Abstract
G protein-coupled receptors (GPCRs) play key physiological roles in numerous tissues, including the heart, and their dysfunction influences a wide range of cardiovascular diseases. Recently, the notion of nuclear localization and action of GPCRs has become more widely accepted. Nuclear-localized receptors may regulate distinct signalling pathways, suggesting that the biological responses mediated by GPCRs are not solely initiated at the cell surface but may result from the integration of extracellular and intracellular signalling pathways. Many of the observed nuclear effects are not prevented by classical inhibitors that exclusively target cell surface receptors, presumably because of their structures, lipophilic properties, or affinity for nuclear receptors. In this topical review, we discuss specifically how angiotensin-II, endothelin, β-adrenergic and opioid receptors located on the nuclear envelope activate signalling pathways, which convert intracrine stimuli into acute responses such as generation of second messengers and direct genomic effects, and thereby participate in the development of cardiovascular disorders.
Stanley Nattel, Terry Hébert and Bruce Allen (shown from left to right) work at the Montreal Heart Institute, University of Montreal and McGill University. The cellular signalling and pathophysiological roles of nuclear-membrane GPCRs constitute one of their major research interests. Their backgrounds are in basic and clinical electrophysiology, molecular pharmacology, and signal-transduction. Artak Tadevosyan and George Vaniotis are doctoral students at the University of Montreal working at the Montreal Heart Institute. Using primary cells, subcellular fractionation, fluorescence imaging, and biochemical assays, their work has contributed to the elucidation of a role for nuclear-membrane localized G protein-coupled receptors in the regulation of gene-expression in normal and diseased hearts.
 |
Introduction
The cardiovascular system is regulated by a diverse array of hormones, neurotransmitters and growth factors, many of which exert their biological effects through the stimulation of G protein-coupled receptors (GPCRs; reviewed in Tang & Insel, 2004; Regard et al. 2008). GPCRs comprise a diverse family of heptahelical transmembrane proteins involved in cellular signalling, and represent the largest class of gene products targeted by therapeutic agents (reviewed in Devi, 2001; Lefkowitz, 2004). Activated by a variety of ligands, including light, neuropeptides, nucleosides/nucleotides, hormones, calcium ions, chemokines, biogenic amines, proteases, lipids and fatty acid mediators, GPCRs transduce these signals into intracellular biochemical responses (see Hermans, 2003). The cellular/physiological responses mediated by the GPCRs result from interactions of the receptor with different heterotrimeric guanine (G) nucleotide-binding proteins composed of α, β and γ subunits. The principal Gα subunits are: Gq, which activates phospholipase C; Gs, which activates adenylyl cyclase (AC); Gi, which inhibits AC; and G12 and G13, which regulate RhoGEF (reviewed in Siehler, 2009). In their inactive state, Gα subunits are bound to GDP. Following receptor activation, Gα subunits undergo conformational changes leading to reduced affinity for, and hence dissociation of, GDP followed by the binding of GTP (Bourne et al. 1991). GTP binding is generally thought to result in dissociation of Gα-GTP from the receptor and the Gβγ complex, at least in vitro (Lambright et al. 1996). However, evidence indicates that this might not always be the case: GPCRs may also simply undergo a series of conformational changes that allow for effector activation (reviewed in Hebert et al. 2006). Both the Gα-GTP and Gβγ complexes can stimulate downstream effector molecules associated with several cellular signalling pathways, thereby modulating the production or release of second messengers (e.g. Ca2+, cGMP, cAMP, diacylglycerol (DAG), nitric oxide (NO), inositol-1,4,5-trisphosphate (IP3)) which ultimately lead to cellular responses (reviewed in Hamm, 1998). Sequestration or metabolism of second messengers, intrinsic GTPase activity of Gα subunits, desensitization, internalization, and down-regulation of receptor responses by second messenger-activated or G protein-coupled receptor kinases (GRKs), β-arrestins, or regulators of G protein signalling (RGS) proteins, negatively regulate the initial wave of GPCR signalling (reviewed in Barren & Artemyev, 2007; Ribas et al. 2007; Kovacs et al. 2009).
In conventional GPCR signalling, receptors localized to the plasma membrane modulate the activity of membrane-localized enzymes and ion channels and/or the abundance or localization of second messengers within the target cell. Research conducted in recent years has revealed that endocytosed GPCRs may regulate distinct signalling pathways, suggesting that the biological responses mediated by GPCRs do not arise solely at the cell surface, but may result from the integration of extracellular and intracellular signalling events (Terrillon & Bouvier, 2004; Hanyaloglu & von Zastrow, 2008; Calebiro et al. 2009; Sorkin & von Zastrow, 2009). Recent experimental evidence suggests both nuclear localization and action of GPCRs. This distinct pool of GPCRs may play central roles in many cellular processes including regulation of gene transcription, ionic homeostasis, cellular proliferation and remodelling (reviewed in Boivin et al. 2008). Intracellular GPCRs may be constitutively active, or may be activated by ligands internalized from the extracellular space or synthesized within the cell. They can regulate signalling pathways distinct from those activated by the same receptor at the cell surface (reviewed in Re, 1999). This new paradigm for cellular signalling adds a further layer of complexity to studies of the function and physiological roles of GPCRs.
Nuclear GPCRs and their intracellular ligands
GPCRs localized to the nuclear membrane
Several GPCRs have now been shown to localize to the nuclear membrane, in a wide variety of different cell systems and tissues (Table 1). There are five major families of GPCRs, and members of the three main classes have been detected on the nuclear membrane. The remaining two classes, which we will not discuss further here, are the adhesion and the frizzled/taste receptor families, respectively (Millar & Newton, 2010).
Table 1.
Nuclear GPCRs in various organ systems
| GPCR subfamilies | Cell/tissue | Species | Method of detection | Function | Reference |
|---|---|---|---|---|---|
| Family 1 | |||||
| Muscarinic acetylcholine receptor (mAChR) | Cornea, corneal epithelial & endothelial cells | Rabbit | Autoradiographic ligand binding, ligand binding assay, functional assays | ↑ DNA and RNA Pol II activity, ↑ nuclear cGMP | Cavanagh & Colley (1989); Lind & Cavanagh (1993, 1995)) |
| Apelin receptor (APJ) | Cerebellum, hypothalamus, transfected cerebellum D283 Med cells | Human | IHC, GFP-CM, Western blot* | Unknown | Lee et al. (2004) |
| Alpha- adrenergic receptor (α-AR) | Neonatal ventricular cardiomyocytes, adult ventricular α1ARKO cardiomyocytes | Rat, murine | Ligand binding assay, GFP-CM, subcellular ligand binding | α-AR; ERK activation, proliferation, survival | Buu et al. (1993); Huang et al. (2007) |
| β-Adrenergic receptor (β-AR) | Adult & neonatal ventricular cardiomyocytes | Rat, murine | Ligand binding, Western blot†, ICC†, functional assays | ↑ Nuclear cAMP, G protein coupling, transcription, PKB activation | Buu et al. (1993); Boivin et al. (2006); Vaniotis et al. (2011b) |
| Angiotensin receptor (ATR) | Vascular endothelial cells (ECs), VSMCs,adult ventricular cardiomyocytes, human adrenal tissue, neuronal cells, hepatocytes,HEK-293T cells, cerebellar cortex | Rat, hamster | Autoradiographic ligand binding, ligand binding assay, GFP-CM, ICC†, Western blot†, IHC†, functional assays | ↑ Nuclear µCa2+½, ERK & p38 activation, tyrosine phosphorylation, G protein coupling, transcription, proliferation | Lee et al. (2004); Re et al. (1984); Robertson & Khairallah (1971); Haller et al. (1999); Cook et al. (2006); Tadevosyan et al. (2010) |
| Cysteinyl leukotriene receptor 1 (CysLT1) | Colon tissue, colorectal carcinomas, Int 407 cells, CaCo-2 cells | Human | IHC†, ICC†, GFP-CM, Western blot†, functional assays | ↑ Nuclear µCa2+½, ERK activation, DNA replication, proliferation | Nielsen et al. (2005) |
| C-X-C chemokine receptor type 4 (CXCR4) | Hepatoma, colorectal cancer HT-29 cells, adenocarcinoma, (NSCLS), HeLa | Human | IHC†, ICC†, flow cytometry | Tumorigenesis | Shibuta et al. (2002); Gobeil et al. (2006a); Spano et al. (2004) |
| Prostaglandin receptor (EPR) | Brain, myometrium, liver, transfected HEK293 & Swiss 3T3, cerebralmicrovessel ECs | Murine, human, porcine, rat | Radioligand binding, ICC†, IHC†, Western blot†, functional assays | ↑ Nuclear µCa2+½, ERK & PKB activation, ↑ nuclear cAMP & IP3, ↑ eNOS mRNA,G-protein coupling, transcription, pro-inflammatory response | Bhattacharya et al. (1998); Bhattacharya et al. (1999); Gobeil et al. (2002); Provost et al. (2010) |
| Endothelin receptor (ETR) | Liver, VSMCs, adult ventricular myocytes, heart, brain, endocardial ECs | Rat, human, canine, sheep | Ligand binding assay, ICC†, Western blot†, radioligand-binding-SDS-PAGE, functional assays | ↑ Nuclear µCa2+½, ↑ phosphorylation, transcription | Hocher et al. (1992); Boivin et al. (2006); Jacques et al. (2005); Vaniotis et al. (2011b) |
| Gonadotropin-releasing hormone Receptor (GnRHR) | Germline, intestine, HEK293HTR-8/SVneo | C. elegans, human, murine | IHC† | Acetylation & phosphorylation of Histone H3 | Vadakkadath Meethal et al. (2006); Re et al. (2010) |
| Lysophosphatidic acid-1-receptor (LPA1R) | Hepatocytes, cerebral microvessel ECs, transfected HTC4 cells | Rat, porcine | Western blot†, ligand binding assay, IHC, ICC, functional assays | ↑ Nuclear µCa2+½, ERK & PKB activation, iNOS activation, transcription | Gobeil et al. (2003, (2006b); Waters et al. (2006) |
| Melanocortin type 2 receptor (MC2R) | Adrenal Y6 cells | Human | Functional assays | Unknown | Doufexis et al. (2007) |
| Melatonin receptor (MT2) | Placental choriocarcinoma cells | Human | ICC* | Unknown | Lanoix et al. (2006) |
| Natural killer receptor (NKR) | Dorsal root ganglia neural cells, HEK 293, Ventral tegmental area (VTA) neurons | Rat | ICC†, IHC†, electron microscopy* | Unknown | Boer & Gontijo (2006); Lessard et al. (2009) |
| Opioid peptide receptor (OPR 1/2) | NG 108–15 neurohybrid cells (OPR1), cardiomyopathic adult ventricular myocytes (OPR2) | Human, hamster | IHC†, ICC†, ligand binding assay, functional assays, transcription | G protein coupling, PKC activation | Belcheva et al. (1993); Ventura et al. (1998) |
| Platelet activating factor receptor (PAFR) | Liver, cerebral microvascular ECs, brain, transfected CHO, HEK293 & COS1cells, brain ECs | Rat, porcine | Flow cytometry, ICC†, IHC†, radio ligand binding assay, GFP-CM, Western blot†, functional assays | ↑ Nuclear DAG, ↑ nuclear µCa2+½,↓ nuclear cAMP, ERK phosphorylation,G protein coupling, transcription, PLC activation. Pro-inflammatory response | Miguel et al. (2001); Marrache et al. (2002); Zhu et al. (2006) |
| Prolactin receptor (PRLR) | Liver, splenocytes | Rat | Functional assays | Mitogenesis | Buckley et al. (1988) |
| Vasoactive intestinal peptide receptor(VPAC) | HT29 cells, breast cancer cells | Human | Ligand binding assay, radioligand-binding-SDS-PAGE | cAMP production | Omary & Kagnoff (1987); Valdehita et al. (2010) |
| Neuropeptide Y1 receptor (Y1R) | Pituitary gland, pituitary cells, endocardial EC | Rat, human | ICC† | Unknown | Chabot et al. (1988); Jacques et al. (2003) |
| Family 2 | |||||
| Parathyroid hormone 1 receptor (PTH1R) | Kidney, liver, gut, uterus, ovary, MC3T3-E1, ROS17/2.8, UMR106 and SaOS-2 cells, osteoclast-like multinucleated cells, LLC-PK1 cells | Rat, murine, human, deer | IHC†, ICC†, Western blot†, far Western blot GFP-CM | DNA synthesis, mitosis | Watson et al. (2000a, b); Faucheux et al. (2002); Pickard et al. (2006) |
| Family 3 | |||||
| Metabotropic glutamate receptor (mGluR) | Neuronal cells, transfected HEK293cells, neonatal striatal neurons | Murine | IHC†, ICC†, Western blot†, functional assays | ↑ Nuclear µCa2+½, transcription, CREB phosphorylation | O’Malley et al. (2003); Jong et al. (2005, 2007) |
Abbreviations for detection methods: IHC, Immunohistochemistry; GFP-CM, green fluorescent protein-confocal microscopy; ICC, immunocytochemistry. Antibodies used for verification of nuclear localisation by Western blotting or immunochemistry were targeted to either *epitope tagged versions of the receptor, or (more commonly) †endogenous receptors.
Class A, or rhodopsin-like receptors, contains 672 family members and are divided into 19 separate subfamilies which account for roughly 85% of all GPCRs (including olfactory receptors), representing the most diverse of the three classes (reviewed in Millar & Newton, 2010). Receptors from this class respond to a variety of different ligands that range from hormones and neurotransmitters to light itself, and all signal through interactions with heterotrimeric G proteins. Class A GPCRs also includes a relatively large number of receptors with no known physiological ligand, referred to as orphan receptors. Receptors in this family have been localized to the nuclear membrane, including members from almost every subfamily such as chemokine receptors (CXCR4), lysophosphatidic acid (LPA) receptors and opioid-like peptide receptors 1/2 (Ventura et al. 1998; Shibuta et al. 2002; Gobeil et al. 2003). Multiple GPCRs have been localized on the nuclear membrane of cardiomyocytes including angiotensin II type 1 receptor (AT1R) (Tadevosyan et al. 2010), angiotensin II type 2 receptor (AT2R, Robertson & Khairallah, 1971; Lee et al. 2004; Tadevosyan et al. 2010), α1AR (Huang et al. 2007), β1-AR, β3-AR, and endothelin B receptor (ETBR, Boivin et al. 2003; Boivin et al. 2006) to name a few (Table 1).
Class B GPCRs are primarily hormone receptors, including receptors for secretin and glucagon (Gkountelias et al. 2010). This family consists of three main subfamilies plus several as yet unclassified receptors. Members of this group have begun to be demonstrated at the level of the nuclear membrane of osteoclast-like cells, as in the case of the parathyroid hormone receptor (Faucheux et al. 2002).
Finally Class C, including metabotropic glutamate (mGlu) and γ-aminobutyric acid (GABA)-B receptors are primarily involved in the central and peripheral nervous systems (Jong et al. 2007; Ribeiro et al. 2010). Interestingly, of the three distinct groups of mGluRs with diverse roles, function and localization, only group I (e.g. mGluR1 and mGluR5) have been demonstrated on nuclear membranes of neuronal cells (Jong et al. 2005; Jong et al. 2007).
GPCR effectors in the nuclear membrane
Most of the downstream molecules normally associated with GPCR signalling at the plasma membrane have also been found in the nucleus (Fig. 1). This includes various G protein subunits: all three major forms of the α subunit as well as βγ complexes (Zhang et al. 2001; Gobeil et al. 2002; Boivin et al. 2005). Furthermore, a number of effector molecules have also been shown to be located at the level of the nucleus, including AC (Yamamoto et al. 1998), PLA2, PLCβ1 and PLCδ1 (Schievella et al. 1995; Freyberg et al. 2001) as well as a variety of ion channels, including Ca2+ (reviewed in Bootman et al. 2009), K+ (Mazzanti et al. 1990; Gobeil et al. 2002; Quesada et al. 2002) and Cl− channels (Tabares et al. 1991; Rousseau et al. 1996; Franco-Obregon et al. 2000). Moreover, the second messengers associated with downstream signalling events, including cAMP (Sastri et al. 2005; Boivin et al. 2006), IP3 and DAG (Kumar et al. 2008), are also present at the level of the nucleus or generated in isolated nuclei. As such, all of the components necessary for GPCR signalling are present at the nuclear membrane. Additionally, several studies have shown that GPCRs and their effectors interact at the nuclear membrane. Nuclear bradykinin type 2, endothelin B, lysophosphatidic acid receptor-1, prostaglandin (EP3), mGlu5 and AT1 receptors have been shown to modulate nucleoplasmic µCa2+½ (Devi, 2001; Gobeil et al. 2002, 2003; Bkaily et al. 2003a,b; Boivin et al. 2003; O’Malley et al. 2003; Savard et al. 2008; Tadevosyan et al. 2010). Consistent with a role in regulation of nucleoplasmic calcium transients, nuclear mGlu5Rs and AT1R also generate nuclear IP3 (Kumar et al. 2008; Tadevosyan et al. 2010). In contrast, nuclear β1-ARs stimulate adenylyl cyclase and increase nuclear µcAMP½ (Boivin et al. 2006). In addition to effectors, many of the systems involved in regulation of GPCR signalling are also be found in the nucleus, including RGS proteins (Burchett, 2003), β-arrestin-1 (Wang et al. 2003), as well as GRKs (Yi et al. 2002; Johnson et al. 2004). This indicates that nuclear GPCR signalling is tightly regulated, in a manner analogous to their cell surface counterparts, and hence reinforces the idea that these receptors are functionally relevant. Additionally, effectors associated with post G protein or G protein-independent GPCR signalling, including mitogen-activated protein kinase (MAPK) and PI3K/PKB pathways, are also present in the nucleus and GPCRs in the nuclear membranes are able to activate these various pathways (Marrache et al. 2002; Gobeil et al. 2006b; Vaniotis et al. 2011a). Furthermore, certain GPCRs, as is the case with the GABAB receptors as well as G proteins themselves, have been shown to interact directly with transcription factors (Nehring et al. 2000; Robitaille et al. 2010; Sato et al. 2011), while nuclear bradykinin B2 and type I gonadotropin releasing hormone receptors appear to be able to regulate histone acetylation (Savard et al. 2008; Re et al. 2010), adding yet another potential method by which nuclear localized GPCR signalling might modulate gene transcription.
Figure 1. Known nuclear angiotensin II, β-adrenergic, endothelin and opioid receptor-activated signalling cascades in the heart.
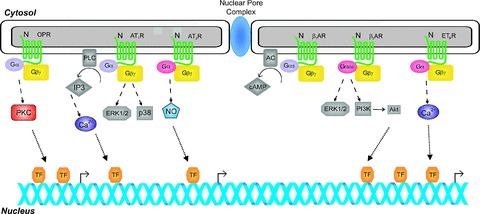
Different receptor systems activate distinct Gα-GTP and Gβγ complexes which stimulate downstream effector molecules modulating the production or release of second messengers (e.g. Ca2+, cAMP, NO, IP3) which may ultimately lead to changes in gene transcription. Considering the potential physiological functions of nuclear GPCRs in various cardiovascular diseases, future studies may provide a foundation for selective pharmacological interventions targeting these systems.
Ligands acting intracellularly
The term ‘intracrine’ ligand was coined to describe intracellular molecules binding to and activating intracellular receptors, including those on the nucleus. A list of known intracrine ligands in cardiovascular physiology is found in Table 2. These ligands are either synthesized within the target cell or are internalized from the extracellular space (Prochiantz & Theodore, 1995; Re, 1999). Enzymes involved in the generation and metabolism of phosphoinositides (Barlow et al. 2010) or processing peptide ligands (e.g. angiotensin converting enzyme) have also been localized to the nuclei of different cell types (Lucero et al. 2010). Further, these intracrine signalling loops are not restricted to GPCRs and may include a number of other classes of receptors including ALK4/ALK5, which are TGF-β superfamily receptors responsive to activin A (Gressner et al. 2008), as well as VEGF receptors (Lee et al. 2007). The potential actions of intracellular ligands include: (1) binding to intracellular membrane receptors and generation of second messengers (e.g. adrenergic, angiotensin, endothelin, insulin: Goldfine et al. 1982; Boivin et al. 2003, 2006; Tadevosyan et al. 2010), (2) binding to nuclei and nucleolus (e.g. angiogenin, fibroblast growth factor (FGF), parathyroid hormone-related protein (PTHrP): Bouche et al. 1987; Moroianu & Riordan, 1994b; Nguyen & Karaplis, 1998; Nguyen et al. 2001), (3) binding to chromatin (e.g. platelet-derived growth factor (PDGF), epidermal growth factor (EGF), nerve growth factor (NGF): Rakowicz-Szulczynska et al. 1986), (4) binding to receptors on the endoplasmic reticulum (ER) immediately following their synthesis (e.g. PDGF-B/v-sis: Fleming et al. 1989; Lee & Donoghue, 1992), and (5) binding to intravesicular receptors with consequent generation of second messengers (e.g. EGF: Wiley et al. 1998).
Table 2.
Intracellular ligands involved in cardiac physiology
| Intracrines | References |
|---|---|
| Angiogenin | Moroianu & Riordan (1994b) |
| Angiotensin II | Re et al. (1984); Erdmann et al. (1996) |
| Angiotensin (l-7) | Camargo de Andrade et al. (2006) |
| Angiotensin converting Enzyme (ACE) | Cristovam et al. (2008) |
| Angiotensinogen | Sherrod et al. (2005) |
| Atrial natriuretic peptide (ANP) | Morel et al. (1988) |
| Brain-derived neurotrophic factor | Marmigere et al. (2001) |
| Dynorphin | Ventura et al. (1998) |
| Endothelial cell growth factor, platelet-derived (PD-ECGF) | Fox et al. (1995); Matsukawa et al. (1996) |
| Endothelin Converting Enzyme | Russell & Davenport (1999) |
| Epidermal growth factor (EGF) | Rakowicz-Szulczynska et al. (1986); Wiley et al. (1998) |
| Erythropoietin | Mtjavila et al. (1991) |
| Fibroblast growth factor (FGF; 1, 2, 3, 10) | Bouche et al. (1987) |
| Gonadotropin | Millar et al. (2004) |
| Growth hormone | Ardail et al. (2010) |
| Hepatoma-derived growth factor | Kishima et al. (2002) |
| Hepatopoietin | Lu et al. (2002) |
| Insulin | Goldfine et al. (1982) |
| Insulin-like growth factor binding Protein (IGFBP; 3, 5, 6) | Schedlich et al. (2000); Iosef et al. (2008) |
| Interferon-y (IFN-y) | Nagy et al. (2000) |
| Interleukins | Clevenger et al. (1990); Curtis et al. (1990) |
| Nerve growth factor (NGF) | Rakowicz-Szulczynska et al. (1986) |
| Neuropeptide Y | Wirth et al. (2005) |
| Oxytocin | Kinsey et al. (2007) |
| Parathyroid hormonerelated protein (PHTrP) | Nguyen & Karaplis (1998) |
| Phospholipase A2-I | Fayard et al. (1998) |
| Platelet-derived growth factor (PDGF) | Rakowicz-Szulczynska et al. (1986) |
| Renin/prorenin | Lee-Kirsch et al. (1999) |
| Thrombospondin-1 | Ashton et al. (2004) |
| Thyroid-releasing hormone (TRH) | Garcia et al. (2000) |
| Transforming growth factor α (TGF-α) | Grasl-Kraupp et al. (2002) |
| Vascular endothelial growth factor (VEGF) | Li & Keller, (2000) |
| WNT13 | Struewing et al. (2006) |
Biosynthesis and actions of intracrine ligands
Hormones and growth factors fated to act within their target cells can be produced in various ways (Fig. 2). Intracrine ligands can be synthesized and targeted to the Golgi apparatus for secretion; however, they may also act intracellularly either before or after secretion (following reuptake; Re, 1999). In the case of peptide agonists, the intracrine gene product might arise from an alternative transcription initiation site, differences in mRNA maturation or translation leading to a gene product lacking secretory signals and consequently active only in the intracellular space (Kiefer et al. 1994; Lee-Kirsch et al. 1999; Xu et al. 2009). Alternative splicing can result in the generation of peptides that lack ER signal sequences or contain an altered hydrophobic sequence. For example, the mitochondrial genome does not contain the gene for renin, and hence the mitochondrial isoform of this peptide must be derived from the nuclear genome and must be synthesized in the cytosolic compartment and imported post-translationally into this subcellular organelle. In fact, the amino-terminal deletion of the ER signal sequence and 10 amino acids of preprorenin leads to trafficking to and retention within the mitochondria (Clausmeyer et al. 1999). A similar process might be envisaged for the nuclear translocation of intracellular ligands. Intramolecular events such as alternative translation initiation sites, the use of internal ribosomal entry sites and protein splicing through inteins that do not require additional enzymes for protein cleavage could also introduce protein variability and be a relevant aspect of intracrine biology and signalling (Amitai et al. 2004). This is particularly true for the Wnt family, where through the use of alternative promoters, alternative translation initiation sites and RNA splicing, three isoforms of Wnt 13 (Wnt 13 A, B, C) are synthesized. One of the isoforms, non-glycosylated Wnt 13B, traffics to the nucleus, increases the β-catenin/T-cell factor activity in HEK293 cells and promotes apoptosis in aortic endothelial cells (Struewing et al. 2006).
Figure 2. Sources of intracrine ligands.
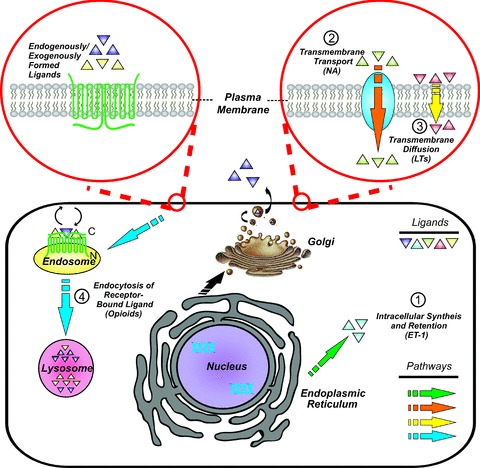
Intracrine systems appear in various forms; hormones, cardiac growth factors, DNA-binding proteins and enzymes can all display intracrine functionality. There are many ways to generate intracrine ligands: (1) ligands can be synthesized within the cell and remain in the cytoplasm where they can be trafficked to organelles such as the nucleus (e.g. ET-1); (2) ligands may pass through channels or pores, or active transport across the plasma membrane (e.g. noradrenaline, NA); (3) ligands sufficiently small may cross the plasma membrane directly, while hydrophobic ligands may freely cross the plasma membrane at will (e.g. leukotrienes, LTs); (4) internalization of endogeneously or exogenously formed ligands following binding to cell-surface receptors and subsequent release from internalized endosomes within the cell (e.g. opioids).
Intracrine ligands have many functional similarities. Many of these ligands regulate the generation of second messengers, translocate and bind to the nucleus, and can be pro-angiogenic or anti-angiogenic (Moroianu & Riordan, 1994a,b; Wakasugi et al. 2002; Tsuji et al. 2005). Intracrine ligands might function in similar or different ways depending on the cell type in question and can serve to amplify or attenuate cellular signals coming from cell membrane events. Infusion of angiotensin-II (Ang-II) increases hepatic and renal angiotensinogen mRNA levels (Kobori et al. 2001a,b) and it has been proposed an amplification system exists whereby increases in intracellular Ang-II results in increased Ang-II expression. Consistent with this, Ang-II up-regulates angiotensinogen and renin expression when administered to isolated hepatic nuclei (Eggena et al. 1993, 1996). However, in isolated rat renal cortical nuclei, Ang-II has no effect on angiotensinogen mRNA, indicating that this system for amplifying the intracellular effects of Ang-II is cell-type specific (Li & Zhuo, 2008). In contrast, extracellular ET-1 reduces ET-1 mRNA levels in endothelial cells, suggesting that extracellular ET-1 may suppress intracellular ET-1 signalling (Farhat et al. 2008). Nuclear binding of PTHrP stimulates proliferation of vascular smooth muscle cells and is implicated in cell cycle progression, whereas its binding to receptors at the plasma membrane suppresses proliferation and mitogenesis (Massfelder et al. 1997; Re, 1999; Tovar Sepulveda et al. 2002). Although still poorly understood, the diverse functions exerted by agonists and hormones acting at intracellular GPCRs suggests that intracrine signalling may play important roles distinct from those of the same receptors activated at the cell surface.
Nuclear GPCRs in the heart
Among the GPCRs currently known to be present on nuclear membranes, few receptors have been studied in cells specifically derived from components of the cardiovascular system. Here, we will review four nuclear GPCRs where function and mode of action has begun to be elucidated.
Renin–angiotensin system (RAS)
The RAS was originally thought to be either a systemic or local loop, where Ang-II is synthesized within the circulatory system or at the tissue level. However, there is now experimental evidence in support of an intracellular RAS. Specific Ang-II binding sites, including the AT1R and AT2R, have been demonstrated on the nuclear membranes in cardiomyocytes (Tadevosyan et al. 2010), hepatocytes, and vascular smooth muscle cell lines (Re et al. 1984; Booz et al. 1992; Cook et al. 2006). Adult cardiac ventricular myocytes express angiotensinogen, angiotensin converting enzyme (ACE) and renin (Malhotra et al. 1999; Tsai et al. 2008). Renin is synthesized as a preproprotein, although two splice variants of renin have been identified that lack exon 1 (Clausmeyer et al. 1999, 2000; Lee-Kirsch et al. 1999; Sinn & Sigmund, 2000). These variants do not possess the precursor domain, which contains a motif that directs preprorenin to the ER and thus the secretory pathway, but instead encode prorenin which remains within the cell. Interestingly, rat heart and lungs express only a prorenin variant, termed 1A renin (Clausmeyer et al. 2000), suggesting an important role for intracellular renin activity in these tissues. In addition, cells are able to internalize and activate circulating prorenin (Peters et al. 2002). Moreover, ACE, which converts angiotensin-I to angiotensin-II, was detected by immunocytochemistry inside nuclei of cultured rat cardiomyocytes and fibroblasts as well as mesangial cells cultured from spontaneously hypertensive rats (Dostal et al. 1992; Camargo de Andrade et al. 2006). Thus, there are means whereby Ang-II can be generated intracellularly. In vascular and cardiac tissue, chymase can replace ACE in the biosynthesis of Ang-II (Miyazaki & Takai, 2006) and chymase immunoreactivity has been detected in cytosolic granules of mast cells, endothelial cells, and mesenchymal interstitial cells of the cardiac interstitium (Urata et al. 1993).
Role of nuclear ATRs in cardiac hypertrophy and remodelling
The role of extracellular Ang-II has been extensively studied in the cardiovascular system, where it is crucial in the progression of cardiovascular remodelling, during the development of heart failure (Xu et al. 2010). Experimental evidence now suggests that intracellular Ang-II (iAng-II) might also be involved in the development of cardiovascular disease. Intracellular renin isoforms are implicated in blood pressure and fluid homeostasis through their potential generation of Ang-II (Lavoie et al. 2006; Schefe et al. 2006). Transduction of neonatal rat ventricular myocytes with an adenoviral construct expressing Ang-II led to the rapid induction of cellular hypertrophy (Baker et al. 2004). Transgenic mice engineered for cardiomyocyte-specific overexpression of rat angiotensinogen, as driven by the mouse α-myosin heavy chain (MHC) promoter, show increased levels of iAng-II and also develop cardiac hypertrophy (Mazzolai et al. 1998). Similarly, mice injected with a plasmid containing an α-MHC-Ang-II construct developed left and right ventricular hypertrophy within 48 h with no change in blood pressure (Baker et al. 2004). Whereas ventricular Ang-II levels were increased in this model, plasma Ang-II concentrations were unchanged and administration of the AT1 antagonist losartan did not prevent hypertrophy, suggesting the effects were a result of intracrine actions of Ang-II (Baker et al. 2004).
There is also accumulating evidence that intracellular Ang II (iAng-II) levels change in various forms of cardiomyopathy, with diabetic cardiomyopathy being the form with the greatest evidence. Ang-II immunoreactivity is increased 3-fold in cardiomyocytes and cardiac endothelial cells from patients with type 2 diabetes and a further 2-fold in diabetic patients with hypertension (Frustaci et al. 2000). High glucose concentrations increase Ang-II synthesis in rat neonatal cardiac ventricular myocytes (Singh et al. 2007), adult rat cardiac ventricular myocytes (Fiordaliso et al. 2001), fibroblasts (Singh et al. 2008a), vascular smooth muscle cells (VSMCs) (Lavrentyev et al. 2007), and mesangial cells (Vidotti et al. 2004). In cardiomyocytes, VSMCs and mesangial cells, the glucose-induced increase in iAng-II production is mediated by chymase rather than ACE. Notably, whereas high glucose increased both extracellular and iAng-II production in cardiac fibroblasts and VSMCs (Lavrentyev et al. 2007; Singh et al. 2008a), only iAng-II production was increased in mesangial cells and cardiomyocytes. In contrast, whereas isoproterenol increased both extracellular and iAng-II production in fibroblasts, only production of extracellular Ang-II was increased in cardiomyocytes. One week following induction of diabetes in rats following streptozotocin treatment, iAng-II levels were elevated in cardiomyocytes and both iAng-II synthesis and cardiac fibrosis were blocked by inhibition of renin, but not ACE or angiotensin receptor blockers (ARBs; Singh et al. 2008b). Many of the observed iAng-II effects are not prevented by ARBs, presumably because of their structures, lipophilic properties, or affinity for intracellular Ang-II receptors. In addition, transgenic mice engineered such that cardiomyocytes chronically secrete Ang-II failed to develop hypertrophy (although fibrosis was evident). In contrast, injection of mice with a plasmid containing a construct directing cardiomyocyte-specific overexpression of Ang II (which was retained within cardiomyocytes) did develop hypertrophy, suggesting that elevated extracellular Ang-II alone is insufficient to induce hypertrophy (van Kats et al. 2001; Baker et al. 2004).
Role in cardiac electrical function
In cardiomyocytes, electrical synchronization and the flow of chemical messages between neighbouring cells is achieved through gap junctions. A decrease in gap junction conductance and alterations in the distribution of connexin 43 are partially implicated in the impairment of impulse propagation and generation of abnormal heart rhythms (Severs, 1994). Dialysis with renin or Ang-II in the presence of enalapril (an ACE inhibitor) decreases gap junction conductance in cardiomyocytes isolated from rat or cardiomyopathic hamster ventricles (De Mello, 1995, 1996). Cell uncoupling and abnormal changes in conduction velocity enhance the generation of re-entrant heart rhythms. Moreover, intracellular injection of Ang-II in single isolated ventricular fibres from failing hearts of cardiomyopathic hamsters shows a significant alteration in resting potential, action potential duration and refractoriness (De Mello, 2011). Intracellular administration of Ang-II to cardiomyocytes significantly reduced inward calcium current (ICa) and increased peak ICa density in the failing hearts of 4-month-old cardiomyopathic hamsters, suggesting that iAng-II may be involved in the regulation of cardiac contractility (De Mello, 1998; De Mello & Monterrubio, 2004). Similarly, dialysis of cardiomyocytes with renin plus angiotensinogen increased L-type Ca2+ currents in the cardiomyopathic hamster, an effect which was abolished by intracellular, but not extracellular, application of losartan (De Mello, 2006).
Role in cardiac gene expression
Although systemic haemodynamic responses to Ang-II often occur in seconds or minutes, the genomic responses to Ang-II, including cellular growth and mitogenic and proliferative effects, occur at later periods ranging from hours and weeks to months. In vascular smooth muscle cells, microinjection of Ang-II rapidly increased nuclear µCa2+½ in both microinjected cells and also in adjacent cells (Haller et al. 1999). Similarly, iAng-II induced IP3-mediated Ca2+ influx, rat aortic contraction, cellular proliferation and activation of cAMP-response element-binding protein (CREB) through phosphorylation of p38 mitogen-activated protein kinase (Haller et al. 1996; Brailoiu et al. 1999; Filipeanu et al. 2001; Cook et al. 2004). Although the molecular mechanisms of gene regulation by nuclear Ang-II receptors are poorly understood, nuclear Ang-II receptors are clearly able to regulate transcriptional events of target genes such as NF-κB, TGF-β1, MCP-1, and NHE-3 (Li & Zhuo, 2008). Moreover, stimulation of cardiac nuclear Ang-II receptors by their cognate ligands modulates NO and IP3, which were shown to participate in fine-tuning the regulation of local calcium release and de novo genomic transcription (Tadevosyan et al. 2011). A sustained increase of nuclear Ca2+ may trigger chromatin activation leading to DNA fragmentation and apoptotic cell death, and hence play a determining role in a variety of pathological and toxicological processes (Orrenius & Nicotera, 1994). It appears that nuclear ionic homeostasis may directly affect cytosolic ionic balance and therefore changes in nuclear receptor expression/activation would directly affect excitation–contraction or excitation–secretion coupling and, in the long term, contribute to diseases such as hypertrophy, hypertension and atherosclerosis.
Endothelin
Endothelin receptors (ETRs) are class A GPCRs. There are two ETRs, ETA and ETB, both of which bind the ligand ET-1 with high affinity. Moreover, these receptors are primarily localized in endothelial cells and cardiomyocytes, where they produce both positive inotropic and chronotropic effects. The ETRs interact primarily with Gαq and signal through the activation of PLC, PLD and PLA2 and the regulation of Ca2+ homeostasis (van Heugten et al. 1994; Husain & Abdel-Latif, 1999). ETB localizes to the nuclear membrane in rat adult cardiac ventricular cardiomyocytes (Boivin et al. 2003) as well as cultured human vascular smooth muscle cells (Bkaily et al. 2003a), while ETA localizes primarily to cell surface membranes in these cells. The precise function of nuclear ETB has yet to be elucidated; however strides have been made into understanding what role they might play in the adult heart.
Role in modulation of nuclear Ca2+ buffering capacity
Nuclear ETB regulates nuclear Ca2+ levels independently of the plasma membrane receptors, potentially via both PTX- and CTX-sensitive G proteins, through a putative R-type Ca2+ channel in human aortic vascular smooth muscle cells (Bkaily et al. 2008). The regulation of nucleoplasmic Ca2+ implies a potential role in both gene transcription and apoptosis. Furthermore, these receptors have been shown to inhibit transcription initiation through an as yet unidentified pathway (Vaniotis et al. 2011b), further reinforcing the notion that nuclear ETB has a physiological and/or pathophysiological role in the cardiovascular system. ET-1 is believed to act in an autocrine/paracrine manner in the ventricular cardiomyocyte (Tirziu et al. 2010). ET-1 can be produced, stored and secreted by adult ventricular cardiomyocytes under basal conditions as well as in response to various stimuli (Thomas et al. 1996). The fact that adult cardiomyocytes have the ability to produce ET-1 internally suggests a possible intracrine mechanism by which these nuclear-localized ETRs may be able to bind ligand and thereby regulate calcium homeostasis and gene expression. In addition, it has been shown that extracellular ET-1 may reduce the intracellular levels of ET-1 mRNA (Farhat et al. 2008), indicating a potential regulatory mechanism wherein extracellular ET-1 actually suppresses intracrine endothelin signalling.
Role in nuclear reactive oxygen species generation
Reactive oxygen species (ROS) are important pathological mediators of cardiovascular diseases (Magder, 2006). ET-1 induces a sustained increase of nuclear ROS in endocardial endothelial cells and in human vascular smooth muscle cells (Provost et al. 2010). In resting and as well as stimulated states, ROS concentrations are higher in the nucleus than in the cytosol and the effect is reversed with glutathione (Go et al. 2007). Nuclear membrane ET-1 receptor generation of nuclear ROS could contribute to modulation of nuclear Ca2+ homeostasis as well as nucleoskeleton reorganization and nuclear hypertrophy. Indeed, intracellular ROS and Ca2+ signalling are intrinsically linked by nitric oxide produced from arginine by NOS (Mazars et al. 2010). Isolated rat liver nuclei treated with an NO donor (SNP) display nuclear Ca2+ mobilization through the sGC/cGMP/PKG pathway, which in turn induces NF-κB activation through protein kinases and subsequent changes in gene expression levels (Gobeil et al. 2006b).
β-Adrenergic receptors
There are three known β-AR subtypes: β1-AR, β2-AR and β3-AR. All three β-ARs are found in the heart, but β1-AR represents the predominant subtype, comprising roughly 70% of the total β-AR density (Tevaearai & Koch, 2004). The principal endogenous agonists for these receptors are noradrenaline and adrenaline; however, the receptors differ with respect to their affinity for these two ligands. β-ARs signal primarily through Gαs/i, and modulate AC activity, and as a result cAMP levels (Xiao et al. 2006). In addition to effects on second messenger generation in isolated nuclei, nuclear β-AR have also been shown to activate additional effectors, including MAPK and PI3K/PKB signalling pathways (Vaniotis et al. 2011b). Recently, β1-AR and β3-AR were shown to localize to the nuclear membrane in adult ventricular myocytes (Boivin et al. 2006).
Role in transcription
Similar to responses seen with plasma membrane receptor activation, β1-AR activated adenylate cyclase and stimulated cAMP formation in isolated nuclei (Boivin et al. 2006). The activation of β3-AR in isolated nuclei evoked a PTX-sensitive increase in transcription initiation (Boivin et al. 2006). Further studies showed that part of this increase is due to the up-regulation of 18S rRNA, whereas a concomitant decrease in mRNA for genes in the NFκB signalling pathway was also observed (Vaniotis et al. 2011b). Furthermore, nuclear β-ARs have been shown to activate the PI3K/PKB pathway (Vaniotis et al. 2011b). These results demonstrated a role for nuclear β-ARs in regulating gene expression and suggest potential roles in both physiological and pathological conditions. Unlike the ETRs, the endogenous ligand of the β-ARs is not produced or secreted by cells expressing β-ARs in the heart. In order for nuclear β-ARs to interact with either of their endogenous ligands, a mechanism for transporting ligand to the nucleus is required. While it remains unclear what this mechanism might be, noradrenaline is rapidly taken up into both neonatal and adult cardiomyocytes (Buu et al. 1993; Wright et al. 2008), and in neonatal rat ventricular cardiomyocytes norepinephrine is transported to the nucleus (Buu et al. 1993).
Nuclear opioid receptors
Cardiogenesis in the developing embryo involves cellular proliferation, differentiation and tissue organization. The role of intracellular opioid receptors and peptides in cellular growth and differentiation has been extensively studied. Dynorphin B, the end-product of the prodynorphin gene, is synthesised in and secreted from cardiomyocytes and acts as a κ-opioid receptor agonist (Ventura et al. 1994). Opioid receptors are present on myocyte nuclei, and binding of dynorphin to these receptors enhances prodynorphin gene expression, activates nuclear protein kinase C (PKC) and primes cardiogenesis in embryonic cells (Ventura et al. 1998; Ventura & Maioli, 2000; Ventura & Branzi, 2006). The nuclear binding of dynorphin B is elevated in cardiomyocytes from cardiomyopathic hamsters and promotes cellular growth and differentiation. The pluripotent embryonic P19 cell line mimics molecular and morphological events that occur during embryonic development, expresses prodynorphin and secretes dynorphin (McBurney et al. 1982). Administration of dynorphin to P19 cells increases expression of cardiac lineage-specific genes involved in normal heart development, including GATA4, Nkx-2.5, cardiac myosin heavy chain and cardiac myosin light chain, and promotes the development of beating colonies of cardiomyocytes (Ventura & Branzi, 2006). Studies also revealed that opioid receptors located on the nuclei of undifferentiated GTR1 cells also increase transcription of cardiac homeobox gene zinc finger containing GATA4 and Csx/Nkx-2.5 and increases prodynorphin gene expression, producing a positive feedback loop (Ventura et al. 2003). These findings suggest that opioid receptors on the nuclear membrane are involved in regulation of embryonic cardiac development.
Intracrine signalling in cardiac angiogenesis
Angiogenesis, the development of new vessels from existing vessels, is involved in the development and progression of ischaemic coronary artery lesions and restenosis (Tabibiazar & Rockson, 2001). The enhancement of angiogenesis through growth factors may represent a therapeutic strategy for cardiac revascularization in cardiovascular disease (Reilly et al. 2005). Proangiogenic factors include angiotensin, endothelin, angiogenin, PDGF, VEGF, bFGF and PTHrP. Many of these factors (e.g. angiogenin, bFGF) exert their angiogenic activity by trafficking to the nucleus and they may act via nuclear receptors, but direct evidence is lacking (Bonnet et al. 1996; Kishimoto et al. 2005). Neamine, which inhibits the angiogenic actions of VEGF and FGF2, has been also shown to block angiogenin trafficking to the nucleus and thus its angiogenic properties (Hu, 2001; Hirukawa et al. 2005; Yoshioka et al. 2006). Certain intracrine agonists traffic along the cytoskeleton, and consequently chemotherapeutic agents that interrupt the cytoskeleton are also antiangiogenic. Interruption of intracellular protein trafficking to the nucleus by interference with the cytoskeleton might be a strategy for the development of antiangiogenic compounds for the treatment of myocardial ischemia.
Conclusions
The discovery of GPCRs on the nuclear membrane and intracellular ligands opens new avenues in the physiology of GPCR signalling. Recent studies have begun to elucidate the roles played by nuclear GPCR signalling in both the healthy cardiovascular system and in response to pathological insults. Several GPCR ligands have now been shown to modulate nucleoplasmic second messenger levels and gene expression when acting on their cognate receptors located on the nuclear envelope. These receptors are well-positioned to play major roles in the effects of prolonged exposure to a specific ligand, while plasma membrane counterparts are likely to play a more pronounced role in acute responses to stimuli. This clearly isn't always the case, as many plasma membrane receptors have also been shown to regulate gene transcription. However, there is evidence suggesting that the profile of genes expressed in response to activation of receptors at the cell surface may differ from that induced by activating their nuclear membrane counterparts (Jong et al. 2009). Moreover, many GPCRs that have been identified at the nucleus are known to be involved in cardiovascular diseases, so it would not be a surprise to discover that their nuclear receptors might play a significant role in disease progression. Our improving understanding of the nature and role of cardiac nuclear GPCRs may have a major impact on our appreciation of physiological control mechanisms and thereby on therapeutic agent development in the future.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research Grants (MGP 6957 and MOP 68929 to S.N., MOP-77791 to B.G.A. and MOP-79354 to T.E.H.), the Fondation de maladies du coeur du Quebec (to B.A.) the Fonds de l’Institut de Cardiologie de Montréal (FICM) and the European-North American Atrial Fibrillation Research Alliance (ENAFRA) award from Fondation Leducq (to S.N.). B.G.A. was a New Investigator of the Heart and Stroke Foundation of Canada and a Senior Scholar of the Fondation de la Recherche en Santé du Québec (FRSQ). T.E.H. holds a Chercheur National award from the FRSQ. S.N. holds the Paul-David Chair in Cardiovascular Electrophysiology. A.T. is recipient of FRSQ-RSCV/HSFQ doctoral scholarship.
Glossary
Abbreviations
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- RAS
renin angiotensin system
- RGS
regulator of G protein signalling
References
- Amitai G, Dassa B, Pietrokovski S. Protein splicing of inteins with atypical glutamine and aspartate C-terminal residues. J Biol Chem. 2004;279:3121–3131. doi: 10.1074/jbc.M311343200. [DOI] [PubMed] [Google Scholar]
- Ardail D, Debon A, Perret-Vivancos C, Biol-N’Garagba MC, Krantic S, Lobie PE, Morel G. Growth hormone internalization in mitochondria decreases respiratory chain activity. Neuroendocrinology. 2010;91:16–26. doi: 10.1159/000268289. [DOI] [PubMed] [Google Scholar]
- Ashton AW, Cheng Y, Helisch A, Ware JA. Thromboxane A2 receptor agonists antagonize the proangiogenic effects of fibroblast growth factor-2: role of receptor internalization, thrombospondin-1, and αvbβ3. Circ Res. 2004;94:735–742. doi: 10.1161/01.RES.0000122043.11286.57. [DOI] [PubMed] [Google Scholar]
- Baker KM, Chernin MI, Schreiber T, Sanghi S, Haiderzaidi S, Booz GW, Dostal DE, Kumar R. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul Pept. 2004;120:5–13. doi: 10.1016/j.regpep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Barlow CA, Laishram RS, Anderson RA. Nuclear phosphoinositides: a signaling enigma wrapped in a compartmental conundrum. Trends Cell Biol. 2010;20:25–35. doi: 10.1016/j.tcb.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barren B, Artemyev NO. Mechanisms of dominant negative G-protein α subunits. J Neurosci Res. 2007;85:3505–3514. doi: 10.1002/jnr.21414. [DOI] [PubMed] [Google Scholar]
- Belcheva M, Barg J, Rowinski J, Clark WG, Gloeckner CA, Ho A, Gao XM, Chuang DM, Coscia C. Novel opioid binding sites associated with nuclei of NG-108–15 neurohybrid cells. J Neuroscience. 1993;13:104–114. doi: 10.1523/JNEUROSCI.13-01-00104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M, Peri KG, Almazan G, Ribeiro-Da-Sylva A, Shichi H, Durocher Y, Abramovitz M, Hou X, Varma DR, Chemtob S. Nuclear localization of prostaglandin E2 receptors. Proc Natl Acad Sci U S A. 1998;95:15792–15797. doi: 10.1073/pnas.95.26.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M, Peri KG, Ribeiro-Da-Sylva A, Almazan G, Shichi H, Hou X, Varma DR, Chemtob S. Localization of functional prostaglandin E2 receptors EP3 and EP4 in the nuclear envelope. J Biol Chem. 1999;274:15719–15724. doi: 10.1074/jbc.274.22.15719. [DOI] [PubMed] [Google Scholar]
- Bkaily G, Choufani S, Avedanian L, Ahmarani L, Nader M, Jacques D, D’Orleans-Juste P, Al Khoury J. Nonpeptidic antagonists of ETA and ETB receptors reverse the ET-1-induced sustained increase of cytosolic and nuclear calcium in human aortic vascular smooth muscle cells. Can J Physiol Pharmacol. 2008;86:546–556. doi: 10.1139/Y08-048. [DOI] [PubMed] [Google Scholar]
- Bkaily G, Choufani S, Sader S, Jacques D, d’Orleans-Juste P, Nader M, Kurban G, Kamal M. Activation of sarcolemma and nuclear membranes ET-1 receptors regulates transcellular calcium levels in heart and vascular smooth muscle cells. Can J Physiol Pharmacol. 2003a;81:654–662. doi: 10.1139/y03-020. [DOI] [PubMed] [Google Scholar]
- Bkaily G, Sleiman S, Stephan J, Asselin C, Choufani S, Kamal M, Jacques D, Gobeil F, D’Orleans-Juste P. Angiotensin II AT1 receptor internalization, translocation and de novo synthesis modulate cytosolic and nuclear calcium in human vascular smooth muscle cells. Can J Physiol Pharmacol. 2003b;81:274–287. doi: 10.1139/y03-007. [DOI] [PubMed] [Google Scholar]
- Boer PA, Gontijo JA. Nuclear localization of SP, CGRP, and NK1R in a subpopulation of dorsal root ganglia subpopulation cells in rats. Cell Mol Neurobiol. 2006;26:191–207. doi: 10.1007/s10571-006-9020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin B, Chevalier D, Villeneuve LR, Rousseau E, Allen BG. Functional endothelin receptors are present on nuclei in cardiac ventricular myocytes. J Biol Chem. 2003;278:29153–29163. doi: 10.1074/jbc.M301738200. [DOI] [PubMed] [Google Scholar]
- Boivin B, Lavoie C, Vaniotis G, Baragli A, Villeneuve LR, Ethier N, Trieu P, Allen BG, Hebert TE. Functional β-adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc Res. 2006;71:69–78. doi: 10.1016/j.cardiores.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Boivin B, Vaniotis G, Allen BG, Hebert TE. G protein-coupled receptors in and on the cell nucleus: a new signaling paradigm? J Recept Signal Transduct Res. 2008;28:15–28. doi: 10.1080/10799890801941889. [DOI] [PubMed] [Google Scholar]
- Boivin B, Villeneuve LR, Farhat N, Chevalier D, Allen BG. Sub-cellular distribution of endothelin signaling pathway components in ventricular myocytes and heart: lack of preformed caveolar signalosomes. J Mol Cell Cardiol. 2005;38:665–676. doi: 10.1016/j.yjmcc.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Bonnet H, Filhol O, Truchet I, Brethenou P, Cochet C, Amalric F, Bouche G. Fibroblast growth factor-2 binds to the regulatory beta subunit of CK2 and directly stimulates CK2 activity toward nucleolin. J Biol Chem. 1996;271:24781–24787. doi: 10.1074/jbc.271.40.24781. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Fearnley C, Smyrnias I, MacDonald F, Roderick HL. An update on nuclear calcium signalling. J Cell Sci. 2009;122:2337–2350. doi: 10.1242/jcs.028100. [DOI] [PubMed] [Google Scholar]
- Booz GW, Conrad KM, Hess AL, Singer HA, Baker KM. Angiotensin-II-binding sites on hepatocyte nuclei. Endocrinology. 1992;130:3641–3649. doi: 10.1210/endo.130.6.1597161. [DOI] [PubMed] [Google Scholar]
- Bouche G, Gas N, Prats H, Baldin V, Tauber JP, Teissie J, Amalric F. Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0-G1 transition. Proc Natl Acad Sci U S A. 1987;84:6770–6774. doi: 10.1073/pnas.84.19.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Filipeanu CM, Tica A, Toma CP, de Zeeuw D, Nelemans SA. Contractile effects by intracellular angiotensin II via receptors with a distinct pharmacological profile in rat aorta. Br J Pharmacol. 1999;126:1133–1138. doi: 10.1038/sj.bjp.0702421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley AR, Crowe PD, Russell DH. Rapid activation of protein kinase C in isolated rat liver nuclei by prolactin, a known hepatic mitogen. Proc Natl Acad Sci U S A. 1988;85:8649–8653. doi: 10.1073/pnas.85.22.8649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchett SA. In through the out door: nuclear localization of the regulators of G protein signaling. J Neurochem. 2003;87:551–559. doi: 10.1046/j.1471-4159.2003.02047.x. [DOI] [PubMed] [Google Scholar]
- Buu NT, Hui R, Falardeau P. Norepinephrine in neonatal rat ventricular myocytes: association with the cell nucleus and binding to nuclear α1- and β-adrenergic receptors. J Mol Cell Cardiol. 1993;25:1037–1046. doi: 10.1006/jmcc.1993.1116. [DOI] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse MJ. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo de Andrade MC, Di Marco GS, de Paulo Castro Teixeira V, Mortara RA, Sabatini RA, Pesquero JB, Boim MA, Carmona AK, Schor N, Casarini DE. Expression and localization of N-domain ANG I-converting enzymes in mesangial cells in culture from spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2006;290:F364–F375. doi: 10.1152/ajprenal.00110.2005. [DOI] [PubMed] [Google Scholar]
- Cavanagh HD, Colley AM. The molecular basis of neurotrophic keratitis. Acta Ophthalmol Suppl. 1989;192:115–134. doi: 10.1111/j.1755-3768.1989.tb07103.x. [DOI] [PubMed] [Google Scholar]
- Chabot JG, Enjalbert A, Pelletier G, Dubois PM, Morel G. Evidence for a direct action of neuropeptide Y in the rat pituitary gland. Neuroendocrinology. 1988;47:511–517. doi: 10.1159/000124963. [DOI] [PubMed] [Google Scholar]
- Clausmeyer S, Reinecke A, Farrenkopf R, Unger T, Peters J. Tissue-specific expression of a rat renin transcript lacking the coding sequence for the prefragment and its stimulation by myocardial infarction. Endocrinology. 2000;141:2963–2970. doi: 10.1210/endo.141.8.7623. [DOI] [PubMed] [Google Scholar]
- Clausmeyer S, Sturzebecher R, Peters J. An alternative transcript of the rat renin gene can result in a truncated prorenin that is transported into adrenal mitochondria. Circ Res. 1999;84:337–344. doi: 10.1161/01.res.84.3.337. [DOI] [PubMed] [Google Scholar]
- Clevenger CV, Sillman AL, Prystowsky MB. Interleukin-2 driven nuclear translocation of prolactin in cloned T-lymphocytes. Endocrinology. 1990;127:3151–3159. doi: 10.1210/endo-127-6-3151. [DOI] [PubMed] [Google Scholar]
- Cook JL, Mills SJ, Naquin R, Alam J, Re RN. Nuclear accumulation of the AT1 receptor in a rat vascular smooth muscle cell line: effects upon signal transduction and cellular proliferation. J Mol Cell Cardiol. 2006;40:696–707. doi: 10.1016/j.yjmcc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Cook JL, Re R, Alam J, Hart M, Zhang Z. Intracellular angiotensin II fusion protein alters AT1 receptor fusion protein distribution and activates CREB. J Mol Cell Cardiol. 2004;36:75–90. doi: 10.1016/j.yjmcc.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Cristovam PC, Arnoni CP, de Andrade MC, Casarini DE, Pereira LG, Schor N, Boim MA. ACE-dependent and chymase-dependent angiotensin II generation in normal and glucose-stimulated human mesangial cells. Exp Biol Med (Maywood) 2008;233:1035–1043. doi: 10.3181/0708-RM-229. [DOI] [PubMed] [Google Scholar]
- Curtis BM, Widmer MB, deRoos P, Qwarnstrom EE. IL-1 and its receptor are translocated to the nucleus. J Immunol. 1990;144:1295–1303. [PubMed] [Google Scholar]
- De Mello WC. The cardiac renin-angiotensin system: its possible role in cell communication and impulse propagation. Cardiovasc Res. 1995;29:730–736. doi: 10.1016/0008-6363(96)88606-8. [DOI] [PubMed] [Google Scholar]
- De Mello WC. Renin-angiotensin system and cell communication in the failing heart. Hypertension. 1996;27:1267–1272. doi: 10.1161/01.hyp.27.6.1267. [DOI] [PubMed] [Google Scholar]
- De Mello WC. Intracellular angiotensin II regulates the inward calcium current in cardiac myocytes. Hypertension. 1998;32:976–982. doi: 10.1161/01.hyp.32.6.976. [DOI] [PubMed] [Google Scholar]
- De Mello WC. Renin increments the inward calcium current in the failing heart. J Hypertens. 2006;24:1181–1186. doi: 10.1097/01.hjh.0000226209.88312.db. [DOI] [PubMed] [Google Scholar]
- De Mello WC. Intracrine action of angiotensin II in the intact ventricle of the failing heart: angiotensin II changes cardiac excitability from within. Mol Cell Biochem. 2011;358:309–315. doi: 10.1007/s11010-011-0981-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello WC, Monterrubio J. Intracellular and extracellular angiotensin II enhance the L-type calcium current in the failing heart. Hypertension. 2004;44:360–364. doi: 10.1161/01.HYP.0000139914.52686.74. [DOI] [PubMed] [Google Scholar]
- Devi LA. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol Sci. 2001;22:532–537. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- Dostal DE, Rothblum KN, Conrad KM, Cooper GR, Baker KM. Detection of angiotensin I and II in cultured rat cardiac myocytes and fibroblasts. Am J Physiol Renal Physiol. 1992;263:C851–C863. doi: 10.1152/ajpcell.1992.263.4.C851. [DOI] [PubMed] [Google Scholar]
- Doufexis M, Storr HL, King PJ, Clark AJ. Interaction of the melanocortin 2 receptor with nucleoporin 50: evidence for a novel pathway between a G-protein-coupled receptor and the nucleus. FASEB J. 2007;21:4095–4100. doi: 10.1096/fj.06-7927com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggena P, Zhu JH, Clegg K, Barrett JD. Nuclear angiotensin receptors induce transcription of renin and angiotensinogen mRNA. Hypertension. 1993;22:496–501. doi: 10.1161/01.hyp.22.4.496. [DOI] [PubMed] [Google Scholar]
- Eggena P, Zhu JH, Sereevinyayut S, Giordani M, Clegg K, Andersen PC, Hyun P, Barrett JD. Hepatic angiotensin II nuclear receptors and transcription of growth-related factors. J Hypertens. 1996;14:961–968. [PubMed] [Google Scholar]
- Erdmann B, Fuxe K, Ganten D. Subcellular localization of angiotensin II immunoreactivity in the rat cerebellar cortex. Hypertension. 1996;28:818–826. doi: 10.1161/01.hyp.28.5.818. [DOI] [PubMed] [Google Scholar]
- Farhat N, Matouk CC, Mamarbachi AM, Marsden PA, Allen BG, Thorin E. Activation of ETB receptors regulates the abundance of ET-1 mRNA in vascular endothelial cells. Br J Pharmacol. 2008;153:1420–1431. doi: 10.1038/bjp.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucheux C, Horton MA, Price JS. Nuclear localization of type I parathyroid hormone/parathyroid hormone-related protein receptors in deer antler osteoclasts: evidence for parathyroid hormone-related protein and receptor activator of NF-κB-dependent effects on osteoclast formation in regenerating mammalian bone. J Bone Miner Res. 2002;17:455–464. doi: 10.1359/jbmr.2002.17.3.455. [DOI] [PubMed] [Google Scholar]
- Fayard JM, Tessier C, Pageaux JF, Lagarde M, Laugier C. Nuclear location of PLA2-I in proliferative cells. J Cell Sci. 1998;111:985–994. doi: 10.1242/jcs.111.7.985. [DOI] [PubMed] [Google Scholar]
- Filipeanu CM, Brailoiu E, Kok JW, Henning RH, De Zeeuw D, Nelemans SA. Intracellular angiotensin II elicits Ca2+ increases in A7r5 vascular smooth muscle cells. Eur J Pharmacol. 2001;420:9–18. doi: 10.1016/s0014-2999(01)01004-4. [DOI] [PubMed] [Google Scholar]
- Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, Anversa P, Kajstura J. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes. 2001;50:2363–2375. doi: 10.2337/diabetes.50.10.2363. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Matsui T, Molloy CJ, Robbins KC, Aaronson SA. Autocrine mechanism for v-sis transformation requires cell surface localization of internally activated growth factor receptors. Proc Natl Acad Sci U S A. 1989;86:8063–8067. doi: 10.1073/pnas.86.20.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SB, Moghaddam A, Westwood M, Turley H, Bicknell R, Gatter KC, Harris AL. Platelet-derived endothelial cell growth factor/thymidine phosphorylase expression in normal tissues: an immunohistochemical study. J Pathol. 1995;176:183–190. doi: 10.1002/path.1711760212. [DOI] [PubMed] [Google Scholar]
- Franco-Obregon A, Wang HW, Clapham DE. Distinct ion channel classes are expressed on the outer nuclear envelope of T- and B-lymphocyte cell lines. Biophys J. 2000;79:202–214. doi: 10.1016/S0006-3495(00)76284-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyberg Z, Sweeney D, Siddhanta A, Bourgoin S, Frohman M, Shields D. Intracellular localization of phospholipase D1 in mammalian cells. Mol Biol Cell. 2001;12:943–955. doi: 10.1091/mbc.12.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- Garcia SI, Porto PI, Martinez VN, Alvarez AL, Finkielman S, Pirola CJ. Expression of TRH and TRH-like peptides in a human glioblastoma-astrocytoma cell line (U-373-MG) J Endocrinol. 2000;166:697–703. doi: 10.1677/joe.0.1660697. [DOI] [PubMed] [Google Scholar]
- Gkountelias K, Papadokostaki M, Javitch JA, Liapakis G. Exploring the binding site crevice of a family B G protein-coupled receptor, the type 1 corticotropin releasing factor receptor. Mol Pharmacol. 2010;78:785–793. doi: 10.1124/mol.110.065474. [DOI] [PubMed] [Google Scholar]
- Go YM, Ziegler TR, Johnson JM, Gu L, Hansen JM, Jones DP. Selective protection of nuclear thioredoxin-1 and glutathione redox systems against oxidation during glucose and glutamine deficiency in human colonic epithelial cells. Free Radic Biol Med. 2007;42:363–370. doi: 10.1016/j.freeradbiomed.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeil F, Fortier A, Zhu T, Bossolasco M, Leduc M, Grandbois M, Heveker N, Bkaily G, Chemtob S, Barbaz D. G-protein-coupled receptors signalling at the cell nucleus: an emerging paradigm. Can J Physiol Pharmacol. 2006a;84:287–297. doi: 10.1139/y05-127. [DOI] [PubMed] [Google Scholar]
- Gobeil F, Jr, Bernier SG, Vazquez-Tello A, Brault S, Beauchamp MH, Quiniou C, Marrache AM, Checchin D, Sennlaub F, Hou X, Nader M, Bkaily G, Ribeiro-da-Silva A, Goetzl EJ, Chemtob S. Modulation of pro-inflammatory gene expression by nuclear lysophosphatidic acid receptor type-1. J Biol Chem. 2003;278:38875–38883. doi: 10.1074/jbc.M212481200. [DOI] [PubMed] [Google Scholar]
- Gobeil F, Zhu T, Brault S, Geha A, Vazquez-Tello A, Fortier A, Barbaz D, Checchin D, Hou X, Nader M, Bkaily G, Gratton JP, Heveker N, Ribeiro-da-Silva A, Peri K, Bard H, Chorvatova A, D’Orleans-Juste P, Goetzl EJ, Chemtob S. Nitric oxide signaling via nuclearized endothelial nitric-oxide synthase modulates expression of the immediate early genes iNOS and mPGES-1. J Biol Chem. 2006b;281:16058–16067. doi: 10.1074/jbc.M602219200. [DOI] [PubMed] [Google Scholar]
- Gobeil FJ, Dumont I, Marrache AM, Vazquez-Tello A, Bernier SG, Abran D, Hou X, Beauchamp MH, Quiniou C, Bouayad A, Choufani S, Bhattacharya M, Molotchnikoff S, Ribeiro-Da-Silva A, Varma DR, Bkaily G, Chemtob S. Regulation of eNOS expression in brain endothelial cells by perinuclear EP3 receptors. Circ Res. 2002;90:682–689. doi: 10.1161/01.res.0000013303.17964.7a. [DOI] [PubMed] [Google Scholar]
- Goldfine ID, Purrello F, Clawson GA, Vigneri R. Insulin binding sites on the nuclear envelope: potential relationship to mRNA metabolism. J Biol Chem. 1982;20:29–39. doi: 10.1002/jcb.240200104. [DOI] [PubMed] [Google Scholar]
- Grasl-Kraupp B, Schausberger E, Hufnagl K, Gerner C, Low-Baselli A, Rossmanith W, Parzefall W, Schulte-Hermann R. A novel mechanism for mitogenic signaling via pro-transforming growth factor α within hepatocyte nuclei. Hepatology. 2002;35:1372–1380. doi: 10.1053/jhep.2002.33203. [DOI] [PubMed] [Google Scholar]
- Gressner OA, Lahme B, Siluschek M, Rehbein K, Weiskirchen R, Gressner AM. Intracrine signalling of activin A in hepatocytes upregulates connective tissue growth factor (CTGF/CCN2) expression. Liver Int. 2008;28:1207–1216. doi: 10.1111/j.1478-3231.2008.01729.x. [DOI] [PubMed] [Google Scholar]
- Haller H, Lindschau C, Erdmann B, Quass P, Luft FC. Effects of intracellular angiotensin II in vascular smooth muscle cells. Circ Res. 1996;79:765–772. doi: 10.1161/01.res.79.4.765. [DOI] [PubMed] [Google Scholar]
- Haller H, Lindschau C, Quass P, Luft FC. Intracellular actions of angiotensin II in vascular smooth muscle cells. J Am Soc Nephrol. 1999;10:S75–S83. [PubMed] [Google Scholar]
- Hamm HE. The many faces of G protein signaling. J Biol Chem. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- Hebert TE, Gales C, Rebois RV. Detecting and imaging protein-protein interactions during G protein-mediated signal transduction in vivo and in situ by using fluorescence-based techniques. Cell Biochem Biophys. 2006;45:85–109. doi: 10.1385/CBB:45:1:85. [DOI] [PubMed] [Google Scholar]
- Hermans E. Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol Ther. 2003;99:25–44. doi: 10.1016/s0163-7258(03)00051-2. [DOI] [PubMed] [Google Scholar]
- Hirukawa S, Olson KA, Tsuji T, Hu GF. Neamine inhibits xenografic human tumor growth and angiogenesis in athymic mice. Clin Cancer Res. 2005;11:8745–8752. doi: 10.1158/1078-0432.CCR-05-1495. [DOI] [PubMed] [Google Scholar]
- Hocher B, Rubens C, Hensen J, Gross P, Bauer C. Intracellular distribution of endothelin-1 receptors in rat liver cells. Biochem Biophys Res Commun. 1992;184:498–503. doi: 10.1016/0006-291x(92)91222-c. [DOI] [PubMed] [Google Scholar]
- Hu GF. Neomycin inhibits the angiogenic activity of fibroblast and epidermal growth factors. Biochem Biophys Res Commun. 2001;287:870–874. doi: 10.1006/bbrc.2001.5668. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wright CD, Merkwan CL, Baye NL, Liang Q, Simpson PC, O’Connell TD. An α1A-adrenergic-extracellular signal-regulated kinase survival signaling pathway in cardiac myocytes. Circulation. 2007;115:763–772. doi: 10.1161/CIRCULATIONAHA.106.664862. [DOI] [PubMed] [Google Scholar]
- Husain S, Abdel-Latif AA. Endothelin-1 activates p38 mitogen-activated protein kinase and cytosolic phospholipase A2 in cat iris sphincter smooth muscle cells. Biochem J. 1999;342:87–96. [PMC free article] [PubMed] [Google Scholar]
- Iosef C, Gkourasas T, Jia CY, Li SS, Han VK. A functional nuclear localization signal in insulin-like growth factor binding protein-6 mediates its nuclear import. Endocrinology. 2008;149:1214–1226. doi: 10.1210/en.2007-0959. [DOI] [PubMed] [Google Scholar]
- Jacques D, Descorbeth M, Abdel-Samad D, Provost C, Perreault C, Jules F. The distribution and density of ET-1 and its receptors are different in human right and left ventricular endocardial endothelial cells. Peptides. 2005;26:1427–1435. doi: 10.1016/j.peptides.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Jacques D, Sader S, Perreault C, Fournier A, Pelletier G, Beck-Sickinger AG, Descorbeth M. Presence of neuropeptide Y and the Y1 receptor in the plasma membrane and nuclear envelope of human endocardial endothelial cells: modulation of intracellular calcium. Can J Physiol Pharmacol. 2003;81:288–300. doi: 10.1139/y02-165. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Scott MG, Pitcher JA. G protein-coupled receptor kinase 5 contains a DNA-binding nuclear localization sequence. Mol Cell Biol. 2004;24:10169–10179. doi: 10.1128/MCB.24.23.10169-10179.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong YJ, Kumar V, Kingston AE, Romano C, O’Malley KL. Functional metabotropic glutamate receptors on nuclei from brain and primary cultured striatal neurons. Role of transporters in delivering ligand. J Biol Chem. 2005;280:30469–30480. doi: 10.1074/jbc.M501775200. [DOI] [PubMed] [Google Scholar]
- Jong YJ, Kumar V, O’Malley KL. Intracellular metabotropic glutamate receptor 5 (mGluR5) activates signaling cascades distinct from cell surface counterparts. J Biol Chem. 2009;184:35827–35838. doi: 10.1074/jbc.M109.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong YJ, Schwetye KE, O’Malley KL. Nuclear localization of functional metabotropic glutamate receptor mGlu1 in HEK293 cells and cortical neurons: role in nuclear calcium mobilization and development. J Neurochem. 2007;101:458–469. doi: 10.1111/j.1471-4159.2006.04382.x. [DOI] [PubMed] [Google Scholar]
- Kiefer P, Acland P, Pappin D, Peters G, Dickson C. Competition between nuclear localization and secretory signals determines the subcellular fate of a single CUG-initiated form of FGF3. EMBO J. 1994;13:4126–4136. doi: 10.1002/j.1460-2075.1994.tb06730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey CG, Bussolati G, Bosco M, Kimura T, Pizzorno MC, Chernin MI, Cassoni P, Novak JF. Constitutive and ligand-induced nuclear localization of oxytocin receptor. J Cell Mol Med. 2007;11:96–110. doi: 10.1111/j.1582-4934.2007.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishima Y, Yamamoto H, Izumoto Y, Yoshida K, Enomoto H, Yamamoto M, Kuroda T, Ito H, Yoshizaki K, Nakamura H. Hepatoma-derived growth factor stimulates cell growth after translocation to the nucleus by nuclear localization signals. J Biol Chem. 2002;277:10315–10322. doi: 10.1074/jbc.M111122200. [DOI] [PubMed] [Google Scholar]
- Kishimoto K, Liu S, Tsuji T, Olson KA, Hu GF. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene. 2005;24:445–456. doi: 10.1038/sj.onc.1208223. [DOI] [PubMed] [Google Scholar]
- Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001a;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001b;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ. Arrestin development: emerging roles for β-arrestins in developmental signaling pathways. Dev Cell. 2009;17:443–458. doi: 10.1016/j.devcel.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Jong YJ, O’Malley KL. Activated nuclear metabotropic glutamate receptor mGlu5 couples to nuclear Gq/11 proteins to generate inositol 1,4,5-trisphosphate-mediated nuclear Ca2+ release. J Biol Chem. 2008;283:14072–14083. doi: 10.1074/jbc.M708551200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. The 2.0 Å crystal structure of a heterotrimeric G protein. Nature. 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- Lanoix D, Ouellette R, Vaillancourt C. Expression of melatoninergic receptors in human placental choriocarcinoma cell lines. Hum Reprod. 2006;21:1981–1989. doi: 10.1093/humrep/del120. [DOI] [PubMed] [Google Scholar]
- Lavoie JL, Liu X, Bianco RA, Beltz TG, Johnson AK, Sigmund CD. Evidence supporting a functional role for intracellular renin in the brain. Hypertension. 2006;47:461–466. doi: 10.1161/01.HYP.0000203308.52919.dc. [DOI] [PubMed] [Google Scholar]
- Lavrentyev EN, Estes AM, Malik KU. Mechanism of high glucose induced angiotensin II production in rat vascular smooth muscle cells. Circ Res. 2007;101:455–464. doi: 10.1161/CIRCRESAHA.107.151852. [DOI] [PubMed] [Google Scholar]
- Lee-Kirsch MA, Gaudet F, Cardoso MC, Lindpaintner K. Distinct renin isoforms generated by tissue-specific transcription initiation and alternative splicing. Circ Res. 1999;84:240–246. doi: 10.1161/01.res.84.2.240. [DOI] [PubMed] [Google Scholar]
- Lee BA, Donoghue DJ. Intracellular retention of membrane-anchored v-sis protein abrogates autocrine signal transduction. J Cell Biol. 1992;118:1057–1070. doi: 10.1083/jcb.118.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Lanca AJ, Cheng R, Nguyen T, Ji XD, Gobeil F, Jr, Chemtob S, George SR, O’Dowd BF. Agonist-independent nuclear localization of the Apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol Chem. 2004;279:7901–7908. doi: 10.1074/jbc.M306377200. [DOI] [PubMed] [Google Scholar]
- Lee TH, Seng S, Sekine M, Hinton C, Fu Y, Avraham HK, Avraham S. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4:e186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol Sci. 2004;25:413–422. doi: 10.1016/j.tips.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Lessard A, Savard M, Gobeil F, Jr, Pierce JP, Pickel VM. The neurokinin-3 (NK3) and the neurokinin-1 (NK1) receptors are differentially targeted to mesocortical and mesolimbic projection neurons and to neuronal nuclei in the rat ventral tegmental area. Synapse. 2009;63:484–501. doi: 10.1002/syn.20627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Keller G. VEGF nuclear accumulation correlates with phenotypical changes in endothelial cells. J Cell Sci. 2000;113:1525–1534. doi: 10.1242/jcs.113.9.1525. [DOI] [PubMed] [Google Scholar]
- Li XC, Zhuo JL. Intracellular ANG II directly induces in vitro transcription of TGF-β1, MCP-1, and NHE-3 mRNAs in isolated rat renal cortical nuclei via activation of nuclear AT1a receptors. Am J Physiol Renal Physiol. 2008;294:C1034–C1045. doi: 10.1152/ajpcell.00432.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind GJ, Cavanagh HD. Nuclear muscarinic acetylcholine receptors in corneal cells from rabbit. Invest Ophtalmol Visual Sci. 1993;34:2943–2952. [PubMed] [Google Scholar]
- Lind GJ, Cavanagh HD. Identification and subcellular distribution of muscarinic acetylcholine receptor-related proteins in rabbit corneal and Chinese hamster ovary cells. Invest Ophthalmol Vis Sci. 1995;36:1492–1507. [PubMed] [Google Scholar]
- Lu C, Li Y, Zhao Y, Xing G, Tang F, Wang Q, Sun Y, Wei H, Yang X, Wu C, Chen J, Guan KL, Zhang C, Chen H, He F. Intracrine hepatopoietin potentiates AP-1 activity through JAB1 independent of MAPK pathway. FASEB J. 2002;16:90–92. doi: 10.1096/fj.01-0506fje. [DOI] [PubMed] [Google Scholar]
- Lucero HA, Kintsurashvili E, Marketou ME, Gavras H. Cell signaling, internalization, and nuclear localization of the angiotensin converting enzyme in smooth muscle and endothelial cells. J Biol Chem. 2010;285:5555–5568. doi: 10.1074/jbc.M109.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magder S. Reactive oxygen species: toxic molecules or spark of life? Crit Care. 2006;10:208. doi: 10.1186/cc3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra R, Sadoshima J, Brosius FC, 3rd, Izumo S. Mechanical stretch and angiotensin II differentially upregulate the renin-angiotensin system in cardiac myocytes In vitro. Circ Res. 1999;85:137–146. doi: 10.1161/01.res.85.2.137. [DOI] [PubMed] [Google Scholar]
- Marmigere F, Choby C, Rage F, Richard S, Tapia-Arancibia L. Rapid stimulatory effects of brain-derived neurotrophic factor and neurotrophin-3 on somatostatin release and intracellular calcium rise in primary hypothalamic cell cultures. Neuroendocrinology. 2001;74:43–54. doi: 10.1159/000054669. [DOI] [PubMed] [Google Scholar]
- Marrache AM, Gobeil FJ, Bernier SG, Stankova J, Rola-Pleszczynski M, Choufani S, Bkaily G, Bourdeau A, Sirois MG, Vazquez-Tello A, Fan L, Joyal JS, Filep JG, Varma DR, Ribeiro-Da-Silva A, Chemtob S. Proinflammatory gene induction by platelet-activating factor mediated via its cognate nuclear receptor. J Immunol. 2002;169:6474–6481. doi: 10.4049/jimmunol.169.11.6474. [DOI] [PubMed] [Google Scholar]
- Massfelder T, Dann P, Wu TL, Vasavada R, Helwig JJ, Stewart AF. Opposing mitogenic and anti-mitogenic actions of parathyroid hormone-related protein in vascular smooth muscle cells: a critical role for nuclear targeting. Proc Natl Acad Sci U S A. 1997;94:13630–13635. doi: 10.1073/pnas.94.25.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa K, Moriyama A, Kawai Y, Asai K, Kato T. Tissue distribution of human gliostatin/platelet-derived endothelial cell growth factor (PD-ECGF) and its drug-induced expression. Biochim Biophys Acta. 1996;1314:71–82. doi: 10.1016/s0167-4889(96)00078-x. [DOI] [PubMed] [Google Scholar]
- Mazars C, Thuleau P, Lamotte O, Bourque S. Cross-talk between ROS and calcium in regulation of nuclear activities. Mol Plant. 2010;3:706–718. doi: 10.1093/mp/ssq024. [DOI] [PubMed] [Google Scholar]
- Mazzanti M, DeFelice LJ, Cohn J, Malter H. Ion channels in the nuclear envelope. Nature. 1990;343:764–767. doi: 10.1038/343764a0. [DOI] [PubMed] [Google Scholar]
- Mazzolai L, Nussberger J, Aubert JF, Brunner DB, Gabbiani G, Brunner HR, Pedrazzini T. Blood pressure-independent cardiac hypertrophy induced by locally activated renin-angiotensin system. Hypertension. 1998;31:1324–1330. doi: 10.1161/01.hyp.31.6.1324. [DOI] [PubMed] [Google Scholar]
- McBurney MW, Jones-Villeneuve EM, Edwards MK, Anderson PJ. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982;299:165–167. doi: 10.1038/299165a0. [DOI] [PubMed] [Google Scholar]
- Miguel BG, Calcerrada MC, Martin L, Catalan RE, Martinez AM. Increase of phosphoinositide hydrolysis and diacylglycerol production by PAF in isolated rat liver nuclei. Prostaglandins Other Lipid Mediat. 2001;65:159–166. doi: 10.1016/s0090-6980(01)00124-1. [DOI] [PubMed] [Google Scholar]
- Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25:235–275. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- Millar RP, Newton CL. The year in G protein-coupled receptor research. Mol Endocrinol. 2010;24:261–274. doi: 10.1210/me.2009-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitjavila MT, Le Couedic JP, Casadevall N, Navarro S, Villeval JL, Dubart A, Vainchenker W. Autocrine stimulation by erythropoietin and autonomous growth of human erythroid leukemic cells in vitro. J Clin Invest. 1991;88:789–797. doi: 10.1172/JCI115378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Takai S. Tissue angiotensin II generating system by angiotensin-converting enzyme and chymase. J Pharmacol Sci. 2006;100:391–397. doi: 10.1254/jphs.cpj06008x. [DOI] [PubMed] [Google Scholar]
- Morel G, Chabot JG, Garcia-Caballero T, Gossard F, Dihl F, Belles-Isles M, Heisler S. Synthesis, internalization, and localization of atrial natriuretic peptide in rat adrenal medulla. Endocrinology. 1988;123:149–158. doi: 10.1210/endo-123-1-149. [DOI] [PubMed] [Google Scholar]
- Moroianu J, Riordan JF. Nuclear translocation of angiogenic proteins in endothelial cells: an essential step in angiogenesis. Biochemistry. 1994a;33:12535–12539. doi: 10.1021/bi00208a001. [DOI] [PubMed] [Google Scholar]
- Moroianu J, Riordan JF. Nuclear translocation of angiogenin in proliferating endothelial cells is essential to its angiogenic activity. Proc Natl Acad Sci U S A. 1994b;91:1677–1681. doi: 10.1073/pnas.91.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G, Pallinger E, Antal-Szalmas P, Aleksza M, Marschalko M, Brozik M, Falus A, Gergely P. Measurement of intracellular interferon-gamma and interleukin-4 in whole blood T lymphocytes from patients with systemic lupus erythematosus. Immunol Lett. 2000;74:207–210. doi: 10.1016/s0165-2478(00)00265-0. [DOI] [PubMed] [Google Scholar]
- Nehring RB, Horikawa HP, El Far O, Kneussel M, Brandstatter JH, Stamm S, Wischmeyer E, Betz H, Karschin A. The metabotropic GABAB receptor directly interacts with the activating transcription factor 4. J Biol Chem. 2000;275:35185–35191. doi: 10.1074/jbc.M002727200. [DOI] [PubMed] [Google Scholar]
- Nguyen M, He B, Karaplis A. Nuclear forms of parathyroid hormone-related peptide are translated from non-AUG start sites downstream from the initiator methionine. Endocrinology. 2001;142:694–703. doi: 10.1210/endo.142.2.7944. [DOI] [PubMed] [Google Scholar]
- Nguyen MT, Karaplis AC. The nucleus: a target site for parathyroid hormone-related peptide (PTHrP) action. J Cell Biochem. 1998;70:193–199. doi: 10.1002/(sici)1097-4644(19980801)70:2<193::aid-jcb5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Nielsen CK, Campbell JI, Ohd JF, Morgelin M, Riesbeck K, Landberg G, Sjolander A. A novel localization of the G-protein-coupled CysLT1 receptor in the nucleus of colorectal adenocarcinoma cells. Cancer Res. 2005;65:732–742. [PubMed] [Google Scholar]
- O’Malley KL, Jong YJ, Gonchar Y, Burkhalter A, Romano C. Activation of metabotropic glutamate receptor mGlu5 on nuclear membranes mediates intranuclear Ca2+ changes in heterologous cell types and neurons. J Biol Chem. 2003;278:28210–28219. doi: 10.1074/jbc.M300792200. [DOI] [PubMed] [Google Scholar]
- Omary MB, Kagnoff MF. Identification of nuclear receptors for VIP on a human colonic adenocarcinoma cell line. Science. 1987;238:1578–1581. doi: 10.1126/science.2825352. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Nicotera P. The calcium ion and cell death. J Neural Transm Suppl. 1994;43:1–11. [PubMed] [Google Scholar]
- Peters J, Farrenkopf R, Clausmeyer S, Zimmer J, Kantachuvesiri S, Sharp MG, Mullins JJ. Functional significance of prorenin internalization in the rat heart. Circ Res. 2002;90:1135–1141. doi: 10.1161/01.res.0000019242.51541.99. [DOI] [PubMed] [Google Scholar]
- Pickard BW, Hodsman AB, Fraher LJ, Watson PH. Type 1 parathyroid hormone receptor (PTH1R) nuclear trafficking: association of PTH1R with importin α1 and β. Endocrinology. 2006;147:3326–3332. doi: 10.1210/en.2005-1408. [DOI] [PubMed] [Google Scholar]
- Prochiantz A, Theodore L. Nuclear/growth factors. Bioessays. 1995;17:39–44. doi: 10.1002/bies.950170109. [DOI] [PubMed] [Google Scholar]
- Provost C, Choufani F, Avedanian L, Bkaily G, Gobeil F, Jacques D. Nitric oxide and reactive oxygen species in the nucleus revisited. Can J Physiol Pharmacol. 2010;88:296–304. doi: 10.1139/Y10-011. [DOI] [PubMed] [Google Scholar]
- Quesada I, Rovira JM, Martin F, Roche E, Nadal A, Soria B. Nuclear KATP channels trigger nuclear Ca2+ transients that modulate nuclear function. Proc Natl Acad Sci U S A. 2002;99:9544–9549. doi: 10.1073/pnas.142039299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowicz-Szulczynska EM, Rodeck U, Herlyn M, Koprowski H. Chromatin binding of epidermal growth factor, nerve growth factor, and platelet-derived growth factor in cells bearing the appropriate surface receptors. Proc Natl Acad Sci U S A. 1986;83:3728–3732. doi: 10.1073/pnas.83.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re M, Pampillo M, Savard M, Dubuc C, McArdle CA, Millar RP, Conn PM, Gobeil F, Jr, Bhattacharya M, Babwah AV. The human gonadotropin releasing hormone type I receptor is a functional intracellular GPCR expressed on the nuclear membrane. PLoS One. 2010;5:e11489. doi: 10.1371/journal.pone.0011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re R. The nature of intracrine peptide hormone action. Hypertension. 1999;34:534–538. doi: 10.1161/01.hyp.34.4.534. [DOI] [PubMed] [Google Scholar]
- Re RN, Vizard DL, Brown J, Bryan SE. Angiotensin II receptors in chromatin fragments generated by micrococcal nuclease. Biochem Biophys Res Commun. 1984;119:220–227. doi: 10.1016/0006-291x(84)91641-3. [DOI] [PubMed] [Google Scholar]
- Regard JB, Sato IT, Coughlin SR. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135:561–571. doi: 10.1016/j.cell.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JP, Grise MA, Fortuin FD, Vale PR, Schaer GL, Lopez J, van Camp JR, Henry T, Richenbacher WE, Losordo DW, Schatz RA, Isner JM. Long-term (2-year) clinical events following transthoracic intramyocardial gene transfer of VEGF-2 in no-option patients. J Interv Cardiol. 2005;18:27–31. doi: 10.1111/j.1540-8183.2005.04026.x. [DOI] [PubMed] [Google Scholar]
- Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F., Jr The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Ribeiro FM, Paquet M, Cregan SP, Ferguson SS. Group I metabotropic glutamate receptor signalling and its implication in neurological disease. CNS Neurol Disord Drug Targets. 2010;9:574–595. doi: 10.2174/187152710793361612. [DOI] [PubMed] [Google Scholar]
- Robertson AL, Khairallah PA. Angiotensin II: rapid localization in nuclei of smooth and cardiac muscle. Science. 1971;172:1138–1139. doi: 10.1126/science.172.3988.1138. [DOI] [PubMed] [Google Scholar]
- Robitaille M, Gora S, Wang Y, Goupil E, Pétrin D, Del Duca D, Villeneuve LR, Allen BG, Laporte SA, Bernard DJ, Hébert TE. Gβγ is a negative regulator of AP-1 mediated transcription. Cell Signal. 2010;22:1254–1266. doi: 10.1016/j.cellsig.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Rousseau E, Michaud C, Lefebvre D, Proteau S, Decrouy A. Reconstitution of ionic channels from inner and outer membranes of mammalian cardiac nuclei. Biophys J. 1996;70:703–714. doi: 10.1016/S0006-3495(96)79610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell FD, Davenport AP. Evidence for intracellular endothelin-converting enzyme-2 expression in cultured human vascular endothelial cells. Circ Res. 1999;84:891–896. doi: 10.1161/01.res.84.8.891. [DOI] [PubMed] [Google Scholar]
- Sastri M, Barraclough DM, Carmichael PT, Taylor SS. A-kinase-interacting protein localizes protein kinase A in the nucleus. Proc Natl Acad Sci U S A. 2005;102:349–354. doi: 10.1073/pnas.0408608102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Hiraoka M, Suzuki H, Bai Y, Kurotani R, Yokoyama U, Okumura S, Cismowski MJ, Lanier SM, Ishikawa Y. Identification of transcription factor E3 (TFE3) as a receptor-independent activator of Gα 16: Gene regulation by nuclear Gα subunit and its activator. J Biol Chem. 2011;286:17766–17776. doi: 10.1074/jbc.M111.219816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savard M, Barbaz D, Belanger S, Muller-Esterl W, Bkaily G, D’Orleans-Juste P, Cote J, Bovenzi V, Gobeil F., Jr Expression of endogenous nuclear bradykinin B2 receptors mediating signaling in immediate early gene activation. J Cell Physiol. 2008;216:234–244. doi: 10.1002/jcp.21398. [DOI] [PubMed] [Google Scholar]
- Schedlich LJ, Le Page SL, Firth SM, Briggs LJ, Jans DA, Baxter RC. Nuclear import of insulin-like growth factor-binding protein-3 and -5 is mediated by the importin beta subunit. J Biol Chem. 2000;275:23462–23470. doi: 10.1074/jbc.M002208200. [DOI] [PubMed] [Google Scholar]
- Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, Pandolfi PP, Ruiz P, Unger T, Funke-Kaiser H. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res. 2006;99:1355–1366. doi: 10.1161/01.RES.0000251700.00994.0d. [DOI] [PubMed] [Google Scholar]
- Schievella AR, Regier MK, Smith WL, Lin LL. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J Biol Chem. 1995;270:30749–30754. doi: 10.1074/jbc.270.51.30749. [DOI] [PubMed] [Google Scholar]
- Severs NJ. Pathophysiology of gap junctions in heart disease. J Cardiovasc Electrophysiol. 1994;5:462–475. doi: 10.1111/j.1540-8167.1994.tb01185.x. [DOI] [PubMed] [Google Scholar]
- Sherrod M, Liu X, Zhang X, Sigmund CD. Nuclear localization of angiotensinogen in astrocytes. Am J Physiol Regul Integr Comp Physiol. 2005;288:R539–546. doi: 10.1152/ajpregu.00594.2004. [DOI] [PubMed] [Google Scholar]
- Shibuta K, Mori M, Shimoda K, Inoue H, Mitra P, Barnard GF. Regional expression of CXCL12/CXCR4 in liver and hepatocellular carcinoma and cell-cycle variation during in vitro differentiation. Jpn J Cancer Res. 2002;93:789–797. doi: 10.1111/j.1349-7006.2002.tb01321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehler S. Regulation of RhoGEF proteins by G12/13-coupled receptors. Br J Pharmacol. 2009;158:41–49. doi: 10.1111/j.1476-5381.2009.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VP, Baker KM, Kumar R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am J Physiol Renal Physiol. 2008a;294:H1675–H1684. doi: 10.1152/ajpheart.91493.2007. [DOI] [PubMed] [Google Scholar]
- Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High-glucose-induced regulation of intracellular ANG II synthesis and nuclear redistribution in cardiac myocytes. Am J Physiol Renal Physiol. 2007;293:H939–H948. doi: 10.1152/ajpheart.00391.2007. [DOI] [PubMed] [Google Scholar]
- Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes. 2008b;57:3297–3306. doi: 10.2337/db08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn PL, Sigmund CD. Identification of three human renin mRNA isoforms from alternative tissue-specific transcriptional initiation. Physiol Genomics. 2000;3:25–31. doi: 10.1152/physiolgenomics.2000.3.1.25. [DOI] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spano JP, Andre F, Morat L, Sabatier L, Besse B, Combadiere C, Deterre P, Martin A, Azorin J, Valeyre D, Khayat D, Le Chevalier T, Soria JC. Chemokine receptor CXCR4 and early-stage non-small cell lung cancer: pattern of expression and correlation with outcome. Ann Oncol. 2004;15:613–617. doi: 10.1093/annonc/mdh136. [DOI] [PubMed] [Google Scholar]
- Struewing IT, Toborek A, Mao CD. Mitochondrial and nuclear forms of Wnt13 are generated via alternative promoters, alternative RNA splicing, and alternative translation start sites. J Biol Chem. 2006;281:7282–7293. doi: 10.1074/jbc.M511182200. [DOI] [PubMed] [Google Scholar]
- Tabares L, Mazzanti M, Clapham DE. Chloride channels in the nuclear membrane. J Membr Biol. 1991;123:49–54. doi: 10.1007/BF01993962. [DOI] [PubMed] [Google Scholar]
- Tabibiazar R, Rockson SG. Angiogenesis and the ischaemic heart. Eur Heart J. 2001;22:903–918. doi: 10.1053/euhj.2000.2372. [DOI] [PubMed] [Google Scholar]
- Tadevosyan A, Maguy A, Villeneuve LR, Babin J, Bonnefoy A, Allen BG, Nattel S. Nuclear-delimited angiotensin receptor-mediated signaling regulates cardiomyocyte gene expression. J Biol Chem. 2010;285:22338–22349. doi: 10.1074/jbc.M110.121749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadevosyan A, Del Duca D, Chen Y, Allen BG, Nattel S. Nuclear angiotensin-II receptor gene expression in cardiac fibroblasts: receptor subtype-specific differential role of IP-3-receptor (IP3R) related and nitric oxide signaling. Circulation. 2011;124:A10257. [Google Scholar]
- Tang CM, Insel PA. GPCR expression in the heart; “new” receptors in myocytes and fibroblasts. Trends Cardiovasc Med. 2004;14:94–99. doi: 10.1016/j.tcm.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Terrillon S, Bouvier M. Receptor activity-independent recruitment of βarrestin2 reveals specific signalling modes. EMBO J. 2004;23:3950–3961. doi: 10.1038/sj.emboj.7600387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevaearai HT, Koch WJ. Molecular restoration of β-adrenergic receptor signaling improves contractile function of failing hearts. Trends Cardiovasc Med. 2004;14:252–256. doi: 10.1016/j.tcm.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Thomas PB, Liu EC, Webb ML, Mukherjee R, Hebbar L, Spinale FG. Exogenous effects and endogenous production of endothelin in cardiac myocytes: potential significance in heart failure. Am J Physiol Renal Physiol. 1996;271:H2629–H2637. doi: 10.1152/ajpheart.1996.271.6.H2629. [DOI] [PubMed] [Google Scholar]
- Tirziu D, Giordano FJ, Simons M. Cell communications in the heart. Circulation. 2010;122:928–937. doi: 10.1161/CIRCULATIONAHA.108.847731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar Sepulveda VA, Shen X, Falzon M. Intracrine PTHrP protects against serum starvation-induced apoptosis and regulates the cell cycle in MCF-7 breast cancer cells. Endocrinology. 2002;143:596–606. doi: 10.1210/endo.143.2.8645. [DOI] [PubMed] [Google Scholar]
- Tsai CT, Lai LP, Hwang JJ, Chen WP, Chiang FT, Hsu KL, Tseng CD, Tseng YZ, Lin JL. Renin-angiotensin system component expression in the HL-1 atrial cell line and in a pig model of atrial fibrillation. J Hypertens. 2008;26:570–582. doi: 10.1097/HJH.0b013e3282f34a4a. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Sun Y, Kishimoto K, Olson KA, Liu S, Hirukawa S, Hu GF. Angiogenin is translocated to the nucleus of HeLa cells and is involved in ribosomal RNA transcription and cell proliferation. Cancer Res. 2005;65:1352–1360. doi: 10.1158/0008-5472.CAN-04-2058. [DOI] [PubMed] [Google Scholar]
- Urata H, Boehm KD, Philip A, Kinoshita A, Gabrovsek J, Bumpus FM, Husain A. Cellular localization and regional distribution of an angiotensin II-forming chymase in the heart. J Clin Invest. 1993;91:1269–1281. doi: 10.1172/JCI116325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadakkadath Meethal S, Gallego MJ, Haasl RJ, Petras SJ, Sgro JY, Atwood CS. Identification of a gonadotropin-releasing hormone receptor orthologue in Caenorhabditis elegans. BMC Evol Biol. 2006;6:1–17. doi: 10.1186/1471-2148-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdehita A, Bajo AM, Fernandez-Martinez AB, Arenas MI, Vacas E, Valenzuela P, Ruiz-Villaespesa A, Prieto JC, Carmena MJ. Nuclear localization of vasoactive intestinal peptide (VIP) receptors in human breast cancer. Peptides. 2010;31:2035–2045. doi: 10.1016/j.peptides.2010.07.024. [DOI] [PubMed] [Google Scholar]
- van Heugten HA, de Jonge HW, Bezstarosti K, Lamers JM. Calcium and the endothelin-1 and α1-adrenergic stimulated phosphatidylinositol cycle in cultured rat cardiomyocytes. J Mol Cell Cardiol. 1994;26:1081–1093. doi: 10.1006/jmcc.1994.1128. [DOI] [PubMed] [Google Scholar]
- van Kats JP, Methot D, Paradis P, Silversides DW, Reudelhuber TL. Use of a biological peptide pump to study chronic peptide hormone action in transgenic mice. Direct and indirect effects of angiotensin II on the heart. J Biol Chem. 2001;276:44012–44017. doi: 10.1074/jbc.M106132200. [DOI] [PubMed] [Google Scholar]
- Vaniotis G, Allen BG, Hebert TE. Nuclear GPCRs in cardiomyocytes – an insider's view of β-adrenergic receptor signaling. Am J Physiol Heart Circ Physiol. 2011b;301:H1754–1764. doi: 10.1152/ajpheart.00657.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaniotis G, Del Duca D, Trieu P, Rohlicek CV, Hebert TE, Allen BG. Nuclear β-adrenergic receptors modulate gene expression in adult rat heart. Cell Signal. 2011a;23:89–98. doi: 10.1016/j.cellsig.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura C, Branzi A. Autocrine and intracrine signaling for cardiogenesis in embryonic stem cells: a clue for the development of novel differentiating agents. Handb Exp Pharmacol. 2006:123–146. [PubMed] [Google Scholar]
- Ventura C, Guarnieri C, Vaona I, Campana G, Pintus G, Spampinato S. Dynorphin gene expression and release in the myocardial cell. J Biol Chem. 1994;269:5384–5386. [PubMed] [Google Scholar]
- Ventura C, Maioli M. Opioid peptide gene expression primes cardiogenesis in embryonal pluripotent stem cells. Circ Res. 2000;87:189–194. doi: 10.1161/01.res.87.3.189. [DOI] [PubMed] [Google Scholar]
- Ventura C, Maioli M, Pintus G, Posadino AM, Tadolini B. Nuclear opioid receptors activate opioid peptide gene transcription in isolated myocardial nuclei. J Biol Chem. 1998;273:13383–13386. doi: 10.1074/jbc.273.22.13383. [DOI] [PubMed] [Google Scholar]
- Ventura C, Zinellu E, Maninchedda E, Fadda M, Maioli M. Protein kinase C signaling transduces endorphin-primed cardiogenesis in GTR1 embryonic stem cells. Circ Res. 2003;92:617–622. doi: 10.1161/01.RES.0000065168.31147.5B. [DOI] [PubMed] [Google Scholar]
- Vidotti DB, Casarini DE, Cristovam PC, Leite CA, Schor N, Boim MA. High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol Renal Physiol. 2004;286:F1039–F1045. doi: 10.1152/ajprenal.00371.2003. [DOI] [PubMed] [Google Scholar]
- Wakasugi K, Slike BM, Hood J, Ewalt KL, Cheresh DA, Schimmel P. Induction of angiogenesis by a fragment of human tyrosyl-tRNA synthetase. J Biol Chem. 2002;277:20124–20126. doi: 10.1074/jbc.C200126200. [DOI] [PubMed] [Google Scholar]
- Wang P, Wu Y, Ge X, Ma L, Pei G. Subcellular localization of β-arrestins is determined by their intact N domain and the nuclear export signal at the C terminus. J Biol Chem. 2003;278:11648–11653. doi: 10.1074/jbc.M208109200. [DOI] [PubMed] [Google Scholar]
- Waters CM, Saatian B, Moughal NA, Zhao Y, Tigyi G, Natarajan V, Pyne S, Pyne NJ. Integrin signalling regulates the nuclear localization and function of the lysophosphatidic acid receptor-1 (LPA1) in mammalian cells. Biochem J. 2006;398:55–62. doi: 10.1042/BJ20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PH, Fraher LJ, Hendy GN, Chung UI, Kisiel M, Natale BV, Hodsman AB. Nuclear localization of the type 1 PTH/PTHrP receptor in rat tissues. J Bone Miner Res. 2000a;15:1033–1044. doi: 10.1359/jbmr.2000.15.6.1033. [DOI] [PubMed] [Google Scholar]
- Watson PH, Fraher LJ, Natale BV, Kisiel M, Hendy GN, Hodsman AB. Nuclear localization of the type 1 parathyroid hormone/parathyroid hormone-related peptide receptor in MC3T3-E1 cells: association with serum-induced cell proliferation. Bone. 2000b;26:221–225. doi: 10.1016/s8756-3282(99)00264-1. [DOI] [PubMed] [Google Scholar]
- Wiley HS, Woolf MF, Opresko LK, Burke PM, Will B, Morgan JR, Lauffenburger DA. Removal of the membrane-anchoring domain of epidermal growth factor leads to intracrine signaling and disruption of mammary epithelial cell organization. J Cell Biol. 1998;143:1317–1328. doi: 10.1083/jcb.143.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth MJ, Patz S, Wahle P. Transcellular induction of neuropeptide Y expression by NT4 and BDNF. Proc Natl Acad Sci U S A. 2005;102:3064–3069. doi: 10.1073/pnas.0404712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CD, Chen Q, Baye NL, Huang Y, Healy CL, Kasinathan S, O’Connell TD. Nuclear α1-adrenergic receptors signal activated ERK localization to caveolae in adult cardiac myocytes. Circ Res. 2008;103:992–1000. doi: 10.1161/CIRCRESAHA.108.176024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao RP, Zhu W, Zheng M, Cao C, Zhang Y, Lakatta EG, Han Q. Subtype-specific α1- and β-adrenoceptor signaling in the heart. Trends Pharmacol Sci. 2006;27:330–337. doi: 10.1016/j.tips.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Xu D, Borges GR, Grobe JL, Pelham CJ, Yang B, Sigmund CD. Preservation of intracellular renin expression is insufficient to compensate for genetic loss of secreted renin. Hypertension. 2009;54:1240–1247. doi: 10.1161/HYPERTENSIONAHA.109.138677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Carretero OA, Liao TD, Peng H, Shesely EG, Liu TS, Yang JJ, Reudelhuber TL, Yang XP. Local angiotensin II aggravates cardiac remodeling in hypertension. Am J Physiol Renal Physiol. 2010;299:H1328–H1338. doi: 10.1152/ajpheart.00538.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Kawamura K, James TN. Intracellular distribution of adenylate cyclase in human cardiocytes determined by electron microscopic cytochemistry. Microsc Res Tech. 1998;40:479–487. doi: 10.1002/(SICI)1097-0029(19980301)40:6<479::AID-JEMT8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Yi XP, Gerdes AM, Li F. Myocyte redistribution of GRK2 and GRK5 in hypertensive, heart-failure-prone rats. Hypertension. 2002;39:1058–1063. doi: 10.1161/01.hyp.0000019130.09167.3b. [DOI] [PubMed] [Google Scholar]
- Yoshioka N, Wang L, Kishimoto K, Tsuji T, Hu GF. A therapeutic target for prostate cancer based on angiogenin-stimulated angiogenesis and cancer cell proliferation. Proc Natl Acad Sci U S A. 2006;103:14519–14524. doi: 10.1073/pnas.0606708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Barr VA, Mo Y, Rojkova AM, Liu S, Simonds WF. Nuclear localization of G protein β5 and regulator of G protein signaling 7 in neurons and brain. J Biol Chem. 2001;276:10284–10289. doi: 10.1074/jbc.M009247200. [DOI] [PubMed] [Google Scholar]
- Zhu T, Gobeil F, Vazquez-Tello A, Leduc M, Rihakova L, Bossolasco M, Bkaily G, Peri K, Varma DR, Orvoine R, Chemtob S. Intracrine signaling through lipid mediators and their cognate nuclear G-protein-coupled receptors: a paradigm based on PGE2, PAF, and LPA1 receptors. Can J Physiol Pharmacol. 2006;84:377–391. doi: 10.1139/y05-147. [DOI] [PubMed] [Google Scholar]


