Abstract
Epigenetic modulation of chromatin states constitutes a vital component of the cellular repertoire of transcriptional regulatory mechanisms. The development of new technologies capable of generating genome-wide maps of chromatin modifications has re-energized the field. We are now poised to determine how species- and tissue-specific patterns of DNA methylation, in concert with other chromatin modifications, function to establish and maintain cell- and tissue-specific patterns of gene expression during normal development, cellular differentiation, and disease. This review addresses our current understanding of the major mechanisms and function of DNA methylation in vertebrates with a historical perspective and an emphasis on what is known about DNA methylation in eye development and disease.
Keywords: DNA methylation, Transcriptional regulation, Ocular cancer, Retina, Lens, Müller glia
Introduction
Research over the past half century has revealed the importance of transcriptional regulatory genes and, by extension, regulation of transcription in the genetics of eye development and disease. However, our understanding of the contribution of epigenetics in these same processes has lagged, particularly when it comes to the visual system. Epigenetics refers to changes in chromatin structure that permanently affect gene expression in a cell or tissue without altering the actual nucleotide sequence. DNA methylation, along with the covalent modifications of histone tails by acetylation and methylation, is a major mechanism of epigenetic gene regulation. Non-coding RNAs (e.g., Xist) also contribute to epigenetic gene regulation by mediating genomic imprinting and heterochromatin formation through recruitment of chromatin-modifying complexes [1–3]. What distinguishes epigenetic gene regulation from simple transcriptional activation or repression is that epigenetic marks within the chromatin structure are maintained in daughter cells following mitosis. Further, epigenetic modifications can be influenced by environment [4], diet [5–7], and aging [8, 9]. This has raised interest in understanding the potential contribution of epigenetics to the etiology of multifactorial and late onset diseases such as glaucoma, age-related macular degeneration, and cataract.
Historical perspectives on DNA methylation
DNA methylation was initially discovered in the 1960's as a mechanism contributing to the strain specificity of bacteriophage infections of E. coli [10]. It was soon apparent that nucleic acid methylation was not unique to prokaryotes, but was also present in eukaryotes [11]. Early interest in the role of DNA methylation was stimulated by findings that various mutagens, including methylmethane sulfonate and dimethylnitrosourea, altered methylation patterns. It was initially thought that the link between DNA methylation and cancer reflected defects in DNA repair, such as defective repair of mutagen-induced methylation and/or from a loss of normal patterns of DNA methylation through overzealous DNA repair [12]. Research to decipher the connection between gene silencing and methylation was subsequently fueled by the finding that transformed cell lines undergo de novo methylation, resulting in permanent gene silencing [13].
Despite extensive research on the role of DNA methylation in cancer, progress in understanding its role in normal development and cellular differentiation has been comparatively slow. A role for DNA methylation in regulating differentiation and in reprogramming somatic nuclei to pluripotency was postulated shortly after the discovery of DNA methylation [14]. Although intriguing, this proposal was made in the absence of clear evidence for methylation or demethylation in eukaryotes. It was several years before methyltransferase activity was detected in eukaryotic cells [15, 16]. The availability of genome databases and development of new technologies capable of generating genome-wide maps of chromatin modifications has re-energized the field.
Patterns of cytosine methylation
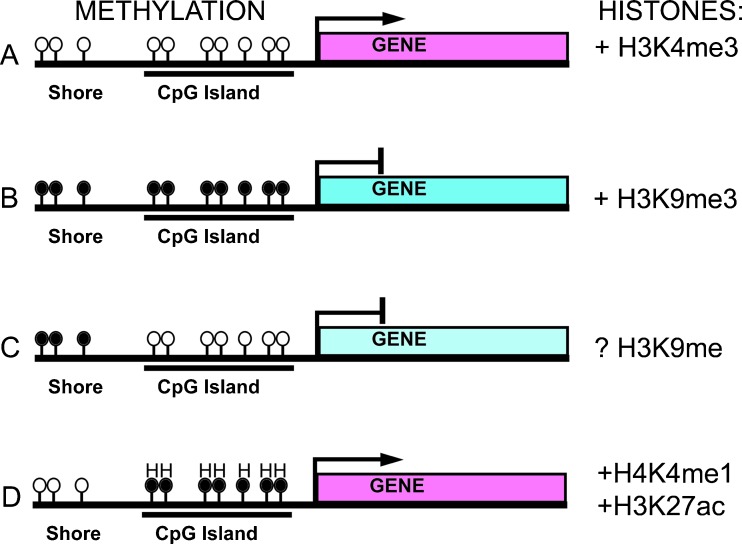
In animal cells, DNA methylation occurs almost exclusively on cytosine residues located within CpG dinucleotides, where the “p” stands for the phosphodiester bond linking the adjacent cytosine and guanosine nucleotides. Non-CpG methylation is well documented in plants [17, 18] and more recently, CpHpG methylation (where H is A, T, or C) has been detected in embryonic stem cells [19, 20]. Non-CpG methylation in vertebrates appears to be restricted to trinucleotide repeat regions. In vertebrates, overall methylation of CpG dinucleotides across the genome is estimated to be 70–80 %. Most of the genome has a lower than expected frequency of CpG dinucleotides, in part because of spontaneous deamination of methylated cytosine to thymidine [21]. In contrast, genomic regions containing higher than expected levels of C + G bases and CpG dinucleotides, known as CpG islands, are typically associated with promoter regions and may extend into the coding and intronic regions [22]. Cytosine methylation varies inversely with CpG density, such that CpG islands are typically hypomethylated, creating a chromatin environment permissive for transcription (Fig. 1a).
Fig. 1.
Effects of DNA methylation and histone modifications in transcription of CpG island-containing genes. a Chromatin with unmethylated cytosine residues (open circles) within CpG islands and adjacent shores is associated with trimethylation of histone H3 on lysine 4 (H3K4me2) and transcriptional activation. b Chromatin with hypermethylated cytosine residues within CpG islands and shores (black circles) is associated with H3K9 trimethylation (H3K9me3) and transcriptional silencing. c Methylation of cytosine residues located in the shores of the CpG island is sufficient for transcriptional repression, even when the CpG island is hypomethylated. Histone modifications associated with methylation in CpG shores has not been determined. d Hydroxymethylcytosine residues (H) are associated with H3K4 methylation (H3K4me1) and H3K27 acetylation and transcriptional activation typical of an open chromatin structure
Covalent modification of methyl cytosine into 5-hydroxymethylcytosine (5-hmC) is another recently identified DNA modification that appears to be abundant in embryonic stem cells and the brain [23, 24]. Conversion of 5-mC to 5-hmC is catalyzed by methylcytosine dioxygenase, which is part of the family of 3-oxoglutarate- and Fe(II)-dependent TET oncogenes [25]. ES cells have a high density of 5-hmC in promoter and enhancer regions, particularly those associated with H3K4me1 and H3K27ac histone modifications and binding sites for pluripotency-regulating transcription factors (e.g., NANOG, OCT4) [26, 27]. This suggests that 5-hmC may be a new marker of active gene regulatory regions (Fig. 1d). Interestingly, bisulfite conversion, the widely used “gold standard” method for analysis of DNA methylation, does not distinguish between 5-mC and 5-hmC [28]. Thus, there is need for caution in interpreting the results of bisulfite sequencing data and new methods will need to be developed to understand the biological significance of this novel chromatin modification.
CpG islands and gene regulation
Approximately 70 % of genes in a vertebrate genome are associated with CpG islands, with the largest single group of CpG island-containing genes consisting of the so-called “housekeeping” genes [22]. These genes are constitutively expressed in all cell types, consistent with the association between CpG island hypomethylation and transcriptional activation. However, not all CpG island-containing genes are ubiquitously expressed. Using a rigorous criteria for identification of CpG islands (>500 bp long, G + C content >50 %, a CpG observed/CpG expected ratio of ≥0.6 and located within 2,000 bp of the transcription start site) reduces the number of identified CpG island-containing genes in the human genome to ∼35 % [29]. Interestingly, analysis of gene ontology terms associated with the 17,820 identified genes revealed high representation of the gene ontology terms development, morphogenesis, synaptogenesis, and neurogenesis. Many CpG island-containing genes associated with neurogenesis also show tissue-restricted and developmentally restricted patterns of expression and genome-wide analysis has revealed dynamic changes in methylation patterns during development [30].
Cell-type-specific analysis has revealed that regulation of transcription by methylation is more complex than a simple on/off switch of hypo or hyper methylation. Modulating promoter activity does not require total CpG methylation and partial methylation can increase as well as decrease promoter activity. For example, our analysis of the effects of DNA methylation on activity of the EphA5 gene showed that in vitro methylation of 10 of 98 CpG dinucleotides within a 2,549 bp promoter construct could reduce activity by 20 %, whereas methylation of a non-overlapping set of 18/98 CpG dinucleotides increased promoter activity by 35 % [31]. Dynamic differences in methylation also occur in CpG dinucleotides located on the “shores” of CpG islands [32]. Analysis of multiple tissue types showed that over 30 % of differentially methylated CpG are located between 0 and 500 bp from a CpG island compared to <10 % located within CpG islands themselves [33] (Fig 1d). Finally, many sites that show tissue-specific variation in methylation during development also have variable methylation during differentiation of induced pluripotent stem cells and in cancer [32, 33].
DNA methyltransferase genes
Establishment of open and closed chromatin structure is regulated by both DNA methylation and histone modification and DNA methylation is inversely correlated with methylation of lysine 4 on histone H3 (H3K4) and positively correlated with H3K9 methylation [34] (Fig 1a, b). This is mediated, in part, through differential recruitment of de novo DNA methyltransferases (DNMT's) and histone remodeling complexes to the chromatin [35]. This section presents a brief overview of the major players involved in modulating DNA methylation of chromatin. Readers interested in more details regarding the molecular mechanisms underlying interactions between histone-modifying complexes and DNA methylation are referred to several excellent reviews [36–38].
Establishment and maintenance of DNA methylation patterns in the mammalian genome rely on the three major DNA methyltransferases: DNMT1, DNMT3a, and DNMT3b. DNMT1 is a maintenance methyltransferase that functions during DNA replication to re-methylate newly synthesized strands of DNA and facilitates cellular inheritance of epigenetic modifications [39]. Embryonic stem cells (ES) lacking Dnmt1 are viable, but have only about 1/3 of the normal CpG methylation levels [40] and Dnmt1 is necessary and sufficient for maintaining the methylation signatures of imprinted genes in the zygote [41]. Mice lacking DNMT1 die in mid-gestation and show genome-wide reductions in DNA methylation, with growth retardation, increased cell death, and a failure of vascular development, although eye development has not been characterized [42]. Conditional knockout of Dnmt1 in the forebrain in mice results in defects in the formation and organization of the barrel cortex, indicating a role for DNA methylation in the formation of sensory maps [43].
DNMT3a and DNMT3b are de novo methytransferases that can initiate methylation of previously unmethylated DNA [44]. DNMT3a is not required for embryonic and fetal development and homozygous Dnmt3a knockout mice appear normal at birth, although they subsequently die around the fourth postnatal week [45]. Loss of Dnmt3b is embryonic lethal in mice and results in a variety of developmental defects, most notably in the neural tube [45]. Lack of DNMT3b in mouse embryonic fibroblasts results in genome-wide DNA hypomethylation, chromosomal breaks, aneuploidy, and polyploidy, suggesting that DNMT3b contributes to maintaining methylation patterns and to genome stability [46]. In somatic cells, DNMT3b has been proposed to maintain the patterns of DNA methylation that silence germ line-specific genes [47]. Mutations in DNMT3b have been identified in some patients with ICF-1 (immunodeficiency-centromeric instability-facial anomalies syndrome 1) and is associated with increased areas of hypomethylation in their genomes [48]. The facial dismorphia in ICF patients includes abnormal epicanthal folds and telcanthus. Interestingly, analysis of gene expression changes in lymphoblastoid cells from ICF patients showed upregulation of multiple genes associated with eye development (e.g., Lhx2, Six3, Ascl1, Isl2, Eomes, EphB1, Sema3b, Robo1) [49].
In addition to the three canonical DNMTs, Dnmt3l (DNA methyltransferase 3-like) encodes a structurally related protein that lacks catalytic activity [50]. DNMT3L interacts directly with H3K4 and recruits DNMT3a to the nucleosome, providing a link between histone modifications and de novo DNA methylation [51, 52]. Both DNMT3L and DNMT3a are essential for establishing methylation patterns associated with imprinted genes in germ line cells [53].
DNMT2 was first identified as a DNA methyltransferase based on sequence similarity, but it lacks the N-terminal regulatory region typical of other DNMTs [54]. DNMT2 has minimal ability to methylate DNA and instead methylates tRNA [55]. Because of these findings, Dnmt2 was recently re-named Trdmt1 (tRNA aspartic acid methyltransferase 1). Homozygous mice lacking catalytic domains of DNMT2 are homozygous viable and fertile, with no obvious morphological defects [55]. In contrast, morpholino knockdown of dnmt2 in zebrafish embryos results in defects in multiple tissues including the eye, without detectible reduction in global DNA methylation [56]. Overexpression of a nuclear-targeted dnmt2 failed to rescue defects in the morphants, whereas overexpression of dnmt2 containing a nuclear export signal kept the protein in the cytoplasm and effected rescue with high efficiency [56].
Methyl CpG-binding proteins
Methylated DNA is bound by methyl Cp-binding protein 2 (MeCp2) and the methyl-binding domain proteins, MBD1, MBD2, and MBD3 [57, 58]. MeCp2 is capable of binding to a single CpG dinucleotide [59]. During replication, the transcriptional repression domain (TRD) of MeCp2 interacts with DNMT1, recruiting it for methylation of cytosine residues on the newly replicated DNA strand [60]. In non-replicating cells, the TRD functions independently as a transcriptional repressor to interpret the methylation signature [61]. MeCp2 also represses indirectly by recruiting co-repressors such as histone deacetylases (HDACs) and SIN3a [62], which remodel the histones and reinforce the chromatin compaction associated with gene silencing. Mutations in MeCp2 have been implicated in the etiology of Rett syndrome, a neurodevelopmental disorder that primarily affects females, beginning in their second year [63]. Despite apparently normal development in the first year and a half, patients with Rett syndrome progressively lose their ability to speak and show severe changes in motor and neurological functions that are often misdiagnosed as autism or multiple sclerosis. In the visual system, patients have substantial refractive errors, but reports on changes in visual function are contradictory [64–66]. However, mice lacking MeCp2 showed defects in the maturation and strengthening of retino-geniculate synapses associated with eye opening and vision-dependent remodeling [67]. This observation is consistent with reported defects in synaptic maturation and plasticity in other portions of the central nervous system associated with loss of MeCp2 [68, 69].
MBD1–3 were discovered based on homology to the methyl-binding domains of MeCp2 [70]. All three MBD's share significant homology within their N-terminal DNA-binding domains, with MBD4 showing the highest sequence similarity to MeCp2. Outside of the MBDs, they are significantly different, with highest sequence similarity between MBD2 and MBD3 (∼70 % amino acid identity) [71]. MBD1–3 are expressed broadly in all mouse tissues examined, although MBD1 and MBD2 transcript levels are lower in ES cells [72].
MBD1 contains several cysteine-rich CxxC domains that share homology to motifs found in DNA methyltransferase and the trithorax-related gene, HRX [73]. The CxxC domain functions as a transcriptional repressor independent of histone deacetylase activity [74]. MBD1 recognizes TCGCA and TGCGCA sequences most efficiently via its MBD; the CxxC domain can recognize a single methylated CpG, but shows no sequence specificity [75]. MBD2 can bind methylated DNA containing a single methylated site, although the efficacy of repression is directly correlated to methylation density [76]. In the genome, MBD2 is stably associated with heavily methylated DNA and recruits a co-repressor complex known as MeCp1 to genomic sites containing 12 or more methylated CpG sites [77]. MBD2/MeCp1 complexes also contain histone deacetylase activity and can be immunoprecipitated by antibodies against HDAC1 and HDAC2 [77]. MBD3 lacks the ability to bind to methylated DNA, but forms a component of the NuRD complex, a large multiunit protein complex containing histone deacetylase and nucleosome remodeling activities [78, 79]. The NuRD-MBD3 complex is subsequently recruited to methylated DNA through interactions with MBD2 [80].
Expression and function of DNA methyltransferases in the eye
In the mouse retina, Dnmt1 is most highly expressed during development with mRNA levels highest at embryonic day 11.5 and showing a progressive decrease with age [81]. DNMT1 protein is detected in most post-mitotic neurons in the inner retina, with strongest immunoreactivity in retinal ganglion cells and amacrine, and lower levels in bipolar and horizontal cells and cone photoreceptors [81]. Consistent with a role in retinal development, morpholino knockdown of dnmt1 in zebrafish embryogenesis results in defects in retinal lamination, loss of melanin pigmentation, a significant down-regulation of interphotoreceptor retinoid-binding protein (irbp/rbp3) expression and defects in the retinal pigmented epithelium (RPE) and photoreceptor differentiation [82].
Dnmt3a is expressed broadly in the embryo at E8.5 and E9.5, whereas Dnmt3b expression is specifically enriched in the neural tube and eyes [45]. Dnmt3a and 3b mRNAs and proteins are both highly expressed in the developing optic cup but retinal expression progressively decreases with age [81]. In the postnatal retina, immunoreactivity for DNMT3a and 3b is most prominent in the ganglion cell layer and inner nuclear layer (in amacrine and horizontal cells), with labeling also present in cone photoreceptors and pigmented epithelium [81]. In contrast, a previous study showed broad immunoreactivity for DNMT3b, but not DNMT1 or DNMT3a in the human retina [83]. The basis for this difference is not known. Ocular phenotypes of mouse knockouts of Dnmt3a or Dnmt3b have not yet been described. However, morpholino knockdown of dnmt3, the zebrafish ortholog of the mammalian Dnmt3b, results in multiple defects in embryos, most notably a reduction in the size of the brain and eye [84]. Compared to dnmt1 morphants, knockdown of dnmt3b resulted in a more severe disorganization of the retinal laminations and a less differentiated retinal phenotype. Although irbp expression was downregulated in dnmt1 and dnmt3 morphants, only dnmt3 morphants lacked expression of other markers of photoreceptor and amacrine cells including crx, neurod, and ascl1a. Intriguingly, both dnmt1 and dnmt3 knockdown resulted in defects in RPE differentiation, with dnmt1 morphants lacking RPE on the dorsal side and dnmt3 morphants lacking RPE on the ventral side of the optic cup [82, 84]. These findings support a potential role for DNA methylation in patterning the developing eyecup, as well as in regulating differentiation of the RPE and photoreceptors.
DNA methylation in ocular cancers
Given the role of DNA methylation in tumorogenesis, it is not surprising that changes in DNA methylation are associated with altered gene expression in ocular tumors. In retinoblastoma, the most common childhood tumor of the retina [85], methylation-sensitive PCR of promoter methylation showed that the tumor suppressor gene, RAS-associated domain family 1A (RASSF1A) was silenced by DNA hypermethylation in 82 % of tumors, but not in adjacent normal retinal tissues [86]. O6-methylguanine-DNA methyltransferase (MGMT), a methyltransferase gene involved in DNA repair, is hypermethylated in a subset of retinoblastoma primary tumors and is associated with earlier disease onset (before age 2) [87]. DNMT1, 3a, and 3b are frequently over-expressed in retinoblastoma and expression levels are positively correlated with pathogenesis [83]. However, increased CpG methylation in retinoblastoma does not simply reflect a generalized hypermethylation, as multiple genes that are silenced by hypermethylation in other cancers (e.g., p14RF, p15INK4b, p16INK4a, or VHL) remain hypomethylated in retinoblastoma tumors [87].
Uveal melanoma is a highly aggressive tumor arising from the pigmented cells of the uvea, including the iris, ciliary body, and choroid [88]. Gene silencing by DNA methylation has been proposed to contribute to the pathophysiology of uveal melanoma, but reports of methylation patterns are contradictory. RASSF1a was found to be hypermethylated in 50 % of archival frozen tumor specimens and in 91 % of uveal melanoma cell lines [89]. In contrast, in 23 uveal melanoma biopsies, only telomerase (hTERT) showed high frequency of hypermethylation (52 %), whereas six additional genes known to be hypermethylated in cutaneous melanoma, including RASSF1, were methylated in less than 15 % of tumor samples [90]. Methodological differences may account for some of the variability in these results; however, determining the role of methylation in the etiology and metastasis of uveal melanoma will require additional research.
DNA methylation in regulation of ocular and retinal gene expression
The body of published research on regulation of ocular gene expression by DNA methylation is small when compared to the vast literature related to methylation in cancer, embryonic stem cells, and other tissues. The earliest studies used DNA digestion by methyl-sensitive restriction endonucleases followed by Southern blot analysis to identify correlations between methylation status and gene expression in ocular vs. non-ocular tissues. Subsequently, bisulfite sequencing enabled identification of all methylated CpG dinuleotides in a region of interest. However, both of these approaches require a priori identification of genes of interest and are relatively slow and labor intensive.
An early study of the role of DNA methylation in regulating ocular genes tested if tissue-specific hyper- or hypomethylation could be responsible for differential expression of chicken delta crystalline (Cryd) in ocular (lens, embryonic neural retina, and RPE) vs. extra ocular (liver, kidney) tissues [91]. Despite some variation in methylation status of the internal cytosine of the CCGG recognition site of HpaII in different tissues, there was no correlation between methylation and mRNA expression. Likewise, lentoid bodies generated from embryonic neural retina showed no correlation between Cryd methylation and mRNA expression [92]. Analysis of a GC rich promoter domain in the gene encoding retinal fatty acid-binding protein (Fabp5) also showed no methylation in retina, where the gene is highly expressed, and in liver, where no transcripts were detectable [93]. However, a role for DNA methylation in lens development was recently demonstrated in zebrafish, where morpholino knockdown of dnmt1 resulted in lens dismorphia, ruptures of the lens capsule and unraveling of lens fibers.
Ten years later, a similar approach found a developmental and tissue-specific decrease in methylation in the promoter of the interphotoreceptor retinoid-binding protein (Rbp3I) gene that correlated with upregulation of mRNA expression during retinal development [94, 95]. A more recent study also implicated DNA methylation in regulating irbp/rbp3I in zebrafish retinas, but showed downregulation of expression following morpholino knockdown of dnmt1, despite a general loss of genome-wide methylation [82]. Nevertheless, defects in cone photoreceptor morphology were also observed following dnmt1 knockdown in zebrafish [96]. This is consistent with preferential detection of DNMT1 protein in cones vs. rods in the mouse retina [81]. Together, results support a role for DNA methylation in photoreceptor development, but suggest that the relationship between methylation and regulation of photoreceptor-specific gene expression may have species-specific differences.
The proposal that genes regulating development of ocular tissues are regulated by differential methylation has been further supported by some recent studies. Analysis of methylation patterns in the whole mouse embryos using methylated DNA immunoprecipitation with microarray analysis (MeDip-on-Chip) identified several genes expressed in the eye [e.g., complexin 4 (Cplx4), a gene encoding a synaptic protein expressed in retina and brain and alpha crystalline-A (Cryaa), a key gene for lens development] as differentially methylated during development. Both genes are hypermethylated in the epiblast around the time of implantation, but are unmethylated specifically in retina and lens respectively in adult mice [97].
Our work has shown a role for DNA methylation in regulation of EphA5 receptor expression in the mouse retina [31]. EphA5 is a member of the Ephrin receptors (Eph) family of tyrosine kinase receptors that regulate multiple patterning events during embryonic development, including the formation of topographical maps in the visual and other sensory systems throughout the CNS [98, 99]. Using bisulfite sequencing of DNA isolated from whole retinas, we found near complete hypomethylation in the proximal EphA5 promoter that did not change from embryogenesis to adults [31]. However, a more detailed analysis of DNA isolated from the nasal or temporal retinas of neonatal mice revealed partial promoter methylation of CpG sites near the transcription start site in a fraction of clones. Methylation was increased in the nasal retina, where EphA5 expression is lowest, compared to the temporal retina where EphA5 expression is highest. However, these studies were performed on DNA samples from full thickness retinal explants, and additional analysis using DNA from purified cell populations are needed before we can attribute the differences in methylation to a particular cell type. We are continuing to pursue the significance of low-level methylation in modulating EphA5 promoter activity to determine how this may contribute to the nasal/temporal gradient of expression in the retina in vivo.
DNA methylation and stem cells
Mechanisms regulating genome demethylation are of great interest, as re-setting the epigenome is critical for pluripotency. Recent studies have shown that induced pluripotent stem cells (iPSCs) can retain characteristics of the cells of origin, predispoising them to re-differentiate along their original lineage [100]. Methylation appears to contribute to this cellular memory, as fully dedifferentiated iPSCs can acquire methylation patterns comparable to embryonic stem cells over time in culture [101]. Pharmacological demethylation can be achieved in vitro using 5-azacytidine (5-Aza) and 5-aza-2′-deoxycytidine (Decitabine), which is used therapeutically for demethylation in some cancers [102, 103]. This may have utility in further enhancing pluripotency.
Our lab is one of several investigating the stem-cell potential of Müller glia in the mammalian retina [104–109]. Although Müller glia are the source of stem cells capable of regenerating all types of retinal neurons in the retinas of teleost fish [110, 111], their regenerative capacity in the retinas of mammals is severely attenuated. We have demonstrated that several genes (e.g., EphA5, EphB1) that are silenced by hypermethylation in conditionally immortalized Müller glia (ImM10 cell line) in vitro, can be upregulated by in vitro demethylation with AzadC [31]. The significance of hypermethylation of this Müller glia cell line and whether similar patterns of methylation are present in other Müller cell lines or in vivo remains to be determined
Conclusions
One of the hurdles in cataloging and interpreting the scope and significance of changes in DNA methylation has been the lack of cell-specific and genome-wide data. Until recently, analysis of methylation patterns has focused on specific genes in cultured cells (particularly tumor derived or immortalized cells) or whole tissues. This approach relies on the assumptions that the researchers have predicted the relevant genes and that methylation patterns are tissue specific, rather than cell-type specific. Since most tissues are composed of multiple cell types, methylation variations that are restricted to a subset of cells within the tissue would be difficult to detect. The use of genome-wide methylation analysis, combined with cell sorting to obtain purified cell populations offers us the unique opportunity to generate comprehensive databases of chromatin modifications of specific cell populations, at distinct developmental stages in both healthy and diseased tissues. Over the next several years, continued use of high content and high-resolution strategies to determine the genome-wide changes in DNA methylation signatures has the potential to reveal new roles for DNA methylation and other epigenetic mechanisms in controlling gene expression in the visual system during development, aging, and disease.
Footnotes
This work supported in part by NIH-R01EY012792.
Several additional articles on DNA methylation in the retina were published after this article went to press and therefore were not cited in this review. Because of their relevance to the subject, the references are provided here.
Livide G, Espistolata MC Amenduni M, Disciglio V, Marozza A, Mencarelli MA, Toti P, Lazzi S, Hadjistilianou T, De Francesco S, D'Ambrosio A, Renieri A, Ariani F. Epigenetic and copy number variation analysis in retinoblastoma by MS-MLPA. Path Oncol Res. 2012, Jan 26. PMID: 22278416
Powell C, Elsaeidi F, Goldman D. Injury-dependent Muller glia and ganglion cell reprogramming during tissue regeneration requires Apobec2a and Apobec2b. J Neurosci 2012;32(3):1096–109. PMID: 22262907
Merbs SL, Khan MA, Hackler L Jr, Oliver VF, Wan J, Qian J, Zack DJ. Cell specific DNA methylation patterns of retina-specific genes. PLoS One 2012, 7(3)e32602. PMID:22403679
Hunter A, Spechler P, Cwanger A, Song Y, Zhang Z, Ying GS, Hunter AK, Dezoeten E, Dunaief J. DNA methylation is associated with altered gene expression in AMD. IOVS, 2012 Mar 12. PMID: 22410570
References
- 1.Costa FF. Non-coding RNAs, epigenetics and complexity. Gene. 2008;410:9–17. doi: 10.1016/j.gene.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Wan LB, Bartolomei MS. Regulation of imprinting in clusters: noncoding RNAs versus insulators. Adv Genet. 2008;61:207–223. doi: 10.1016/S0065-2660(07)00007-7. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Qu L. Non-coding RNAs and the acquisition of genomic imprinting in mammals. Sci Chin C Life Sci. 2009;52:195–204. doi: 10.1007/s11427-009-0035-2. [DOI] [PubMed] [Google Scholar]
- 4.Waterland RA. Is epigenetics an important link between early life events and adult disease? Horm Res. 2009;71(Suppl 1):13–16. doi: 10.1159/000178030. [DOI] [PubMed] [Google Scholar]
- 5.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132:2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- 6.Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr. 2002;132:2333S–2335S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- 7.Tost J. DNA methylation: an introduction to the biology and the disease-associated changes of a promising biomarker. Methods Mol Biol (Clifton, NJ) 2009;507:3–20. doi: 10.1007/978-1-59745-522-0_1. [DOI] [PubMed] [Google Scholar]
- 8.Bollati V, Schwartz J, Wright R, Litonjua A, Tarantini L, et al. Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mech Ageing Dev. 2009;130:234–239. doi: 10.1016/j.mad.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez DG, Nalls MA, Gibbs JR, Arepalli S, Brug M, et al. Distinct DNA methylation changes highly correlated with chronological age in the human brain. Hum Mol Genet. 2011;20:1164–1172. doi: 10.1093/hmg/ddq561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold M, Hausmann R, Maitra U, Hurwitz J. The enzymatic methylation of Rna and DNA. 8. Effects of bacteriophage infection on the activity of the methylating enzymes. Proc Natl Acad Sci USA. 1964;52:292–297. doi: 10.1073/pnas.52.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hattman S, Kenny C, Berger L, Pratt K. Comparative study of DNA methylation in three unicellular eucaryotes. J Bacteriol. 1978;135:1156–1157. doi: 10.1128/jb.135.3.1156-1157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laird PW, Jaenisch R. DNA methylation and cancer. Hum Mol Genet. 1994;3 Spec No:1487-1495. [DOI] [PubMed]
- 13.Reilly JG, Thomas CA, Jr, Sen A. DNA methylation in mouse cells in culture as measured by restriction enzymes. Biochim Biophys Acta (BBA)—Gene Struct Expr. 1982;697:53–59. doi: 10.1016/0167-4781(82)90044-6. [DOI] [PubMed] [Google Scholar]
- 14.Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187:226–232. doi: 10.1126/science.1111098. [DOI] [PubMed] [Google Scholar]
- 15.Gruenbaum Y, Cedar H, Razin A. Substrate and sequence specificity of a eukaryotic DNA methylase. Nature. 1982;295:620–622. doi: 10.1038/295620a0. [DOI] [PubMed] [Google Scholar]
- 16.Bestor TH, Ingram VM. Two DNA methyltransferases from murine erythroleukemia cells: purification, sequence specificity, and mode of interaction with DNA. Proc Natl Acad Sci USA. 1983;80:5559–5563. doi: 10.1073/pnas.80.18.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta R, Nagarajan A, Wajapeyee N. Advances in genome-wide DNA methylation analysis. Biotechniques. 2010;49:iii–xi. doi: 10.2144/000113493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He G, Elling AA, Deng XW. The epigenome and plant development. Annu Rev Plant Biol. 2011;62:411–435. doi: 10.1146/annurev-arplant-042110-103806. [DOI] [PubMed] [Google Scholar]
- 19.Ramsahoye BH, Biniszkiewicz D, Lyko F, Clark V, Bird AP, Jaenisch R. Non-CpG methylation is prevalent in embryonic stem cells and may be mediated by DNA methyltransferase 3a. Proc Natl Acad Sci USA. 2000;97:5237–5242. doi: 10.1073/pnas.97.10.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyachenko OV, Schevchuk TV, Kretzner L, Buryanov YI, Smith SS. Human non-CG methylation: are human stem cells plant-like? Epigenetics. 2010;5:569–572. doi: 10.4161/epi.5.7.12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nabel CS, Manning SA, Kohli RM. The curious chemical biology of cytosine: deamination, methylation, and oxidation as modulators of genomic potential. ACS Chem Biol. 2011;7(1):20–30. doi: 10.1021/cb2002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saxonov S, Berg P, Brutlag DL. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc Natl Acad Sci USA. 2006;103:1412–1417. doi: 10.1073/pnas.0510310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song CX, Szulwach KE, Fu Y, Dai Q, Yi C, et al. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat Biotechnol. 2010;29:68–72. doi: 10.1038/nbt.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W, Liu M. Distribution of 5-hydroxymethylcytosine in different human tissues. J Nucleic Acids. 2011;2011:870726. doi: 10.4061/2011/870726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pastor WA, Pape UJ, Huang Y, Henderson HR, Lister R, et al. Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells. Nature. 2011;473:394–397. doi: 10.1038/nature10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szulwach KE, Li X, Li Y, Song CX, Han JW, et al. Integrating 5-hydroxymethylcytosine into the epigenomic landscape of human embryonic stem cells. PLoS Genet. 2011;7:e1002154. doi: 10.1371/journal.pgen.1002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Y, Pastor WA, Shen Y, Tahiliani M, Liu DR, Rao A. The behaviour of 5-hydroxymethylcytosine in bisulfite sequencing. PLoS One. 2010;5:e8888. doi: 10.1371/journal.pone.0008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson PN, Bohme U, Lopez R, Mundlos S, Nurnberg P. Gene-Ontology analysis reveals association of tissue-specific 5′ CpG-island genes with development and embryogenesis. Hum Mol Genet. 2004;13:1969–1978. doi: 10.1093/hmg/ddh207. [DOI] [PubMed] [Google Scholar]
- 30.Song F, Mahmood S, Ghosh S, Liang P, Smiraglia DJ, Nagase H, Held WA. Tissue specific differentially methylated regions (TDMR): changes in DNA methylation during development. Genomics. 2009;93:130–139. doi: 10.1016/j.ygeno.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petkova TD, Seigel GM, Otteson DC. A role for DNA methylation in regulation of EphA5 receptor expression in the mouse retina. Vis Res. 2011;51:260–268. doi: 10.1016/j.visres.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doi A, Park IH, Wen B, Murakami P, Aryee MJ, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet. 2009;41:1350–1353. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41:178–186. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimoto H, Vertino PM, Cheng X. Molecular coupling of DNA methylation and histone methylation. Epigenomics. 2011;2:657–669. doi: 10.2217/epi.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datta J, Majumder S, Bai S, Ghoshal K, Kutay H, et al. Physical and functional interaction of DNA methyltransferase 3A with Mbd3 and Brg1 in mouse lymphosarcoma cells. Cancer Res. 2005;65:10891–10900. doi: 10.1158/0008-5472.CAN-05-1455. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 37.Ikegami K, Ohgane J, Tanaka S, Yagi S, Shiota K. Interplay between DNA methylation, histone modification and chromatin remodeling in stem cells and during development. Int J Dev Biol. 2009;53:203–214. doi: 10.1387/ijdb.082741ki. [DOI] [PubMed] [Google Scholar]
- 38.Cheng X, Blumenthal RM. Coordinated chromatin control: structural and functional linkage of DNA and histone methylation. Biochemistry. 2010;49:2999–3008. doi: 10.1021/bi100213t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hermann A, Gowher H, Jeltsch A. Biochemistry and biology of mammalian DNA methyltransferases. Cell Mol Life Sci. 2004;61:2571–2587. doi: 10.1007/s00018-004-4201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsumura A, Hayakawa T, Kumaki Y, Takebayashi S, Sakaue M, et al. Maintenance of self-renewal ability of mouse embryonic stem cells in the absence of DNA methyltransferases Dnmt1, Dnmt3a and Dnmt3b. Genes Cells. 2006;11:805–814. doi: 10.1111/j.1365-2443.2006.00984.x. [DOI] [PubMed] [Google Scholar]
- 41.Hirasawa R, Chiba H, Kaneda M, Tajima S, Li E, Jaenisch R, Sasaki H. Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev. 2008;22:1607–1616. doi: 10.1101/gad.1667008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-F. [DOI] [PubMed] [Google Scholar]
- 43.Golshani P, Hutnick L, Schweizer F, Fan G. Conditional Dnmt1 deletion in dorsal forebrain disrupts development of somatosensory barrel cortex and thalamocortical long-term potentiation. Thalamus Relat Syst. 2005;3:227–233. doi: 10.1017/S1472928807000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen T, Ueda Y, Dodge JE, Wang Z, Li E. Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Mol Cell Biol. 2003;23:5594–5605. doi: 10.1128/MCB.23.16.5594-5605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/S0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 46.Dodge JE, Okano M, Dick F, Tsujimoto N, Chen T, et al. Inactivation of Dnmt3b in mouse embryonic fibroblasts results in DNA hypomethylation, chromosomal instability, and spontaneous immortalization. J Biol Chem. 2005;280:17986–17991. doi: 10.1074/jbc.M413246200. [DOI] [PubMed] [Google Scholar]
- 47.Walton EL, Francastel C, Velasco G. Maintenance of DNA methylation: Dnmt3b joins the dance. Epigenetics 6. 2011. [DOI] [PubMed]
- 48.Hagleitner MM, Lankester A, Maraschio P, Hulten M, Fryns JP, et al. Clinical spectrum of immunodeficiency, centromeric instability and facial dysmorphism (ICF syndrome) J Med Genet. 2008;45:93–99. doi: 10.1136/jmg.2007.053397. [DOI] [PubMed] [Google Scholar]
- 49.Jin B, Tao Q, Peng J, Soo HM, Wu W, et al. DNA methyltransferase 3B (DNMT3B) mutations in ICF syndrome lead to altered epigenetic modifications and aberrant expression of genes regulating development, neurogenesis and immune function. Hum Mol Genet. 2008;17:690–709. doi: 10.1093/hmg/ddm341. [DOI] [PubMed] [Google Scholar]
- 50.Jeltsch A. Molecular enzymology of mammalian DNA methyltransferases. Curr Top Microbiol Immunol. 2006;301:203–225. doi: 10.1007/3-540-31390-7_7. [DOI] [PubMed] [Google Scholar]
- 51.Jia D, Jurkowska RZ, Zhang X, Jeltsch A, Cheng X. Structure of Dnmt3a bound to Dnmt3L suggests a model for de novo DNA methylation. Nature. 2007;449:248–251. doi: 10.1038/nature06146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ooi SK, Qiu C, Bernstein E, Li K, Jia D, et al. DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature. 2007;448:714–717. doi: 10.1038/nature05987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hata K, Okano M, Lei H, Li E. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development. 2002;129:1983–1993. doi: 10.1242/dev.129.8.1983. [DOI] [PubMed] [Google Scholar]
- 54.Schaefer M, Lyko F. Solving the Dnmt2 enigma. Chromosoma. 2009;119:35–40. doi: 10.1007/s00412-009-0240-6. [DOI] [PubMed] [Google Scholar]
- 55.Goll MG, Kirpekar F, Maggert KA, Yoder JA, Hsieh CL, et al. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–398. doi: 10.1126/science.1120976. [DOI] [PubMed] [Google Scholar]
- 56.Rai K, Chidester S, Zavala CV, Manos EJ, James SR, et al. Dnmt2 functions in the cytoplasm to promote liver, brain, and retina development in zebrafish. Genes Dev. 2007;21:261–266. doi: 10.1101/gad.1472907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bogdanovic O, Veenstra GJ. DNA methylation and methyl-CpG binding proteins: developmental requirements and function. Chromosoma. 2009;118:549–565. doi: 10.1007/s00412-009-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Defossez PA, Stancheva I. Biological functions of methyl-CpG-binding proteins. Prog Mol Biol Transl Sci. 2011;101:377–398. doi: 10.1016/B978-0-12-387685-0.00012-3. [DOI] [PubMed] [Google Scholar]
- 59.Nan X, Meehan RR, Bird A. Dissection of the methyl-CpG binding domain from the chromosomal protein MeCP2. Nucleic Acids Res. 1993;21:4886–4892. doi: 10.1093/nar/21.21.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kimura H, Shiota K. Methyl-CpG-binding protein, MeCP2, is a target molecule for maintenance DNA methyltransferase, Dnmt1. J Biol Chem. 2003;278:4806–4812. doi: 10.1074/jbc.M209923200. [DOI] [PubMed] [Google Scholar]
- 61.Yu F, Thiesen J, Stratling WH. Histone deacetylase-independent transcriptional repression by methyl-CpG-binding protein 2. Nucleic Acids Res. 2000;28:2201–2206. doi: 10.1093/nar/28.10.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 63.Nan X, Bird A. The biological functions of the methyl-CpG-binding protein MeCP2 and its implication in Rett syndrome. Brain Dev. 2001;23(Suppl 1):S32–37. doi: 10.1016/S0387-7604(01)00333-3. [DOI] [PubMed] [Google Scholar]
- 64.Kalmanchey R. Evoked potentials in the Rett syndrome. Brain Dev. 1990;12:73–76. doi: 10.1016/S0387-7604(12)80181-1. [DOI] [PubMed] [Google Scholar]
- 65.Saunders KJ, McCulloch DL, Kerr AM. Visual function in Rett syndrome. Dev Med Child Neurol. 1995;37:496–504. doi: 10.1111/j.1469-8749.1995.tb12037.x. [DOI] [PubMed] [Google Scholar]
- 66.Tetzchner S, Jacobsen KH, Smith L, Skjeldal OH, Heiberg A, Fagan JF. Vision, cognition and developmental characteristics of girls and women with Rett syndrome. Dev Med Child Neurol. 1996;38:212–225. doi: 10.1111/j.1469-8749.1996.tb15083.x. [DOI] [PubMed] [Google Scholar]
- 67.Noutel J, Hong YK, Leu B, Kang E, Chen C. Experience-dependent retinogeniculate synapse remodeling is abnormal in MeCP2-deficient mice. Neuron. 2011;70:35–42. doi: 10.1016/j.neuron.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Setoguchi H, Namihira M, Kohyama J, Asano H, Sanosaka T, Nakashima K. Methyl-CpG binding proteins are involved in restricting differentiation plasticity in neurons. J Neurosci Res. 2006;84:969–979. doi: 10.1002/jnr.21001. [DOI] [PubMed] [Google Scholar]
- 69.Kavalali ET, Nelson ED, Monteggia LM. Role of MeCP2, DNA methylation, and HDACs in regulating synapse function. J Neurodev Disord. 2011;3:250–256. doi: 10.1007/s11689-011-9078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hendrich B, Abbott C, McQueen H, Chambers D, Cross S, Bird A. Genomic structure and chromosomal mapping of the murine and human Mbd1, Mbd2, Mbd3, and Mbd4 genes. Mamm Genome. 1999;10:906–912. doi: 10.1007/s003359901112. [DOI] [PubMed] [Google Scholar]
- 71.Rodriguez-Osorio N, Wang H, Rupinski J, Bridges SM, Memili E. Comparative functional genomics of mammalian DNA methyltransferases. Reprod Biomed Online 20:243-255. [DOI] [PubMed]
- 72.Hendrich B, Bird A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol. 1998;18:6538–6547. doi: 10.1128/mcb.18.11.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Voo KS, Carlone DL, Jacobsen BM, Flodin A, Skalnik DG. Cloning of a mammalian transcriptional activator that binds unmethylated CpG motifs and shares a CXXC domain with DNA methyltransferase, human trithorax, and methyl-CpG binding domain protein 1. Mol Cell Biol. 2000;20:2108–2121. doi: 10.1128/MCB.20.6.2108-2121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ng HH, Jeppesen P, Bird A. Active repression of methylated genes by the chromosomal protein MBD1. Mol Cell Biol. 2000;20:1394–1406. doi: 10.1128/MCB.20.4.1394-1406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clouaire T, Las Heras JI, Merusi C, Stancheva I. Recruitment of MBD1 to target genes requires sequence-specific interaction of the MBD domain with methylated DNA. Nucleic Acids Res. 2010;38:4620–4634. doi: 10.1093/nar/gkq228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boyes J, Bird A. Repression of genes by DNA methylation depends on CpG density and promoter strength: evidence for involvement of a methyl-CpG binding protein. EMBO J. 1992;11:327–333. doi: 10.1002/j.1460-2075.1992.tb05055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ng HH, Zhang Y, Hendrich B, Johnson CA, Turner BM, et al. MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nat Genet. 1999;23:58–61. doi: 10.1038/12659. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y, Ng HH, Erdjument-Bromage H, Tempst P, Bird A, Reinberg D. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999;13:1924–1935. doi: 10.1101/gad.13.15.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saito M, Ishikawa F. The mCpG-binding domain of human MBD3 does not bind to mCpG but interacts with NuRD/Mi2 components HDAC1 and MTA2. J Biol Chem. 2002;277:35434–35439. doi: 10.1074/jbc.M203455200. [DOI] [PubMed] [Google Scholar]
- 80.Jiang CL, Jin SG, Pfeifer GP. MBD3L1 is a transcriptional repressor that interacts with methyl-CpG-binding protein 2 (MBD2) and components of the NuRD complex. J Biol Chem. 2004;279:52456–52464. doi: 10.1074/jbc.M409149200. [DOI] [PubMed] [Google Scholar]
- 81.Nasonkin IO, Lazo K, Hambright D, Brooks M, Fariss R, Swaroop A. Distinct nuclear localization patterns of DNA methyltransferases in developing and mature mammalian retina. J Comp Neurol. 2011;519:1914–1930. doi: 10.1002/cne.22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rai K, Nadauld LD, Chidester S, Manos EJ, James SR, et al. Zebra fish Dnmt1 and Suv39h1 regulate organ-specific terminal differentiation during development. Mol Cell Biol. 2006;26:7077–7085. doi: 10.1128/MCB.00312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qu Y, Mu G, Wu Y, Dai X, Zhou F, et al. Overexpression of DNA methyltransferases 1, 3a, and 3b significantly correlates with retinoblastoma tumorigenesis. Am J Clin Pathol. 2010;134:826–834. doi: 10.1309/AJCPHGQ69FXDFWII. [DOI] [PubMed] [Google Scholar]
- 84.Rai K, Jafri IF, Chidester S, James SR, Karpf AR, Cairns BR, Jones DA. Dnmt3 and G9a cooperate for tissue-specific development in zebrafish. J Biol Chem. 2009;285:4110–4121. doi: 10.1074/jbc.M109.073676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mahoney MC, Burnett WS, Majerovics A, Tanenbaum H. The epidemiology of ophthalmic malignancies in New York State. Ophthalmology. 1990;97:1143–1147. doi: 10.1016/s0161-6420(90)32445-4. [DOI] [PubMed] [Google Scholar]
- 86.Choy KW, Lee TC, Cheung KF, Fan DS, Lo KW, et al. Clinical implications of promoter hypermethylation in RASSF1A and MGMT in retinoblastoma. Neoplasia. 2005;7:200–206. doi: 10.1593/neo.04565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choy KW, Pang CP, To KF, Yu CB, Ng JS, Lam DS. Impaired expression and promotor hypermethylation of O6-methylguanine-DNA methyltransferase in retinoblastoma tissues. Investig Ophthalmol Vis Sci. 2002;43:1344–1349. [PubMed] [Google Scholar]
- 88.Sato T, Han F, Yamamoto A. The biology and management of uveal melanoma. Curr Oncol Rep. 2008;10:431–438. doi: 10.1007/s11912-008-0066-z. [DOI] [PubMed] [Google Scholar]
- 89.Maat W, Velden PA, Out-Luiting C, Plug M, Dirks-Mulder A, Jager MJ, Gruis NA. Epigenetic inactivation of RASSF1a in uveal melanoma. Investig Ophthalmol Vis Sci. 2007;48:486–490. doi: 10.1167/iovs.06-0781. [DOI] [PubMed] [Google Scholar]
- 90.Moulin AP, Clement G, Bosman FT, Zografos L, Benhattar J. Methylation of CpG island promoters in uveal melanoma. Br J Ophthalmol. 2008;92:281–285. doi: 10.1136/bjo.2007.127035. [DOI] [PubMed] [Google Scholar]
- 91.Bower DJ, Errington LH, Cooper DN, Morris S, Clayton RM. Chicken lens delta-crystallin gene expression and methylation in several non-lens tissues. Nucleic Acids Res. 1983;11:2513–2527. doi: 10.1093/nar/11.9.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Errington LH, Cooper DN, Clayton RM. The pattern of DNA methylation in the delta-crystallin genes in transdifferentiating neural retina cultures. Differ Res Biol Divers. 1983;24:33–38. doi: 10.1111/j.1432-0436.1983.tb01299.x. [DOI] [PubMed] [Google Scholar]
- 93.Bisgrove DA, Monckton EA, Godbout R. Involvement of AP-2 in regulation of the R-FABP gene in the developing chick retina. Mol Cell Biol. 1997;17:5935–5945. doi: 10.1128/mcb.17.10.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liou GI, Wang M, Matragoon S. Timing of interphotoreceptor retinoid-binding protein (IRBP) gene expression and hypomethylation in developing mouse retina. Dev Biol. 1994;161:345–356. doi: 10.1006/dbio.1994.1036. [DOI] [PubMed] [Google Scholar]
- 95.Boatright JH, Nickerson JM, Borst DE. Site-specific DNA hypomethylation permits expression of the IRBP gene. Brain Res. 2000;887:211–221. doi: 10.1016/S0006-8993(00)02990-5. [DOI] [PubMed] [Google Scholar]
- 96.Tittle RK, Sze R, Ng A, Nuckels RJ, Swartz ME, et al. Uhrf1 and Dnmt1 are required for development and maintenance of the zebrafish lens. Dev Biol. 2010;350:50–63. doi: 10.1016/j.ydbio.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Borgel J, Guibert S, Li Y, Chiba H, Schubeler D, et al. Targets and dynamics of promoter DNA methylation during early mouse development. Nat Genet. 2010;42:1093–1100. doi: 10.1038/ng.708. [DOI] [PubMed] [Google Scholar]
- 98.Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr Opin Cell Biol. 2004;16:580–589. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 99.Scicolone G, Ortalli AL, Carri NG. Key roles of Ephs and ephrins in retinotectal topographic map formation. Brain Res Bull. 2009;79:227–247. doi: 10.1016/j.brainresbull.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 100.Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:1117–1119. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nishino K, Toyoda M, Yamazaki-Inoue M, Fukawatase Y, Chikazawa E, et al. DNA methylation dynamics in human induced pluripotent stem cells over time. PLoS Genet. 2011;7:e1002085. doi: 10.1371/journal.pgen.1002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patra SK, Bettuzzi S. Epigenetic DNA-(cytosine-5-carbon) modifications: 5-aza-2′-deoxycytidine and DNA-demethylation. Biochemistry (Mosc) 2009;74:613–619. doi: 10.1134/S0006297909060042. [DOI] [PubMed] [Google Scholar]
- 103.Szmigielska-Kaplon A, Robak T. Hypomethylating agents in the treatment of myelodysplastic syndromes and myeloid leukemia. Curr Cancer Drug Targets. 2011;11(7):837–848. doi: 10.2174/156800911796798940. [DOI] [PubMed] [Google Scholar]
- 104.Fischer AJ, Reh TA. Potential of Muller glia to become neurogenic retinal progenitor cells. Glia. 2003;43:70–76. doi: 10.1002/glia.10218. [DOI] [PubMed] [Google Scholar]
- 105.Lawrence JM, Singhal S, Bhatia B, Keegan DJ, Reh TA, et al. MIO-M1 cells and similar Muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25(8):2033–43. doi: 10.1634/stemcells.2006-0724. [DOI] [PubMed] [Google Scholar]
- 106.Florian C, Langmann T, Weber BH, Morsczeck C. Murine Muller cells are progenitor cells for neuronal cells and fibrous tissue cells. Biochem Biophys Res Commun. 2008;374:187–191. doi: 10.1016/j.bbrc.2008.06.119. [DOI] [PubMed] [Google Scholar]
- 107.Giannelli SG, Demontis GC, Pertile G, Rama P, Broccoli V. Adult human Muller glia cells are a highly efficient source of rod photoreceptors. Stem Cells. 2010;29:344–356. doi: 10.1002/stem.579. [DOI] [PubMed] [Google Scholar]
- 108.Otteson DC, Phillips MJ. A conditional immortalized mouse Muller glial cell line expressing glial and retinal stem-cell genes. Investig Ophthalmol Vis Sci. 2010;51:5991–6000. doi: 10.1167/iovs.10-5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Phillips MJ, Otteson DC. Differential expression of neuronal genes in Muller glia in two- and three-dimensional cultures. Invest Ophthalmol Vis Sci. 2011;52(3):1439–49. doi: 10.1167/iovs.10-6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Otteson DC, Hitchcock PF. Stem cells in the teleost retina: persistent neurogenesis and injury-induced regeneration. Vis Res. 2003;43:927–936. doi: 10.1016/S0042-6989(02)00400-5. [DOI] [PubMed] [Google Scholar]
- 111.Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–7040. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]