Abstract
Homeostatic immune regulatory mechanisms can mediate premature termination of therapy-induced antitumor T-effector cell responses. Administration of cyclophosphamide (CY) prior to intratumoral IL-12 and GM-CSF delivery blocked post-treatment T-suppressor cell rebound via elimination of the pre-existing T-suppressor cell pool, allowed repeated activation of antitumor cytotoxic T-cells and resulted in the cure of advanced spontaneous tumors.
Keywords: Interleukin-12, chemoimmunotherapy, chronic treatment, immune regulation, spontaneous tumors
Immune Activation and Homeostatic Counter-Regulation in Cancer Immunotherapy
Majority of immune-based cancer therapies are designed to induce long-term tumor-specific T-cell responses. In theory, the initial effector T-cell response is expected to eliminate established disease with the subsequent memory phase controlling recurrence. In practice however, induction of antitumor cytotoxic T-cell activity has not readily translated to effective tumor kill and relapse-free survival, particularly in the clinical setting.1 Studies in the past decade have revealed that the disconnect between the ability to promote antitumor immunity and effective disease eradication is associated with numerous immunological roadblocks including impaired trafficking and infiltration of lymphocytes into tumors, rapid inactivation of infiltrating effector cells by the tumor and the upregulation of T-cell intrinsic negative regulatory checkpoint molecules.2 Whereas these findings underline the challenges associated with achieving and maintaining effective T-cell activity in the tumor microenvironment, others have demonstrated that such hurdles can be overcome by extraneous immune manipulation.3 Thus, combination approaches involving immune activation with concurrent blocking of tumor-mediated immune suppressive and/or regulatory mechanisms are now being tested in patients.3
Studies in our laboratory demonstrated that local and sustained delivery of IL-12 and GM-CSF to tumors can effectively reverse immune suppression in the tumor microenvironment leading to the induction of both local and systemic antitumor T-cell immunity and tumor eradication.4 Longer-term monitoring of mice however, revealed that the effector T-cell activity was limited to a 5 to 7 d window and was rapidly countered by suppressor T-cell resurgence.5 Further analysis demonstrated that an IFNγ-dependent switch in Dendritic cell (DC) function in the TDLN was responsible for the post-therapy regulatory cell expansion.6 More specifically, the IL-12-IFNγ axis was shown to promote the expression of the tolerogenic enzyme indoleamine 2,3 dioxygenase (IDO) in post-treatment DC and skew their function toward T-suppressor cell expansion.6 Importantly, repeated treatment of persistent tumors resulted in the exacerbation of the regulatory rebound, which ultimately led to complete loss of cytotoxic T-cell activity.5 These findings identified post-treatment homeostatic immune regulation as a critical impediment to successful tumor immune therapy (Fig. 1A). This observation is not unique to our model as a similar expansion of tumor-specific T-suppressor cells following repeated tumor vaccination has also been reported by Zhou et al.7
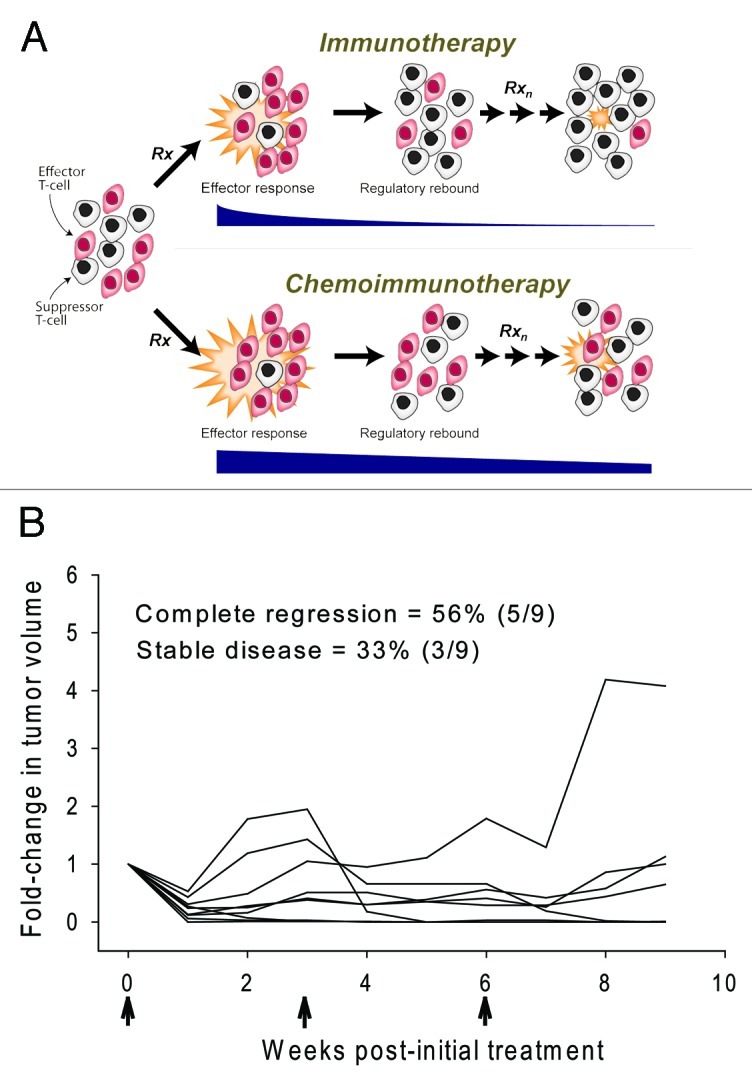
Figure 1. Chronic chemoimmunotherapy as long-term maintenance therapy. (A) CY-mediated modulation of homeostatic immune regulation. Repeated immune stimulation results in progressive intensification of the regulatory rebound and the inability to restore T-effector cell activity. Preferential depletion of the pre-existing T-suppressor cell pool with CY tempers the regulatory rebound allowing for repeated rescue of cytotoxic T-cell activity. Black horizontal bars are representative of the intensity of the antitumor response. (B) Chronic chemoimmunotherapy achieves complete cure of advanced spontaneous mammary tumors. FVBneuN mice bearing established (100–200 mm3) mammary tumors were treated with a single ip injection of CY followed by a single intratumoral injection of IL-12 and GM-CSF microspheres as described9 except that D-1MT was added to the treatment regimen (2 mg/ml in 0.1 ml saline injected intratumorally twice a week for the duration of the study). Microspheres were administered every 3 weeks for a total of 3 cycles (arrows). Each line represents an individual mouse (n = 9).
Controlling Counter-Regulation with Chemoimmunotherapy
When administered prior to immune therapy select chemotherapeutics such as CY potentiate antitumor immunity via multiple mechanisms including preferential depletion of T-suppressor cells, creation of a lymphopenia-associated cytokine sink favorable to expansion of treatment-induced effector cells and direct augmentation of tumor immunogenicity.8 To this end we recently tested the ability of CY to temper post-therapy regulatory surge via preferential depletion of the pre-existing T-suppressor cell pool in the tumor-draining lymph nodes (TDLN). This approach successfully diminished the intensity of the regulatory surge and extended the antitumor T-effector cell activity window.9 More importantly, when administered repeatedly, chemoimmunotherapy promoted the complete cure of established spontaneous mammary tumors in 45% of her-2/neu transgenic FVBneuN mice.9 Further analysis revealed that tumor cure was associated with the ability to repeatedly block counter-regulation thus allowing for long-term maintenance of antitumor cytotoxic T-cell activity9 (Figure 1A).
In separate studies we examined whether targeting a different checkpoint in the feedback inhibitory loop, i.e., blocking of the tolerogenic switch in post-treatment TDLN DC, could be similarly effective. To this end, administration of an inhibitor of IDO, 1-methyl-d-tryptophan (D-1MT) concurrent with IL-12 and GM-CSF, suppressed the tolerogenic switch and the subsequent suppressor T-cell expansion resulting in enhanced tumor eradication.10 More recently, we tested whether combining chemoimmunotherapy with D-1MT, thereby targeting both the source (pre-existing T-suppressors) and the prime facilitator (DC) of the regulatory surge, could further enhance efficacy. The data shown in Figure 1B demonstrate that this strategy achieved either long-term tumor arrest or complete cure in 89% of the mice further underlining the critical role of homeostatic regulation in short-circuiting antitumor T-effector cell activity.
Clinical Potential, Pitfalls and Future Studies
Whereas the above data were obtained in our specific pre-clinical model, we expect this strategy to be applicable to all treatments that are designed to promote TH1 responses. A further advantage is the fact that both CY and D-1MT have been or are currently being evaluated in cancer patients, which should facilitate the clinical development of this concept. Finally, the proposed approach could be particularly useful in the long-term management of persistent and/or inoperable tumors which are resistant to traditional therapy.
Although the ability to block counter-regulation allows for repeated activation of antitumor cytotoxic T-cells, whether such activity can be maintained indefinitely is an important question. For example, monitoring of CD8+ T-cell cytotoxicity during chronic treatment with CY + IL-12/GM-CSF revealed that the intensity of the cytotoxic response gradually declined.9 It is well-known that repeated exposure to antigen can result in the exhaustion of a clonal T-cell response. In addition, preferential depletion of T-suppressors by CY is not absolute and some loss of tumor-specific CD8+ T-cells also occurs every time CY is administered. Finally, inhibition of counter-regulation is transient and regulatory cells eventually return. Whether any one (or a combination) of the above mechanisms are responsible for the inability to fully restore cytotoxic activity after each cycle of treatment is not yet known. Additional studies that are designed to define the ultimate limits of chronic chemoimmunotherapy and the mechanisms that underlie such limitations are currently underway.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/19369
References
- 1.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 2.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–61. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 3.Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev. 2011;241:104–18. doi: 10.1111/j.1600-065X.2011.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill HC, Conway TF, Jr., Sabel MS, Jong YS, Mathiowitz E, Bankert RB, et al. Cancer immunotherapy with interleukin 12 and granulocyte-macrophage colony-stimulating factor-encapsulated microspheres: coinduction of innate and adaptive antitumor immunity and cure of disseminated disease. Cancer Res. 2002;62:7254–63. [PubMed] [Google Scholar]
- 5.Nair RE, Kilinc MO, Jones SA, Egilmez NK. Chronic immune therapy induces a progressive increase in intratumoral T suppressor activity and a concurrent loss of tumor-specific CD8+ T effectors in her-2/neu transgenic mice bearing advanced spontaneous tumors. J Immunol. 2006;176:7325–34. doi: 10.4049/jimmunol.176.12.7325. [DOI] [PubMed] [Google Scholar]
- 6.Harden JL, Gu T, Kilinc MO, Rowswell-Turner RB, Virtuoso LP, Egilmez NK. Dichotomous effects of IFN-γ on dendritic cell function determine the extent of IL-12-driven antitumor T cell immunity. J Immunol. 2011;187:126–32. doi: 10.4049/jimmunol.1100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–36. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65:8059–64. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 9.Rowswell-Turner RB, Harden JL, Nair RE, Gu T, Kilinc MO, Egilmez NK. Chronic chemoimmunotherapy achieves cure of spontaneous murine mammary tumors via persistent blockade of posttherapy counter-regulation. J Immunol. 2011;187:4109–18. doi: 10.4049/jimmunol.1101136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu T, Rowswell-Turner RB, Kilinc MO, Egilmez NK. Central role of IFNgamma-indoleamine 2,3-dioxygenase axis in regulation of interleukin-12-mediated antitumor immunity. Cancer Res. 2010;70:129–38. doi: 10.1158/0008-5472.CAN-09-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]


