form of serpinin (serpinin-RRG; Ala29Gly) was unable to affect myocardial performance at various concentrations tested. Both the cardiac effects of pGlu-serpinin and serpinin require a β1-adrenergic receptor (β1-AR)/AD/cAMP/PKA pathway. These data uncover the cardiocirculatory properties of serpinin and pGlu-serpinin, providing new relevant insights into the way these CgA-derived peptides can finely tune the heart's response to β-adrenergic stimuli.
MATERIALS AND METHODS
Analysis of rat heart peptides by HPLC and ELISA
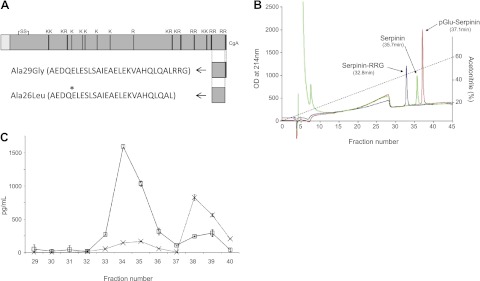
Rat hearts were homogenized in ice-cold T-per lysis buffer (Pierce, Rockland, IL, USA) supplemented with fresh inhibitors. The homogenate was centrifuged at 13,000 rpm for 20 min, and the subsequent supernatant was purified through C18 reverse phase Sep-pak cartridges (Peninsula, Belmont, CA, USA). The eluate was subsequently separated on a C18 HPLC column as described previously (18). Fractions were collected, lyophilized, and reconstituted in EIA buffer and assayed by direct ELISA using an antibody that detects serpinin-like peptides, as described previously (18), or a sandwich ELISA specific for pGlu-serpinin, as described previously (19). Under these conditions, standard serpinin (Ala26Leu) eluted in fraction 36, pGlu23Leu eluted in fraction 38, and Ala29Gly eluted in fraction 34.
Animals
Male Wistar rats (Harlan Laboratories Srl, Udine, Italy), weighing 180–220 g, were housed (4 rats/cage) in a ventilated cage rack system under standard conditions. Animals had access to food and water ad libitum. The investigation conformed to the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, rev. 1996).
Drugs
Serpinin peptides [wild-type (WT)-Ala26Leu, pGlu23Leu, Ala29Gly], were custom synthesized by Phoenix Pharmaceuticals, Inc., (Burlingame, CA, USA). Nebivolol, a β-1 adrenoreceptor antagonist, was kindly provided by Menarini S.p.A. (Florence, Italy). CGP20712A, a β-1 adrenoreceptor antagonist; MDL 12330 A, an AD inhibitor; KT-5720, a PKA inhibitor; thapsigargin, a noncompetitive inhibitor of sarcoplasmic reticulum Ca2+-ATPase (SERCA); milrinone, a phosphodiesterase 3 (PDE3) inhibitor; and tyramine, an activator of norepinephrine (NE) outflow from sympathetic nerve endings were obtained from Sigma-Aldrich (Milan, Italy). All drug-containing solutions were freshly prepared before experimentation.
Isolated heart preparation
Rats were anesthetized with ethyl carbamate (2 mg/g body weight, i.p.); the hearts were rapidly excised and then transferred to ice-cold buffered Krebs-Henseleit solution (KHS). As described previously (15), the aorta was immediately cannulated with a glass cannula and connected to Langendorff apparatus to start perfusion at constant flow rate (12 ml/min). Briefly, the apex of the left ventricle (LV) was pierced to avoid fluid accumulation. A water-filled latex balloon, connected to a BLPR gauge (WPI, Inc., Sarasota, FL, USA), was inserted through the mitral valve into the LV, allowing isovolumic contraction and continuous mechanical parameter recording. Another pressure transducer was located just above the aorta and recorded coronary pressure (CP). The perfusion solution consisted of a modified nonrecirculating KHS containing (in mM) 113 NaCl, 4.7 KCl, 25 NaHCO3, 1.2 MgSO4, 1.8 CaCl2, 1.2 KH2PO4, 11 glucose, 1.1 mannitol, 5 Na-pyruvate (pH 7.4; 37°C; 95%O2–5%CO2). Hemodynamic parameters were assessed using a PowerLab data acquisition system (ADInstruments, Basile, Italy) and analyzed using Chart software (ADInstruments).
Basal conditions
Heart performance was evaluated from the left ventricular pressure (LVP; mmHg; index of contractile activity), the rate-pressure product [RPP; heart rate (HR)×LVP, 104 mmHg·beats/min; index of cardiac work), the maximal value of the first derivative of LVP (mmHg/s; index of the maximal rate of LV contraction), the time to peak tension of isometric twitch (TTP; an assessment of inotropism). For lusitropism, the maximal rate of LVP decline [−(LVdP/dt)max, mmHg/s], the half-time relaxation (HTR; s; the time required for tension to fall from the peak to 50%), and the T/−T ratio [obtained by +(LVdP/dt)max/−(LVdP/dt)max] were calculated. Mean CP was calculated by averaging values obtained during several cardiac cycles (15).
Experimental protocols
Each heart was allowed to stabilize for 20 min, then baseline parameters were recorded. After stabilization, hearts were randomly assigned to one of the groups described below.
Group 1 (isolated and perfused Langendorff rat heart): hearts were stabilized, and dose-response curves for Ala26Leu (n=7), pGlu23Leu (n=7), or Ala29Gly (n=7) were generated.
Group 2 (isolated rat papillary muscle): papillary muscles were stabilized and either 11 nM (n=6) or 1 and 33 nM (n=5) of pGlu23Leu was administered.
Groups 3–7 (isolated and perfused Langendorff rat heart, n=6/inhibitor): hearts were perfused with pGlu23Leu plus one of the following inhibitors (100 nM): nebivolol, CGP20712A, MDL12330A, KT5720, or milrinone.
Groups 8–12 (isolated and perfused Langendorff rat heart; n=6/inhibitor): hearts were perfused with Ala26Leu plus one of the following inhibitors (100 nM): nebivolol, CGP20712A, MDL12330A, KT5720, or milrinone.
Groups 12 and 13 (isolated and perfused Langendorff rat heart): hearts were perfused with pGlu23Leu (n=6) or Ala26Leu (n=6) plus thapsigargin (100 nM).
Group 14 (isolated and perfused Langendorff rat heart; n=3): hearts were perfused with pGlu23Leu to determine NE concentration by HPLC.
Serpinin-stimulated preparations
Repetitive exposure of each heart to serpinin (WT-Ala26Leu, pGlu23Leu, or Ala29Gly) at a single concentration (33 nM) revealed an absence of desensitization (data not shown). Thus, concentration-response curves were generated by perfusing cardiac preparations with KHS supplemented with increasing concentrations (1–165 nM) of WT-Ala26Leu, pGlu23Leu, or Ala29Gly for 10 min.
Isolated papillary muscle
Papillary muscle dissection and mounting were performed as described previously (4). Briefly, rat papillary muscles were dissected free from the LV under a stereomicroscope and superfused with oxygenated (100% O2) Tyrode solution (composition, in mM: 154 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 5.5 d-glucose, and5 HEPES; pH adjusted to 7.38 with NaOH) at 37°C. Papillary muscles were driven at constant frequency (120 beats/min) with a pair of electrodes connected to a stimulator (302-T Anapulse; W. P. Instruments, New Haven, CT, USA) via a stimulus isolator (model 305-R; W. P. Instruments) operating in constant-current mode. Isometric twitches were evaluated by a transducer (model 60-2997; Harvard Instruments, Cambridge, MA, USA) and continuously acquired and recorded by a PowerMac computer using the Labview software (National Instruments, Austin, TX, USA). Before each experiment, papillary muscles were equilibrated in Tyrode solution for 20 min, then treated with different concentrations of pGlu-serpinin(1–33 nM) for 10 min. All solutions containing drugs were prepared immediately before the experiments.
Sympathetic nerve termini involvement
To exclude the possibility that serpinin could act indirectly by eliciting NE efflux from intracardiac sympathetic nerve endings, the hearts were pretreated with tyramine alone (1 mM) for 30 min (for details, see ref. 20) and subsequently exposed to pGlu-serpinin (1–165 nM). Additional experiments were performed on isolated rat hearts exposed to pGlu-serpinin (165 nM), in which NE concentrations were determined both in the perfusates and in cardiac tissue homogenates using HPLC with electrochemical detection, as described previously (20).
AR involvement
To obtain information on the involvement of β1 ARs on the inotropic and lusitropic effects induced by serpinin or pGlu-serpinin, cardiac preparations, stabilized for 20 min with KHS, were perfused with 100 nM of either Nebivolol or CGP20712A for 10 min and then washed out with KHS. After returning to control conditions, each heart was perfused with KHS containing a single concentration of serpinin or pGlu-serpinin (11 nM) plus 100 nM of either nebivolol or CGP20712A for an additional 10 min.
G-protein-coupled receptor (GPCR) signaling assays
Luciferase assays were done as described previously (21–23) with slight modifications. In brief, cells were transfected in 96-well plates cells at ∼80% confluency under serum-free conditions using Dulbecco's Eagle medium. HEK293 cells were transfected using polyethylenimine transfection reagent, prepared as described previously (24) and used at a final concentration of 2 μg/ml. The following cDNAs were cotransfected: 4 ng/well of mouse β1 adrenergic receptor or empty vector, 5 ng/well of the luciferase gene Luc2CP (Promega, Madison, WI, USA) under the control of a 6× multimerized cAMP response element, and 10 ng/well β-galactosidase as an expression control. At 20 h after transfection, cells were stimulated with the indicated concentrations of serpinins or isoproterenol. Following 4 h of stimulation, luciferase activity was quantified using Steadylite plus reagent (Perkin-Elmer, Waltham, MA, USA). Luciferase activity in each well was normalized to corresponding β-galactosidase levels as described previously (23).
Interaction between β1-ARs and serpinin
To describe the receptor interaction between serpinin and β1-ARs further, dose-response curves were generated by perfusing the heart preparations with KHS enriched with increasing concentrations of serpinin or pGlu-serpinin (1–165 nM) alone. These curves were then compared to those obtained by exposing other cardiac preparations to the same perfusion medium containing increasing concentrations of serpinin or pGlu-serpinin (1–165 nM) plus a single concentration (100 nM) of either nebivolol or CGP20712A.
AC/cAMP/PKA/PDE3-pathway involvement
Hearts were stabilized for 20 min with KHS and perfused with 11 nM serpinin or pGlu-serpinin for 10 min, and then the peptides were washed out with KHS. After returning to control conditions, each heart was perfused with KHS containing either the AC inhibitor MDL12330A, a PKA blocker (KT5720), or the specific PDE3 inhibitor milrinone. Subsequently, the hearts were exposed to the specific signaling inhibitor plus 11 nM of serpinin or pGlu-serpinin.
Calcium involvement
To evaluate the involvement of intracellular calcium in the cardiac action of serpinin or pGlu-serpinin, hearts were pretreated with KHS enriched with thapsigargin (100 nM) and then exposed for 10 min to serpinin or pGlu-serpinin (11 nM).
Statistics
Data are expressed as means ± se. Since each heart represents its own control, the statistical significance of differences within a group was assessed using the ANOVA test (P<0.05). Comparison between groups was made by using a 1-way ANOVA followed by the Bonferroni correction for post hoc t tests. The concentration-response curves of the LVP stimulation induced by serpinin or pGlu-serpinin alone and by serpinin or pGlu-serpinin plus either nebivolol or CGP20712A were fitted using GraphPad Prism 4.02 (GraphPad, San Diego, CA, USA). This provided, for each curve, the −log of the concentration (molar) that induced the 50% of the effect (EC50) of serpinin or pGlu-serpinin alone and by serpinin or pGlu-serpinin plus either nebivolol or CGP20712A. Differences were considered to be statistically significant at values of P < 0.05.
Measurements of cAMP
Acid extracts of frozen heart tissue (200–300 mg of endocardial tissue and cardiomyocytes from ventricles), with and without serpinin treatment were treated with 6% trichloroacetic acid at 0°C and centrifuged at 10,000 g for 10 min. The supernatant was extracted 3 times with 3 ml of diethyl ether saturated with water, and the aqueous phase was collected and stored at −80°C. cAMP concentrations were measured using a commercial enzyme immunoassay (Biotrak enzyme immunoassay system; Amersham Biosciences, Piscataway, NJ, USA).
Western blotting
To evaluate differences in protein phosphorylation, cardiac ventricular homogenates were used. Cardiac ventricles, obtained after perfusion with KHS alone and a single concentration of serpinin or pGlu-serpinin, were homogenized in ice-cold RIPA buffer (Sigma-Aldrich) containing a mixture of protease inhibitors (1 mM aprotinin, 20 mM phenylmethylsulfonyl fluoride, and 200 mM sodium orthovanadate). The homogenates were centrifuged at 200 g for 10 min at 4°C to remove debris. The protein concentration was determined using the Bradford reagent (Sigma-Aldrich) according to the manufacturer's recommendations. Proteins were separated on 8% SDS-PAGE gels, transferred to membrane, blocked with nonfat dried milk,and incubated overnight at 4°C with polyclonal rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1:1000 in TBS-T containing 5% nonfat dry milk. Peroxidase-inked anti-rabbit secondary antibody (Santa Cruz Biotechnology) was diluted 1:2000 in TBS-T containing 5% nonfat dry milk.
RESULTS
Detection of serpinin peptides in the heart by HPLC and ELISA
CgA was found to be processed to different serpinin-related peptides at the C terminus in rat hearts (see Fig. 1A). To identify these peptides, synthetic serpinin standards were separated on the HPLC first to identify their elution profiles. Serpinin eluted in fraction 36, whereas pGlu-serpinin eluted in fraction 38. The extended serpinin peptide, Ala29Gly, eluted in fraction 34 (Fig. 1B). The concentrated and partially purified peptide extract from rat heart was applied to the C18 column and, after reconstitution of the fractions, was assayed for serpinin immunoreactivity. Two peaks were identified that were consistent with the Ala29Gly extended serpinin in fraction 34 and pGlu-serpinin in fraction 38. A small shoulder in fraction 35 and signal in fraction 36 are consistent with the presence of a small amount of Ala26Leu (Fig. 1C). When assayed by the sandwich ELISA for pGlu-serpinin specifically, the predominant peak identified was in fraction 38, consistent with its identification as pGlu-serpinin (Fig. 1C). The concentration of pGlu-serpinin was 103.8 ± 14.7 pg/g rat heart.
Figure 1.
A) Peptide standards serpinin, serpinin-RRG, and pGlu-serpinin were custom synthesized by Phoenix Pharmaceuticals. Asterisk indicates the glutamate residue that becomes cyclized to form pyroglutamate. B) Standards (1–3 μg) were applied separately to the C18 reverse-phase HPLC column described in Materials and Methods. Their elution profiles were monitored by optical density (OD) at 214 nm. Serpinin-RRG eluted at 32.8 min, serpinin at 35.7 min, and pGlu-serpinin at 37.1 min. These elution times equate to fractions 34, 36, and 38 for serpinin-RRG, serpinin, and pGlu-serpinin, respectively. C) The C18 cartridge-purified rat heart extract was applied to the same HPLC analysis; 1 ml fractions were collected, lyophilized and assayed by ELISA for serpinin immunoreactivity (IR) and also for pGlu-serpinin specifically. Note the peak of serpinin-IR (open squares) in fractions 34/35 and 38/39, consistent with the elution profile of serpinin-RRG and pGlu-serpinin standards, respectively. Note also the peak of pGlu-serpinin only in the fractions where pGlu-serpinin standard eluted. A blank run was performed prior to the heart sample, and the fractions were collected and assayed in the same way. There was no signal in any fraction, demonstrating that the HPLC system was not contaminated by residual standards.
Basal conditions—Langendorff isolated heart
The cardiac parameters, indicated in Materials and Methods, were obtained after 20 min equilibration and are indicated in Table 1. Endurance and stability of the preparations, analyzed by measuring the performance variables every 10 min, showed that the heart was stable up to 180 min.
Table 1.
Cardiac parameters under basal conditions
| Parameter | Value |
|---|---|
| LVP (mmHg) | 89 ± 3 |
| HR (beats/min) | 280 ± 7 |
| EDVP (mmHg) | 5–8 |
| RPP (mmHg · beats/min) | 2.5 ± 0.1 ×104 |
| +(LVdP/dt)max (mmHg/s) | 2492 ± 129 |
| −(LVdP/dt)max (mmHg/s) | −1663 ± 70 |
| TTP (s) | 0.08 ± 0.01 |
| HTR (s) | 0.05 ± 0.01 |
| T/−T (mmHg/s) | 1.498 ± 1.84 |
| CP (mmHg) | 63 ± 3 |
| Weight of heart (g) | 1.2 ± 0.2 |
| Weight of left ventricle (g) | 0.7 ± 0.05 |
| Weight of animal (g) | 230 ± 10 |
| Pressure perfusion (mmHg) | 100 |
LVP, left ventricular pressure; HR, heart rate; EDVP, end-diastolic ventricular pressure; RPP, rate-pressure product; +(LVdP/dt)max, maximal rate of left ventricular contraction; −(LVdP/dt)max, maximal rate of left ventricular pressure decline; TTP, time to peak tension of isometric twitch; HTR, half time relaxation; T/−T, ratio obtained by +(LVdP/dt)max/−(LVdP/dt)max; CP, coronary pressure.
Serpinin peptide-induced inotropism and lusitropism
Langendorff perfused rat heart
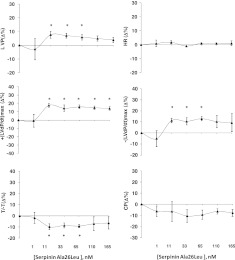
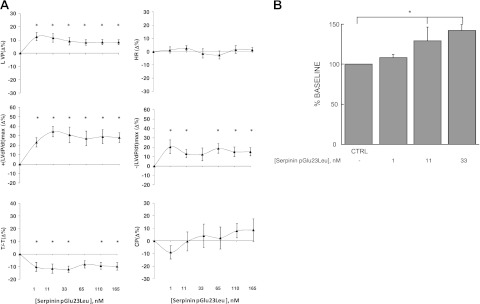
To determine in hemodynamic terms (intraventricular pressure, etc.) whether serpinin, pGlu-serpinin, or serpinin-Ala29Gly affects mammalian basal cardiac performance, rat cardiac preparations were exposed to increasing concentrations of either serpinin, pGlu-serpinin or serpinin-Ala29Gly to generate concentration-response curves. The effects of all three peptides remained stable until 10 min. Accordingly, cardiac parameters were measured at 5 min. Serpinin (1–165 nM) caused a significant concentration-dependent positive inotropic effect on LVP beginning at 11 nM. This peptide also caused a significant increase of +(LVdP/dt)max beginning at 11 nM, without modifying HR (Fig. 2). The analysis of lusitropic parameters revealed a significant increase of −(LVdP/dt)max and a reduction of T/−T. The peptide induced vasodilation, which reduced CP (Fig. 2). Treatment with pGlu-serpinin (1–165 nM) caused a significant increase of LVP and +(LVdP/dt)max, starting from 1 nM (Fig. 3A), which was more potent than that of serpinin. pGlu-serpinin induced a significant increase in −(LVdP/dt)max and T/−T, without changes to HR and CP (Fig. 3A). The treatment with serpinin-Ala29Gly did not modify cardiac performance at any concentration tested (1–165 nM; data not shown). Hence, our present study has focused mainly on serpinin and pGlu-serpinin.
Figure 2.
Isolated and perfused Langendorff rat heart. Dose-dependent response curves of serpinin Ala26Leu (1 to 165 nM) on HR; myocardial parameters LVP, RPP, and +(LVdP/dt)max; and lusitropic parameters −(LVdP/dt)max, T/−T, and CP on Langendorff perfused rat heart preparation. See Results for basal values. Percentage changes were evaluated as means ± se of 7 experiments. *P < 0.05 vs. control; 1-way ANOVA.
Figure 3.
A) Isolated and perfused Langendorff rat heart. Dose-dependent response curves of serpinin pGlu23Leu (1–165 nM) on HR; myocardial parameters LVP, RPP, and +(LVdP/dt)max; and lusitropic parameters −(LVdP/dt)max, and T/−T, and CP on Langendorff perfused rat heart preparation. See Results for basal values. Percentage changes were evaluated as means ± se of 7 experiments. *P < 0.05 vs. control; 1-way ANOVA. B) Isolated rat papillary muscle. While ineffective at 1 nM, higher concentrations (11 and 33 nM) of pGlu-serpinin enhanced contractile force in a concentration-dependent manner. Data are expressed as mean ± sem percentage of baseline values from 6 (11 nM) or 5 (1 and 33 nM) experiments/group. *P < 0.05.
Isolated papillary muscle
The isolated papillary muscle was used to measure the direct contractile effect of serpinin in terms of tension development and changes in muscle length. This preparation also allows an evaluation that is independent of alterations in heartbeat frequency and coronary flow. We observed that a low concentration (1 nM) of pGlu-serpinin had little effect on myocardial contractility, whereas higher concentrations (11 and 33 nM) induced a positive inotropic effect (∼25 and 40% over control, respectively; Fig. 3B), which reached a peak after 3–4 min, then slowly decreased, partially persisting at the end of treatment (10 min). The inotropic effect of pGlu-serpinin was completely reverted after washout in Tyrode solution.
Involvement of NE efflux from sympathetic nerve termini
In sympathetic nerve endings, several stimuli may evoke an efflux of NE (21). To exclude the possibility that serpinin could act indirectly through such a mechanism, isolated Langendorff hearts were pretreated with tyramine alone (1 μM). It is known that tyramine significantly enhances NE outflow by displacing the amine from its storage vesicles through a calcium-independent mechanism (25). After 30 min of tyramine exposure, the hearts were perfused with pGlu-serpinin (1–165 nM). The results showed that pGlu-serpinin-dependent positive inotropism and lusitropism were unaffected by tyramine pretreatment [the effects on LVP measured at 165 nM were +10.5±1.9; on +(LVdP/dt)max, +28.05±5.07; on −(LVdP/dt)max, +15.28±4.3], thus indicating that these actions are independent of NE outflow from sympathetic nerve termini. In addition, the effect of pGlu-serpinin (165 nM) on NE release was also evaluated in isolated perfused rat hearts. Under basal conditions, the amounts of NE detected in the coronary perfusate were comparable to those reported in the literature (20) for the same preparation (358.3±57 ng/ml). Treatment with pGlu-serpinin did not significantly modify either the amounts of NE present in the coronary perfusate (108% of basal value) or the total NE content present in cardiac tissue homogenates (111% with respect to untreated hearts).
Involvement of β1-ARs, AD, PKA, and PDEs
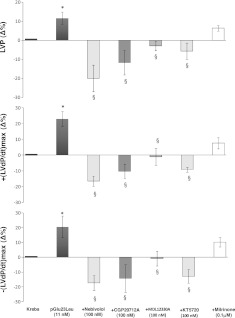
To verify the involvement of β1-ARs, Langendorff cardiac preparations were perfused with KHS containing either nebivolol, an antagonist of β1-ARs (26), or the more selective β1-AR antagonist CGP20712A (27), in the presence of serpinin (Supplemental Fig. S1) or pGlu-serpinin (Fig. 4). Both inhibitors (at 100 nM) abolished the positive inotropism [i.e., LVP and +(LVdP/dt)max] and lusitropism [i.e., −(LVdP/dt)max] of both serpinin (Supplemental Fig. S1) and pGlu-serpinin (Fig. 4). MDL123330A is a specific inhibitor of AD activity (28). To verify the involvement of AD in the mechanism of action of the peptides, the hearts were perfused with serpinin or pGlu-serpinin in the presence of MDL123330A at 100 nM. MDL123330A abolished the inotropic and lusitropic effects of both serpinin (Supplemental Fig. S1) and pGlu-serpinin (Fig. 4). The involvement of the cAMP/PDE3 signaling in the serpinin-dependent cardioactivity was examined by perfusing the cardiac preparations with either KT5720 (100 nM) or milrinone (0.1 μM) in the presence of serpinin or pGlu-serpinin. The positive inotropic effects induced by serpinin (Supplemental Fig. S1) and pGlu-serpinin (Fig. 4) on LVP, +(LVdP/dt)max, and −(LVdP/dt)max were abolished by pretreatment with KT5720 and but were unchanged by milrinone.
Figure 4.
Effects of serpinin pGlu23Leu (11 nM) alone and serpinin pGlu23Leu in presence of 100 nM nebivolol, CGP20712A, MDL12330A, KT5720, or milrinone on LVP, +(LVdP/dt)max, and −(LVdP/dt)max. *P < 0.05, 1-way ANOVA; §P < 0.05, 1-way ANOVA with Bonferroni's post hoc test (n=6).
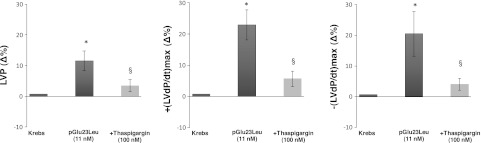
Intracellular calcium involvement
To elucidate whether SERCA2a plays a role in serpinin-induced cardiac response, hearts were exposed to thapsigargin (100 nM; SERCA2a inhibitor) in the presence of either serpinin or pGlu-serpinin. Serpinin (Supplemental Fig. S2) or pGlu-serpinin-dependent (Fig. 5) positive inotropism [LVP, +(LVdP/dt)max] and lusitropism [−(LVdP/dt)max] were abolished by treatment with thapsigargin (100 nM). In all experiments, the antagonist concentration was selected on the basis of the results of preliminary dose-response curves as the first dose that does not significantly affect cardiac performance (see Table 2).
Figure 5.
Effects of werpinin pGlu23Leu (11 nM) alone and serpinin pGlu23Leu in the presence of thapsigargin on LVP, +(LVdP/dt)max, and −(LVdP/dt)max. *P < 0.05, 1-way ANOVA; §P < 0.05, 1-way ANOVA with Bonferroni's post hoc test (n=6).
Table 2.
Effect of each inhibitor administered alone on LVP
| Inhibitor | Biological activity | LVP (Δ%) |
|---|---|---|
| Nebivolol (100 nM) | Selective β1-AR antagonist | 0.53 ± 0.46 |
| CGP20712A (100 nM) | More selective β1-AR antagonist | −2.41 ± 1.12 |
| MDL 12330A (100 nM) | Selective AC inhibitor | −3.1 ± 2.6 |
| KT5720 (100 nM) | Selective PKA inhibitor | −5.9 ± 3.7 |
| Milrinone (0.1 μM) | Selective PDE3 inhibitor | 3.1 ± 2.1 |
| Thapsigargin (100 nM) | Selective SERCA2a inhibitor | 4.7 ± 2.8 |
Serpinin and pGlu-serpinin interaction with β1-ARs
To verify the interaction between serpinin or pGlu-serpinin with β1-ARs, heart preparations were perfused with KHS containing increasing concentrations of both serpinin (Supplemental Fig. S3A) and pGlu-serpinin (Supplemental Fig. S3B) (1–165 nM) either alone or in combination with a single concentration (100 nM) of either nebivolol or CGP20712A. Serpinin stimulation induced a significant increase of LVP. Analysis of the percentage of LVP variations provided the EC50 values in the presence of either increasing concentrations of serpinin or pGlu-serpinin alone or of serpinin or pGlu-serpinin plus nebivolol or CGP20712A, respectively. The EC50 values (log M) of either serpinin alone or pGlu-serpinin alone were −10.25 ± 1.2 (r2=0.30) and −10.07 ± 0.5 (r2=0.67), respectively; serpinin plus nebivolol or CGP20712A were −10.35 ± 2.58 (r2=0.07) and −9.77 ± 0.85 (r2=0.41), respectively; and pGlu-serpinin plus nebivolol or CGP20712A were −8.2 ± 1.9 (r2=0.43) and −7.08 ± 2.52 (r2=0.60), respectively. These results indicate a noncompetitive antagonism of nebivolol or CGP20712A against serpinin and pGlu-serpinin stimulation. To evaluate the interaction between serpinin or pGlu-serpinin with the β1-AR active site, HEK293 cells transfected with β1-AR were analyzed by luciferase activity. Data revealed no specific binding on the active site of the receptor (Supplemental Fig. S4).
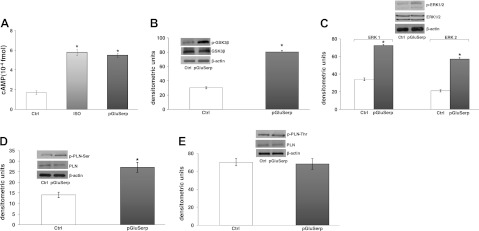
cAMP involvement
To verify the involvement of cAMP in the mechanism of action of pGlu-serpinin, intracellular cAMP was measured in rat heart extracts. Hearts treated with pGlu-serpinin at 33 nM showed a significant increase in cAMP levels, in agreement with the positive inotropism. This effect was similar to that induced by isoproteronol stimulation (Fig. 6A).
Figure 6.
A) cAMP concentrations in heart extracts. Percentage changes were evaluated as means ± se of 4 experiments. *P < 0.05, **P < 0.01 vs. control; §P < 0.05 between groups. B–E) Immunoblots showing phosphorylation of GSK3-β (B), ERK1/2 (C), p-PLN-Ser (D), and p-PLN-Thr (E) in cardiac ventricle (n=4/group).
Protein phosphorylation
It is known that positive inotropism and lusitropism associate with phosphorylation of various cellular proteins, including ERK1/2, GSK3β, phospholamban (PLN) Ser16, or PLN Thr17 (27). To assess the contribution of these proteins in the cardiac effects induced by pGlu-serpinin, Western blot analyses were performed on homogenates of cardiac tissue treated with pGlu-serpinin. Figure 6 shows that pGlu-serpinin induced an increase in phosphorylation of GSK3β, ERK1/2, and PLN(Ser16). pGlu-serpinin-dependent inotropism and lusitropism did not modify the phosphorylation of PLN at Thr17 (Fig. 6B–E).
DISCUSSION
Very recently, it has been shown that CgA is cleaved at the C-terminal dibasic amino acid sites, most probably by prohormone convertase 1 (PC1) and/or PC2 in CgA-containing secretory cells, as well as in chromaffin cells, to form serpinin-related peptides (18, 19, 30). These include serpinin, pGlu-serpinin, and C-terminally extended serpinin-RRG. In corticotrophs, on stimulation, serpinin is released to up-regulate, in an autocrine manner, PN-1-dependent granule biogenesis via a cAMP-PKA-Sp1 pathway, to replenish released granules (18). Here we report in the rat heart, the presence of the CgA C-terminally derived fragments, pGlu-serpinin (103.8±14.7 pg/g), serpinin-RRG, and in lesser amounts, standard serpinin (Fig. 1). The higher levels of serpinin-RRG compared to serpinin would suggest that the processing of the last pair of basic residues of serpinin-RRG may be limiting; also, the relatively lower levels of serpinin compared to pGlu-serpinin would suggest that the N terminus of serpinin is processed quickly to generate the pyroglutaminated moiety by glutamyl cyclase. These findings add new information to the intracardiac processing of CgA. In the rat heart, following the first documentation of CgA storage in cardiomyocytes by Steiner et al. (7), we showed the presence of endogenous N-terminal CgA-derived peptides (CgA4–113, CgA1–124, CgA1–135 and CgA1–199) containing the vasostatin sequence (29). Furthermore, more recently, Biswas et al. (9) provided evidence for the generation of CST-containing peptides. Of note, myocardial CgA (>0.5 μg/g) was also found in the failing human heart (8). Given our present findings that pGlu-serpinin is a powerful positive inotropic agent, while previous studies showed that the N-terminally derived vasostatin peptides and CST have negative inotropic effects (17), we hypothesize that in response to a specific stimulus elicited under normal or stressful conditions, CgA may be differentially processed in the heart to generate different peptides that could act in a paracrine/autocrine manner in opposing directions to maintain cardiac homeostasis (31). Thus, stimulus-activated differential processing of CgA in the heart may be very important for optimal physiological function. Another highly significant and novel result of the present study is the demonstration that pGlu-serpinin and serpinin act at nanomolar range as β-adrenergic-like agonists, similar to the intracardiac sympathetic neurotransmitters and/or circulating CAs. Like these agents, they increase cellular levels of cAMP, thereby exerting remarkable effects on myocardial mechanical performance. This is demonstrated by both the hemodynamic analysis (intraventricular pressure changes) obtained with the Langendorff whole-heart preparation, and the myocardial behavior studied with the papillary muscle preparation (in which contractility is described in terms of tension development and changes in muscle length). The serpinin peptides strikingly enhance inotropy (the rate and extent of tension development during systole), hence increasing stroke volume, and at the same time accelerate myocardial relaxation, thus shortening the overall duration of diastole, i.e., lusitropy. Since β-adrenergic stimulation also increases HR (tachycardia), the β-adrenergic-cAMP-dependent interplay between inotropy and lusitropy ensures the heart to relax from a stronger contraction in a shorter time, allowing it to adequately fill during the abbreviated diastole (32). Notably, the experiments performed on the isolated papillary muscle also suggest a direct peptide action, independent from any possible alteration in cardiac rhythm and coronary flow rate. Consistent with the results obtained by both tyramine treatment and the direct detection of NE release, this action appears also independent from an influence exerted at the level of sympathetic nerve termini.
As for other CgA-derived peptides, no direct binding partner or receptor has been identified for serpinin or pGlu-serpinin. The peptide might bind to a GPCR, a mechanism common for cAMP elevation (18). Both the β1 antagonist results and the preliminary luciferase binding data (Supplemental Fig. S4) suggest that serpinin and pGlu-serpinin interact as allosteric modulators of the β-AR independently from the ligand binding site, thereby activating the classical physiological cascade of reactions set into motion by the β-adrenergic agonists. In fact, serpinin and pGlu-serpinin increased the cardiac cAMP levels, while both their induced inotropic and lusitropic effects were abolished by selective inhibition of AD and PKA with MDL123330A and Kt5720, respectively. Consistent with this AD-cAMP signaling, the major downstream targets, PKAs, were activated. These kinases phosphorylate a number of proteins, including SERCA and its associated regulatory protein, PLN, which are both central to inotropic and lusitropic regulation (32). SERCA-dependent Ca2+ uptake within SR promotes the removal of this cation during diastole, thus affecting relaxation and the subsequent contraction (33). Consistent with this role of the SR Ca2+ fluxes, the functional suppression of SR activity by thapsigargin inhibition of SERCA, abolished the pGlu-serpinin-elicited inotropy and lusitropy. It is well known that Ca2+ sensitivity for SERCA is enhanced by PKA-induced phosphorylation of PLN (34), hence contributing to the βAR-dependent inotropic and lusitropic effects. Accordingly, pGlu-serpinin induced PLN phosphorylation at Ser16, the residue, which is a downstream target of the β-adrenergic-PKA cascade (34).
We also detected pGlu-serpinin-PKA-induced phosphorylation of ERK1/2 and GSK3β. Both phosphorylated proteins mediate PGE2 and EP4 signaling in neonatal ventricular myocytes (35) and are components of the protective reperfusion injury signaling kinase (RISK) pathway involved in myocardial protection against ischemia-reperfusion injury in rodents (36, 37). Indeed, they might contribute to the pGlu-serpinin-induced antiapoptotic effects against radical oxygen species reported in cultured cerebral neurons by Koshimizu et al. (19).
CgA and its derived cardiosuppressive and antiadrenergic peptides, VS-1 and CST, were suggested as cardiac counterregulators in zero steady-state error homeostasis, particularly under intense excitatory stimuli, e.g., CA-induced myocardial stress. The antiadrenergic effects may be due to an endothelial PI3K/Akt/p-eNOS pathway rather than to a modulation of the receptor coupling in the cardiomyocytes (CST, ref. 38; VS-1, refs. 39, 40). Our understanding is now enriched by the present discovery that the novel cardiac C-terminal CgA-derived peptide pGlu-serpinin influences myocardial inotropy and lusitropy through β-1-dependent signaling. This stimulates the working hypothesis that spatiotemporally concerted processing of these peptides can orchestrate “whip-brake ” connection-integration networks that may be particularly important under perturbed cardiac homeostasis (16). In addition, the finding that pGlu-serpinin acts on β1-AR in a functional manner; i.e., independent of a direct classical interaction with the AR active site, may represent an alternative to direct adrenoreceptor stimulation. In fact, it is well established that prolonged, long-term stimulation of β-AR directly leads to detrimental (maladaptive) cardiac hypertrophy and, eventually, heart failure. pGlu-serpinin and serpinin may allow the separation of beneficial from detrimental pathways related to downstream targets of β-AR signaling, being potentially helpful under specific clinical conditions, when direct adrenergic stimulation can be undesirable.
Supplementary Material
Acknowledgments
The authors thank Dr. G. Mengozzi and M. Lucchiari (Dipartimento di Diagnostica di Laboratorio, Azienda Ospedaliera Universitaria San Giovanni Battista di Torino, Turin, Italy) for their precious support in NE detection. The authors also thank Prof. Alan Kopin and Mr. Benjamin Harwood (Molecular Pharmacology Research Center, Tufts Medical Center, Boston, MA, USA) for their help with the GPCR signaling assays.
This research was supported by grants from Ministero dell'Università e Ricerca Scientifica e Tecnologica (ex60%), the Italian National Institute of Cardiovascular Research (INRC), and the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U.S. National Institutes of Health).
The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AD
- adenylate cyclase
- AR
- adrenergic receptor
- CA
- catecholamine
- CgA
- chromogranin A
- CHGA
- human chromogranin A gene
- Chga
- rodent chromogranin A gene
- CP
- coronary pressure
- CST
- catestatin
- GPCR
- G-protein-coupled receptor
- HR
- heart rate
- KHS
- Krebs-Henseleit solution
- LV
- left ventricle
- LVP
- left ventricular pressure
- NE
- norepinephrine
- PC1/2
- prohormone convertase 1/2
- PDE3
- phosphodiesterase 3
- pGlu-serpinin
- pyroglutamate serpinin (pGlu23Leu)
- PKA
- protein kinase A
- PN-1
- protease nexin-1
- PLN
- phospholamban
- SERCA
- sarcoplasmic reticulum Ca2+-ATPase
- serpinin
- standard serpinin (Ala26Leu)
- serpinin-RRG
- C-terminally extended serpenin (Ala26Leu)
- VS-1
- vasostatin 1
REFERENCES
- 1. Winkler H., Fischer-Colbrie R. (1992) The chromogranins A and B: the first 25 years and future perspectives. Neuroscience 49, 497–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Helle K. B., Corti A., Metz-Boutigue M. H., Tota B. (2007) The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cell. Mol. Life Sci. 64, 2863–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tatemoto K., Efendic S., Mutt V., Makk G., Feistner G. J., Barchas J. D. (1986) Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature 324, 476–478 [DOI] [PubMed] [Google Scholar]
- 4. Cerra M. C., Gallo M. P., Angelone T., Quintieri A. M., Pulerà E., Filice E., Guérold B., Shooshtarizadeh P., Levi R., Ramella R., Brero A., Boero O., Metz-Boutigue M. H., Tota B., Alloatti G. (2008) The homologous rat chromogranin A1-64 (rCGA1-64) modulates myocardial and coronary function in rat heart to counteract adrenergic stimulation indirectly via endothelium-derived nitric oxide. FASEB J. 22, 3992–4004 [DOI] [PubMed] [Google Scholar]
- 5. Mahapatra N. R., O'Connor D. T., Vaingankar S. M., Hikim A. P., Mahata M., Ray S., Staite E., Wu H., Gu Y., Dalton N., Kennedy B. P., Ziegler M. G., Ross J., Mahata S. K. (2005) Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J. Clin. Invest. 115, 1942–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gayen J. R., Zhang K., Ramachandrarao S. P., Mahata M., Chen Y., Kim H-S., Naviaux R. K., Sharma K., Mahata S. K., O'Connor D. T. (2010) Role of reactive oxygen species in hyperadrenergic hypertension: Biochemical, physiological, and pharmacological evidence from targeted ablation of the chromogranin A gene. Circ. Cardiovasc. Genet. 3, 414–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steiner H. J., Weiler R., Ludescher C., Schmid K. W., Winkler H. (1990) Chromogranins A and B are co-localized with atrial natriuretic peptides in secretory granules of rat heart. J. Histochem. Cytochem. 38, 845–850 [DOI] [PubMed] [Google Scholar]
- 8. Pieroni M., Corti A., Tota B., Curnis F., Angelone T., Colombo B., Cerra M. C., Bellocci F., Crea F., Maseri A. (2007) Myocardial production of chromogranin A in human heart: a new regulatory peptide of cardiac function. Eur. Heart J. 28, 1117–1127 [DOI] [PubMed] [Google Scholar]
- 9. Biswas N., Curello E., O'Connor D. T., Mahata S. K. (2010) Chromogranin/secretogranin proteins in murine heart: myocardial production of chromogranin A fragment catestatin (Chga(364–384)). Cell Tissue Res. 342, 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Connor D. T., Bernstein K. N. (1984) Radioimmunoassay of chromogranin A in plasma as a measure of exocytotic sympathoadrenal activity in normal subjects and patients with pheochromocytoma. N. Engl. J. Med. 311, 764–770 [DOI] [PubMed] [Google Scholar]
- 11. Stridsberg M., Husebye E. S. (1997) Chromogranin A and chromogranin B are sensitive circulating markers for phaeochromocytoma. Eur. J. Endocrinol. 136, 67–73 [DOI] [PubMed] [Google Scholar]
- 12. Ceconi C., Ferrari R., Bachetti T., Opasich C., Volterrani M., Colombo B., Parrinello G., Corti A. (2002) Chromogranin A in heart failure: a novel neurohumoral factor and a predictor for mortality. Eur. Heart J. 23, 967–974 [DOI] [PubMed] [Google Scholar]
- 13. Jansson A. M., Røsjø H., Omland T., Karlsson T., Hartford M., Flyvbjerg A., Caidahl K. (2009) Prognostic value of circulating chromogranin A levels in acute coronary syndromes. Eur. Heart J. 30, 25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Røsjø H., Masson S., Latini R., Flyvbjerg A., Milani V., La Rovere M. T., Revera M., Mezzani A., Tognoni G., Tavazzi L., Omland T., and GISSI-HF Investigators (2010) Prognostic value of chromogranin A in chronic heart failure: data from the GISSI-Heart Failure trial. Eur. J. Heart Fail. 12, 549–556 [DOI] [PubMed] [Google Scholar]
- 15. Angelone T., Quintieri A. M., Brar B. K., Limchaiyawat P. T., Tota B., Mahata S. K., Cerra M. C. (2008) The antihypertensive chromogranin A peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology 149, 4780–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tota B., Cerra M. C., Gattuso A. (2010) Catecholamines, cardiac natriuretic peptides and chromogranin A: evolution and physiopathology of a ‘whip-brake’ system of the endocrine heart. J. Exp. Biol. 213(Pt. 18), 3081–3103 [DOI] [PubMed] [Google Scholar]
- 17. Mazza R., Imbrogno S., Tota B. (2010) The interplay between chromogranin A-derived peptides and cardiac natriuretic peptides in cardioprotection against catecholamine-evoked stress. Regul. Pept. 165, 86–94 [DOI] [PubMed] [Google Scholar]
- 18. Koshimizu H., Cawley N. X., Kim T., Yergey A. L., Loh Y. P. (2011a) Serpinin: a novel chromogranin a-derived, secreted Peptide up-regulates protease nexin-1 expression and granule biogenesis in endocrine cells. Mol. Endocrinol. 25, 732–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koshimizu H., Cawley N. X., Yergy A. L., Loh Y. P. (2011b) Role of pGlu-Serpinin, a novel chromogranin A-derived peptide in inhibition of cell death. J. Mol. Neurosci. 2011b; DOI 10.1007/s12031-011-9521-7 [DOI] [PMC free article] [PubMed]
- 20. Bott-Flügel L., Bernshausen A., Schneider H., Luppa P., Zimmermann K., Albrecht-Küpper B., Kast R., Laugwitz K. L., Ehmke H., Knorr A., Seyfarth M. (2011) Selective attenuation of norepinephrine release and stress-induced heart rate increase by partial adenosine A1 agonism. PLoS One 6, e18048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al-Fulaij M. A., Ren Y., Beinborn M., Kopin A. S. (2007) Identification of amino acid determinants of dopamine 2 receptor synthetic agonist function. J. Pharmacol. Exp. Ther. 321, 298–307 [DOI] [PubMed] [Google Scholar]
- 22. Fortin J. P., Zhu Y., Choi C., Beinborn M., Nitabach M. N., Kopin A. S. (2009) Membrane-tethered ligands are effective probes for exploring class B1 G protein-coupled receptor function. Proc. Natl. Acad. Sci. U. S. A. 106, 8049–8054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hearn M. G., Ren Y., McBride E. W., Reveillaud I., Beinborn M., Kopin A. S. (2002) A Drosophila dopamine 2-like receptor: molecular characterization and identification of multiple alternatively spliced variants. Proc. Natl. Acad. Sci. U. S. A. 99, 14554–14559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zaric V., Weltin D., Erbacher P., Remy J. S., Behr J. P., Stephan D. (2004) Effective polyethylenimine-mediated gene transfer into human endothelial cells. J. Gene Med. 6, 176–184 [DOI] [PubMed] [Google Scholar]
- 25. Chahine R., Nadeau R., Lamontagne D., Yamaguchi N., de Champlain J. (1994) Norepinephrine and dihydroxyphenylglycol effluxes from sympathetic nerve endings during hypoxia and reoxygenation in the isolated rat heart. Can. J. Physiol. Pharmacol. 72, 595–601 [DOI] [PubMed] [Google Scholar]
- 26. Gao Y., Vanhoutte P. M. (2012) Nebivolol: an endothelium-friendly selective β1-adrenoceptor blocker. J. Cardiovasc. Pharmacol. 59, 16–21 [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez-Muñoz C., Fuente T., Medin-Aguerre S., Herńandez-Cascales J. (2011) The increase in rat ventricular automaticity induced by salbutamol is mediated through β(1)- but not β(2)-adrenoceptors: role of phosphodiesterases. Life Sci. 88, 1095–1101 [DOI] [PubMed] [Google Scholar]
- 28. De Arcangelis V., Liu S., Zhang D., Soto D., Xiang Y. K. (2010) Equilibrium between adenylyl cyclase and phosphodiesterase patterns adrenergic agonist dose-dependent spatiotemporal cAMP/protein kinase A activities in cardiomyocytes. Mol. Pharmacol. 78, 340–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mohammadi K., Liu L., Tian J., Kometiani P., Xie Z., Askari A. J. (2003) Positive inotropic effect of ouabain on isolated heart is accompanied by activation of signal pathways that link Na+/K+-ATPase to ERK1/2. Cardiovasc. Pharmacol. 41, 609–614 [DOI] [PubMed] [Google Scholar]
- 30. Hook V., Bark S., Gupta N., Lortie M., Lu W. D., Bandeira N., Funkelstein L., Wegrzyn J., O'Connor D. T., Pevzner P. (2010) Neuropeptidomic components generated by proteomic functions in secretory vesicles for cell-cell communication. AAPS J. 12, 635–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Glattard E., Angelone T., Strub J. M., Tota B., Aunis D., Metz-Boutigue M. H., Goumon Y. (2006) Identification and characterization of Vasostatin-containing peptides in rat heart. FEBS J. 273, 3311–3321 [DOI] [PubMed] [Google Scholar]
- 32. Katz A. M. (1990) Inotropic and lusitropic abnormalities in heart failure. Eur. Heart J. 11(Suppl. A), 27–31 [DOI] [PubMed] [Google Scholar]
- 33. Satoh K., Matsu-Ura T., Enomoto M., Nakamura H., Michikawa T., Mikoshiba K. (2011) Highly cooperative dependence of sarco/endoplasmic reticulum calcium ATPase (SERCA) 2a pump activity on cytosolic calcium in living cells. J. Biol. Chem. 286, 20591–20599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mattiazzi A., Vittone L., Mundiña-Weilenmann C. (2007) Ca2+/calmodulin-dependent protein kinase: a key component in the contractile recovery from acidosis. Cardiovasc. Res. 73, 648–656 [DOI] [PubMed] [Google Scholar]
- 35. He Q., Harding P., LaPointe M. C. (2010) PKA, Rap1, ERK1/2, and p90RSK mediate PGE2 and EP4 signaling in neonatal ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 298, H136–H143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Das A., Salloum F. N., Xi L., Rao Y. J., Kukreja R. C. (2009) ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. Am. J. Physiol. Heart Circ. Physiol. 296, H1236–H1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Penna C., Perrelli M. G., Raimondo S., Tullio F., Merlino A., Moro F., Geuna S., Mancardi D., Pagliaro P. (2009) Postconditioning induces an anti-apoptotic effect and preserves mitochondrial integrity in isolated rat hearts. Biochim. Biophys. Acta 1787, 794–801 [DOI] [PubMed] [Google Scholar]
- 38. Bassino E., Fornero S., Gallo M. P., Ramella R., Mahata S. K., Tota B., Levi R., Alloatti G. (2011) A novel catestatin-induced antiadrenergic mechanism triggered by the endothelial PI3K-eNOS pathway in the myocardium. Cardiovasc. Res. 91, 617–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cappello S., Angelone T., Tota B., Pagliaro P., Penna C., Rastaldo R., Corti A., Losano G., Cerra M. C. (2007) Human recombinant chromogranin A-derived vasostatin-1 mimics preconditioning via an adenosine/nitric oxide signaling mechanism. Am. J. Physiol. Heart Circ. Physiol. 293, H719–H727 [DOI] [PubMed] [Google Scholar]
- 40. Gallo M. P., Levi R., Ramella R., Brero A., Boero O., Tota B., Alloatti G. (2007) Endothelium-derived nitric oxide mediates the antiadrenergic effect of human vasostatin-1 in rat ventricular myocardium. Am. J. Physiol. Heart Circ. Physiol. 292, H2906–H2912 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.