Abstract
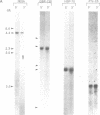
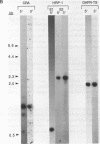
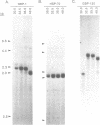
A novel set of reaction conditions for mung bean nuclease has been described in which Plasmodium genes were specifically excised as intact fragments from purified DNA. We have now determined that under the new conditions mung bean nuclease cleaves precisely at sites outside of the coding region of every P. falciparum gene for which the extent of the protein coding region in genomic DNA is known. We conclude that this enzyme activity is probably a general one for P. falciparum genes. Introns are not specifically cleaved, although one gene contained a cleavage site within an intron. There is no direct relationship between dA.dT-richness and sites of cleavage under these conditions. Also contrary to the expectations of a model based on cleavage at denaturation bubbles, there was no general relationship between the concentration of the DNA denaturant, formamide, and the size of the resulting gene-containing fragments. Thus, the data strongly suggest the involvement of an altered DNA structure near gene boundaries in determining the recognition sites for this enzyme activity.
Full text
PDF






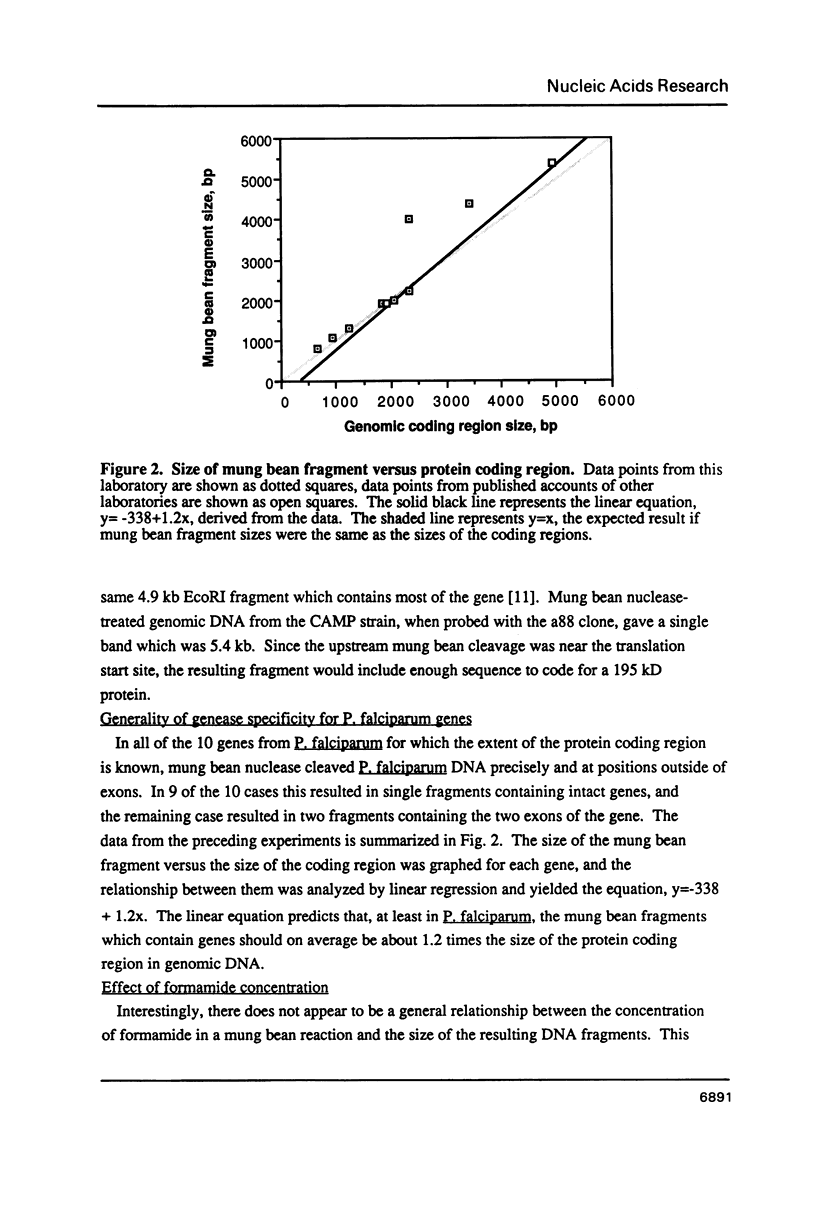
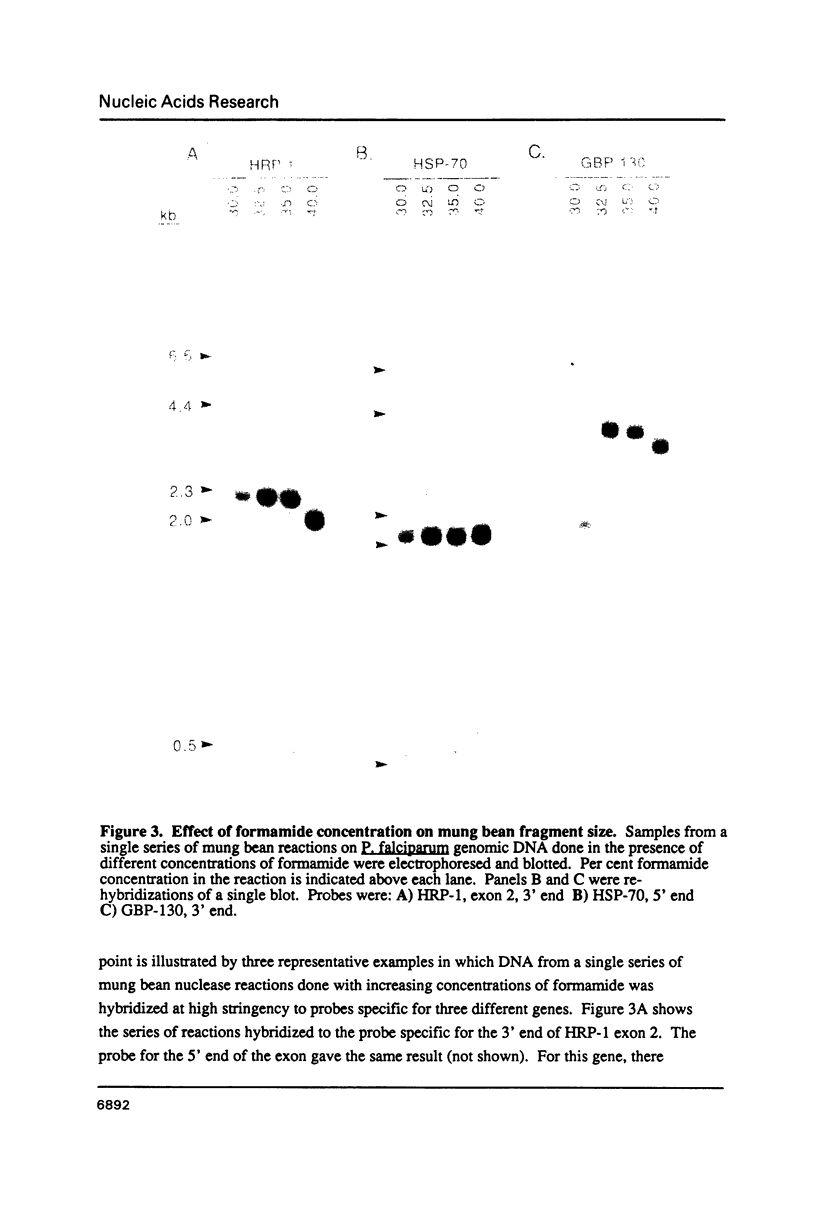




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam R. D., Aggarwal A., Lal A. A., de La Cruz V. F., McCutchan T., Nash T. E. Antigenic variation of a cysteine-rich protein in Giardia lamblia. J Exp Med. 1988 Jan 1;167(1):109–118. doi: 10.1084/jem.167.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman Y., Rice D., Grosschedl R., Baltimore D. Two regulatory elements for immunoglobulin kappa light chain gene expression. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7041–7045. doi: 10.1073/pnas.81.22.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A. E., Favaloro J. M., Burkot T. R., Culvenor J. G., Crewther P. E., Brown G. V., Anders R. F., Coppel R. L., Kemp D. J. A repetitive antigen of Plasmodium falciparum that is homologous to heat shock protein 70 of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8713–8717. doi: 10.1073/pnas.83.22.8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. H., Brentano S. T., Donelson J. E. Mung bean nuclease cleaves preferentially at the boundaries of variant surface glycoprotein gene transpositions in trypanosome DNA. J Biol Chem. 1986 Aug 5;261(22):10352–10358. [PubMed] [Google Scholar]
- Bzik D. J., Li W. B., Horii T., Inselburg J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8360–8364. doi: 10.1073/pnas.84.23.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986 Apr 11;45(1):3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- Dame J. B., McCutchan T. F. Cloning and characterization of a ribosomal RNA gene from Plasmodium berghei. Mol Biochem Parasitol. 1983 Jul;8(3):263–279. doi: 10.1016/0166-6851(83)90048-8. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Favaloro J. M., Coppel R. L., Corcoran L. M., Foote S. J., Brown G. V., Anders R. F., Kemp D. J. Structure of the RESA gene of Plasmodium falciparum. Nucleic Acids Res. 1986 Nov 11;14(21):8265–8277. doi: 10.1093/nar/14.21.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Lockyer M. J., Odink K. G., Sandhu J. S., Riveros-Moreno V., Nicholls S. C., Hillman Y., Davey L. S., Tizard M. L., Schwarz R. T. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature. 1985 Sep 19;317(6034):270–273. doi: 10.1038/317270a0. [DOI] [PubMed] [Google Scholar]
- Johnson A. M., Illana S., Dubey J. P., Dame J. B. Toxoplasma gondii and Hammondia hammondi: DNA comparison using cloned rRNA gene probes. Exp Parasitol. 1987 Jun;63(3):272–278. doi: 10.1016/0014-4894(87)90173-1. [DOI] [PubMed] [Google Scholar]
- Kochan J., Perkins M., Ravetch J. V. A tandemly repeated sequence determines the binding domain for an erythrocyte receptor binding protein of P. falciparum. Cell. 1986 Mar 14;44(5):689–696. doi: 10.1016/0092-8674(86)90834-2. [DOI] [PubMed] [Google Scholar]
- Kohwi-Shigematsu T., Kohwi Y. Poly(dG)-poly(dC) sequences, under torsional stress, induce an altered DNA conformation upon neighboring DNA sequences. Cell. 1985 Nov;43(1):199–206. doi: 10.1016/0092-8674(85)90024-8. [DOI] [PubMed] [Google Scholar]
- Kohwi-Shigematsu T., Manes T., Kohwi Y. Unusual conformational effect exerted by Z-DNA upon its neighboring sequences. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2223–2227. doi: 10.1073/pnas.84.8.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski D. Changes in site specificity of single-strand-specific endonucleases on supercoiled PM2 DNA with temperature and ionic environment. Nucleic Acids Res. 1984 Sep 25;12(18):7071–7086. doi: 10.1093/nar/12.18.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski D., Kroeker W. D., Laskowski M., Sr Mung bean nuclease I. Physical, chemical, and catalytic properties. Biochemistry. 1976 Oct 5;15(20):4457–4463. doi: 10.1021/bi00665a019. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Hansen J. L., Dame J. B., Mullins J. A. Mung bean nuclease cleaves Plasmodium genomic DNA at sites before and after genes. Science. 1984 Aug 10;225(4662):625–628. doi: 10.1126/science.6330899. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., de la Cruz V. F., Lal A. A., Gunderson J. H., Elwood H. J., Sogin M. L. Primary sequences of two small subunit ribosomal RNA genes from Plasmodium falciparum. Mol Biochem Parasitol. 1988 Feb;28(1):63–68. doi: 10.1016/0166-6851(88)90181-8. [DOI] [PubMed] [Google Scholar]
- Muhich M. L., Simpson L. Specific cleavage of kinetoplast minicircle DNA from Leishmania tarentolae by mung bean nuclease and identification of several additional minicircle sequence classes. Nucleic Acids Res. 1986 Jul 11;14(13):5531–5556. doi: 10.1093/nar/14.13.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls S. C., Hillman Y., Lockyer M. J., Odink K. G., Holder A. A. An S antigen gene from Plasmodium falciparum contains a novel repetitive sequence. Mol Biochem Parasitol. 1988 Feb;28(1):11–19. doi: 10.1016/0166-6851(88)90174-0. [DOI] [PubMed] [Google Scholar]
- Sharma Y. D., Kilejian A. Structure of the knob protein (KP) gene of Plasmodium falciparum. Mol Biochem Parasitol. 1987 Nov;26(1-2):11–16. doi: 10.1016/0166-6851(87)90124-1. [DOI] [PubMed] [Google Scholar]
- Sheflin L. G., Kowalski D. Altered DNA conformations detected by mung bean nuclease occur in promoter and terminator regions of supercoiled pBR322 DNA. Nucleic Acids Res. 1985 Sep 11;13(17):6137–6154. doi: 10.1093/nar/13.17.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheflin L. G., Kowalski D. Mung bean nuclease cleavage of a dA + dT-rich sequence or an inverted repeat sequence in supercoiled PM2 DNA depends on ionic environment. Nucleic Acids Res. 1984 Sep 25;12(18):7087–7104. doi: 10.1093/nar/12.18.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D., Woollett G., Bergin-Cartwright M., Kay D., Scaife J. A malaria protein exported into a new compartment within the host erythrocyte. EMBO J. 1987 Feb;6(2):485–491. doi: 10.1002/j.1460-2075.1987.tb04779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walliker D., Quakyi I. A., Wellems T. E., McCutchan T. F., Szarfman A., London W. T., Corcoran L. M., Burkot T. R., Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987 Jun 26;236(4809):1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- Weber J. L., Leininger W. M., Lyon J. A. Variation in the gene encoding a major merozoite surface antigen of the human malaria parasite Plasmodium falciparum. Nucleic Acids Res. 1986 Apr 25;14(8):3311–3323. doi: 10.1093/nar/14.8.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems T. E., Howard R. J. Homologous genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6065–6069. doi: 10.1073/pnas.83.16.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]