Abstract
Neurons establish their unique morphology by elaborating multiple neurites that subsequently form axons and dendrites. Neurite initiation entails significant surface area expansion, necessitating addition to the plasma membrane. We report that regulated membrane delivery coordinated with the actin cytoskeleton is crucial for neuritogenesis, and identify two independent pathways that use distinct exocytic and cytoskeletal machinery to drive neuritogenesis. One pathway employs Ena/VASP-regulated actin dynamics coordinated with VAMP2-mediated exocytosis, and involves a novel role for Ena/VASP in exocytosis. A second mechanism occurs in the presence of laminin through integrin-dependent activation of FAK and src, and utilizes coordinated activity of the Arp2/3 complex and VAMP7-mediated exocytosis. We conclude that neuritogenesis can be driven by two distinct pathways that differentially coordinate cytoskeletal dynamics and exocytosis. These regulated changes and coordination of cytoskeletal and exocytic machinery may be utilized in other physiological contexts involving cell motility and morphogenesis.
Introduction
During cortical development, neurons sprout multiple neurites, growth cone-tipped processes that are the precursors of axons and dendrites. In culture, cortical neurons progress through several morphological stages (Dotti et al., 1988), starting with the extension of F-actin rich lamellipodia and filopodia (Dehmelt et al., 2003). Within 24 hours, neurons elaborate multiple neurites, a process that requires both F-actin and microtubule (MT) dynamics. Cortical neurons from mice lacking all three members of the Ena/VASP family of actin regulators fail to form axons in vivo (Kwiatkowski et al., 2007) due to a block in neuritogenesis (Dent et al., 2007; Kwiatkowski et al., 2007). Ena/VASP-deficient neurons lack filopodia, which are essential for cortical neuritogenesis (Dent et al., 2007; Lebrand et al., 2004). Filopodia induction by Ena/VASP-independent methods rescues neuritogenesis (Dent et al., 2007). Despite the striking neuritogenesis defect in the cortex of Ena/VASP null animals, neurons elsewhere in the developing embryo, such as those the retina and dorsal root ganglia form neurites and axons (Dent et al., 2007; Kwiatkowski et al., 2007), indicating that neuritogenesis can also occur through Ena/VASP-independent mechanisms. Interestingly, even Ena/VASP-deficient cortical neurons can form neurites under the right conditions: a small fraction of Ena/VASP-deficient cortical neurons migrate out of the cortex, reach the pial membrane, and extend axons back into the cortex (Dent et al., 2007). Therefore, the Ena/VASP-independent neuritogenesis pathway appears to be triggered by non-cell autonomous environmental factors such as the extracellular matrix (ECM).
The locations in which Ena/VASP-deficient neurons form neurites and axons contain the ECM component laminin, (LN) (Dent et al., 2007); the cortex, however, contains only small amounts of LN, Since cortical neurons normally grow in an environment with little LN, they are usually cultured without LN. When cultured on LN, Ena/VASP-deficient cortical neurons form neurites, confirming LN can trigger Ena/VASP-independent neuritogenesis. Attachment to LN is mediated by transmembrane receptors, including the integrin family (Buck and Horwitz, 1987). Integrin-ECM attachment activates signaling pathways that modulate cellular processes including cytoskeletal dynamics (Geiger et al., 2001). The mechanisms driving LN-dependent neuritogenesis, including the role(s) of integrin activation and signaling are unknown.
Neuritogenesis results in a rapid and large increase in surface area (Pfenninger, 2009), that requires rapid insertion of new membrane and proteins into the plasma membrane. Regulated delivery via exocytosis is required for other neuronal morphogenic events that involve increases in surface area (Futerman and Banker, 1996; Lanzetti, 2007; Martinez-Area et al., 2000; Pfenninger, 2009; Tang, 2001; Tojima et al., 2007) though a role for regulated exocytosis in neuritogenesis has not been established. Exocytic vesicles move along the cytoskeleton to the cell periphery (Schroer, 1992; Tsaneva-Atanasova et al., 2009), where the exocyst complex tethers them to the membrane (EauClaire and Guo, 2003; Murthy et al., 2003; TerBush et al., 1996). Membrane fusion is mediated by a vesicle (v-SNARE) complexing with target SNAREs (t-SNAREs) in the destination membrane (Hong, 2005; Tang, 2001). Many SNARE proteins are brain enriched (Malsam et al., 2008) and several v-SNAREs have specific neuronal functions. Vesicle associated membrane protein 2 (VAMP2, synaptobrevin) is implicated in growth cone chemoattraction (Tojima et al., 2007) and synaptic function (Schoch et al., 2001; Wang and Tang, 2006), but not neurite elongation (Osen-Sand et al., 1996); VAMP7 (tetanus insensitive, TI-VAMP) is implicated in neurite elongation (Alberts et al., 2006; Martinez-Area et al., 2000).
Here we focus on mechanisms underlying Ena/VASP- and LN-dependent neurite initiation and discover they are 2 mutually exclusive pathways. We find neuritogenesis requires both actin dynamics and exocytosis, however, the Ena/VASP- and LN-dependent modes utilize distinct pairs of an actin regulatory protein and a v-SNARE. On LN, integrin signaling triggers the concomitant switch in cytoskeletal and exocytic machinery driving neuritogenesis. Therefore, the ECM exerts a context-dependent influence over cell shape and behavior by inducing a coordinated switch in the cytoskeletal and exocytic machinery used to initiate neurite formation, a hallmark of nervous system development.
Results
Integrin Activation Supports Ena/VASP-independent Neuritogenesis
Neuritogenesis and axon outgrowth are blocked in cortical neurons genetically null for all 3 Ena/VASP proteins (Dent et al., 2007; Kwiatkowski et al., 2007). Due to the complexity of obtaining triple null embryos from timed pregnancies, we utilized a well-established method to inhibit Ena/VASP. This approach exploits the highly specific interaction of the EVH1 domain of Ena/VASP with the ligand motif DFPPPPXDE (FP4) attached to a mitochondrial targeting sequence (FP4Mito) to deplete Ena/VASP from sites of function and sequester Ena/VASP on the mitochondrial surface, blocking function (Bear et al., 2000). This phenocopies the defects observed in cells genetically null for Ena/VASP, including fibroblasts, neurons, and endothelial cells; a control form of the construct (AP4Mito) has no effect on Ena/VASP localization or phenotype (Bear et al., 2000; Bear et al., 2002; Dent et al., 2007; Furman et al., 2007; Lebrand et al., 2004). The strategy is also effective in vivo in Drosophila (Gates et al., 2007). To investigate LN-dependent (Ena/VASP-independent) neuritogenesis, we plated FP4Mito expressing (EnaA/ASP neutralized; “e/v”), cortical neurons on LN. Ena/VASP-dependent neuritogenesis was studied in AP4Mito expressing control neurons (CON) plated on poly-D-lysine (PDL, Fig 1A).
Figure 1. α3β1 and α7β1 integrins mediate LN-dependent neuritogenesis.
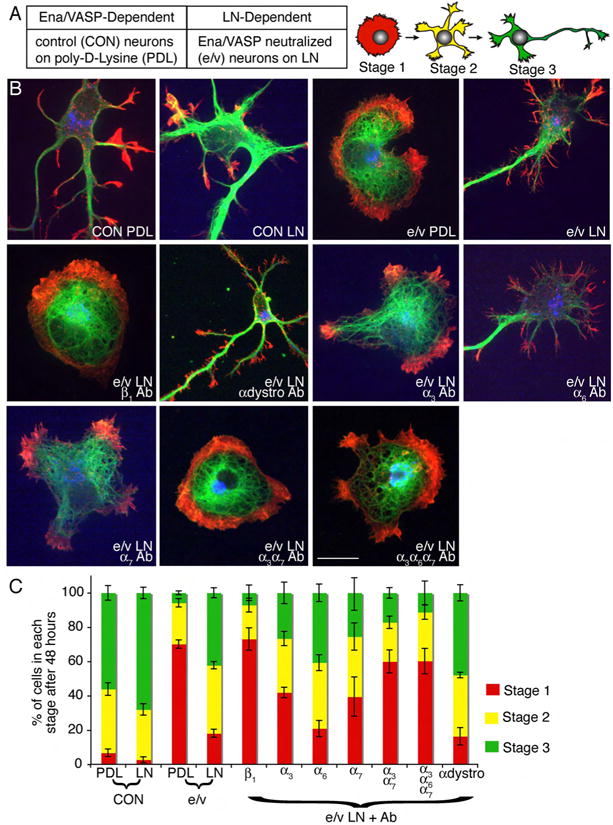
(A) Conditions used to study Ena/VASP-dependent and LN-dependent neuritogenesis and schematic of neuronal morphologies. (B) Representative images of Embryonic day (E)14.5 cortical neurons cultured for 48 hours, expressing AP4Mito (CON) or FP4Mito (e/v) (blue) plated on PDL or LN. Neurons were stained for βIII tubulin (green) and phalloidin (red), β1 α3 and α7 integrin function-blocking antibodies (Ab, 1:1000) decreased neurite formation, while α6 integrin (1:1000) and α-dystroglycan (α-dystro, 1:200) function-blocking antibodies did not. (C) Percentage of cells in each morphological stage +/- SEM. n ≥ 3 independent experiments/treatment, ≥ 25 neurons/experiment. Scale bar is 10 μm.
We first identified the LN receptor required for Ena/VASP-independent neurite initiation. Candidate receptors include α3β1, α6β1, α7β1integrins and α-dystroglycan (Flanagan et al., 2006; Gorecki et al., 1994; Hynes, 2002; Ivins et al., 1998; Plantman et al., 2008). Due to the rapid kinetics of neurite formation and the postmitotic state of neurons, siRNA was not effective at depleting protein levels during neuritogenesis. Therefore, we used function-blocking antibodies to LN receptors. After 48 hours, the morphology of neurons was classified (Fig 1A) as lacking neurites (stage 1), having one or more neurites (stage 2), or having one neurite/axon at least twice as long as any other (stage 3). CON neurons formed neurites on both PDL and LN (Fig 1), while e/v neurons failed to form neurites on PDL (p<.05 Fig 1), equivalent to null neurons (Dent et al., 2007). As expected, this was rescued by LN (Fig 1A, p<.05). A β1 integrin function-blocking antibody inhibited neurite formation by e/v neurons on LN (Fig 1, p<.05), while a function-blocking antibody against αdystroglycan did not. Blocking either β1 integrin or αdystroglycan had no effect on neurite initiation by CON neurons grown either on PDL or LN (Fig S1A), indicating that loss of Ena/VASP exposes a requirement for integrin activation during neuritogenesis.
β1 integrin dimerizes with (α3, α6, and α7 integrin to form LN receptors (Hynes, 2002). Function-blocking antibodies against α3 and α7 integrin partially reduced neuritogenesis in e/v neurons on LN (p<.05 Fig1), while an α6 integrin function-blocking antibody did not. Using the α3 and α7 integrin antibodies together phenocopied β1 integrin inhibition; addition of the α6 integrin antibody did not reduce neuritogenesis further (Fig 1), indicating LN-dependent neuritogenesis was mediated by α3β1 and α7β1 integrin heterodimers.
Unlike LN, fibronectin (FN) and collagen do not rescue cortical neuritogenesis in the absence of Ena/VASP (Dent et al., 2007). Western blots of cortical lysates indicated cortical neurons expressed only trace amounts of α5 and αv integrins (not shown), which dimerize with β1 or β3 integrin to form fibronectin (FN) receptors (Hynes, 2002). Ectopic expression of α5 integrin rescued neuritogenesis in e/v neurons on FN (p<.05 Fig S1) but not on PDL, therefore integrin-mediated neuritogenesis likely results from a general integrin-signaling pathway that depends on the integrin repertoire expressed by neurons and the local ECM composition.
FAK, src, and Rac function in LN-dependent neuritogenesis
To identify signaling molecules regulating neuritogenesis, we utilized pharmacological perturbations or dominant negative mutants (Fig 2). Surprisingly, inhibition of molecules implicated in axon specification and guidance, such as phosphoinositide 3 kinase (PI3K), phospholipase D (PLD), protein kinase A (PKA), and protein kinase C (PKC) (Han et al., 2007; Shen et al., 2002; Wolf et al., 2008; Yoon et al., 2005) did not affect neuritogenesis in e/v neurons on LN (Fig 2AB) or CON neurons (Fig S1). In contrast, inhibition of focal adhesion kinase (FAK) by its noncatalytic domain, FRNK (Schaller et al., 1993) or src with 50 nM PP2 (Ohnishi et al., 2001) decreased neurite initiation in e/v neurons on LN (p<.05 Fig 2AB), but not in CON neurons on PDL (Fig S1A). Conversely, constitutively active src (srcY527F) rescued neuritogenesis in e/v neurons on PDL (p<.05 Fig 2AB). Therefore, src and FAK were necessary for LN-dependent neuritogenesis, but dispensable for Ena/VASP mediated neuritogenesis. In the absence of Ena/VASP, activated src was sufficient to support neuritogenesis and bypass seed the requirement for LN.
Figure 2. FAK, Src, and Rac activity are necessary for LN-dependent neuritogenesis.

(A) E14.5 e/v cortical neurons cultured 48 hours expressing FP4Mito (blue), and either the FAK inhibitor (FRNK, green), constitutively active Src (Y527F, green) or treated with the indicated drugs, plated on PDL or LN. Neurons not expressing FRNK or SrcY527F were stained for βIII tubulin (green) and phalloidin (Red). (B) Quantification of morphologies, as in Fig 1. Targeted molecules include PI3K, (50 μM LY294002, LY), PKA (20 μM H-89), PKC (1 μM GF109230X, GF), Src (50 nM PP2), PLD (0.3% 1-butanol, 1-b). n≥ 3 independent experiments/treatment, ≥25 neurons/experiment. Scale bar is 10 μm (C) E14.5 neurons cultured 48 hours, expressing AP4Mito (CON) or FP4Mito (e/v) (blue) plated on PDL or LN. Red is phalloidin and green indicates either anti-myc (for Rac mutants), GFP (for Cdc42 mutants) or βIII tubulin, in neurons not expressing a mutant. (D) Quantification of morphologies +/- SEM. n ≥ 3 independent experiments/treatment, ≥25 neurons/experiment. Scale bar is 10 μm.
Rho family GTPases are intermediates coordinating integrin signaling and cytoskeletal dynamics. Rac and Cdc42 in particular regulate neuronal morphogenesis and membrane protrusion (Brouns et al., 2001; Gallo et al., 2002; Miyashita et al., 2004; Nobes and Hall, 1995; Pommereit and Wouters, 2007; Schaefer et al., 2008). Expression of dominant negative Cdc42N17 (Ridley and Hall, 1992; Ridley et al., 1992) caused only a slight reduction of neurite initiation in CON neurons on PDL (Fig 2CD p<.05), but did not affect e/v neurons on LN. Constitutively active Cdc42Q61L expression did not rescue neuritogenesis in e/v neurons on PDL, although it induced filopodia formation (Fig 2CD); it is possible these filopodia were unable to support neuritogenesis due to confounding secondary effects.
RacN17 expression in CON neurons on PDL reduced neurite formation slightly (Fig 2 p<.05). In contrast, RacN17 expression or treatment with a Rac inhibitor, NSC-23766, (Gao et al., 2004) reduced neuritogenesis substantially in e/v neurons on LN (p<.05 Fig 2CD). RacQ61L, however, failed to rescue neuritogenesis in e/v neurons on PDL. These data suggest Cdc42 and Rac play minor roles in Ena/VASP-dependent neuritogenesis. In contrast, Rac activity was required for LN-dependent neuritogenesis, although unlike activated src, active Rac was insufficient for Ena/VASP-independent neuritogenesis.
LN Switches the Actin Regulators Mediating Neuritogenesis
We next asked whether other types of actin regulatory proteins compensated for the absence of Ena/VASP in LN-mediated neuritogenesis. Ena/VASP proteins promote F-actin polymerization by protecting the fast growing ends of F-actin from capping and can cluster the ends of actin filaments (Applewhite et al., 2007; Bachmann et al., 1999; Barzik et al., 2005; Bear et al., 2002; Breitsprecher et al., 2008; Schirenbeck et al., 2006) and mediate filopodia formation (Dent et al., 2007; Gupton and Gertler, 2007; Lebrand et al., 2004). Previously we found that ectopic expression Myosin X or mDia2, proteins that drive Ena/VASP-independent filopodia formation rescued neuritogenesis in e/v neurons, however, neither is detectably expressed in cortical neurons at this developmental stage (Dent et al., 2007). The Arp2/3 complex can be activated downstream of Rac, nucleates and branches F-actin, and has been implicated in axon guidance and neuronal morphology (Korobova and Svitkina, 2008; Strasser et al., 2004; Withee et al., 2004). We used the CA domain of the Arp2/3 activator, N-WASP, to reduce Arp2/3 activity during neuritogenesis. CA binds Arp2/3, blocks its activation in vitro (Rohatgi et al., 1999) and attenuates its activity in cells (May et al., 2000; May et al., 1999). CA expression in CON neurons on PDL did not reduce neurite formation, although it reduced the number of stage 3 neurons (Fig 3), indicating Arp2/3 may play a role in axon specification. However, CA expression reduced neuritogenesis in e/v neurons on LN (p<.05 Fig 3). Therefore Arp2/3 is required for LN-dependent but not Ena/VASP– dependent neurite initiation, revealing a novel switch in the roles of actin regulators or a requirement for specific types of F-actin supramolecular structures for LN-dependent neuritogenesis.
Figure 3. The Arp2/3 complex functions in LN-dependent neuritogenesis.
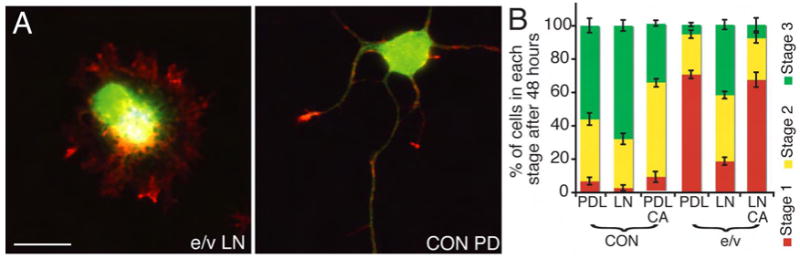
(A) E14.5 neurons cultured 48 hours, expressing mCherry-CA (green) and either AP4Mito (CON) or FP4Mito (e/v) (blue) plated on PDL or LN. Phalloidin staining is red. Scale bar is 10 μm. (B) Quantification of morphologies +/- SEM. n ≥ 3 independent experiments/treatment, n ≥25 neurons/experiment.
An Exocytic Switch on LN
We next examined the regulation of membrane delivery during the rapid increase in surface area that occurs during neuritogenesis (Pfenninger, 2009; Vega and Hsu, 2001); while exocytosis is likely involved in this process, it is not clear which of the v-SNAREs mediate vesicle fusion during neuritogenesis. The v-SNARE VAMP2 functions in growth cone chemoattraction (Tojima et al., 2007), but not neurite elongation (Osen-Sand et al., 1996). We utilized tetanus neurotoxin (TeNT), to cleave and inhibition exocytosis mediated by VAMP1, VAMP2, and VAMP3 (Fig S2, (Sikorra et al., 2008; Verderio et al., 1999)). Treatment with TeNT blocked neuritogenesis in CON neurons on PDL (p<.05 Fig 4). This block was reversible (Fig S2) and as previously reported, TeNT treatment did not affect outgrowth once neurites had formed (Fig S2E-F,(Osen-Sand et al., 1996). VAMP2 is expressed highly in cortical neurons at this stage (Fig S2), while closely related v-SNAREs, VAMP1 and VAMP3 are not detectable (Schoch et al., 2001), suggesting VAMP2-mediated exocytosis is essential for Ena/VASP-dependent neuritogenesis.
Figure 4. VAMP2 and VAMP7 are required for Ena/VASP-dependent and LN-dependent, Ena/VASP-independent neuritogenesis, respectively.

(A) neurons expressing AP4Mito (CON) or FP4Mito (e/v) (blue) plated on PDL or LN and cultured 48 hours. (B) Quantification of neuronal morphologies +/- SEM. n ≥ 3 independent experiments/treatment, n ≥ 25 neurons per experiment. 50 nM tetanus neurotoxin (TeNT) and expression of the NH2 domain of VAMP7 (NH2-V7) ere used to block VAMP2 and VAMP7-mediated exocytosis, respectively. Overexpression of Exo70 or VAMP7 but not VAMP2 nor VAMP3 rescued neuritogenesis in e/v neurons on PDL. VAMP expression is green. Phalloidin is red; neurons not expressing VAMP were stained for βIII tubulin (green). Scale bar is 10 μm. (C) Summary of molecules implicated in Ena/VASP-dependent or LN-dependent neuritogenesis.
Surprisingly, TeNT did not block neuritogenesis on LN, indicating attachment to LN also bypasses the necessity for VAMP2 during neuritogenesis. This suggested LN triggered neurons to use another v-SNARE. VAMP7 was a logical candidate: it is present in the cortex (Malsam et al., 2008), Fig S2) and is insensitive to TeNT (Galli et al., 1998). The NH2-terminal fragment of VAMP7 blocks VAMP7 SNARE complex formation (Martinez-Area et al., 2000) and, as expected, its expression reduced VAMP7-mediated, but not VAMP2-mediated exocytosis (Fig S2G). VAMP7 inhibition blocked neurite formation in e/v neurons on LN (Fig 4 p<.05) but not CON neurons on PDL (Fig S1A). Together these data indicate that exocytosis is required for neuritogenesis and that LN induced a switch in the key v-SNARE mediating exocytosis: VAMP2 was required for Ena/VASP-dependent neuritogenesis; VAMP7 was necessary for LN-dependent neuritogenesis.
Since VAMP2 inhibition phenocopied loss of Ena/VASP activity, we wondered if the neuritogenesis defect in e/v neurons involved reduced exocytosis. Exo70 is a component of the exocyst complex that when overexpressed drives filopodia formation, membrane protrusion, vesicle tethering, and secretion (Liu et al., 2009; Zuo et al., 2006). Exo70 overexpression restored neuritogenesis (p<.05 Fig 4), as did VAMP7 (p<.05 Fig 4), whereas overexpression of VAMP2 or VAMP3 failed to rescue neuritogenesis of e/v neurons (Fig 4). These data indicate that the Ena/VASP-dependent defect may result from reduced exocytosis. Coexpression of the inhibitory VAMP7 NH2-fragment impaired the ability of exogenous Exo70 to drive neuritogenesis in e/v neurons (Fig 4 p<.05), indicating Exo70 rescued neuritogenesis through a mechanism partially dependent on VAMP7. Furthermore, in this system, VAMP7 function was necessary and sufficient for Ena/VASP-independent neuritogenesis.
Vesicle Dynamics and Exocytosis are Altered on LN
VAMP2 and VAMP7 localize to distinct vesicle populations in cortical neurons (Fig S3). Since the ECM can switch both the v-SNAREs and F-actin regulators required for neuritogenesis, we hypothesized the dynamics of v-SNARE-containing vesicles would be sensitive to substrate as well. To characterize vesicle dynamics, we performed live cell total internal reflection fluorescence microscopy (TIR-FM, (Axelrod et al., 1983) of GFP-tagged v-SNAREs expressed at low levels (Fig 5A-D), which localize with endogenous proteins (Fig S3). In CON neurons on PDL, VAMP2 containing vesicles dynamically explored the cell periphery and filopodia, while on LN, most vesicles remained static in central regions. VAMP7 containing vesicles exhibited inverse dynamics, visiting the periphery frequently on LN (Movie 1). Quantification of velocity showed no change on PDL or LN (Fig 5C). However, VAMP2 containing vesicles moved more directionally on PDL than LN, while VAMP7-containing vesicles were more directional on LN (t-tests, p<.05, Fig 5C). Dual spectral imaging of VAMP2 and VAMP7 confirmed these distinct dynamics (Fig 5D, Movie 2). Therefore directional vesicle movement occurs for a v-SNARE when it is required for neurite initiation.
Figure 5. VAMP2 and VAMP7 containing vesicles display distinct dynamics and fusion on PDL and LN.
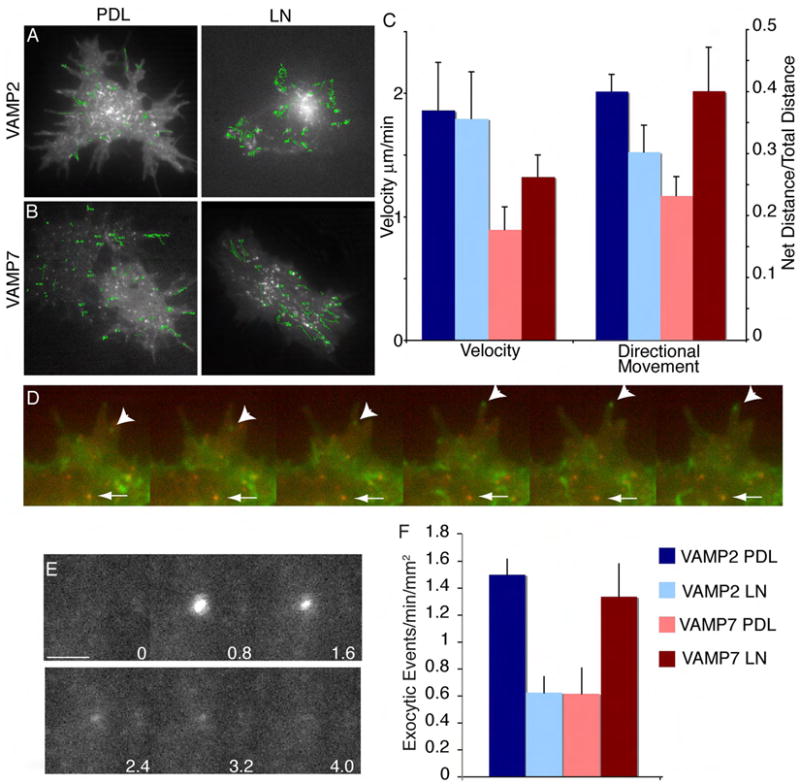
Frames from time lapse of E14.5 CON cortical neurons expressing either GFP-VAMP2 (A) or GFP-VAMP7 (B) plated on PDL or LN imaged 6 hours after plating. Green tracks indicate vesicle position over time. (C) Quantification of vesicle speed and directionality of vesicle movement +/- SEM. Directionality was calculated by dividing the net distance traveled by the total distance traveled by each vesicle; a higher ratio indicates more directional vesicle movement. n ≥ 5 cells/condition, ≥ 30 vesicles/cell, imaged in ≥ 5 consecutive frames. Scale bar is 5 μm. (D) Frames from a time lapse of E14.5 CON neurons expressing GFP-VAMP2 and mcherry-VAMP7. Arrowhead indicates a VAMP2-containg vesicle moving into a filopodia, while the arrow indicates a VAMP7-containing vesicle remaining stationary in a more central region. (E-F) E14.5 CON neurons expressing VAMP2-pHluroin or VAMP7-pHluorin were plated on PDL or LN. After 6 hours, vesicle fusion events on the basal cell membrane were imaged by TIR-FM. (E) Representative images from a time-lapse demonstrate a vesicle fusion event. The fluorescence intensity of pHluorin is low when it is within the vesicle lumen (t=0s). Upon fusion with the plasma membrane, pHluorin is exposed to the higher cytosolic pH, and there is a rapid increase in fluorescence intensity (t=0.8s), followed by a rapid diffusion of fluorescence as the molecules disperse within the plasma membrane (t=1.6-2.4s). The frequency of vesicle fusion +/- SEM is shown in (F). n ≥15 cells/condition. Scale bar is 1 μm.
The observations described above led us to hypothesize that Ena/VASP affects vesicle dynamics. Ena/VASP inhibition did not affect the velocities of VAMP2 or VAMP7-containing vesicles on PDL or LN. However on PDL, Ena/VASP inhibition caused VAMP2-containing vesicles to no longer pause at the periphery. In contrast, VAMP7-containing vesicle dynamics appeared unaffected by Ena/VASP inhibition (Movie 3), suggesting Ena/VASP or associated F-actin may be important for dynamics or docking of VAMP2, but not VAMP7-containing vesicles.
VAMP2 and VAMP7-mediated exocytosis were measured in stage one neurons using TIR-FM of pH-sensitive pHluorin-tagged VAMP2 and VAMP7 ((Alberts et al., 2006; Miesenbock et al., 1998), Fig 5EF, Movie 4). VAMP2-mediated exocytosis occurred three fold more frequently in CON neurons on PDL than on LN (p<.05). In contrast, VAMP7-mediated exocytosis was more frequent on LN (p<.05 Fig 5F, Movie 5). The high frequency of VAMP2 and VAMP7-mediated exocytosis on PDL and LN, respectively correlated with their increased directionality and requirement in context-dependent neuritogenesis, indicating attachment to LN switches the dynamics and function of exocytic machinery.
Exocytic Fusion and the Cytoskeleton
Since the relationship between cytoskeletal dynamics and exocytosis is complex (Becker and Hart, 1999; Sokac and Bement, 2006; Valentijn et al., 2000) and exocytosis and cytoskeletal dynamics are crucial for neuritogenesis in cortical neurons (Dehmelt et al., 2003; Dent et al., 2007), we hypothesized they were coordinated. To determine how dampening of actin dynamics affects exocytosis, we treated CON neurons with the F-actin capping drug cytochalasin D (CD) at a concentration that blocks filopodia and neurite formation, but does not affect growth cone dynamics once neurites/axons have formed (Dent and Kalil, 2001; Dent et al., 2007). Since VAMP2 is required on PDL and VAMP7 is required for LN-dependent neuritogenesis, we analyzed vesicle fusion on these respective substrates. The frequencies of VAMP2 and VAMP7-mediated exocytosis were significantly decreased by long term and acute CD treatment (p<.05 Movie 6-7 Fig 6B, Fig S4), indicating exocytosis mediated by these v-SNAREs requires proper F-actin dynamics.
Figure 6. Cytoskeletal dependence of VAMP-mediated vesicle fusion.

(A) Control, CD, and nocodazole treated E14.5 CON neurons stained with phalloidin (red) and βIII tubulin (green) 6 hours after plating. Scale bar is 5 μm (B) Frequency of vesicle fusion +/- SEM in E14.5 neurons expressing either VAMP2-pHluroin or VAMP7-pHluorin plated on PDL or LN, respectively, as in Fig 6. VAMP2 and VAMP7 fusion events were significantly reduced by CD treatment (p<.05), but not nocodazole treatment, although VAMP7 events showed an insignificant reduction (t-test, p=.1). VAMP2-mediated fusion, but not VAMP7-mediated fusion, was reduced in e/v neurons. Inhibition of the Arp2/3 complex (CA) significantly reduced VAMP7-mediated fusion but not VAMP2-mediated fusion. The low frequency of VAMP2-mediated exocytic fusion in e/v neurons was rescued by expression of mDia2 (e/v mDia2). n ≥ 15 cells/condition.
To determine if MT dynamics function in exocytosis, we treated CON neurons with nocodazole, at a concentration that dampens MT dynamics and blocks neuritogenesis, but leaves polymer levels intact (Dent et al., 2007). Neither VAMP2 nor VAMP7-mediated fusion were significantly affected by long term treatment (Movie 6, Fig 6B), however both were slightly reduced by acute treatment (Movie 7, Fig S4), indicating a complex relationship between MT dynamics and exocytosis.
The Cytoskeletal and Exocytic Switches Are Coordinated and Mutually Exclusive
We hypothesized Ena/VASP or Arp2/3 activity is required for context-dependent (i.e. Ena/VASP vs. LN) exocytosis. We imaged VAMP2-phluorin and VAMP7-phluorin CON neurons following Ena/VASP or Arp2/3 inhibition. VAMP2-mediated exocytosis on PDL was attenuated significantly by Ena/VASP but not Arp2/3 inhibition (Fig 6B, p<.05 Movie 8). In contrast VAMP7-mediated fusion on LN was unaffected by Ena/VASP inhibition, but reduced by CA expression. Therefore, respectively, VAMP2 and VAMP7-mediated exocytosis requires Ena/VASP and Arp2/3 activity and/or the type of F-actin structures, formed by these two actin regulators. Secondly, the switches in cytoskeletal and exocytic machinery following attachment to LN are coordinated,.
mDia2 nucleates linear F-actin filaments, drives filopodia formation (Copeland et al., 2004; Harris et al., 2006), and rescues filopodia formation and neuritogenesis in the absence of Ena/VASP (Dent et al., 2007). Expression of mDia2 in e/v neurons also rescued the frequency of VAMP2-mediated vesicle fusion (p<.05 Fig 6B, Movie 8), suggesting F-actin bundles and/or filopodia, structures produced by either mDia2 or Ena/VASP, are required for VAMP2-mediated exocytosis, and that mDia2 may support neurite initiation in e/v neurons by rescuing VAMP2 mediated exocytosis.
VAMP7-Mediated Exocytosis Occurs Downstream of LN-dependent Signaling
Our data suggest that 1) specific F-actin structures or remodeling proteins may specify or facilitate the specific v-SNARE driving neuritogenesis and 2) activation of integrin-dependent signaling pathways stimulates VAMP7-mediated vesicle fusion. We hypothesized VAMP7 overexpression might stimulate exocytosis and neuritogenesis independently of the signaling pathway, as in e/v neurons on PDL, perhaps through a mechanism similar to that of Exo70 overexpression. We overexpressed VAMP7 in e/v neurons on LN when molecules required for LN-dependent neurite initiation were inhibited (Fig 7A-B). VAMP7 overexpression rescued neuritogenesis blocked by inhibition of FAK, src, Rac, and Arp2/3 (t-tests, p<.05), suggesting VAMP7 functions in LN-dependent neuritogenesis downstream of FAK, src, Rac, and Arp2/3. However VAMP7 was unable to rescue neuritogenesis blocked by CD treatment (Fig S5), indicating intact actin dynamics mediated by Arp2/3 and likely another actin regulators such as cofilin, DAAM1, or Cordon blue (Ahuja et al., 2007; Aizawa et al., 2001; Chen et al., 2006; Matusek et al., 2008), may be involved.
Figure 7. VAMP7 rescues neuritogenesis downstream of FAK, Src, Rac, and Arp2/3 activity.

(A) E14.5 neurons cultured 48 hours expressing FP4Mito (e/v) (blue) +/- GFP-VAMP7 (green) were plated on PDL or LN. Phalloidin staining is shown in red. Neurons not expressing GFP-VAMP7 are stained for βIII tubulin (green). Scale bar is 10 μm. (B) Quantification of morphologies, as in Figure 1. n ≥ 3 independent experiments/treatment, n ≥ 25 neurons per experiment. VAMP7 overexpression rescues neuritogenesis of e/v neurons on LN with inhibited FAK (FRNK), Arp2/3 (CA), Rac (RacN17) or Src (PP2). (C) Frequency of VAMP7-mediated vesicle fusion +/_ SEM in CON E14.5 neurons expressing VAMP7-pHluorin, and either FRNK or treated with 50 nM PP2 plated on LN.
To assess directly whether VAMP7 functions downstream of integrin signaling, we imaged exocytic events on LN and found FAK or src inhibition (both of which block LN-dependent neuritogenesis), decreased VAMP7-mediated exocytosis (p<.05 Fig 8C, Fig S4, Movie 9), indicating they regulate VAMP7-mediated exocytosis in this context.
Discussion
We have identified two mutually exclusive, parallel pathways that mediate neuritogenesis. Both pathways require specific coordinated sets of F-actin and exocytic machinery. We found exocytosis is required for neurite initiation, and is mediated by a specific pairing of v-SNARE and F-actin regulator. Cortical neurons normally require the activities of Ena/VASP (Dent et al., 2007) and VAMP2. Attachment to LN rendered Ena/VASP and VAMP2 dispensable, and revealed a requirement for Arp2/3 and VAMP7 downstream of integrin, FAK, src, and Rac (Fig 4). Interestingly, VAMP2-mediated exocytosis required Ena/VASP while VAMP7-mediated exocytosis required Arp2/3. This may indicate a preference of v-SNARE containing vesicles for specific F-actin structures: linear and/or bundled versus branched F-actin. These changes occurred downstream of integrin signaling, raising the possibility that similar conversion mechanisms may be employed for neurite initiation by other neuronal types, for other steps in neuronal development, such as axon outgrowth and chemoattraction, or in shape changes and motility of non-neuronal cells.
An Unexpected Role for Ena/VASP in Exocytosis
Our data indicate Ena/VASP proteins or associated F-actin structures function in VAMP2-mediated exocytosis and that an exocytosis defect contributed to a neuritogenesis block in e/v neurons. Both Ena/VASP and VAMP2 inhibition blocked neuritogenesis. and e/v neurons had reduced VAMP2 exocytic fusion. Neuritogenesis in e/v neurons was rescued by overexpression of Exo70 or VAMP7, but not VAMP2, indicating that VAMP2 function requires Ena/VASP or the type of structures produced by Ena/VASP. Introducing mDia2, which promotes F-actin polymerization and filopodia formation, rescued the frequency of VAMP2-mediated exocytosis in e/v cells, correlating with the ability of mDia2 to rescue e/v neuritogenesis (Dent et al., 2007). Together these results lead us to propose that VAMP2-mediated exocytosis requires F-actin structures typically formed by Ena/VASP. Thus, a critical role of Ena/VASP in neuritogenesis involves VAMP2-mediated exocytosis. In contrast to VAMP2, VAMP7-mediated exocytosis required Arp2/3 but not Ena/VASP, suggesting VAMP7 may rely on branched F-actin for vesicle fusion. VAMP2 has been implicated in growth cone chemoattraction (Tojima et al., 2007), while VAMP7 is required for axon elongation (Martinez-Area et al., 2000). It is possible that a mechanism similar to the regulated switch in v-SNAREs and actin regulatory proteins that we identified here may also be utilized at other times during neuronal morphogenesis to regulate exocytosis and therefore membrane extension and movement.
Integrin Activation Switches the Actin and Exocytic Machinery Driving Shape Change
Overexpression of α5 integrin and attachment to FN rescued neuritogenesis in e/v neurons. Activated src, a common target of integrin signaling, also rescued neuritogenesis in e/v neurons, indicating the changes in cytoskeletal and exocytic machinery may be triggered by a canonical integrin signaling pathway, leading to neurite initiation, or other morphological changes, depending upon the ECM composition and integrin repertoire present.
In the cortex, neurons are packed tightly and not in contact with significant amounts of LN or many other types of common ECM components that activate integrin signaling--likely the reason why neurons lacking Ena/VASP exhibit a cortex-specific neuritogenesis defect in vivo. In fact, the relevant substrate for neurons undergoing neuritogenesis in the cortex remains unknown. However, Ena/VASP-deficient neurons elsewhere in the brain and body contact substantial amounts of integrin ligands including the ectopic cortical mutant neurons that reach the pial membrane, dorsal root ganglia and retinal neurons, and form neurites in the absence of Ena/VASP. The context-dependent, mutually exclusive pathways may therefore be employed to regulate distinct cell behaviors in different environments in both physiological and pathological conditions.
As a result of a canonical integrin-signaling pathway, we found an unexpected switch in exocytic and cytoskeletal machinery. On LN, Arp2/3 is likely activated downstream of integrin, src, FAK, and Rac. We anticipate this alters the role of Arp2/3 in neuronal morphology seen previously (Korobova and Svitkina, 2008; Pinyol et al., 2007; Strasser et al., 2004). Src, FAK, Rack and Arp2/3 share several binding and activity partners, including paxillin, cortactin, p130Cas, p190RhoGAP, Nck and Grb2 (Brouns et al., 2001; Brown et al., 2005; Brunton et al., 2004; Rohatgi et al., 2001; Tehrani et al., 2007; Vuori and Ruoslahti, 1995), which could coordinate FAK and src activity with Rac and Arp2/3 activation (Rohatgi et al., 2001; Tehrani et al., 2007).
VAMP7-mediated exocytosis was stimulated by LN and dampened by src, FAK, or Arp2/3 inhibition. This may involve interaction between Arp2/3 and Exo70 (Liu et al., 2009; Zuo et al., 2006). Exo70 has been found to activate or coactivate Arp2/3 mediated actin polymerization (Liu et al., 2009), is involved in SNARE complex assembly and stabilization (Wiederkehr et al., 2004), and is a component of the exocyst complex, which is integral in vesicle trafficking during neuronal morphogenesis (Murthy et al., 2003). Exo70 overexpression rescued neuritogenesis in e/v neurons on PDL, similar to VAMP7 overexpression, and Exo70-mediated neuritogenesis was dependent upon VAMP7 activity. VAMP7 overexpression also rescued neuritogenesis in e/v neurons on LN following FAK, src, Rac, or Arp2/3 inhibition; inhibition of these same molecules decreased the frequency of VAMP7 exocytic events. We propose that VAMP7 is downstream of these molecules in LN-dependent neuritogenesis pathway.
While the entire signaling pathway is not yet elucidated, the small GTPase Ral is an intriguing candidate molecule that may connect Arp2/3, the exocyst complex, and VAMP7 to integrin signaling. Exocyst-driven filopodia formation occurs downstream of RalA activity, which binds to Sec5 of the exocyst complex (Sugihara et al., 2002). Ral is activated by a number of molecules including PI3K, which we find does not function in LN-dependent neuritogenesis, PDK1 (Tian et al., 2002), and BCAR3, which forms a complex with Cas, Src, and FAK (Gotoh et al., 2000), some of which we have implicated in neuritogenesis. Ral is also regulated by direct phosphorylation by an unknown kinase whose activity is negatively regulated by the tumor suppressor phosphatase PP2A (Sablina et al., 2007). PP2A associates with β1 integrin and is involved in bidirectional integrin regulation. Future work will be needed to understand the entire regulatory pathways that links ECM to neuritogenesis. Such information may make it possible to manipulate the pathway to drive neuritogenesis in a spatiotemporally controlled manner and therefore be of value in designing therapies to rebuild a cortex damaged by injury or disease.
Methods
Cortical neuron culture and transfection
All mouse procedures were approved by the MIT Committee on Animal Care. Cortical neuron cultures were prepared from embryonic day (E) 14.5 mice, as previously described (Dent et al., 2007; Kwiatkowski et al., 2007). Standard methods were used for western blotting, fixation and immunocytochemistry (Dent et al., 2007). Additional details are available in supplemental material.
Microscopy
Immunofluoresence images were acquired on an ORCA-ER CCD camera (Hamamatsu) using a 60× 1.4NA Plan Apo (DIC–fluor) Nikon objective on an inverted TE300 microscope (Nikon) equipped with dual Ludl filter wheels for excitation and emission. Timelapse images of neurons were acquired on an inverted Nikon TE2000U microscope modified to allow for through-the-objective multispectral TIR-FM using a 100× 1.45NA objective. The wavelength and intensity of the 2W multi-line laser (Coherent) were controlled with an AOTF. Laser light was focused at the aperture plane and directed to the coverslip by a dichromatic mirror (Chroma), and the laser angle was adjusted manually with a micrometer. Since neurons were imaged only 6 hours after plating and were not tightly adhered to the coverslips, a thick TIRF illumination field of approximately 200 nm was used. Fluorescence emission was controlled with a filter wheel devise containing narrow bandpass emission filters (Sutter Instruments, Novato, CA). Images were acquired on a CoolSnap HQ2 CCD camera (Photometries, Tucson, AZ). Neurons were imaged in Neurobasal media supplemented with B27 and kept at 37°C, 5% CO2 in an incubation chamber (Solent) fitted for the microscope. Vesicle dynamics were imaged at 3s intervals for 5 minutes. Exocytic fusion events were imaged at 0.8s intervals for 5-8 minutes.
Image Analysis and Statistics
All images were collected, measured, and compiled with Metamorph imaging software (Molecular Devices). Morphological stage was quantified after 48 hours in culture; neurites were considered any narrow and consolidated extension proximal to cell body. Stage 1 neurons lack neurites, stage 2 have one or more minor neurites, and stage 3 have one neurite at least twice as long as any other. Vesicle dynamics were tracked using the “Track Points” function in Metamorph. Vesicle fusion events were specified by the appearance of a vesicle, a bright increase in fluorescence intensity (> 3 fold over background), followed by a rapid diffusion (< 2s). All statistical tests were performed with AnalyzelT software (Leeds, UK). ANOVA with a Tukey post-hoc test were used to determine significance, unless otherwise noted in cases of a standard t-test.
Acknowledgments
We thank R. Hynes, L. Machesky, J. Parsons, T. Galli, P. De Camilli, and W. Guo for generously sharing reagents. We thank: R. Hynes (MIT), B. Eickholt (Kings College) and D. Yarar (Whitehead Institute) for thoughtful conversation. We also thank J. Tadros for assistance with molecular cloning. We appreciate advice, comments and discussion from Gertler lab members. S.G. was supported by a Jane Coffin Childs fellowship. This work was supported by NIH grant GM68678 and funds from the Stanley Medical Research Institute (F.B.G).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahuja R, Pinyol R, Reichenbach N, Custer L, Klingensmith J, Kessels MM, Qualmann B. Cordon-bleu is an actin nucleation factor and controls neuronal morphology. Cell. 2007;131:337–350. doi: 10.1016/j.cell.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci. 2001;4:367–373. doi: 10.1038/86011. [DOI] [PubMed] [Google Scholar]
- Alberts P, Rudge R, Irinopoulou T, Danglot L, Gauthier-Rouviere C, Galli T. Cdc42 and actin control polarized expression of TI-VAMP vesicles to neuronal growth cones and their fusion with the plasma membrane. Mol Biol Cell. 2006;17:1194–1203. doi: 10.1091/mbc.E05-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applewhite DA, Barzik M, Kojima SI, Svitkina TM, Gertler FB, Borisy GG. Ena/VASP Proteins Have an Anti-Capping Independent Function in Filopodia Formation. Mol Biol Cell. 2007 doi: 10.1091/mbc.E06-11-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D, Thompson NL, Burghardt TP. Total internal inflection fluorescent microscopy. J Microsc. 1983;129(Pt 1):19–28. doi: 10.1111/j.1365-2818.1983.tb04158.x. [DOI] [PubMed] [Google Scholar]
- Bachmann C, Fischer L, Walter U, Reinhard M. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J Biol Chem. 1999;274:23549–23557. doi: 10.1074/jbc.274.33.23549. [DOI] [PubMed] [Google Scholar]
- Barzik M, Kotova TI, Higgs HN, Hazelwood L, Hanein D, Gertler FB, Schafer DA. Ena/VASP proteins enhance actin polymerization in the presence of barbed end capping proteins. J Biol Chem. 2005;280:28653–28662. doi: 10.1074/jbc.M503957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JE, Loureiro JJ, Libova I, Fassler R, Wehland J, Gertler FB. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell. 2000;101:717–728. doi: 10.1016/s0092-8674(00)80884-3. [DOI] [PubMed] [Google Scholar]
- Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, et al. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–521. doi: 10.1016/s0092-8674(02)00731-6. [DOI] [PubMed] [Google Scholar]
- Becker KA, Hart NH. Reorganization of filamentous actin and myosin-II in zebrafish eggs correlates temporally and spatially with cortical granule exocytosis. J Cell Sci. 1999;112(Pt 1):97–110. doi: 10.1242/jcs.112.1.97. [DOI] [PubMed] [Google Scholar]
- Breitsprecher D, Kiesewetter AK, Linkner J, Urbanke C, Resch GP, Small JV, Faix J. Clustering of VASP actively drives processive, WH2 domain-mediated actin filament elongation. EMBO J. 2008;27:2943–2954. doi: 10.1038/emboj.2008.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouns MR, Matheson SF, Settleman J. pl90 RhoGAP is the principal Src substrate in brain and regulates axon outgrowth, guidance and fasciculation. Nat Cell Biol. 2001;3:361–367. doi: 10.1038/35070042. [DOI] [PubMed] [Google Scholar]
- Brown MC, Cary LA, Jamieson JS, Cooper JA, Turner CE. Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness. Mol Biol Cell. 2005;16:4316–4328. doi: 10.1091/mbc.E05-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton VG, MacPherson IR, Frame MC. Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochim Biophys Acta. 2004;1692:121–144. doi: 10.1016/j.bbamcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Buck CA, Horwitz AF. Integrin, a transmembrane glycoprotein complex mediating cell-substratum adhesion. J Cell Sci Suppl. 1987;8:231–250. doi: 10.1242/jcs.1987.supplement_8.13. [DOI] [PubMed] [Google Scholar]
- Chen TJ, Gehler S, Shaw AE, Bamburg JR, Letourneau PC. Cdc42 participates in the regulation of ADF/cofilin and retinal growth cone filopodia by brain derived neurotrophic factor. J Neurobiol. 2006;66:103–114. doi: 10.1002/neu.20204. [DOI] [PubMed] [Google Scholar]
- Copeland JW, Copeland SJ, Treisman R. Homo-oligomerization is essential for F-actin assembly by the formin family FH2 domain. J Biol Chem. 2004;279:50250–50256. doi: 10.1074/jbc.M404429200. [DOI] [PubMed] [Google Scholar]
- Dehmelt L, Smart FM, Ozer RS, Halpain S. The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation. J Neurosci. 2003;23:9479–9490. doi: 10.1523/JNEUROSCI.23-29-09479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Kalil K. Axon branching requires interactions between dynamic microtubules and actin filaments. J Neurosci. 2001;21:9757–9769. doi: 10.1523/JNEUROSCI.21-24-09757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Kwiatkowski AV, Mebane LM, Philippar U, Barzik M, Rubinson DA, Gupton S, Van Veen JE, Furman C, Zhang J, et al. Filopodia are required for cortical neurite initiation. Nat Cell Biol. 2007;9:1347–1359. doi: 10.1038/ncb1654. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EauClaire S, Guo W. Conservation and specialization. The role of the exocyst in neuronal exocytosis. Neuron. 2003;37:369–370. doi: 10.1016/s0896-6273(03)00059-x. [DOI] [PubMed] [Google Scholar]
- Flanagan LA, Rebaza LM, Derzic S, Schwartz PH, Monuki ES. Regulation of human neural precursor cells by laminin and integrins. J Neurosci Res. 2006;83:845–856. doi: 10.1002/jnr.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman C, Sieminski AL, Kwiatkowski AV, Rubinson DA, Vasile E, Bronson RT, Fassler R, Gertler FB. Ena/VASP is required for endothelial barrier function in vivo. J Cell Biol. 2007;179:761–775. doi: 10.1083/jcb.200705002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman AH, Banker GA. The economics of neurite outgrowth--the addition of new membrane to growing axons. Trends Neurosci. 1996;19:144–149. doi: 10.1016/s0166-2236(96)80025-7. [DOI] [PubMed] [Google Scholar]
- Galli T, Zahraoui A, Vaidyanathan VV, Raposo G, Tian JM, Karin M, Niemann H, Louvard D. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol Biol Cell. 1998;9:1437–1448. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G, Yee HF, Jr, Letourneau PC. Actin turnover is required to prevent axon retraction driven by endogenous actomyosin contractility. J Cell Biol. 2002;158:1219–1228. doi: 10.1083/jcb.200204140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates J, Mahaffey JP, Rogers SL, Emerson M, Rogers EM, Sottile SL, Van Vactor D, Gertler FB, Peifer M. Enabled plays key roles in embryonic epithelial morphogenesis in Drosophila. Development. 2007;134:2027–2039. doi: 10.1242/dev.02849. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Gorecki DC, Derry JM, Barnard EA. Dystroglycan: brain localisation and chromosome mapping in the mouse. Hum Mol Genet. 1994;3:1589–1597. doi: 10.1093/hmg/3.9.1589. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Cai D, Tian X, Feig LA, Lerner A. pl30Cas regulates the activity of AND-34, a novel Ral, Rap1, and R-Ras guanine nucleotide exchange factor. J Biol Chem. 2000;275:30118–30123. doi: 10.1074/jbc.M003074200. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Sci STKE. 2007;2007:re5. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- Han J, Han L, Tiwari P, Wen Z, Zheng JQ. Spatial targeting of type II protein kinase A to filopodia mediates the regulation of growth cone guidance by cAMP. J Cell Biol. 2007;176:101–111. doi: 10.1083/jcb.200607128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ES, Rouiller I, Hanein D, Higgs HN. Mechanistic Differences in Actin Bundling Activity of Two Mammalian Formins, FRL1 and mDia2. J Biol Chem. 2006;281:14383–14392. doi: 10.1074/jbc.M510923200. [DOI] [PubMed] [Google Scholar]
- Hong W. SNAREs and traffic. Biochim Biophys Acta. 2005;1744:493–517. [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ivins JK, Colognato H, Kreidberg JA, Yurchenco PD, Lander AD. Neuronal receptors mediating responses to antibodyactivated laminin-1. J Neurosci. 1998;18:9703–9715. doi: 10.1523/JNEUROSCI.18-23-09703.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, Svitkina T. Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol Biol Cell. 2008;19:1561–1574. doi: 10.1091/mbc.E07-09-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski AV, Rubinson DA, Dent EW, Edward van Veen J, Leslie JD, Zhang J, Mebane LM, Philippar U, Pinheiro EM, Burds AA, et al. Ena/VASP Is Required for neuritogenesis in the developing cortex. Neuron. 2007;56:441–455. doi: 10.1016/j.neuron.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Lanzetti L. Actin in membrane trafficking. Curr Opin Cell Biol. 2007;19:453–458. doi: 10.1016/j.ceb.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Dent EW, Strasser GA, Lanier LM, Krause M, Svitkina TM, Borisy GG, Gertler FB. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron. 2004;42:37–49. doi: 10.1016/s0896-6273(04)00108-4. [DOI] [PubMed] [Google Scholar]
- Liu J, Yue P, Artym VV, Mueller SC, Guo W. The Role of the Exocyst in MMP Secretion and Actin Dynamics during Tumor Cell Invadopodia Formation. Mol Biol Cell. 2009 doi: 10.1091/mbc.E08-09-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malsam J, Kreye S, Sollner TH. Membrane fusion: SNAREs and regulation. Cell Mol Life Sci. 2008;65:2814–2832. doi: 10.1007/s00018-008-8352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arca S, Alberts P, Zahraoui A, Louvard D, Galli T. Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J Cell Biol. 2000;149:889–900. doi: 10.1083/jcb.149.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusek T, Gombos R, Szecsenyi A, Sanchez-Soriano N, Czibula A, Pataki C, Gedai A, Prokop A, Rasko I, Mihaly J. Formin proteins of the DAAM subfamily play a role during axon growth. J Neurosci. 2008;28:13310–13319. doi: 10.1523/JNEUROSCI.2727-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May RC, Caron E, Hall A, Machesky LM. Involvement of the Arp2/3 complex in phagocytosis mediated by FcgammaR or CR3. Nat Cell Biol. 2000;2:246–248. doi: 10.1038/35008673. [DOI] [PubMed] [Google Scholar]
- May RC, Hall ME, Higgs HN, Pollard TD, Chakraborty T, Wehland J, Machesky LM, Sechi AS. The Arp2/3 complex is essential for the actin-based motility of Listeria monocytogenes. Curr Biol. 1999;9:759–762. doi: 10.1016/s0960-9822(99)80337-6. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Miyashita M, Ohnishi H, Okazawa H, Tomonaga H, Hayashi A, Fujimoto TT, Furuya N, Matozaki T. Promotion of neurite and filopodium formation by CD47: roles of integrins, Rac, and Cdc42. Mol Biol Cell. 2004;15:3950–3963. doi: 10.1091/mbc.E04-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M, Garza D, Scheller RH, Schwarz TL. Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron. 2003;37:433–447. doi: 10.1016/s0896-6273(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Ohnishi H, Yamamori S, Ono K, Aoyagi K, Kondo S, Takahashi M. A src family tyrosine kinase inhibits neurotransmitter release from neuronal cells. Proc Natl Acad Sci U S A. 2001;98:10930–10935. doi: 10.1073/pnas.191368198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osen-Sand A, Staple JK, Naldi E, Schiavo G, Rossetto O, Petitpierre S, Malgaroli A, Montecucco C, Catsicas S. Common and distinct fusion proteins in axonal growth and transmitter release. J Comp Neurol. 1996;367:222–234. doi: 10.1002/(SICI)1096-9861(19960401)367:2<222::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Pfenninger KH. Plasma membrane expansion: a neuron's Herculean task. Nat Rev Neurosci. 2009;10:251–261. doi: 10.1038/nrn2593. [DOI] [PubMed] [Google Scholar]
- Pinyol R, Haeckel A, Ritter A, Qualmann B, Kessels M. Regulation of N-WASP and the Arp2/3 complex by Abp1 controls neuronal morphology. PloS one. 2007;2:e400. doi: 10.1371/journal.pone.0000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantman S, Patarroyo M, Fried K, Domogatskaya A, Tryggvason K, Hammarberg H, Cullheim S. Integrin-laminin interactions controlling neurite outgrowth from adult DRG neurons in vitro. Mol Cell Neurosci. 2008;39:50–62. doi: 10.1016/j.mcn.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Pommereit D, Wouters FS. An NGF-induced Exo70-TC10 complex locally antagonises Cdc42-mediated activation of N-WASP to modulate neurite outgrowth. J Cell Sci. 2007;120:2694–2705. doi: 10.1242/jcs.03475. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Nollau P, Ho HY, Kirschner MW, Mayer BJ. Nck and phosphatidylinositol 4,5-bisphosphate synergistically activate actin polymerization through the N-WASP-Arp2/3 pathway. J Biol Chem. 2001;276:26448–26452. doi: 10.1074/jbc.M103856200. [DOI] [PubMed] [Google Scholar]
- Sablina AA, Chen W, Arroyo JD, Corral L, Hector M, Bulmer SE, DeCaprio JA, Hahn WC. The tumor suppressor PP2A Abeta regulates the RalA GTPase. Cell. 2007;129:969–982. doi: 10.1016/j.cell.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AW, Schoonderwoert VT, Ji L, Mederios N, Danuser G, Forscher P. Coordination of actin filament and microtubule dynamics during neurite outgrowth. Dev Cell. 2008;15:146–162. doi: 10.1016/j.devcel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Parsons JT. Autonomous expression of a noncatalytic domain of the focal adhesion-associated protein tyrosine kinase ppl25FAK. Mol Cell Biol. 1993;13:785–791. doi: 10.1128/mcb.13.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirenbeck A, Arasada R, Bretschneider T, Stradal TE, Schleicher M, Faix J. The bundling activity of vasodilator-stimulated phosphoprotein is required for filopodium formation. Proc Natl Acad Sci U S A. 2006;103:7694–7699. doi: 10.1073/pnas.0511243103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Schroer TA. Motors for fast axonal transport. Curr Opin Neurobiol. 1992;2:618–621. doi: 10.1016/0959-4388(92)90028-j. [DOI] [PubMed] [Google Scholar]
- Shen Y, Mani S, Donovan SL, Schwob JE, Meiri KF. Growth-associated protein-43 is required for commissural axon guidance in the developing vertebrate nervous system. J Neurosci. 2002;22:239–247. doi: 10.1523/JNEUROSCI.22-01-00239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorra S, Henke T, Galli T, Binz T. Substrate recognition mechanism of VAMP/synaptobrevin-cleaving clostridial neurotoxins. J Biol Chem. 2008;283:21145–21152. doi: 10.1074/jbc.M800610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokac AM, Bement WM. Kiss-and-coat and compartment mixing: coupling exocytosis to signal generation and local actin assembly. Mol Biol Cell. 2006;17:1495–1502. doi: 10.1091/mbc.E05-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser GA, Rahim NA, VanderWaal KE, Gertler FB, Lanier LM. Arp2/3 is a negative regulator of growth cone translocation. Neuron. 2004;43:81–94. doi: 10.1016/j.neuron.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Sugihara K, Asano S, Tanaka K, Iwamatsu A, Okawa K, Ohta Y. The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat Cell Biol. 2002;4:73–78. doi: 10.1038/ncb720. [DOI] [PubMed] [Google Scholar]
- Tang BL. Protein trafficking mechanisms associated with neurite outgrowth and polarized sorting in neurons. J Neurochem. 2001;79:923–930. doi: 10.1046/j.1471-4159.2001.00674.x. [DOI] [PubMed] [Google Scholar]
- Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proc Natl Acad Sci U S A. 2007;104:11933–11938. doi: 10.1073/pnas.0701077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- Tian X, Rusanescu G, Hou W, Schaffhausen B, Feig LA. PDK1 mediates growth factor-induced Ral-GEF activation by a kinase-independent mechanism. EMBO J. 2002;21:1327–1338. doi: 10.1093/emboj/21.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojima T, Akiyama H, Itofusa R, Li Y, Katayama H, Miyawaki A, Kamiguchi H. Attractive axon guidance involves asymmetric membrane transport and exocytosis in the growth cone. Nat Neurosci. 2007;10:58–66. doi: 10.1038/nn1814. [DOI] [PubMed] [Google Scholar]
- Tsaneva-Atanasova K, Burgo A, Galli T, Holcman D. Quantifying neurite growth mediated by interactions among secretory vesicles, microtubules, and actin networks. Biophys J. 2009;96:840–857. doi: 10.1016/j.bpj.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn JA, Valentijn K, Pastore LM, Jamieson JD. Actin coating of secretory granules during regulated exocytosis correlates with the release of rab3D. Proc Natl Acad Sci U S A. 2000;97:1091–1095. doi: 10.1073/pnas.97.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega IE, Hsu SC. The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J Neurosci. 2001;21:3839–3848. doi: 10.1523/JNEUROSCI.21-11-03839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C, Coco S, Bacci A, Rossetto O, De Camilli P, Montecucco C, Matteoli M. Tetanus toxin blocks the exocytosis of synaptic vesicles clustered at synapses but not of synaptic vesicles in isolated axons. J Neurosci. 1999;19:6723–6732. doi: 10.1523/JNEUROSCI.19-16-06723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuori K, Ruoslahti E. Tyrosine phosphorylation of pl30Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J Biol Chem. 1995;270:22259–22262. doi: 10.1074/jbc.270.38.22259. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang BL. SNAREs in neurons-beyond synaptic vesicle exocytosis (Review) Mol Membr Biol. 2006;23:377–384. doi: 10.1080/09687860600776734. [DOI] [PubMed] [Google Scholar]
- Wiederkehr A, De Craene JO, Ferro-Novick S, Novick P. Functional specialization within a vesicle tethering complex: bypass of a subset of exocyst deletion mutants by Seclp or Sec4p. J Cell Bioli. 2004;67:875–887. doi: 10.1083/jcb.200408001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withee J, Galligan B, Hawkins N, Garriga G. Caenorhabditis elegans WASP and Ena/VASP proteins play compensatory roles in morphogenesis and neuronal cell migration. Genetics. 2004;167:1165–1176. doi: 10.1534/genetics.103.025676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AM, Lyuksyutova AI, Fenstermaker AG, Shafer B, Lo CG, Zou Y. Phosphatidylinositol-3-kinase-atypical protein kinase C signaling is required for Wnt attraction and anterior-posterior axon guidance. J Neurosci. 2008;28:3456–3467. doi: 10.1523/JNEUROSCI.0029-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon MS, Yon C, Park SY, Oh DY, Han AH, Kim DS, Han JS. Role of phospholipase Dl in neurite outgrowth of neural stem cells. Biochem Biophys Res Commun. 2005;329:804–811. doi: 10.1016/j.bbrc.2005.02.087. [DOI] [PubMed] [Google Scholar]
- Zuo X, Zhang J, Zhang Y, Hsu SC, Zhou D, Guo W. Exo70 interacts with the Arp2/3 complex and regulates cell migration. Nat Cell Biol. 2006;8:1383–1388. doi: 10.1038/ncb1505. [DOI] [PubMed] [Google Scholar]


