Abstract
Wolbachia are widespread and abundant intracellular symbionts of arthropods and filarial nematodes. Their symbiotic relationships encompass obligate mutualism, commensalism, parasitism, and pathogenicity. A consequence of these diverse associations is that Wolbachia encounter a wide range of host cells and intracellular immune defense mechanisms of invertebrates, which they must evade to maintain their populations and spread to new hosts. Here we show that autophagy, a conserved intracellular defense mechanism and regulator of cell homeostasis, is a major immune recognition and regulatory process that determines the size of Wolbachia populations. The regulation of Wolbachia populations by autophagy occurs across all distinct symbiotic relationships and can be manipulated either chemically or genetically to modulate the Wolbachia population load. The recognition and activation of host autophagy is particularly apparent in rapidly replicating strains of Wolbachia found in somatic tissues of Drosophila and filarial nematodes. In filarial nematodes, which host a mutualistic association with Wolbachia, the use of antibiotics such as doxycycline to eliminate Wolbachia has emerged as a promising approach to their treatment and control. Here we show that the activation of host nematode autophagy reduces bacterial loads to the same magnitude as antibiotic therapy; thus we identify a bactericidal mode of action targeting Wolbachia that can be exploited for the development of chemotherapeutic agents against onchocerciasis, lymphatic filariasis, and heartworm.
Keywords: Brugia malayi, innate immunity, chemotherapy, helminth, endosymbiont
Wolbachia is a widespread and abundant endosymbiotic bacterium of arthropods and filarial nematodes that resides in vacuoles of host germline and somatic cells. Wolbachia show a diverse variety of symbiotic associations with their host, ranging from obligate mutualism in filarial nematodes to commensal, parasitic, or pathogenic associations in insects and other arthropod hosts (1–5).
In filarial nematodes Wolbachia is obligatory for normal larval growth and development, embryogenesis, and survival of adult worms (1). Although the molecular basis of this mutualistic relationship remains unknown, a comparison of host and bacterial genomes suggests that intact biosynthetic pathways for haem, nucleotides, riboflavin, and FAD may be among the contributions of the bacteria to the biology of the nematode host (6–8). The biological processes most sensitive to Wolbachia loss include larval growth and development and embryogenesis in adult females. These processes have a high metabolic demand because of the rapid growth, development, and organogenesis of the nematode and are associated with the rapid expansion of Wolbachia populations following larval infection of mammalian hosts and in reproductively active adult females (9). Loss of Wolbachia results in extensive apoptosis of germline and somatic cells of embryos, microfilariae, and fourth-stage (L4) larvae, presumably because of the lack of provision of an essential nutrient or metabolite required to prevent apoptosis of these cells and tissues (10); thus apoptosis due to loss of Wolbachia accounts for some of the antifilarial activities of antibiotic therapy.
Therefore we wished to investigate the mechanisms responsible for the regulation of Wolbachia population growth to determine if activation of host nematode defense could be turned against the host’s symbiont, targeting Wolbachia for chemotherapeutic treatments as an alternative to antibiotics. Our studies revealed that periods of rapid population growth and expansion were accompanied by activation of the autophagy pathway and that chemical and genetic manipulation of this pathway could regulate bacterial populations directly at a level equivalent to that achieved with antibiotic treatment. We then extended our observation to other Wolbachia symbiotic relationships and showed that both parasitic and pathogenic strains of Wolbachia also could be regulated by insect autophagy, demonstrating that this mechanism is a common one for the control and regulation of Wolbachia populations.
Results
Initiation and Activation of Autophagy by Wolbachia in Brugia malayi.
ATG8a is a major autophagosomal maturation marker and serves as a biomarker of autophagy activation in eukaryotic cells. This protein has two main forms: (i) a cytosol-associated form, which comprises a reservoir pool of protein, and (ii) a cleaved membrane-associated form located on the phagosomal membranes (11, 12). We used antibodies generated to detect human ATG8a (LC3), which has 85.56% homology with the related protein in Brugia malayi. We detected no other proteins with the same sequence in the nematode and Wolbachia protein databases. ATG8a was observed by confocal microscopy throughout the lateral chord cytoplasm of B. malayi adult females and was associated with areas where Wolbachia reside (Fig. 1 A–D). This pattern of ATG8a distribution was not observed in Acanthocheilonema viteae, a Wolbachia-free filarial nematode (Fig. 1 E and F).
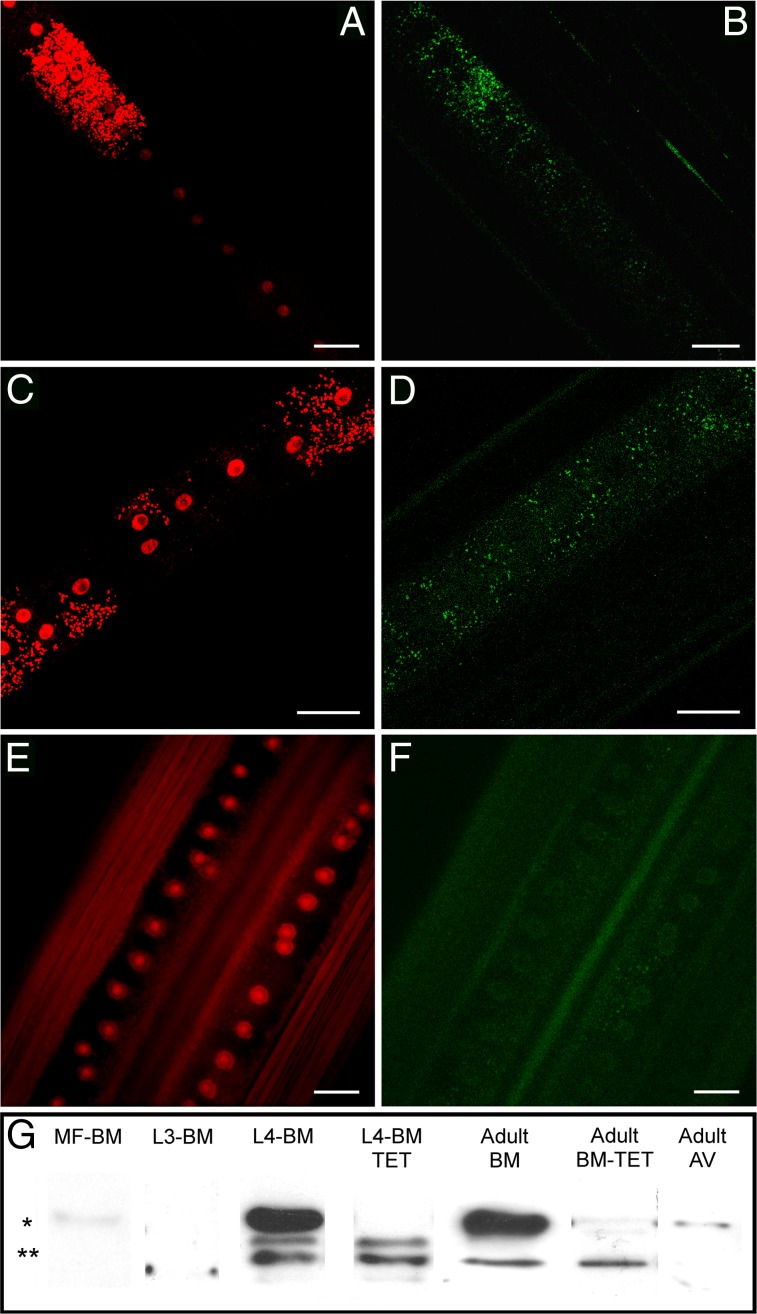
Fig. 1.
Association of ATG8a expression and Wolbachia in the filarial nematode B. malayi. (A–F) ATG8a (green in B, D, and F) colocalized with Wolbachia clusters (small red spots in A and C; large red structures are nematode nuclei) throughout the lateral chord cytoplasm of adult female B. malayi (A–D) and is absent from naturally Wolbachia-free A. viteae (E and F). (Scale bars: 50 μm. (G) Western blot (composite image) of the ATG8a protein in B. malayi (BM) and A. viteae (AV). MF-BM, B. malayi microfilaria; L3-BM, B. malayi L3; L4-BM, B. malayi L4; L4-BM-TET, B. malayi L4 treated with tetracycline in vivo for 14 d; Adult-BM, protein extract from untreated adult females; Adult-BM-TET protein extract from adult females treated with tetracycline in vivo for 6 wk. *ATG8a cytosolic form; **cleaved membrane-associated forms.
Next we studied the expression of ATG8a protein in Wolbachia-infected B. malayi, tetracycline-treated B. malayi, and A. viteae (a Wolbachia-free filarial nematode) during different life-cycle developmental stages, which experience different rates of Wolbachia population growth. Protein extracts from microfilaria or mosquito vector-derived third-stage larvae (L3), the stages that show the lowest ratio and rate of bacterial growth (9), had either no or a minor signal of the cytosol-associated form of ATG8a (Fig. 1G). In contrast, both forms of ATG8a were expressed abundantly in 14-d-old L4 larvae and adult stages. Tetracycline depletion of Wolbachia resulted in the loss of the abundant cytosolic form of ATG8a, and only activated forms were detected, showing that there was no new production of ATG8a protein following depletion of Wolbachia (Fig. 1G). In A. viteae adult female worms, only minor signals of the cytosolic form of ATG8a were detected.
Next we investigated the gene expression of atg8a, a major marker of autophagy initiation, during the life-cycle stages [microfilariae, L3, L4 (14-d-old), and adults] of B. malayi. No expression of atg8a was observed in microfilaria, in which the number and ratio of Wolbachia is the lowest of all life-cycle stages (9). Expression of atg8a in L3 larvae was detectable and was used as a basal level for comparison with the gene expression in other stages. An 11- to 14-fold increase in atg8a expression was observed in L4 (14-d-old) larvae and adult worms compared with L3 larvae (P < 0.003) (Fig. S1A).
Together these results confirm that the activation of autophagy in B. malayi is dependent on the presence of Wolbachia and is markedly elevated and activated during periods in which the bacterial population grows rapidly and in the developmental stages with the highest bacterial density.
Regulation of Autophagy Controls Wolbachia Populations in B. malayi.
Next we investigated whether regulators of autophagy affected Wolbachia growth in B. malayi. Rapamycin, which acts by inhibiting the suppressor target of rapamycin (TOR), was used as an activator of autophagy (13, 14). We treated microfilaria, L3 larvae, and L4 (14-d-old) larvae in vitro with rapamycin (5 μM final concentration) for 5 d. The Wolbachia number was lower in all treated stages (39% in microfilaria, 26% in L3 larvae, 41% in L4 larvae) than in DMSO-treated controls (Fig. 2). Treatment of adult female worms for 7 d with rapamycin resulted in more than a two times reduction in Wolbachia loads; this reduction is similar in magnitude to that achieved using doxycycline, the current gold standard for antiwolbachial treatment, (Fig. 2E).
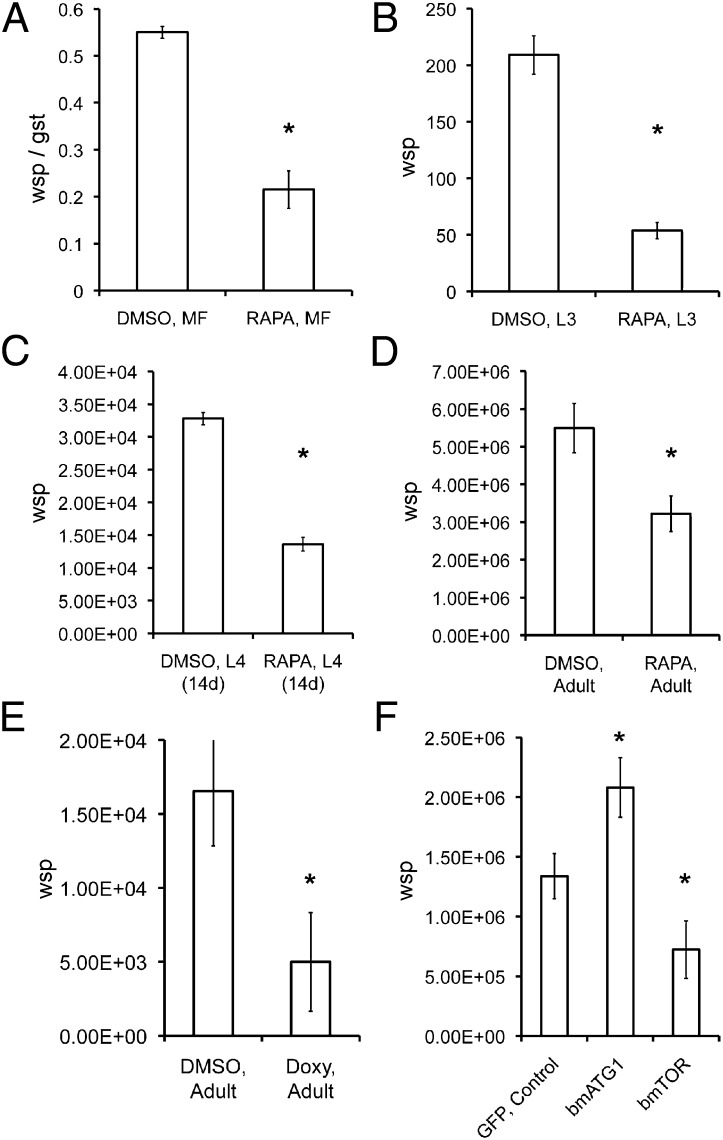
Fig. 2.
qPCR analysis of Wolbachia numbers in B. malayi after in vitro treatment with rapamycin (A–D), doxycycline (E), or siRNA (F). (A) Ratio of wsp/gst in microfilaria after 5 d of treatment with rapamycin (RAPA). (B) Number of wsp copies in L3 larvae treated for 5 d. (C) Number of wsp copies in L4 larvae treated for 5 d. (D) Number of wsp copies per worm in adult females treated with rapamycin or DMSO (control) for 7 d. (E) Number of wsp copies per worm in adult females treated with doxycycline (Doxy) and DMSO (control). (F) Number of wsp copies per worm in adult females treated with siRNA (bmTOR or bmATG1) or GFP as a control. *P < 0.001.
In parallel we used siRNA silencing (siTOR) designed specifically to inhibit the expression of B. malayi target of rapamycin (bmTOR) in the nematode. In adult female worms, a significant reduction (P < 0.001) of Wolbachia number was observed after 7 d of treatment with siTOR compared with siGFP-treated controls (Fig. 2E), showing that suppression of bmTOR and activation of autophagy results in reduced bacterial density. Next we used siRNA to silence ATG1, a key regulator of autophagy initiation. In this experiment silencing of ATG1 and inhibition of autophagy led to a significant increase in Wolbachia numbers in adult worms (Fig. 2F).
Thus, the pharmacological or genetic activation and suppression of autophagy directly regulate Wolbachia populations in B. malayi.
Cellular Mechanism of bmTOR Inhibition in B. malayi.
To confirm that autophagy was induced by the inhibition of TOR and to investigate further the mechanism by which Wolbachia is eliminated from B. malayi, we fixed treated and control adult females for transmission electron microscopy (TEM) 2 d after treatment with rapamycin or siTOR. The cytoplasm of hypodermal chord and embryonic cells contained numerous primary and mature lysosomes and phagolysosomes in samples treated with rapamycin. The number of lysosomes was 3.6 times higher in the rapamycin-treated samples than in the control samples (P < 0.001) Fig. 3A and Fig. S2A). In the cytoplasm of hypodermal chords from treated samples we observed numerous lysosomes surrounding Wolbachia and fused with the bacterial vacuole (Fig. 3B). These observations were reproduced using siRNA-bmTOR treatment, which inhibits TOR synthesis. Phagolysosomes containing digested material, including bacteria-like structures, were found in the cytoplasm of hypodermal chord cells confirming that bacteria were recognized and digested by the activation of autophagy. Therefore, activation of autophagy increased maturation of phagosomes containing bacteria and resulted in their fusion with lysosomes. Nuclear structure in the hypodermal chord cells remained intact.
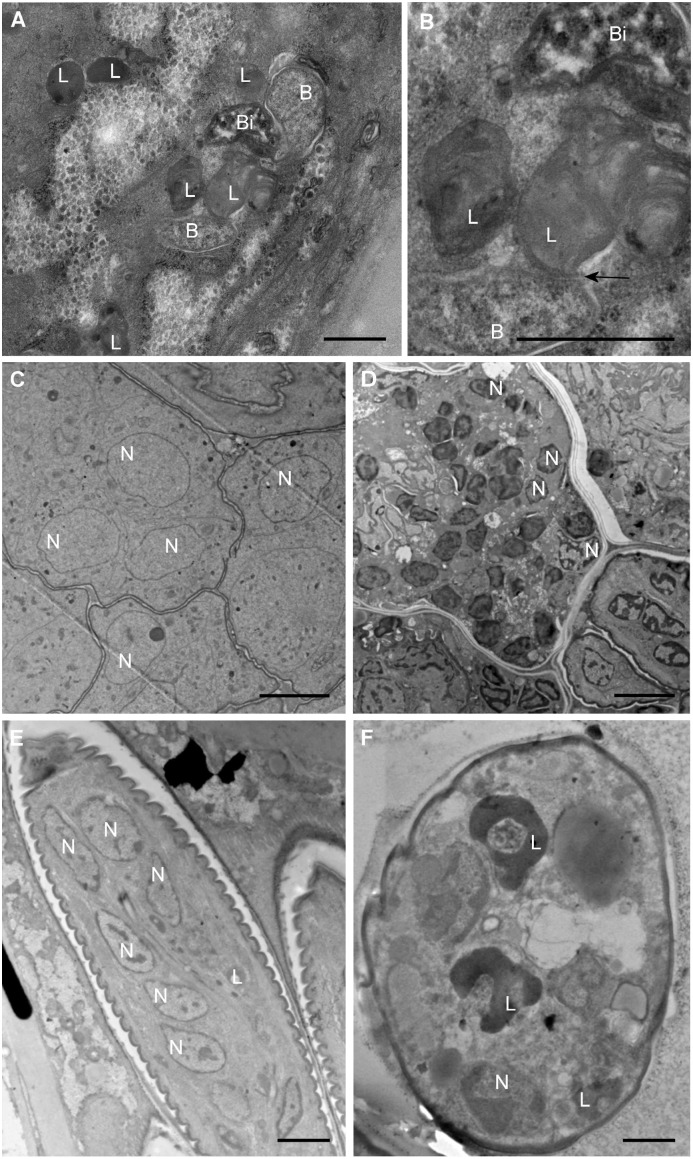
Fig. 3.
Morphological effects on B. malayi treated with rapamycin. Micrographs of hypodermal chord cells (A and B), developing embryos (C and D), and stretched microfilaria (E and F) in the uterus of adult females treated with rapamycin and control. A, B, D, and F show rapamycin-treated samples; C and E show control samples. The arrow in B indicates the fusion of the lysosome and bacteria. B, bacteria; Bi, degenerated bacteria; L, lysosomes; N, nuclei. (Scale bars: 1 μm in A and B; 15 μm in C–F.)
Blockage of embryogenesis caused by extensive apoptosis is one of the major biological processes affected by the depletion of Wolbachia (10). We observed significant morphological alterations of embryonic cells in adult females treated with rapamycin or siRNA-bmTOR. There were dramatic changes of cytoplasm density, with the presence of large vacuoles, mature lysosomes, and clusters of proteins suggesting active digestive processes (Fig. 3 D and F); these changes were not observed during filarial embryogenesis in control samples (Fig. 3 C and E). Eighty percent of the nuclei from treated embryos and stretched microfilaria were fragmented, with condensed chromatin and loss of nuclear membrane integrity, events that occur soon after depletion of Wolbachia from B. malayi (10), suggesting that activation of apoptotic cell death was induced in the embryos after the treatment with rapamycin (Fig. S2B). Such phenotypic outcomes are not observed in Caenorhabditis elegans treated with rapamycin, which instead promotes reproductive development and increased lifespan (15), suggesting that our observations in B. malayi are caused by Wolbachia depletion. In conclusion, inhibition of TOR induced typical intracellular events consistent with the activation of autophagy in B. malayi adult females, resulting in a reduction of Wolbachia populations and subsequent induction of apoptosis in embryos.
ATG8a Localization on the Bacterial Vacuole and in the Cell Wall and Matrix of Wolbachia.
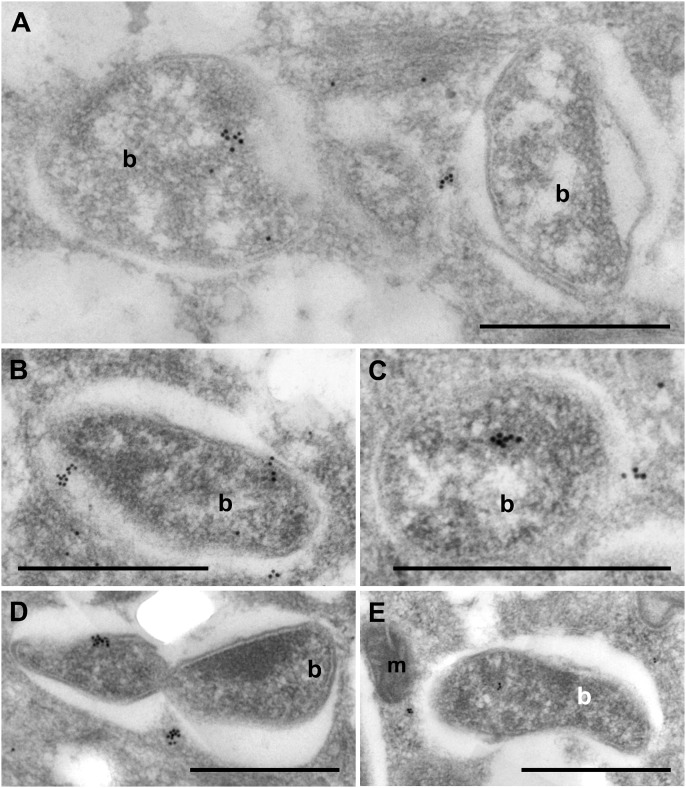
To establish and maintain population levels necessary for a mutualistic symbiotic relationship, Wolbachia must evade or subvert autophagosomal destruction. Immuno-TEM of ATG8a protein localized a single or a few discreet cluster(s) on the vacuoles containing Wolbachia (Fig. 4). Immunogold labeling also was localized to the bacterial cell wall (Fig. 4 B and D) and within the bacterial matrix (Fig. 4 A, C, D, and E). This observation suggests a possible mechanism whereby Wolbachia either recruits or modifies the ATG8a host nematode protein to promote bacterial survival and evasion of autophagy. A BLAST search of ATG8a peptide against the translated wBm genome revealed no homology to explain cross-reactivity of antibodies or production of a mimic bacterial protein. However, this result does not exclude the possibility of a 3D homolog of ATG8a synthesized by bacteria.
Fig. 4.
Ultrastructural localization of ATG8a protein in B. malayi and Wolbachia on the bacterial vacuole (A–E), bacterial cell wall (B and D), and in the bacterial cell matrix (A, B, C, E). b, bacteria; m, mitochondria. (Scale bars: 1 μm.)
Autophagy Controls Wolbachia Populations in Insects.
Autophagy regulates Wolbachia from the mosquito Aedes albopictus.
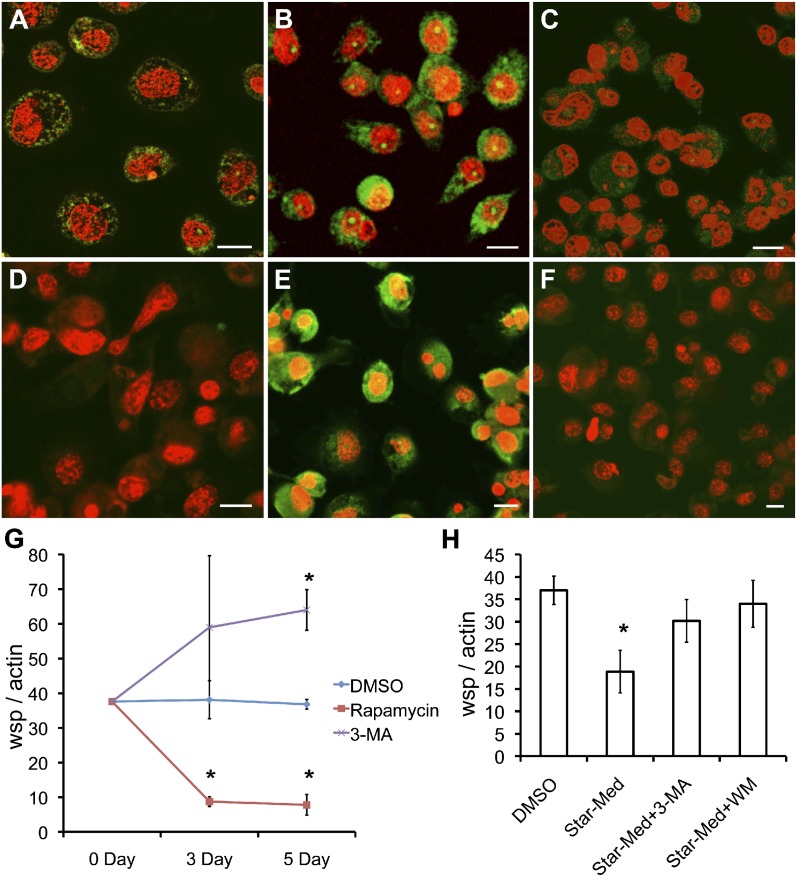
To determine whether regulation of bacterial populations by autophagy extends to other types of Wolbachia that parasitize insects and arthropods, we used the mosquito cell line C6/36 infected with Wolbachia from the mosquito Aedes albopictus (wAlbB). We incubated infected C6/36 (wAlbB) cells and noninfected C6/36 (NI) cells with compounds overnight and processed samples for immunofluorescent localization of ATG8a. ATG8a was observed in C6/36 (wAlbB) cells under standard culture conditions and increased in intensity after induction of autophagy by treatment with rapamycin (Fig. 5 A and B). ATG8a was not commonly observed in C6/36 (NI) cells during standard culture (Fig. 5D) but showed the same pattern of increased intensity after treatment with rapamycin as seen in the infected C6/36 (wAlbB) cells (Fig. 5E). Suppression of autophagy by treatment with 3-methyladenine (3-MA) almost completely eliminated the signal from the cytoplasm of infected and noninfected mosquito cells (Fig. 5 C and F). To confirm that induction of autophagy in C6/36 (wAlbB) cells by rapamycin led to an increase in the maturation of phagosomes, we calculated the number of cells that displayed lysosomal activity. Approximately 90% of cells treated with rapamycin showed high lysosomal activity, compared with 10% of control cells (Fig. S2C).
Fig. 5.
Regulation of autophagy controls ATG8a expression and Wolbachia load in A. albopictus C6/36 cells. (A–F) Detection of ATG8a (green) in C6/36 (wAlbB) cells (A–C) and uninfected C6/36 cells (D–F) during the treatment. (A and D) Control (DMSO-treated) cells. (B and E) Rapamycin-induced cells display up-regulated ATG8a signals. (C and F) 3-MA–treated cells show suppressed expression of ATG8a. (Scale bars: 5 μm). (G and H) qPCR analysis of Wolbachia (WSP) and host actin gene copies in mosquito cells after treatment. (G) Ratio of wsp:actin in the C6/36 (wAlbB) cells treated with rapamycin, 3-MA, or DMSO (control). (H) Ratio of wsp:actin in the C6/36 (wAlbB) cells exposed to starvation (Star-Med) and treated with autophagy inhibitors Wortmannin (WM) or 3-MA. *P < 0.001.
Treatment with rapamycin significantly reduced the number of wAlbB in C6/36 cells at days 3 and 5 after treatment as compared with the control (Fig. 5G). Suppression of autophagy with 3-MA, led to an increase in bacteria numbers that became significant 5 d after treatment (Fig. 5G). Using a different approach to induce autophagy, we subjected C3/36 (wAlbB) cells to starvation by culture in the absence of FCS supplementation for 2 h every second day of culture over a 5-d period (a starvation that did not affect the rate of mosquito cell growth). Induction of starvation resulted in a significant reduction in numbers of Wolbachia (Fig. 5H). Addition of inhibitors of autophagy—3-MA or wortmannin—restored the numbers of Wolbachia to levels equivalent to those in control cells, confirming that autophagy is responsible for reduced bacterial numbers following starvation (Fig. 5H).
Both these approaches confirm that Wolbachia populations are regulated by autophagy in C6/36 mosquito cells.
Autophagy regulates Wolbachia in Drosophila cells.
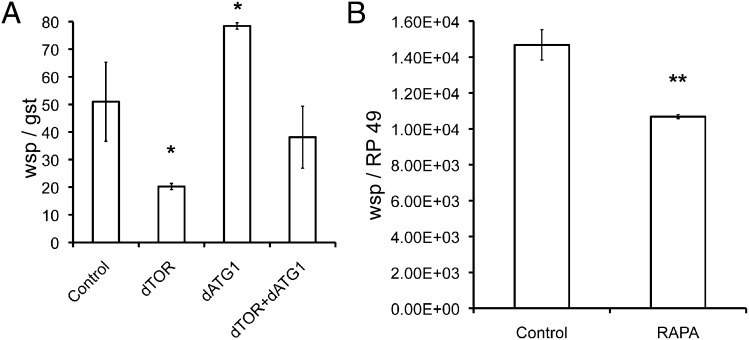
The PC15 cell line infected with the pathogenic strain wMelPop was derived from naturally infected Drosophila melanogaster (w1118) and was provided by W. Sullivan (University of California, Santa Cruz, CA). We used this cell line for siRNA treatment to block specific autophagy protein synthesis. Cells were treated with siTOR targeted to Drosophila TOR, and siATG1 targeted to the ATG1 protein, either singly or in combination. In PC15 (wMelPop) cells, Wolbachia number was reduced by 45% 5 d after treatment with siTOR (Fig. 6A) and by 95% on day 9 after treatment as compared with control samples. Activation of autophagy through inhibition of TOR can be blocked by suppression of ATG1. There was no effect on Wolbachia number in Drosophila cells after 5 d of treatment with siTOR and siATG1 treatment in combination (Fig. 6A). This result confirms that activation of autophagy by TOR inhibition could be suppressed by the absence of a downstream partner (ATG1) in the same signaling pathway. Moreover, Wolbachia numbers increased in PC15 cells treated with only siATG1 molecules (Fig. 6A). This observation allows us to conclude that Wolbachia (wMelPop) is under autophagy control during cell-line cultivation and that the suppression of autophagy results in an increase in wMelPop populations.
Fig. 6.
Autophagy activation controls Wolbachia populations in Drosophila. (A) Effects of siRNA treatment on wMelPop populations in Drosophila cells (PC15). Ratio of wsp:RP49 in PC15 (wMelPop) cells. (B) Reduction of the wsp:RP49 ratio in D. melanogaster (w1118) naturally infected with wMelPop and treated with rapamycin (RAPA).
Increased expression of atg8a in D. melanogaster infected with wMelPop.
wMelPop has a pathogenic effect on D. melanogaster, shortening its lifespan. Here we investigate the role of autophagy in protecting the host against pathogenic Wolbachia in this Wolbachia/Drosophila association. The expression of the atg8a gene in D. melanogaster was compared in wMelPop-infected D. melanogaster (w1118) and Wolbachia-free D. melanogaster (w1118). A threefold (P < 0.05, n = 3) increase in atg8a gene expression in infected female flies was detected using a housekeeping gene (RP49) for normalization of the data (Fig. S1B). All results were confirmed using another housekeeping gene (actin) for normalization of the data.
Rapamycin decreases Wolbachia (wMelPop) populations in D. melanogaster.
Rapamycin was added to the standard food given to Drosophila. D. melanogaster females infected with wMelPop receiving the rapamycin-supplemented or standard food (as the control) laid new embryos overnight; then the adult flies were eliminated from the vial. The next generation was collected 3 d later, and quantitative PCR (qPCR) was used to determine the number of bacteria, which was reduced by 30% in Drosophila treated with rapamycin as compared with the control (Fig. 6B).
Chemical inducers of autophagy as potential antiwolbachial therapeutics.
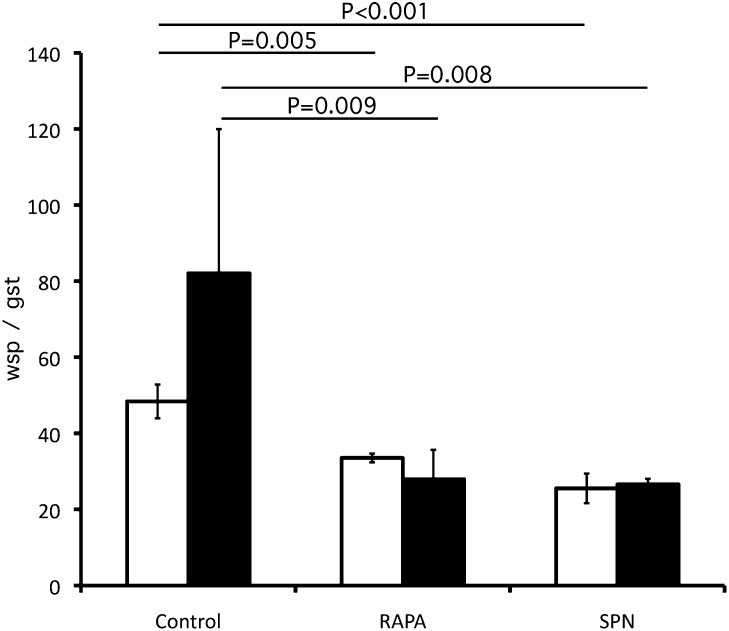
To test whether drugs that induce the activation of autophagy could lead to a reduction in Wolbachia populations in vivo, we treated gerbils infected with B. malayi with rapamycin and spermidine. Rapamycin and spermidine extend the lifespan of yeast, flies, and worms and have beneficial effects on the health of rodents (16, 17). Rapamycin slows tumorigenesis and extends lifespan in mice (17, 18), and spermidine leads to enhanced resistance to oxidative stress and decreased cell death (17, 19). In the first experiment gerbils were infected with L3 larvae and divided into three groups (n = 3 per group). The first group acted as a vehicle control and received 50 μL DMSO (20%)/EtOH (10%, vol/vol) in PBS by s.c. injection; the second group was injected s.c. daily for 14 d with 50 μL rapamycin [5 mg/kg in DMSO (20%)/EtOH (10%) (vol/vol)] in PBS. The third group received 30 mM spermidine in drinking water, which was changed daily, also for 14 d. Worms (L4 larvae) were collected after 14 d, and Wolbachia loads were analyzed by qPCR. In a second experiment gerbils (n = 4 per group) were treated as in the first experiment but for a period of 35 d; then Wolbachia loads of adult worms were analyzed by qPCR. Wolbachia loads in parasites treated with rapamycin or spermidine for 14 d were reduced by 30.7% and 47.3%, respectively, in L4 larvae as compared with the untreated control (Fig. 7). In adult females, treatment with rapamycin or spermidine reduced Wolbachia loads by 66–68% for both groups as compared with the control. These results provide a proof of concept that drug-induced activation of autophagy is as effective as antibiotic therapy in reducing Wolbachia populations in vivo and identify a bactericidal mode of action that can be exploited in the discovery and development of antifilarial treatments.
Fig. 7.
qPCR analysis of Wolbachia number in B. malayi after in vivo treatment with rapamycin (RAPA) or spermidine (SPN) and in controls. Reductions of Wolbachia were seen in worms treated with inducers of autophagy. White bars indicate wsp:gst ratio in L4 larvae (treated for 14 d), black bars indicate wsp:gst ratio in adult females treated for 35 d.
Discussion
Here we show that autophagy is a key regulator of Wolbachia populations in diverse host–symbiont relationships that range from mutualism to pathogenicity. Infection and expansion of Wolbachia populations activate the autophagy pathway, acting as a conserved immune recognition process across a wide range of invertebrate hosts. The genetic manipulation of the TOR–Atg1 signaling pathway or pharmacological activation or suppression of autophagy regulates Wolbachia loads in all host organisms and cells investigated.
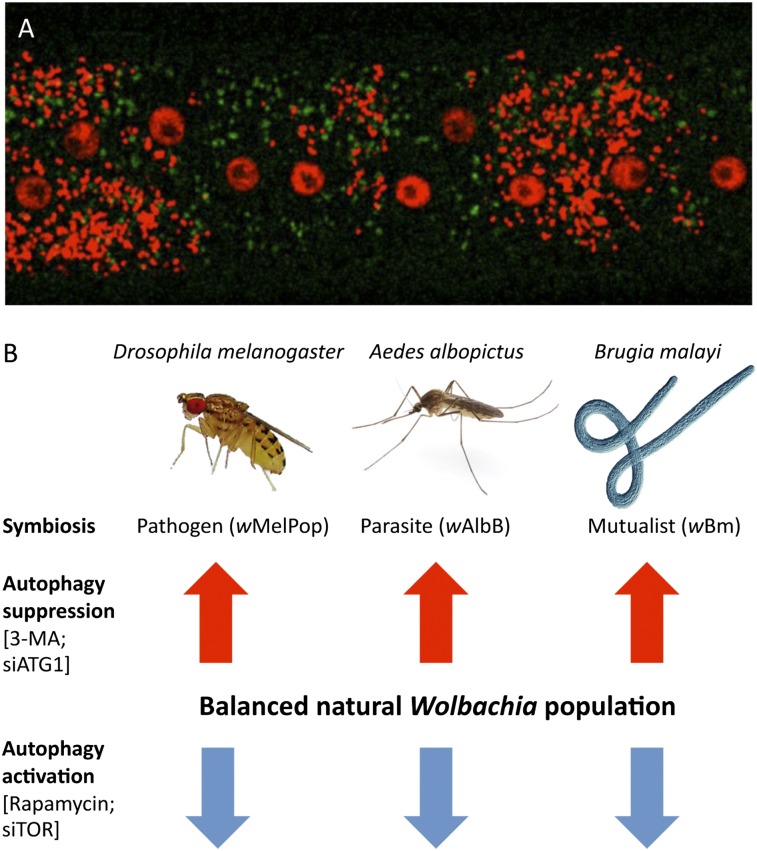
The activation of autophagy through initiation and elongation steps was associated with Wolbachia infection dynamics and was up-regulated during periods of rapid bacterial growth and population expansion. The cellular distribution of the clustering of the major autophagosomal protein marker ATG8a was associated closely with Wolbachia distribution in nematode and insects cells. We show that activation of autophagy by Wolbachia is a process common to all three Wolbachia–host associations studied: (i) Wolbachia from B. malayi (wBm), which has a mutualistic association with the host filarial nematode; (ii) wAlbB from the mosquito Aedes albopictus, a commensal/parasitic strain that induces cytoplasmic incompatibility; and (iii) wMelPop, which has a pathogenic effect on Drosophila, decreasing the lifespan of the host.
Recognition and activation of autophagy by Wolbachia in filarial nematodes demonstrates that, even when a host has become entirely dependent on Wolbachia for growth, development, and survival, Wolbachia still is recognized as a foreign invader and must circumvent the host intracellular defense system to survive. This phenomenon is shared with other mutualistic bacterial host symbioses (20, 21). As in other host–symbiont relationships, there must be a balance among (i) accommodating the symbiont to provide sufficient essential factors that serve the mutualistic association, (ii) regulating the symbiont population to avoid unnecessary fitness costs and pathogenicity; and (iii) retaining an intact host defense against other related pathogens.
Activation of nematode autophagy can increase the lifespan of C. elegans and protection from bacterial infection (22), illustrating the dual key roles played by this process in cell homeostasis and host defense. The filarial nematodes that host Wolbachia are renowned for their longevity of 10–15 y (23). Removal of Wolbachia with antibiotics such as doxycycline leads to a rapid blockage in embryogenesis and larval development that is associated with a Wolbachia-mediated prevention of cell apoptosis, probably through the provision of essential factors necessary for the most metabolically demanding periods of the nematode's development (10). However, this process does not appear to account for the more long-term consequences on adult worm survival, because apoptosis is confined to embryonic and larval somatic cells and adult female germline cells but does not occur in most adult somatic cells (10). After Wolbachia depletion with antibiotics, it takes 1–2 y before the adult worms die prematurely (23). It is intriguing to speculate that the activation of autophagy by Wolbachia may contribute to this extended lifespan of filarial nematodes and that the depletion of Wolbachia sentences the adult worms to a shorter lifespan and one more typical of an adult nematode.
To survive and serve as an essential mutualist, nematode Wolbachia must have developed a mechanism to evade autophagy-mediated removal from the cell. Other Ricketsiales, such as Anaplasma phagocytophilum, subvert the autophagy system to grow and replicate in early autophagosomes but prevent their maturation to late autophagosomes and fusion with lysosomes (24). Activation of autophagy with rapamycin favors A. phagocytophilum infection and growth, and the inhibition of autophagy with 3-MA arrests their growth (24). This effect is in stark contrast to our observations with Wolbachia, in which activation of autophagy leads to the elimination of bacteria and its inhibition promotes population expansion, highlighting important differences in the mechanisms by which these closely related bacteria avoid autophagy-mediated destruction. Our results show that induction of autophagy through TOR-Atg1 results in an increase in the number of lysosomes, that Wolbachia-containing vacuoles can fuse with lysosomes, leading to their elimination, and that the inhibition of autophagy and lysosomal activity by 3-MA increases the number of Wolbachia in host organisms and cells. The mechanism by which Wolbachia populations maintain their levels may depend on a fine balance between the rate of population growth and the rate of elimination by autophagy. One process that might contribute to maintaining this balance is the possible modification or mimicry of key autophagy proteins by the bacteria that block or delay autophagosomal maturation. Our observation of the localization of ATG8a antibody reactivity to components within the bacterial matrix and membranes may be one example of such modification or mimicry by which Wolbachia masks or subverts host ATG8a function. We are exploring this possibility with further experimental approaches.
Transcriptional analysis of two other Wolbachia symbioses—a feminizing association in the woodlouse, Armadillidium vulgare, and obligate symbiosis in the parasitoid wasp, Asobara tabida—provide further evidence for regulation of the autophagy pathway by Wolbachia. In the isopod atg7 and atg12 were underexpressed in infected ovaries, and autophagy genes were down-regulated in the wasp association, suggesting widespread regulation of autophagy by Wolbachia is required for bacterial survival (25, 26).
Autophagy is not the only host-defense mechanism that can be activated by Wolbachia. Natural and experimental infections of Drosophila and mosquitoes with the overreplicating and life-shortening wMelPop strain can induce up-regulation of host immune responses and inhibit microbial infection with viruses, protozoa, and helminth parasites (27–30). Nevertheless, not all Wolbachia–host associations lead to activation of host immunity, and among the strains that do not activate host immunity are natural strains infecting Drosophila and Aedes aegypti (31, 32). The induction of host defense and protection from microbial infection therefore is strain dependent and appears to be restricted to strains that have a high replication rate and widespread tissue tropisms (29, 31, 33). Alternately, it has been suggested that the metabolic demands of such overreplicating bacteria may prevent microbial infection and transmission through competition for host cell resources (27).
Although the mechanism by which Wolbachia protect host insects from microbial infection remains to be fully resolved, our result suggests that autophagy activation and manipulation is a mechanism that might contribute to this phenomenon. The enhanced activation of autophagy by rapidly replicating bacteria such as wBm during larval development and in adult worm populations and induced by wMelPop in Drosophila also may influence the successful infection and transmission of viruses. For example, the requirement of arboviruses (Dengue and Chikungunya) for an intact host autophagy system and their use of autophagosomes for successful replication and transmission (34, 35) may be blocked by Wolbachia-mediated manipulation of autophagosomal maturation, a hypothesis we are testing currently.
Finally, the use of antibiotics such as doxycycline to target Wolbachia elimination from filarial nematodes has emerged as a promising approach to the treatment and control of onchocerciasis and lymphatic filariasis (23). Antiwolbachial therapy is more effective than existing standard antifilarial drugs because of the permanent sterilization of adult worms and long-term macrofilaricidal effects. However, widespread mass administration of doxycycline is compromised by the relatively lengthy course of treatment (4 wk) and the exclusion of pregnant women and children <9 y of age. These barriers stimulated the formation of the Anti-Wolbachia (A·WOL) consortium (http://www.a-wol.net) to search for drugs active against Wolbachia that overcome these restrictions. Our observation that activation of nematode autophagy with drugs and small molecules leads to reductions of Wolbachia populations similar in magnitude to those achieved with gold-standard antibiotics such as doxycycline, both in vitro and in vivo in animal models, provides important proof of concept of a bactericidal mode of action that could be exploited for the discovery and development of drugs against filarial diseases. This proof of concept can stimulate the search for drugs that preferentially activate host nematode autophagy as an alternative approach to the elimination of this essential symbiont.
In conclusion, we have described how the regulation of Wolbachia populations is under the control of host autophagy and show that, to ensure their survival, the bacteria must manipulate or modulate this process through mechanisms that are distinct from those adopted by closely related bacteria. All Wolbachia/host associations studied, ranging from mutualism through to pathogenicity, display similarities in the activation of autophagy, which is associated particularly with overreplicating strains and periods of rapid replication and population expansion. In filarial nematodes, which host a mutualistic association with Wolbachia, the activation of host nematode autophagy provides a bactericidal mode of action to target Wolbachia for the development of chemotherapeutic agents against filarial diseases and in insects may represent an alternative host-defense mechanism to account for Wolbachia-mediated protection against viruses and other microbial pathogens.
Materials and Methods
Parasite Material.
B. malayi was maintained in the peritoneal cavity of gerbils (Meriones unguiculatus). Parasites originally were obtained from TRS Labs, and the life cycle was maintained in house at the Liverpool School of Tropical Medicine. L3 larvae were collected from Ae. aegypti mosquitoes. Microfilaria, L4 larvae, and adult worms were collected from the peritoneal cavities using preheated (37 °C) standard culture medium (RPMI-1640 supplemented with 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM l-glutamine, 2.5 mg/mL amphotericin B, and 25 mM Hepes) (GIBCO). Individual worms were frozen at −80 °C for future extraction of protein/RNA/DNA. The remaining worms were cultivated in 24-well plates with either rapamycin (Sigma) at a final concentration of 5 μM for 5 d or siRNA for 7 d (37 °C, 5% CO2). Adults were incubated individually with 10 worms per experimental group; L3 and L4 larvae with 8–10 worms per group, and microfilaria with 10,000 worms per experimental group. To treat worms with specific siRNA (45 mg/mL), freshly prepared siRNA molecules were dissolved in 0.5 mL nonsupplemented RPMI-1640 (without FBS), and worms were incubated at 37 °C, 5% CO2 for 2 h (36). Then the medium was replaced with culture medium (supplemented with heat-inactivated FBS) containing 45 mg/mL siRNA. Worms were cultivated for 7 d before collection. After chemical or siRNA treatment, all worms were washed individually with PBS and stored for future analysis.
To obtain Wolbachia-depleted parasites, gerbils infected with L3 larvae were treated with tetracycline administered in drinking water (2.5 mg/mL final concentration) for 14 d (for L4 larvae). Gerbils with adult worm infections were treated for 6 wk. Worms were collected 2 wk following treatment, as described above, washed, and frozen for future analysis.
For in vivo treatment, gerbils (n = 3 or 4 animals per group) infected with L3 larvae were treated with (i) rapamycin injected s.c. in a concentration of 5 mg/kg every day for 14 d for analysis of L4 larvae or for 35 d to collect adults. (ii) Spermidine was delivered in drinking water (30 mM final concentration) daily for 14 for L4 larvae and 35 d adult worms. (iii) Control animals received vehicle solution (DMSO 20%/EtOH 10% in PBS) by s.c. following the regime used in the rapamycin group. Worms were collected as described above, washed, and frozen for future analysis.
All animal experiments were carried out in strict accordance with the Animals Scientific (Procedures Act) 1986 (UK) under a license granted by the Home Office (London). Experimental procedures were reviewed and approved by the Animal Welfare Committee, Liverpool School of Tropical Medicine and the Home Office (London).
Drosophila Maintenance.
D. melanogaster (w1118) naturally infected with wMelPop and D. melanogaster (w1118 NI) were maintained at 25 °C and a 12-h dark/light regime. Agar-yeast standard food was changed every 20 d (37).
To study the effect of rapamycin on the Wolbachia loads in Drosophila, 6-d-old females were placed overnight to lay new eggs in vials containing standard food (as the control) or food supplemented with rapamycin (5 μM). Then adult flies were removed from the vials. The new generation was collected from vials on day 5 and frozen for future analysis of Wolbachia population by qPCR.
Mosquitoes and Drosophila Cell Lines.
Mosquito cell line C6/36 (NI), originally uninfected with Wolbachia, was established from Ae. albopictus. The cell line C6/36 (wAlbB) was infected with wAlbB derived from Aa23 (Ae. albopictus) at the Liverpool School of Tropical Medicine and was cultivated successfully for 4 y in the laboratory (38, 39). The cell lines were cultured routinely in 25-cm2 plastic culture flasks at 26 °C in 5 mL of Leibovitz-15 medium consisting 10% of heat-inactivated FBS, 50 U/mL penicillin, 50 mg/mL streptomycin, and 2 mM l-glutamine. Cells were transferred into a new flask every 4–5 d.
One day before the experiments, cells were transferred to a 96-well plate at 10,000 cells per well. On the next day the medium and nonattached cells were removed, and fresh medium with compounds was added. Rapamycin (5 μM) and 3-MA (100 mM) were used to treat C6/36 cells for 3 and 5 d, respectively. For the starvation experiment, cells were cultivated in medium without FBS for 2 h; then the medium was replaced with standard medium. This procedure was repeated every day during the 5-d experiment. To suppress autophagy in the starved cells, medium was supplemented with 3-MA (100 mM) or Wortmannin (10 μM). At the end of the experiment cells were washed twice with PBS, and DNA was extracted using a Promega DNA-extraction kit following the manufacturer’s instructions.
Drosophila PC15 (wMelPop) cells were derived from naturally infected D. melanogaster (w1118) females. The cell line was cultured routinely in 25-cm2 plastic culture flasks at 26 °C in 5 mL of Schneider’s insect medium consisting of 10% heat-inactivated FBS, 50 U/mL penicillin, and 50 mg/mL streptomycin. Cells were transferred into a new flask routinely once every 10 d. One day before the experiments, cells were transferred to a 96-well plate at 10,000 cells per well. On the next day the medium and nonattached cells were removed, medium without FBS and containing 20 mg siRNA was added in the wells, and cells were cultivated for 2 h. Then the modified medium was replaced with standard medium containing 20 mg siRNA. This procedure was repeated three times during the 7-d period of cell cultivation. At the end of the experiment (on day 7) cells were washed twice with PBS, and DNA was extracted as described above.
Production of RNA for dsRNA and siRNA.
Total RNA was extracted from B. malayi or D. melanogaster adult females by a TRIzol-based method (40). Purified RNA was treated with 1 U DNase I (Epicentre) at 37 °C for 30 min followed by inactivation by EDTA. Treated RNA (5 μg) was used as a template for cDNA synthesis performed by SuperScript III (Invitrogen). The cDNA template was amplified by PCR using specific primers containing the T7 promoter sequence (Table S1), and the product was used as a template for T7 RNA polymerase to synthesize the dsRNA by the HiScribe T7 in vitro transcription kit (New England BioLabs). Quality and integrity of dsRNA was checked by standard agarose gel electrophoresis. siRNA (18–25 bp) corresponding to the specific target was produced by digesting transcribed dsRNA with the ShortCut RNase III kit (New England BioLabs) following the manufacturer’s instructions. The siRNA was quantified by comparison on agarose gel to siRNA standard (New England BioLabs). RNA of the green fluorescent protein (gfp) gene was used as a control for siRNA treatment. The RNA was extracted from Drosophila flies containing gfp-gene insertion using the methods and procedures used for the production of experimental siRNA.
Gene Expression.
Total RNA was extracted from 10,000 microfilaria, five L3 larvae, five L4 (14-d old) larvae, five male or female adults, or individual female Drosophila. Then cDNA synthesis was performed as described above. Specific primers for detection of atg8a gene expression level were designed by tehePrimerPrimier 4.0 program using cDNA of atg8a B. malayi or D. melanogaster as templates (Table S1). All amplifications and fluorescence quantifications were performed by a Bio-Rad Chromo 4 real-time PCR Detector (Bio-Rad). A ΔΔCt-based method was used to analyze atg8 levels and the gst gene of B. malayi or the RP49 gene of Drosophila for normalization (10, 41, 42). All comparisons were replicated with at least three biological repeats with three technical replicates for each repeat.
Western Blot.
B. malayi worms (microfilaria, L4 larvae, and adults) were collected from treated and untreated gerbils. L3 larvae were obtained by dissection of infected mosquitoes. All samples were washed three times in PBS and lysed with 50 μL of Tissue Extraction Reagent (Invitrogen). The concentration of the proteins was estimated by bicinchoninic acid assay (Invitrogen) following the manufacturer’s instructions. Lysates of worms were mixed with LDS sample buffer (NuPAGE; Invitrogen), boiled, and run in 12% PAGE. Protein was transferred to nitrocellulose membranes and used in the Western blot as previously described (43). Western blot detection of ATG8a was performed using anti-ATG8a (LC3-II) antibody (Invitrogen and New England BioLabs). Western blot was performed using three independent protein samples analyzed in parallel.
DNA and qPCR.
DNA was extracted from worms, mosquito or Drosophila cells, and Drosophila flies by using the QIAGenes Expression Kit (QIAGEN) following the manufacturer’s instructions. Wolbachia numbers were quantified by qPCR using a single-copy gene: wsp (for Wolbachia) as previously described (9). To estimate the dynamics of the bacterial populations in B. malayi microfilaria, we calculated the ratio of single-copy genes wsp and gst (B. malayi) (9, 10); to estimate Wolbachia loads in the cells, we calculated the ratio of wsp:actin for C6/36 cells and wsp:RP 49 for Drosophila to standardize the data (39).
Microscopy.
B. malayi adult females were fixed using 4% formaldehyde in PBS with 0.05% Triton-X100 (PBST) for 20 min for confocal microscopy analysis of ATG8a protein localization. During fixation, worms were cut to improve distribution of the fixative. Samples then were washed three times in PBST and treated with RNase A (100 mg/mL) overnight at 4 °C (10). The following day, samples were washed in PBS and blocked with 5% BSA for 15 min and incubated overnight at 4 °C with anti-Atg8a (LC3-II) antibody (Invitrogen) diluted 1:200. Secondary antibody labeled with FITC was used at 1:500. After incubation with antibodies samples were costained with propidium iodide for 20 min to visualize DNA (host nuclei and Wolbachia) and were viewed with an LSM 5 Pascal confocal microscope (Zeiss).
For TEM, worms were fixed with 2.5% glutaraldehyde for 2 h. During the fixation worms were cut into ∼5-mm pieces. After fixation, samples were washed three times in PBS and postfixed by 4% OsO4 for 1 h. Samples then were washed and dehydrated using a series of ethanol concentrations (50–100%) with a final wash of acetone. Samples were embedded in plastic (Agar 100) and prepared for sectioning. Ultrathin sections were contrasted with uranyl acetate (1%) and lead citrate and then were analyzed under the Tecnai G2 Spirit BioTWIN TEM by the TEM unit, University of Liverpool (Liverpool, UK).
For immuno-TEM worms were fixed by 4% paraformaldehyde dissolved in PBS for 4 h at 4 °C. During fixation, worms were cut. After fixation, samples were washed in PBS (three times on ice) and dehydrated in a series of ethanol concentrations (50–100%) on ice. Dehydrated samples were embedded in Lowicryl Gold plastic resin. Ultrathin sections were blocked by 5% BSA and incubated with primary anti-Atg8a (LC3-II) antibody (Invitrogen) diluted 1:200 in 1% BSA overnight at 4 °C. On the next day, sections were washed three times in PBS and incubated with secondary antibody labeled with 10-nm gold particles. Sections then were washed with water and contrasted by uranyl acetate (1%) and lead citrate and then were analyzed under the Tecnai G2 Spirit BioTWIN TEM by the TEM unit, University of Liverpool (Liverpool, UK).
C6/36 cell lines were grown on glass overnight and then were fixed by 4% formaldehyde in PBST for 20 min for confocal microscopy analysis of ATG8a protein localization. After fixation, cells were washed three times in PBS and processed as described above. Stained cells were investigated under an LSM 5 Pascal confocal microscope (Zeiss).
Statistical Analysis.
Differences between means were analyzed using one-way ANOVA with Dunnett’s multiple comparison tests as appropriate. For in vitro experiments, means were obtained from 8–10 biological replicates for qPCR and from three biological replicates for qRT-PCR. For in vivo experiments, means were obtained from three or four biological replicates. Analysis of each biological replicate was performed in triplicate. A Dunn–Šidák adjustment was made for multiple comparisons, using a normal P value of P = 0.006 for individual tests to provide an overall significance (α) level of 0.05. All analyses were performed using the PASW Statistics 17 statistical computer program (IBM).
Supplementary Material
Acknowledgments
We thank Prof. W. Sullivan, Dr. A. Debec, Dr. L. Serbus, and Dr. C. Casper Lindley (University of California, Santa Cruz) for providing PC15 Drosophila cell lines. This work was supported by a grant from the Bill and Melinda Gates Foundation awarded to the Liverpool School of Tropical Medicine as part of the Anti-Wolbachia consortium.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 9684 (volume 109, number 25).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203519109/-/DCSupplemental.
References
- 1.Taylor MJ, Bandi C, Hoerauf A. Wolbachia bacterial endosymbionts of filarial nematodes. Adv Parasitol. 2005;60:245–284. doi: 10.1016/S0065-308X(05)60004-8. [DOI] [PubMed] [Google Scholar]
- 2.Dedeine F, Boulétreau M, Vavre F. Wolbachia requirement for oogenesis: Occurrence within the genus Asobara (Hymenoptera, Braconidae) and evidence for intraspecific variation in A. tabida. Heredity (Edinb) 2005;95:394–400. doi: 10.1038/sj.hdy.6800739. [DOI] [PubMed] [Google Scholar]
- 3.Werren JH, Baldo L, Clark ME. Wolbachia: Master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 4.Ferree PM, Avery A, Azpurua J, Wilkes T, Werren JH. A bacterium targets maternally inherited centrosomes to kill males in Nasonia. Curr Biol. 2008;18:1409–1414. doi: 10.1016/j.cub.2008.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMeniman CJ, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- 6.Foster J, et al. The Wolbachia genome of Brugia malayi: Endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005;3:e121. doi: 10.1371/journal.pbio.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu B, et al. The heme biosynthetic pathway of the obligate Wolbachia endosymbiont of Brugia malayi as a potential anti-filarial drug target. PLoS Negl Trop Dis. 2009;3:e475. doi: 10.1371/journal.pntd.0000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slatko BE, Taylor MJ, Foster JM. The Wolbachia endosymbiont as an anti-filarial nematode target. Symbiosis. 2010;51:55–65. doi: 10.1007/s13199-010-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGarry HF, Egerton GL, Taylor MJ. Population dynamics of Wolbachia bacterial endosymbionts in Brugia malayi. Mol Biochem Parasitol. 2004;135:57–67. doi: 10.1016/j.molbiopara.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Landmann F, Voronin D, Sullivan W, Taylor MJ. Anti-filarial activity of antibiotic therapy is due to extensive apoptosis after Wolbachia depletion from filarial nematodes. PLoS Pathog. 2011;7:e1002351. doi: 10.1371/journal.ppat.1002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen IA, Attardo GM, Roy SG, Raikhel AS. Target of rapamycin-dependent activation of S6 kinase is a central step in the transduction of nutritional signals during egg development in a mosquito. J Biol Chem. 2005;280:20565–20572. doi: 10.1074/jbc.M500712200. [DOI] [PubMed] [Google Scholar]
- 14.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Meléndez A, Levine B. Autophagy in C. elegans. WormBook, ed The C. elegans Research Community, 10.1895/wormbook.1.7.1. Available at http://www.wormbook.org.
- 16.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenberg T, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 18.Liu M, et al. Antitumor activity of rapamycin in a transgenic mouse model of ErbB2-dependent human breast cancer. Cancer Res. 2005;65:5325–5336. doi: 10.1158/0008-5472.CAN-04-4589. [DOI] [PubMed] [Google Scholar]
- 19.Madeo F, Eisenberg T, Büttner S, Ruckenstuhl C, Kroemer G. Spermidine: A novel autophagy inducer and longevity elixir. Autophagy. 2010;6:160–162. doi: 10.4161/auto.6.1.10600. [DOI] [PubMed] [Google Scholar]
- 20.Anselme C, et al. Identification of the weevil immune genes and their expression in the bacteriome tissue. BMC Biol. 2008;6:43. doi: 10.1186/1741-7007-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor M, Mediannikov O, Raoult D, Greub G. Endosymbiotic bacteria associated with nematodes, ticks and amoebae. FEMS Immunol Med Microbiol. 2012;64:21–31. doi: 10.1111/j.1574-695X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 22.Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- 23.Taylor MJ, Hoerauf A, Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376:1175–1185. doi: 10.1016/S0140-6736(10)60586-7. [DOI] [PubMed] [Google Scholar]
- 24.Niu H, Yamaguchi M, Rikihisa Y. Subversion of cellular autophagy by Anaplasma phagocytophilum. Cell Microbiol. 2008;10:593–605. doi: 10.1111/j.1462-5822.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 25.Kremer N, et al. Influence of Wolbachia on host gene expression in an obligatory symbiosis. BMC Microbiol. 2012;12(Suppl 1):S7. doi: 10.1186/1471-2180-12-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chevalier F, et al. Feminizing Wolbachia: A transcriptomics approach with insights on the immune response genes in Armadillidium vulgare. BMC Microbiol. 2012;12(Suppl 1):S1. doi: 10.1186/1471-2180-12-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreira LA, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 28.Kambris Z, Cook PE, Phuc HK, Sinkins SP. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science. 2009;326:134–136. doi: 10.1126/science.1177531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frentiu FD, Robinson J, Young PR, McGraw EA, O’Neill SL. Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PLoS ONE. 2010;5:e13398. doi: 10.1371/journal.pone.0013398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kambris Z, et al. Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathog. 2010;6:e1001143. doi: 10.1371/journal.ppat.1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong ZS, Hedges LM, Brownlie JC, Johnson KN. Wolbachia-mediated antibacterial protection and immune gene regulation in Drosophila. PLoS ONE. 2011;6:e25430. doi: 10.1371/journal.pone.0025430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan X, et al. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2012;109:E23–E31. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popovici J, et al. Assessing key safety concerns of a Wolbachia-based strategy to control dengue transmission by Aedes mosquitoes. Mem Inst Oswaldo Cruz. 2010;105:957–964. doi: 10.1590/s0074-02762010000800002. [DOI] [PubMed] [Google Scholar]
- 34.Lee YR, et al. Autophagic machinery activated by dengue virus enhances virus replication. Virology. 2008;374:240–248. doi: 10.1016/j.virol.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krejbich-Trotot P, et al. Chikungunya triggers an autophagic process which promotes viral replication. Virol J. 2011;8:432. doi: 10.1186/1743-422X-8-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ford L, et al. Functional analysis of the cathepsin-like cysteine protease genes in adult Brugia malayi using RNA interference. PLoS Negl Trop Dis. 2009;3:e377. doi: 10.1371/journal.pntd.0000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voronin DA, Bochernikov AM, Baricheva EM, Zakharov IK, Kiseleva EV. [Influence of Drosophila melanogaster genotype on biological effects of endosymbiont Wolbachia (stamm wMelPop)] Tsitologiia. 2009;51:335–345. Russian. [PubMed] [Google Scholar]
- 38.Turner JD, et al. Wolbachia endosymbiotic bacteria of Brugia malayi mediate macrophage tolerance to TLR- and CD40-specific stimuli in a MyD88/TLR2-dependent manner. J Immunol. 2006;177:1240–1249. doi: 10.4049/jimmunol.177.2.1240. [DOI] [PubMed] [Google Scholar]
- 39.Voronin D, Tran-Van V, Potier P, Mavingui P. Transinfection and growth discrepancy of Drosophila Wolbachia strain wMel in cell lines of the mosquito Aedes albopictus. J Appl Microbiol. 2010;108:2133–2141. doi: 10.1111/j.1365-2672.2009.04621.x. [DOI] [PubMed] [Google Scholar]
- 40.Knox DP, Geldhof P, Visser A, Britton C. RNA interference in parasitic nematodes of animals: A reality check? Trends Parasitol. 2007;23:105–107. doi: 10.1016/j.pt.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 41.LaLonde M, et al. A role for Phospholipase D in Drosophila embryonic cellularization. BMC Dev Biol. 2006;6:60. doi: 10.1186/1471-213X-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matta BP, Bitner-Mathé BC, Alves-Ferreira M. Getting real with real-time qPCR: A case study of reference gene selection for morphological variation in Drosophila melanogaster wings. Dev Genes Evol. 2011;221:49–57. doi: 10.1007/s00427-011-0356-6. [DOI] [PubMed] [Google Scholar]
- 43.Johnston KL, et al. Lipoprotein biosynthesis as a target for anti-Wolbachia treatment of filarial nematodes. Parasit Vectors. 2010;3:99. doi: 10.1186/1756-3305-3-99. [DOI] [PMC free article] [PubMed] [Google Scholar]