Abstract
OBJECTIVE:
To examine circumstances of allergic reactions to foods in a cohort of preschool-aged children.
METHODS:
We conducted a prospective, 5-site observational study of 512 infants aged 3 to 15 months with documented or likely allergy to milk or egg, and collected data prospectively examining allergic reactions.
RESULTS:
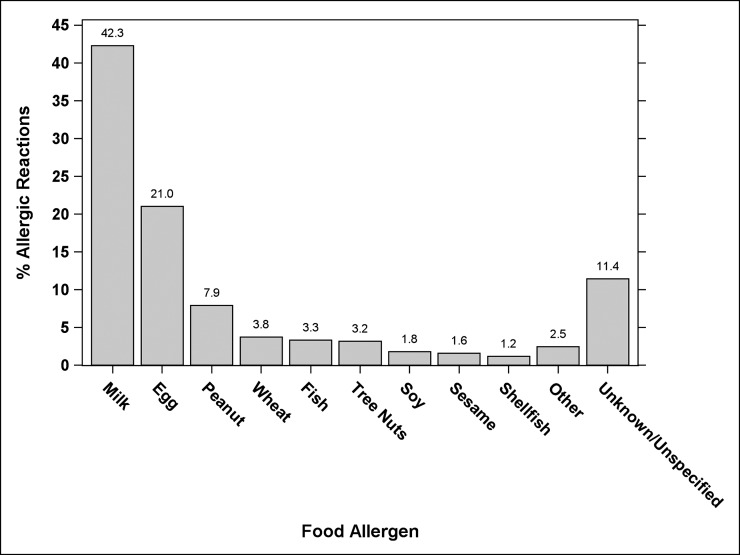
Over a median follow-up of 36 months (range: 0–48.4), the annualized reaction rate was 0.81 per year (367/512 subjects reporting 1171 reactions [95% confidence interval: 0.76–0.85]). Overall, 269/512 (52.5%) reported >1 reaction. The majority of reactions (71.2%) were triggered by milk (495 [42.3%]), egg (246 [21.0%]), and peanut (93 [7.9%]), with accidental exposures attributed to unintentional ingestion, label-reading errors, and cross-contact. Foods were provided by persons other than parents in 50.6% of reactions. Of 834 reactions to milk, egg, or peanut, 93 (11.2%) were attributed to purposeful exposures to these avoided foods. A higher number of food allergies (P < .0001) and higher food-specific immunoglobulin E (P < .0001) were associated with reactions. Of the 11.4% of reactions (n = 134) that were severe, 29.9% were treated with epinephrine. Factors resulting in undertreatment included lack of recognition of severity, epinephrine being unavailable, and fears about epinephrine administration.
CONCLUSIONS:
There was a high frequency of reactions caused by accidental and nonaccidental exposures. Undertreatment of severe reactions with epinephrine was a substantial problem. Areas for improved education include the need for constant vigilance, accurate label reading, avoidance of nonaccidental exposure, prevention of cross-contamination, appropriate epinephrine administration, and education of all caretakers.
Keywords: food allergy, IgE-mediated allergic reaction, epinephrine
What’s Known on This Subject:
Infants and children with diagnosed food allergy are at risk for acute, potentially life-threatening symptoms. Limited data are available on the frequency, severity, and circumstances of reactions and caretaker medical response.
What This Study Adds:
This study describes food allergy reaction frequency, circumstances, and response. Pitfalls that may inform improved anticipatory guidance included lack of vigilance, misreading ingredient labels, allergen cross-contact, nonaccidental allergen feeding, and underutilization of epinephrine for severe reactions.
Allergic reactions to foods affect up to 8% of children.1 Food allergy appears to be increasing in prevalence,2 can be severe,1 and potentially fatal.3 Management requires avoidance of the trigger foods and treatment of anaphylaxis promptly with epinephrine.4 Errors in daily management can result in allergic reactions and undertreatment of severe reactions.5–16
The primary aim of this study was to determine the frequency and circumstances of allergic reactions to foods, and treatment responses, in a prospective study of infants and preschool-aged children with likely egg or milk allergies. The cohort we examined is participating in an observational study on the natural course of milk/egg allergy and is being observed for the development of peanut allergy.17,18 Egg, milk, and peanut allergies are a focus of this study because they represent the most common food allergies in this age group,1 and participants were instructed to avoid these foods if tolerance was not documented. However, we include data on allergic reactions to any foods to examine the full scope of the problem. Understanding the circumstances of allergic reactions can potentially inform educational and treatment strategies for food allergy management in young children.
Methods
Study Participants
Characteristics of the participants have been reported.17,18 Briefly, 512 infants, ages 3 to 15 months, were enrolled at 5 US sites (New York, NY; Baltimore, MD; Little Rock, AR; Denver, CO; and Durham, NC) by advertisement through pediatric and allergy practices. Infants fulfilled at least 1 of 2 enrollment criteria: (1) convincing allergic reaction to milk and/or egg with a positive prick skin test to the trigger food(s) (n = 308) and/or (2) moderate to severe atopic dermatitis and a positive prick skin test to milk and/or egg (n = 204).17,19 Of 616 children originally presenting for screening in the study, 104 were not enrolled at screening for previously documented peanut allergy because the aim of the cohort was to follow children for development of this allergy.17,18 Nonetheless, 68.8% of the cohort were already sensitized to peanut, with 26.6% fulfilling a serologic diagnosis.20 Subjects were classified as allergic according to the clinical history and food-specific immunoglobulin E (IgE) levels.17
Study procedures were approved by a data safety monitoring board and local institutional review boards, and written consents were obtained. Participants were scheduled for a clinical evaluation at 6-month intervals for 2 visits, and then yearly, with telephone contacts between each visit. Participants received written and verbal food allergen avoidance advice for the specific foods to which they were allergic and written emergency plans with prescriptions for self-injectable epinephrine. Advice regarding introduction of foods followed 2000 American Academy of Pediatrics guidelines active at the time of enrollment.21
Documentation of Acute Allergic Reactions and Severity Grading
A structured 36-item questionnaire, modified from ones used previously,5,6,14 was used to obtain details about the reaction including the symptoms and their timing, the trigger, route of exposure, person providing the food, circumstances of exposure (accidental, nature of accident or error, purposefulness, etc), and detailed response to the reaction symptoms. A reaction was included in the study if IgE-mediated symptoms (eg, urticaria, angioedema, wheezing, etc) developed within 2 hours of ingestion. Participants were instructed to contact the study site at the time of reactions. Additionally, participants were queried at every clinical visit and telephone follow-up, as described above. Nonaccidental (purposeful) exposures were those where the food was knowingly given. Due to the subjects’ ages, reactions resulting from children self-feeding the culprit food were considered accidental. Reactions during medically supervised feedings were excluded. Reaction severity was graded based upon Perry et al16 as mild (skin and/or oral symptoms and/or upper respiratory symptoms but not all 3 organ systems); moderate (skin, oral, and upper respiratory symptoms, or gastrointestinal symptoms); and severe (lower respiratory symptoms, cardiovascular symptoms or a combination of skin, oral, upper respiratory and gastrointestinal symptoms).
Statistical Methods
The annualized rate of reactions was calculated by summing the number of reactions across subjects and dividing by the number of follow-up years. To identify associations, univariate Poisson regression analysis was performed to examine demographic/laboratory factors where the number of reactions was the outcome variable; each factor was tested as a predictor, with the number of follow-up years as the offset.
Pairwise comparisons were made to evaluate whether purposeful exposure was similarly common for milk (or egg) versus peanut. Information from cases where reactions to both foods were observed (McNemar’s test) was combined with information from isolated food reactions (Fisher’s exact test) by using Fisher’s method for combining P values.
To evaluate potential escalation of severity with repeated exposures to milk, egg, or peanut, severity from the first reaction was subtracted from the severity of the second (mild = 1, moderate = 2, severe = 3), and the difference was analyzed by using the signed rank test.
Repeated measures analyses, which took into account multiple reactions from the same subject, were performed to compare outcomes for milk or egg compared with peanut by using a generalized linear model with a multinomial distribution (3 severity grades, by using PROC GENMOD SAS 9.2; SAS Institute, Inc, Cary, NC).
Results
Subject Characteristics
A total of 512 children with a median of 35.5 months (range: 0–48.4) follow-up were included (1 infant died and 1 discontinued participation in the month after enrollment). Demographic characteristics of the participants have been previously reported.17,18 Based on previously defined criteria from baseline data and food-specific IgE,17 173 subjects had no confirmed baseline food allergy, 278 had 1 (milk or egg), and 53 had both. Where allergy diagnosis was uncertain, families were instructed to avoid egg, milk, or peanut pending medically supervised oral food challenges, or per advice regarding introduction of foods following the 2000 American Academy of Pediatrics guidelines active at the time of enrollment (eg, peanut to be introduced after age 3 years).21 Over the period of observation, 97.9% of participants were retained.
Allergic Reactions for All Foods
A total of 1171 reactions were reported by 367 (71.7%) subjects. More than 1 reaction was reported by 269 participants (52.5%). Table 1 shows the percent of subjects experiencing reactions, and Fig 1 shows the percent of allergic reactions to each food. The annualized rates of reactions were 0.81 per year overall (95% confidence interval [CI]: 0.76–0.85), with milk 0.34 per year (95% CI: 0.31–0.37), egg 0.17 per year (95% CI: 0.15–0.19), and peanut 0.06 per year (95% CI: 0.05–0.08), as shown in Table 2. The median time between a reaction and the time it was reported was 26 days (range, 0–382; 95% CI: 23–29).
TABLE 1.
Percent of Subjects With Acute Allergic Reactions According to Baseline Features
| N | % of Subjects With Reactions | |||||
|---|---|---|---|---|---|---|
| All | Milk | Egg | Peanut | Other | ||
| Overall | ||||||
| All subjects | 512 | 71.7 | 42.4 | 30.3 | 13.5 | 35.2 |
| Age, mo | ||||||
| 3–5 | 65 | 61.5 | 38.5 | 21.5 | 13.8 | 32.3 |
| 6–8 | 128 | 69.5 | 37.5 | 24.2 | 10.9 | 38.3 |
| 9–12 | 182 | 76.9 | 50.0 | 34.1 | 11.5 | 37.9 |
| 13–15 | 137 | 71.5 | 38.7 | 35.0 | 18.2 | 29.9 |
| Gender | ||||||
| Boy | 345 | 74.2 | 45.8 | 29.9 | 13.6 | 33.0 |
| Girl | 167 | 66.5 | 35.3 | 31.1 | 13.2 | 39.5 |
| Race | ||||||
| White | 380 | 72.4 | 43.9 | 32.1 | 12.1 | 33.7 |
| African American | 78 | 67.9 | 35.9 | 30.8 | 17.9 | 38.5 |
| Asian | 44 | 65.9 | 40.9 | 15.9 | 15.9 | 31.8 |
| Other | 10 | 100 | 40.0 | 20.0 | 20.0 | 80.0 |
| Sitea | ||||||
| National Jewish Health | 99 | 77.8 | 40.4 | 34.3 | 18.2 | 34.3 |
| Duke University | 103 | 47.6 | 33.0 | 11.7 | 5.8 | 6.8 |
| Johns Hopkins University | 109 | 70.6 | 45.9 | 24.8 | 9.2 | 38.5 |
| Mount Sinai Medical Center | 107 | 80.4 | 43.9 | 31.8 | 11.2 | 58.9 |
| University of Arkansas | 94 | 83.0 | 48.9 | 51.1 | 24.5 | 36.2 |
| Baseline no. of food allergiesa | ||||||
| None (no milk, no egg) | 173 | 66.5 | 28.3 | 26.0 | 13.3 | 37.6 |
| 1 (either milk or egg) | 278 | 72.3 | 45.0 | 29.5 | 13.7 | 33.1 |
| 2 (both milk and egg) | 53 | 83.0 | 71.7 | 45.3 | 11.3 | 35.8 |
| Household incomea | ||||||
| <$50 000 | 86 | 74.4 | 39.5 | 43.0 | 19.8 | 33.7 |
| $50 000–$99 000 | 135 | 66.7 | 40.0 | 25.2 | 13.3 | 30.4 |
| ≥$100 000 | 215 | 75.3 | 48.4 | 27.0 | 10.7 | 39.1 |
| Maternal education | ||||||
| High school or less | 56 | 69.6 | 30.4 | 41.1 | 21.4 | 30.4 |
| Some college/college degree | 245 | 74.3 | 44.5 | 27.8 | 13.1 | 35.9 |
| Graduate degree | 208 | 69.2 | 42.8 | 30.3 | 11.5 | 35.6 |
| Paternal educationa | ||||||
| High school or less | 90 | 74.4 | 37.8 | 44.4 | 22.2 | 33.3 |
| Some college/college degree | 209 | 75.1 | 45.5 | 28.7 | 13.4 | 36.8 |
| Graduate degree | 206 | 68.9 | 42.2 | 26.2 | 9.7 | 35.0 |
| Baseline milk IgE, kUA/Lb | ||||||
| <0.35 | 194 | 52.1 | 13.4 | 25.8 | 14.4 | 25.8 |
| ≥0.35–2 | 111 | 73.9 | 39.6 | 34.2 | 10.8 | 32.4 |
| ≥2–5 | 64 | 79.7 | 51.6 | 37.5 | 15.6 | 40.6 |
| ≥5 | 134 | 93.3 | 81.3 | 28.4 | 12.7 | 47.0 |
| Baseline egg IgE, kUA/Lb | ||||||
| <0.35 | 129 | 53.5 | 32.6 | 13.2 | 4.7 | 22.5 |
| ≥0.35–2 | 119 | 62.2 | 30.3 | 23.5 | 9.2 | 30.3 |
| ≥2–5 | 81 | 82.7 | 48.1 | 43.2 | 18.5 | 30.9 |
| ≥5 | 174 | 85.6 | 54.6 | 40.2 | 20.1 | 48.9 |
| Baseline peanut IgE, kUA/Lb | ||||||
| <0.35 | 198 | 57.6 | 32.3 | 23.2 | 4.5 | 23.2 |
| ≥0.35–2 | 105 | 71.4 | 41.0 | 30.5 | 11.4 | 37.1 |
| ≥2–5 | 60 | 78.3 | 41.7 | 41.7 | 16.7 | 36.7 |
| ≥5 | 140 | 87.9 | 57.1 | 33.6 | 25.7 | 48.6 |
| Baseline atopic dermatitis | ||||||
| None | 41 | 61.0 | 43.9 | 17.1 | 4.9 | 24.4 |
| Mild | 50 | 72.0 | 46.0 | 36.0 | 6.0 | 26.0 |
| Moderate | 258 | 70.9 | 44.6 | 31.4 | 14.7 | 34.1 |
| Severe | 163 | 75.5 | 37.4 | 30.1 | 16.0 | 42.3 |
| Have siblings | ||||||
| No | 223 | 75.3 | 44.8 | 35.0 | 14.3 | 33.2 |
| Yes | 289 | 68.9 | 40.5 | 26.6 | 12.8 | 36.7 |
| Breastfeeding history | ||||||
| Never | 73 | 75.3 | 41.1 | 43.8 | 20.5 | 31.5 |
| Yes, currently | 177 | 70.1 | 49.2 | 24.3 | 8.5 | 38.4 |
| Yes, but no longer breastfeeding | 262 | 71.8 | 38.2 | 30.5 | 14.9 | 34.0 |
| Parental history of atopy | ||||||
| Neither parent | 75 | 66.7 | 46.7 | 29.3 | 12.0 | 28.0 |
| 1 parent | 205 | 68.8 | 38.0 | 30.2 | 12.7 | 35.1 |
| Both parents | 223 | 75.8 | 44.4 | 29.6 | 14.3 | 38.1 |
kUA/L, kilounits of antibody per liter.
P value from Poisson regression for all reactions was <.0005.
P value from Poisson regression was <.0001 for the following risk factor analyses: baseline milk IgE for milk reactions, baseline egg IgE for egg reactions, and baseline peanut IgE for peanut reactions.
FIGURE 1.
Allergic reactions per food allergen.
TABLE 2.
Annualized Rates of Allergic Reactions
| Food | Annualized Rate of Reactions | 95% CI |
|---|---|---|
| All foods | 0.81 | 0.76–0.85 |
| Milk | 0.34 | 0.31–0.37 |
| Egg | 0.17 | 0.15–0.19 |
| Peanut | 0.06 | 0.05–0.08 |
| Other | 0.23 | 0.21–0.26 |
We evaluated baseline demographic and laboratory data (Table 1) to determine characteristics that may be associated with reactions. Significant associations as shown in Table 1 included clinical site, higher number of baseline food allergies, paternal education of high school or less, being in lower or higher than median income brackets, and higher baseline allergen-specific IgE. Other baseline characteristics that were not associated include age, atopic dermatitis, breastfeeding history, parental atopy, and having siblings.
Circumstances of Reactions to Milk, Egg, and Peanut
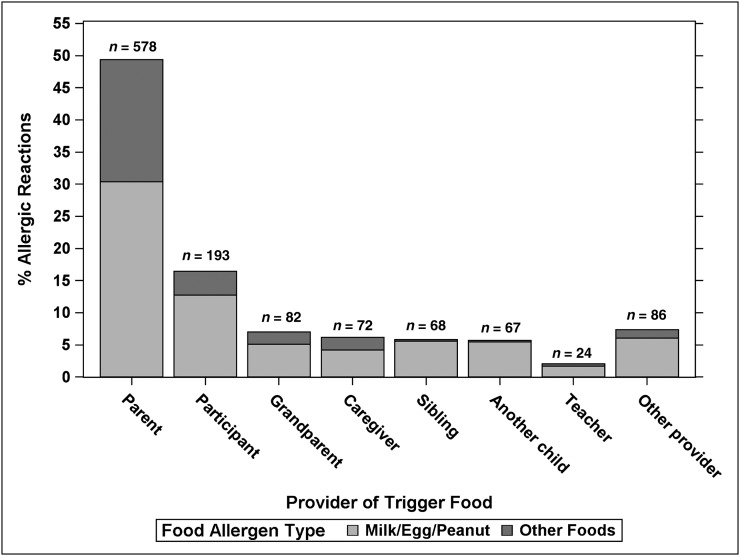
Of the 834 reactions to milk, egg, or peanut, 729 (87.4%) were due to accidental exposures. Causes of accidental reactions included the following: unintentional ingestions (eg, purely accidental such as forgetfulness, reduced supervision, not checking a product, etc), 473 (64.9%); label reading error, 115 (15.8%); cross-contamination, 110 (15.1%); error in preparation, 30 (4.1%); and manufacturer labeling error, 1 (0.1%). Of 729 accidental exposures, parents were most often providers of the food (36.2%), followed by the patients (26.5%), and then various relatives and caregivers (Fig 2). Regarding reactions categorized as being due to nonaccidental ingestion of avoided foods (n = 93), parents were the most common providers (87.1%), followed by grandparents (9.7%), and others (3.2%). There were 12 reactions (1.4%) categorized as purposeful but not occurring during avoidance of milk, egg, or peanut; examples included new onset reactions or reactions to foods containing larger allergen amounts than previously tolerated. Nonaccidental exposure, compared with accidental, was more likely to occur for milk (P = .03) or egg (P = .01) compared with peanut.
FIGURE 2.
Allergic reactions per individual who provided the participant with the food.
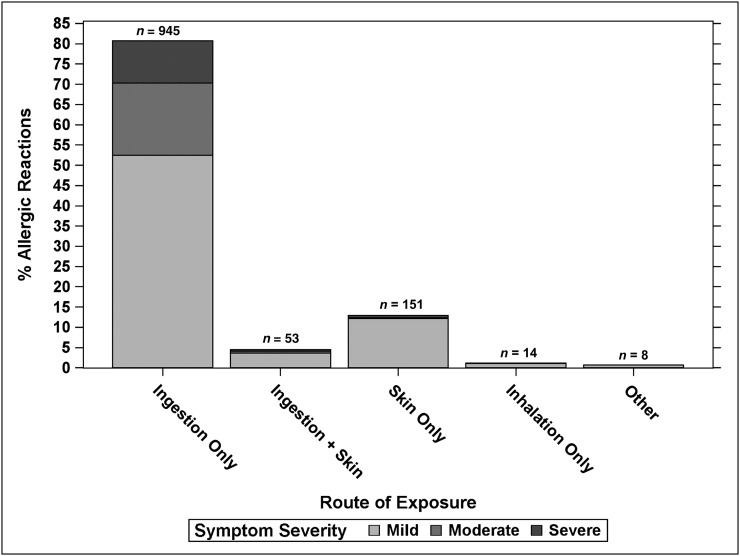
Routes of exposure were characterized as ingestion alone (80.7%), skin alone (12.9%), ingestion plus skin (4.5%), inhalation alone (1.2%), or other (0.7%). The severity of reactions from all foods with respect to route of exposure is shown in Fig 3, indicating that ingestion was the primary route causing severe reactions.
FIGURE 3.
Allergic reactions according to route of exposure. Other exposures included 3 allergic reactions with other combinations, 1 unknown exposure, and 4 by injection (egg in influenza vaccine).
Severity and Treatment of Milk, Egg, and Peanut Reactions
Reaction severity for milk, egg, and peanut is shown in Table 3. The severity of reactions ranked from highest to lowest was peanut > milk > egg, where the difference between peanut and egg was statistically significant (P = .005), but the difference between peanut and milk was not (P = .06). Table 3 also summarizes the medications used to treat reactions. Parents treated 58.9% of milk, egg, or peanut reactions, other persons 4.9%, teachers 3.4%, and school nurses 0.2%. Overall, there was no treatment provided in 21.3% of reactions.
TABLE 3.
Symptom Severity, Medical Treatments, and Reasons for Not Using Epinephrine for Acute Allergic Reactions by Food Allergen Type
| All | Food Allergen | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Milk | Egg | Peanut | Other | |||||||
| N | % | N | % | N | % | N | % | N | % | |
| Total reactions | 1171 | 100.0 | 495 | 100.0 | 246 | 100.0 | 93 | 100.0 | 337 | 100.0 |
| Symptom severity | ||||||||||
| Mild | 821 | 70.1 | 347 | 70.1 | 189 | 76.8 | 57 | 61.3 | 228 | 67.7 |
| Moderate | 216 | 18.4 | 103 | 20.8 | 29 | 11.8 | 17 | 18.3 | 67 | 19.9 |
| Severe | 134 | 11.4 | 45 | 9.1 | 28 | 11.4 | 19 | 20.4 | 42 | 12.5 |
| No treatment given | 250 | 21.3 | 111 | 22.4 | 54 | 22.0 | 14 | 15.1 | 71 | 21.1 |
| Epinephrine given | 52 | 4.4 | 15 | 3.0 | 6 | 2.4 | 7 | 7.5 | 24 | 7.1 |
| Antihistamines given | 877 | 74.9 | 370 | 74.7 | 179 | 72.8 | 75 | 80.6 | 253 | 75.1 |
| IV/oral steroids given | 66 | 5.6 | 19 | 3.8 | 11 | 4.5 | 11 | 11.8 | 25 | 7.4 |
| Topical steroids given | 118 | 10.1 | 39 | 7.9 | 37 | 15.0 | 15 | 16.1 | 27 | 8.0 |
| Asthma medication given | 37 | 3.2 | 17 | 3.4 | 7 | 2.8 | 3 | 3.2 | 10 | 3.0 |
| IV fluids given | 6 | 0.5 | 2 | 0.4 | 1 | 0.4 | 1 | 1.1 | 2 | 0.6 |
| Other treatment given | 15 | 1.3 | 4 | 0.8 | 3 | 1.2 | 2 | 2.2 | 6 | 1.8 |
| Reason epinephrine not given for treated reactions (n = 869) | ||||||||||
| Reaction not severe enough | 783 | 90.1 | 338 | 91.6 | 166 | 89.2 | 60 | 83.3 | 219 | 90.5 |
| Reaction not recognized | 31 | 3.6 | 13 | 3.5 | 9 | 4.8 | 2 | 2.8 | 7 | 2.9 |
| Not available | 15 | 1.7 | 6 | 1.6 | 4 | 2.2 | 3 | 4.2 | 2 | 0.8 |
| Other | 19 | 2.2 | 7 | 1.9 | 2 | 1.1 | 1 | 1.4 | 9 | 3.7 |
| Unknown | 21 | 2.4 | 5 | 1.4 | 5 | 2.7 | 6 | 8.3 | 5 | 2.1 |
When comparing first and second same-food reactions (milk: 123; egg: 58; peanut: 19), no differences were detected for milk, P = .20, egg, P = .63, or peanut, P = .13, indicating no escalation of severity. However, when the first reaction to milk was compared with the fourth from the same subject (n = 48), an increase in severity was found (P = .002); this increase was not seen for any other comparisons (first to third, P = .09; second to third, P = .23; third to fourth, P = .56). Too few subjects had 3 or more egg or peanut reactions to make similar comparisons.
Circumstances of Epinephrine Administration
Of 52 reactions treated with epinephrine (Table 3), triggers were milk (15), peanut (7), egg (6), wheat (4), fish (3), shrimp (1), walnut (1), others (4), and uncertain food (11). Severity grading of 52 reactions treated with epinephrine were severe (40), moderate (4), and mild (8). Data were analyzed to assess epinephrine use according to reaction severity, with all severe reactions warranting epinephrine administration based on current guidelines.4 Of the 1171 reactions, 134 (11.4%) were severe, and the minority (40/134 [29.9%]) were treated with epinephrine.
There were 65 reactions in which epinephrine was not given during a reaction, even though caretakers admitted in retrospect that epinephrine was warranted. The reasons for not injecting epinephrine included the following: reaction not recognized (47.7%), epinephrine unavailable (23.1%), too afraid (12.3%), waiting for more symptoms (6.2%), and unsure if needed (3.1%).
Discussion
This is the first large-scale, multicenter, prospective study evaluating the frequency and circumstances of reactions due to multiple foods in children with a certain or likely diagnosis, having received avoidance advice. Our cohort comprised infants and preschool-aged children enrolled with likely milk or egg allergy, at risk for peanut allergy, a common clinical presentation of food allergy for this age group.17,18 We describe a large number of reactions (1171), their circumstances, and treatment response. Key findings and their implications are highlighted in Table 4.
TABLE 4.
Key Study Findings and Management Implications for Infants/Young Children With Proven or Likely Egg or Milk Allergy
| Observation | Clinical Implication for Anticipatory Guidance/Education |
|---|---|
| The annualized rate or reactions for all foods was 0.81 (95% CI: 0.76–0.85). | The high rate of reactions suggests the need for increased education to avoid reactions. |
| Most (64.9%) accidental allergic reactions to milk, egg, or peanut were attributed to lack of vigilance (failure to check ingredients, forgetfulness, child taking the food, etc). | Emphasize need for supervision, checking ingredients for each meal/snack. |
| Additional common errors in accidental milk, egg, or peanut allergic reactions include misreading labels (15.8%), cross-contact in meal preparation (15.1%). | Educate about label-reading, avoiding allergens in meal preparation/restaurant meals. |
| Half (50.6%) of all allergic reactions were attributed to food not provided by parents, including relatives and teachers. | Education should be given to all caretakers, not just parents. |
| Purposeful trial of avoided milk, egg, or peanut accounted for 11.2% of allergic reactions to these foods. | Family should discuss allergen re-introduction before attempting on their own. |
| Overall, only 29.9% of reactions with severe symptoms were treated with epinephrine. | Emphasize the symptoms that warrant treatment with epinephrine. |
| Almost all severe reactions (94.8%) were attributed to ingestion rather than other routes of exposure (skin, inhalation). | Emphasis should be placed on avoidance of oral exposure (including transfer from hand to mouth in young children). |
The few previous studies about rates of reactions typically focus on older children and evaluate single foods.5,20,22 In a 1-year retrospective study of 3 year olds with milk allergy (n = 88) in Spain, 40% had 53 reactions,22 compared with 42.2% over the period of our study. In a retrospective study of 252 Canadian children (mean age 8.1 years) with peanut allergy, an annual rate of 14.3% was observed,20 compared with 6% in our group. However, direct comparisons are limited by the different age groups and study design. Advantages of our study include a large cohort, a prospective design, capturing reactions to multiple foods, and a prolonged observation period. Caregivers in the current investigation were not only aware of their child’s food allergies, they also received standardized milk, egg, and peanut avoidance advice. Despite instruction, 72% of participants experienced a reaction. It should be noted that reactions to all foods were collected in the study, but we chose to primarily focus on milk, egg, and peanut because these were the foods to which we had confirmed allergy in the former 2 with testing and advised families on avoidance of all 3 allergens unless tolerance was confirmed. The majority of reactions being to milk/egg might be expected because these are ubiquitous foods and the infants/children had these common allergies.
The authors of previous studies have identified specific pitfalls in management but have methodological differences from our study.5–7 Literature regarding food-allergic reactions and response to symptoms primarily include retrospective chart reviews,10,11 patient recall,6,12–14 or oral food challenge data.15,16 Errors have been identified including misreading labels, poor communication in restaurants, cross-contact of allergens, and lack of vigilance.5–7 In our comprehensive prospective study, we identified a number of pitfalls, including novel ones (purposeful exposure, high frequency of reactions from food not provided by parents), and their relative frequency, that warrant attention for anticipatory guidance and have implications for education (Table 4).
Nonaccidental (purposeful) ingestion of known food allergens has so far only been addressed and reported for teenagers with risk-taking behaviors.8,9 An unexpected, worrisome finding in this study is that 11% of milk, egg, or peanut reactions resulted from these exposures. In some cases, reactions occurred to a food that was given in a larger amount than before, which is a nuance worth considering when taking a medical history of young children with possible food allergies. Reasons for these exposures need further exploration but may reflect parental testing for resolution of allergy. A preemptive discussion of the risks of purposeful exposure is advisable.
As reported previously,20,23 there is a hesitancy to administer epinephrine for anaphylaxis. We found 65 reactions where the caretaker failed to administer epinephrine even though they felt it was indicated. Education about treatment and reassuring caretakers about the safety of administering epinephrine24 is indicated because some caretakers reported being afraid to use it.
We identified a number of baseline factors that were associated with having reactions (Table 1). An association with higher food-specific IgE supports observations that more sensitive children are at risk25 but should not be construed to warrant different care instructions based upon IgE levels. We found a higher rate of reactions among the children with a greater number of food allergies, a finding that might be expected based upon probability. These and other associations (eg, income, paternal education) must be interpreted with caution as they may reflect factors unique to the study population but provide results of interest for future studies on risk factors.
There is often concern that casual exposure, such as skin contact or inhalation (eg, via boiling milk), might trigger severe reactions.7,26,27 However, we found that the vast majority of severe reactions were caused by isolated ingestion, which emphasizes the increased risks associated with ingestion compared with inhalation or skin contact. Although severe reactions were more likely to be caused by peanut, our results indicate that severe reactions can involve many other foods.
A common public perception is that reaction severity increases with repeated allergen exposure, a notion that remains controversial.20,28–30 We found no statistical evidence that reactions worsen with second exposure to the same foods. For milk, there was some suggestion that additional exposures had worsening severity; there were too few repeated reactions to egg and peanut to address severity escalation. Our data support the notion that subsequent reaction severity is not easily predicted, but more research is needed.
Limitations of the study include the possibility that not all reactions were captured, that parental reports included inaccuracies about details, recall bias since not all reactions were reported immediately, and parents might have been reluctant to report reactions to purposeful exposures. The generalizability of the results is affected by the enrollment criteria (a convenience sample with likely egg/milk allergy without known peanut allergy), and demographic factors (mostly a middle class, white population). Nonetheless, the study focused upon the most common allergies in this age group, following standard avoidance instructions. We suspect that children with fewer visits for food allergy may have higher rates of reactions than we observed. Our relatively lower rate of peanut reactions could be attributed to the enrollment criteria excluding known peanut allergy, but given that most participants were sensitized and many likely allergic, the lower rate could also reflect more vigilance; however, further studies would be needed to determine this possibility.
Conclusions
The results of this prospective study revealed a high frequency of reactions from accidental and nonaccidental exposures. Based upon the characteristics of the reactions, areas requiring improved education include anticipatory guidance on persistent vigilance, accurate label reading, prevention of cross-contamination, avoidance of purposeful exposures, and appropriate treatment of allergic reactions. Subsequent reaction severity was not easily predicted, and ingestion, rather than skin or inhalational exposure, was responsible for almost all severe reactions. With these data, the pediatrician can provide management instructions and anticipatory guidance with new insights on potential pitfalls. Additionally, the data provide guidance toward improved educational programs that should include all caregivers. Educational materials are available at www.cofargroup.org.31
Acknowledgments
We thank the additional site investigators: A. M. Scurlock, A. Liu, D. Y. M. Leung, R. Lindblad; and the coordinators and supporters: D. Brown, L. Talarico, S. Noone, K. Mudd, S. Driggers, P. Steele, J. Kamilaris, S. Carlisle, T. Hubbart, A. Hiegel, L. Christie, M. Groetch, J. Slinkard, J. Stone, S. Leung, K. Morgan, and K. Brown-Engelhardt. We thank M. Plaut, MD, Medical Officer for the program, and J. Poyser. We thank the families who kindly participated.
Footnotes
- CI
- confidence interval
- IgE
- immunoglobulin E
All authors participated in the design of the study, acquisition of data, and read and approved the final article; Drs Fleischer, Perry, Atkins, Stablein, and Sicherer and Ms Henning drafted the first article; and Dr Stablein and Ms Henning performed the statistical analysis. Drs Fleischer, Stablein, and Sicherer had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or the National Institutes of Health.
FINANCIAL DISCLOSURE: Drs Fleischer and Atkins are consultants for Sunovion. Dr Wood is a consultant to the Asthma and Allergy Foundation of America and is on the Medical Advisory Board of the Food Allergy & Anaphylaxis Network. Dr Burks is a consultant for ActoGeniX NV, Intelliject, McNeil Nutritionals, Novartis, and Schering-Plough; is a minority stockholder in Allertein and MastCell, Inc; is on the advisory board for Dannon Co Probiotics; is on the expert panel for Nutricia; has provided legal consultation services/expert witness testimony in cases related to food allergy; is on the Medical Board of Directors for the Food Allergy & Anaphylaxis Network; is a Dermatological Allergy Committee member for American College of Allergy, Asthma, and Immunology; is a Study Section member for the National Institutes of Health; Hypersensitivity, Autoimmune, and Immune-mediated Disorders; serves on the reviewer board for the Journal of Allergy and Clinical Immunology; and is a member of the Food and Drug Administration. Dr Jones serves on the Medical Advisory Board for the Food Allergy & Anaphylaxis Network and is a steering committee member for Sanofi-Aventis. Dr Sampson is on the Medical Board of Directors for the Food Allergy & Anaphylaxis Network; is a consultant to the Asthma and Allergy Foundation of America; is a consultant for Allertein, LLC; is a consultant/scientific advisor for the Food Allergy Initiative; and is 45% owner of Herb Springs, LLC. Dr Sicherer is a consultant for the Food Allergy Initiative and is an advisor for the Food Allergy & Anaphylaxis Network. Dr Perry, Ms Henning, and Dr Stablein have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institutes of Health (NIH)-National Institute of Allergy and Infectious Diseases (NIAID) U19AI066738 and U01AI066560. The project was also supported by grants UL1 RR025780 (National Jewish Health), UL1 RR029887 (Mount Sinai School of Medicine), UL1 RR029884 (University of Arkansas for Medical Sciences), UL1 RR024128 (Duke University Medical Center), and UL1 RR025005 (Johns Hopkins University School of Medicine) from the National Center for Research Resources, a component of the NIH. Dr Fleischer received grant support from the NIH-NIAID. Dr Perry received research support from the NIH-National Heart, Lung, and Blood Institute and the Marion B. Lyon Award. Dr Atkins received grant support from the NIH. Dr Wood received grant support from the NIH. Dr Burks received grant support from the NIH, the Food Allergy & Anaphylaxis Network, and the Wallace Research Foundation. Dr Jones received research support from the National Peanut Board, the NIH-NIAID, the Food Allergy Initiative, and Dyax Corp. Dr Stablein received grant support from the NIH. Dr Sampson received research support from the NIH-NIAID and the Food Allergy Initiative. Dr Sicherer received research support from the NIH-NIAID and the Food Allergy Initiative. Funded by the National Institutes of Health (NIH).
References
- 1.Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1). Available at: www.pediatrics.org/cgi/content/full/128/1/e9. [DOI] [PubMed] [Google Scholar]
- 2.Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009;124(6):1549–1555 [DOI] [PubMed] [Google Scholar]
- 3.Bock SA, Muñoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007;119(4):1016–1018 [DOI] [PubMed] [Google Scholar]
- 4.Boyce JA, Assa’ad A, Burks AW, et al. NIAID-Sponsored Expert Panel . Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(suppl 6):S1–S58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sicherer SH, Burks AW, Sampson HA. Clinical features of acute allergic reactions to peanut and tree nuts in children. Pediatrics. 1998;102(1). Available at: www.pediatrics.org/cgi/content/full/102/1/e6. [DOI] [PubMed] [Google Scholar]
- 6.Furlong TJ, DeSimone J, Sicherer SH. Peanut and tree nut allergic reactions in restaurants and other food establishments. J Allergy Clin Immunol. 2001;108(5):867–870 [DOI] [PubMed] [Google Scholar]
- 7.Greenhawt MJ, McMorris MS, Furlong TJ. Self-reported allergic reactions to peanut and tree nuts occurring on commercial airlines. J Allergy Clin Immunol. 2009;124(3):598–599 [DOI] [PubMed] [Google Scholar]
- 8.Monks H, Gowland MH, MacKenzie H, et al. How do teenagers manage their food allergies? Clin Exp Allergy. 2010;40(10):1533–1540 [DOI] [PubMed] [Google Scholar]
- 9.Sampson MA, Muñoz-Furlong A, Sicherer SH. Risk-taking and coping strategies of adolescents and young adults with food allergy. J Allergy Clin Immunol. 2006;117(6):1440–1445 [DOI] [PubMed] [Google Scholar]
- 10.Rudders SA, Banerji A, Corel B, Clark S, Camargo CA, Jr. Multicenter study of repeat epinephrine treatments for food-related anaphylaxis. Pediatrics. 2010;125(4). Available at: www.pediatrics.org/cgi/content/full/e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross MP, Ferguson M, Street D, Klontz K, Schroeder T, Luccioli S. Analysis of food-allergic and anaphylactic events in the National Electronic Injury Surveillance System. J Allergy Clin Immunol. 2008;121(1):166–171 [DOI] [PubMed] [Google Scholar]
- 12.Järvinen KM, Sicherer SH, Sampson HA, Nowak-Wegrzyn A. Use of multiple doses of epinephrine in food-induced anaphylaxis in children. J Allergy Clin Immunol. 2008;122(1):133–138 [DOI] [PubMed] [Google Scholar]
- 13.Eigenmann PA, Zamora SA. An internet-based survey on the circumstances of food-induced reactions following the diagnosis of IgE-mediated food allergy. Allergy. 2002;57(5):449–453 [DOI] [PubMed] [Google Scholar]
- 14.Sicherer SH, Furlong TJ, DeSimone J, Sampson HA. The US Peanut and Tree Nut Allergy Registry: characteristics of reactions in schools and day care. J Pediatr. 2001;138(4):560–565 [DOI] [PubMed] [Google Scholar]
- 15.Järvinen KM, Amalanayagam S, Shreffler WG, et al. Epinephrine treatment is infrequent and biphasic reactions are rare in food-induced reactions during oral food challenges in children. J Allergy Clin Immunol. 2009;124(6):1267–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry TT, Matsui EC, Conover-Walker MK, Wood RA. Risk of oral food challenges. J Allergy Clin Immunol. 2004;114(5):1164–1168 [DOI] [PubMed] [Google Scholar]
- 17.Sicherer SH, Wood RA, Stablein D, et al. Immunologic features of infants with milk or egg allergy enrolled in an observational study (Consortium of Food Allergy Research) of food allergy. J Allergy Clin Immunol. 2010;125(5):1077–1083, e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sicherer SH, Wood RA, Stablein D, et al. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. J Allergy Clin Immunol. 2010;126(6):1191–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101(3). Available at: www.pediatrics.org/cgi/content/full/101/3/e8. [DOI] [PubMed] [Google Scholar]
- 20.Yu JW, Kagan R, Verreault N, et al. Accidental ingestions in children with peanut allergy. J Allergy Clin Immunol. 2006;118(2):466–472 [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics. Committee on Nutrition . Hypoallergenic infant formulas. Pediatrics. 2000;106(2 pt 1):346–349 [PubMed] [Google Scholar]
- 22.Boyano-Martínez T, García-Ara C, Pedrosa M, Díaz-Pena JM, Quirce S. Accidental allergic reactions in children allergic to cow’s milk proteins. J Allergy Clin Immunol. 2009;123(4):883–888 [DOI] [PubMed] [Google Scholar]
- 23.Nowak-Wegrzyn A, Conover-Walker MK, Wood RA. Food-allergic reactions in schools and preschools. Arch Pediatr Adolesc Med. 2001;155(7):790–795 [DOI] [PubMed] [Google Scholar]
- 24.Kemp SF, Lockey RF, Simons FE, World Allergy Organization ad hoc Committee on Epinephrine in Anaphylaxis . Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy. 2008;63(8):1061–1070 [DOI] [PubMed] [Google Scholar]
- 25.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107(5):891–896 [DOI] [PubMed] [Google Scholar]
- 26.Simonte SJ, Ma S, Mofidi S, Sicherer SH. Relevance of casual contact with peanut butter in children with peanut allergy. J Allergy Clin Immunol. 2003;112(1):180–182 [DOI] [PubMed] [Google Scholar]
- 27.Perry TT, Conover-Walker MK, Pomés A, Chapman MD, Wood RA. Distribution of peanut allergen in the environment. J Allergy Clin Immunol. 2004;113(5):973–976 [DOI] [PubMed] [Google Scholar]
- 28.Vander Leek TK, Liu AH, Stefanski K, Blacker B, Bock SA. The natural history of peanut allergy in young children and its association with serum peanut-specific IgE. J Pediatr. 2000;137(6):749–755 [DOI] [PubMed] [Google Scholar]
- 29.Sicherer SH, Furlong TJ, Muñoz-Furlong A, Burks AW, Sampson HA. A voluntary registry for peanut and tree nut allergy: characteristics of the first 5149 registrants. J Allergy Clin Immunol. 2001;108(1):128–132 [DOI] [PubMed] [Google Scholar]
- 30.Hourihane JO, Kilburn SA, Dean P, Warner JO. Clinical characteristics of peanut allergy. Clin Exp Allergy. 1997;27(6):634–639 [PubMed] [Google Scholar]
- 31.Sicherer SH, Vargas PA, Groetch ME, et al. Development and validation of educational materials for food allergy. J Pediatr. 2012;160(4):651–656 [DOI] [PMC free article] [PubMed] [Google Scholar]