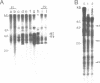Abstract
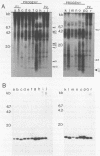
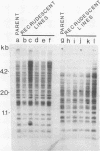
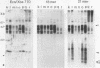
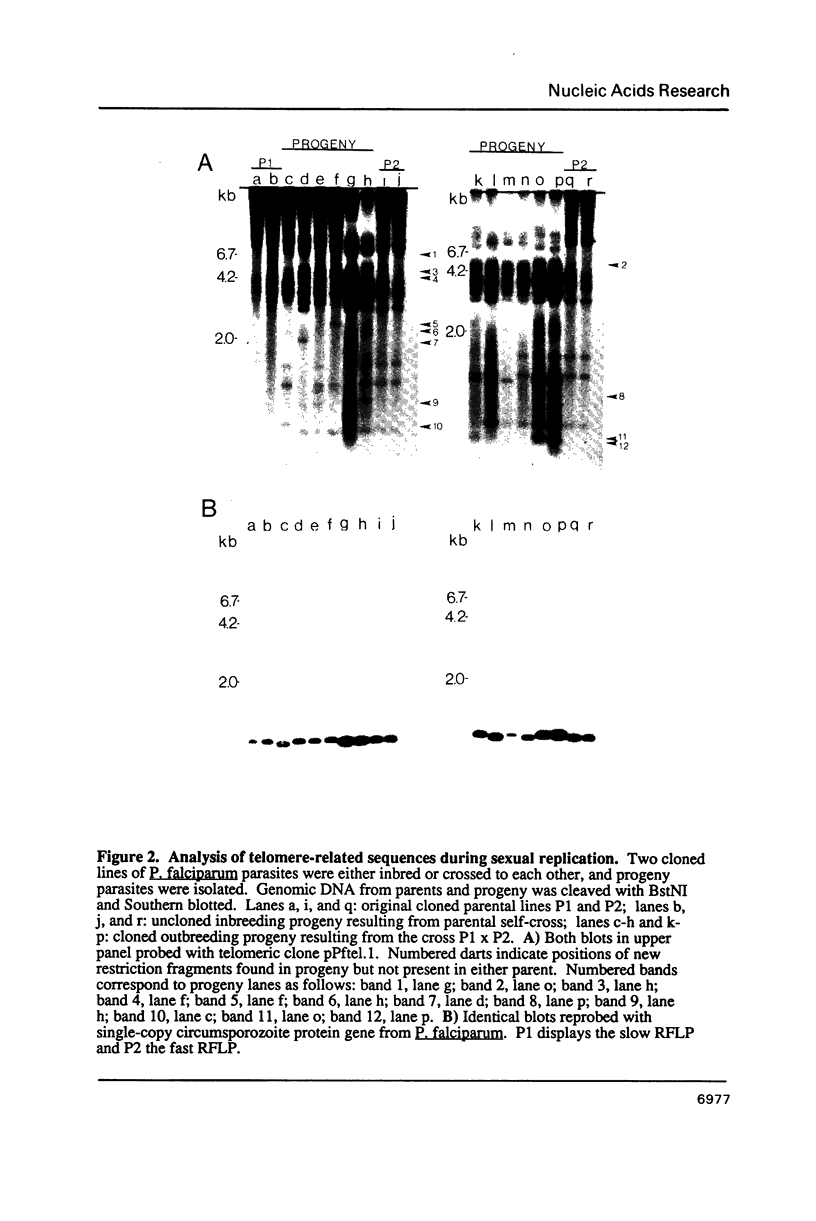
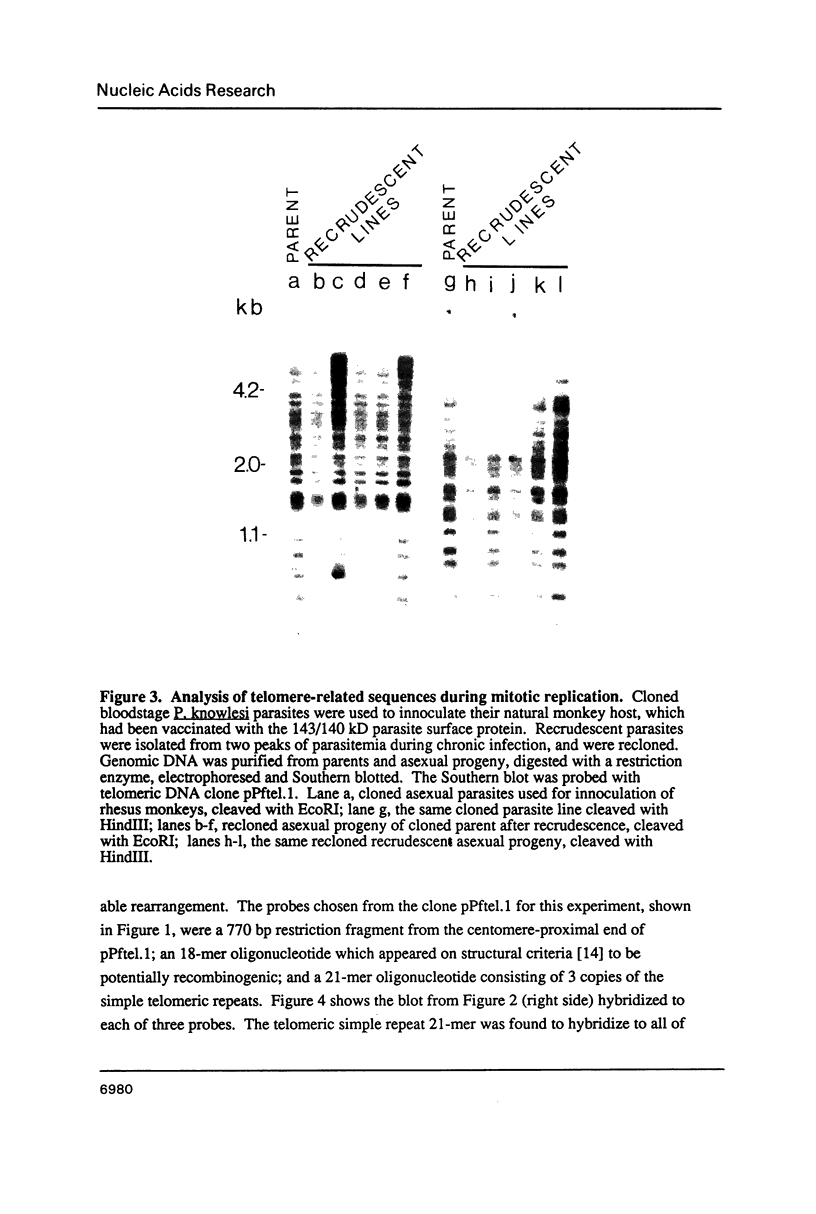
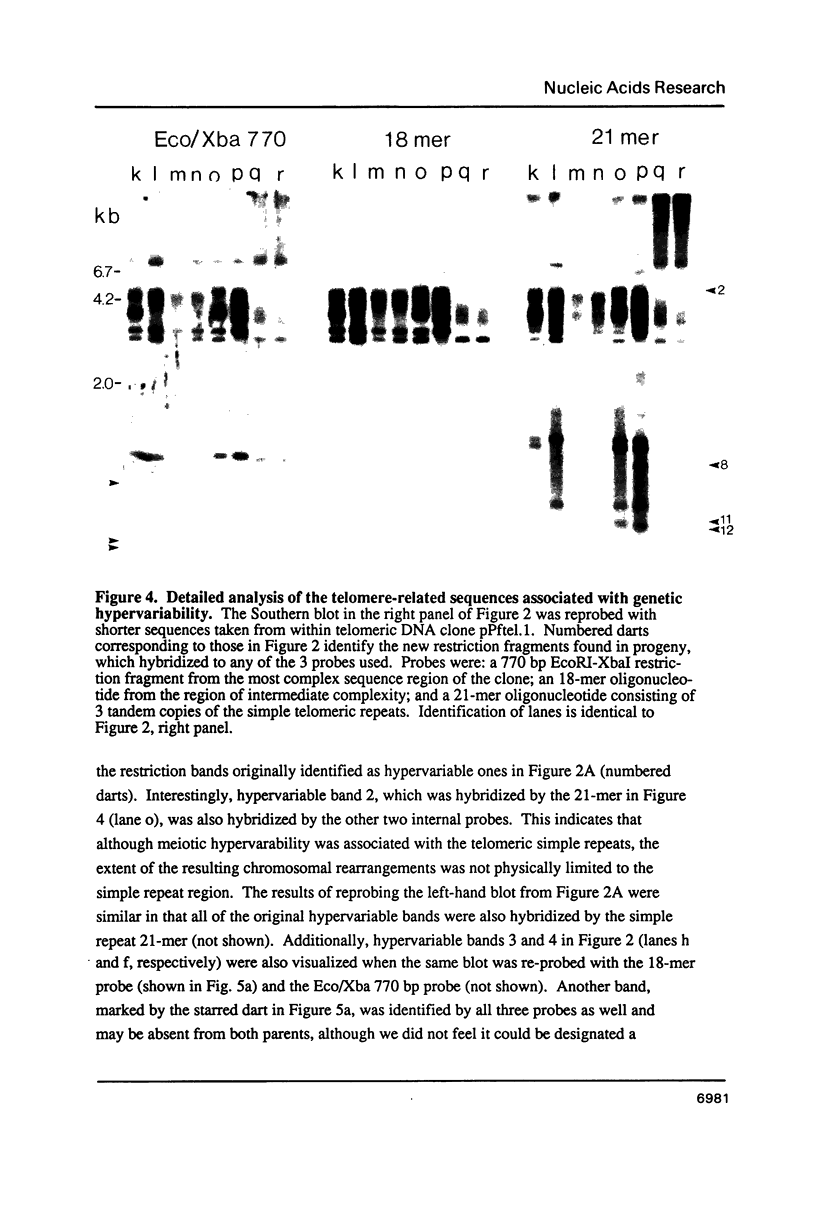
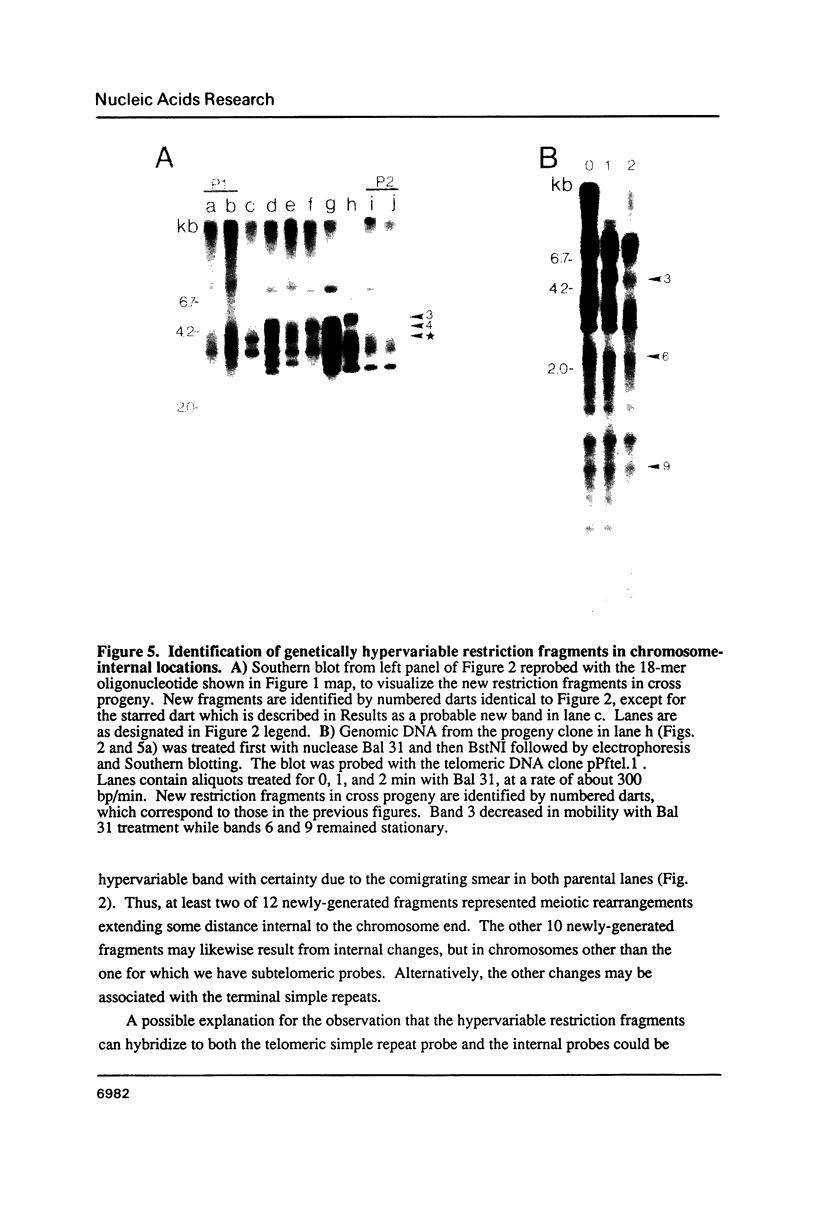
Sequences related to those near chromosome telomeres in the human malaria parasite, Plasmodium falciparum, were extremely unstable during a genetic cross between two different clonal genotypes. Many progeny of the heterologous cross displayed telomere-homologous restriction fragments found in neither parent. A significant number of the new fragments resulted from rearrangements at chromosome-internal locations which were bounded by more complex tracts of DNA sequence. The same instability was not seen to arise during an inbreeding cross, nor during mitotic replication of parasites. Thus, a form of genetic hypervariability results from molecular events which occur during meiotic reduction and is apparent only in a cross between heterologous strains of parasite. Since other sequences were entirely stable under the same conditions, it appears that chromosome-internal blocks of telomeric sequences in the P. falciparum genome may designate conditionally unstable chromosomal domains. We discuss some potential implications of these findings for the population biology of P. falciparum.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernardi G., Olofsson B., Filipski J., Zerial M., Salinas J., Cuny G., Meunier-Rotival M., Rodier F. The mosaic genome of warm-blooded vertebrates. Science. 1985 May 24;228(4702):953–958. doi: 10.1126/science.4001930. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Organization of the SUC gene family in Saccharomyces. Mol Cell Biol. 1983 Mar;3(3):351–359. doi: 10.1128/mcb.3.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran L. M., Forsyth K. P., Bianco A. E., Brown G. V., Kemp D. J. Chromosome size polymorphisms in Plasmodium falciparum can involve deletions and are frequent in natural parasite populations. Cell. 1986 Jan 17;44(1):87–95. doi: 10.1016/0092-8674(86)90487-3. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Saint R. B., Coppel R. L., Brown G. V., Anders R. F., Kemp D. J. Conserved sequences flank variable tandem repeats in two S-antigen genes of Plasmodium falciparum. Cell. 1985 Apr;40(4):775–783. doi: 10.1016/0092-8674(85)90337-x. [DOI] [PubMed] [Google Scholar]
- Dame J. B., McCutchan T. F. Cloning and characterization of a ribosomal RNA gene from Plasmodium berghei. Mol Biochem Parasitol. 1983 Jul;8(3):263–279. doi: 10.1016/0166-6851(83)90048-8. [DOI] [PubMed] [Google Scholar]
- Enea V., Galinski M., Schmidt E., Gwadz R., Nussenzweig R. S. Evolutionary profile of the circumsporozoite gene of the Plasmodium cynomolgi complex. J Mol Biol. 1986 Apr 20;188(4):721–726. doi: 10.1016/s0022-2836(86)80017-1. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Galinski M. R., Arnot D. E., Cochrane A. H., Barnwell J. W., Nussenzweig R. S., Enea V. The circumsporozoite gene of the Plasmodium cynomolgi complex. Cell. 1987 Jan 30;48(2):311–319. doi: 10.1016/0092-8674(87)90434-x. [DOI] [PubMed] [Google Scholar]
- Horowitz H., Thorburn P., Haber J. E. Rearrangements of highly polymorphic regions near telomeres of Saccharomyces cerevisiae. Mol Cell Biol. 1984 Nov;4(11):2509–2517. doi: 10.1128/mcb.4.11.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Thompson J. K., Walliker D., Corcoran L. M. Molecular karyotype of Plasmodium falciparum: conserved linkage groups and expendable histidine-rich protein genes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7672–7676. doi: 10.1073/pnas.84.21.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz F. W., Hudson D. E., Coon H. G., Miller L. H. Vaccination-induced variation in the 140 kD merozoite surface antigen of Plasmodium knowlesi malaria. J Exp Med. 1987 Feb 1;165(2):359–367. doi: 10.1084/jem.165.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Dame J. B., Gwadz R. W., Vernick K. D. The genome of Plasmodium cynomolgi is partitioned into separable domains which appear to differ in sequence stability. Nucleic Acids Res. 1988 May 25;16(10):4499–4510. doi: 10.1093/nar/16.10.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan T. F., Hansen J. L., Dame J. B., Mullins J. A. Mung bean nuclease cleaves Plasmodium genomic DNA at sites before and after genes. Science. 1984 Aug 10;225(4662):625–628. doi: 10.1126/science.6330899. [DOI] [PubMed] [Google Scholar]
- Michels C. A., Needleman R. B. The dispersed, repeated family of MAL loci in Saccharomyces spp. J Bacteriol. 1984 Mar;157(3):949–952. doi: 10.1128/jb.157.3.949-952.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pologe L. G., Ravetch J. V. A chromosomal rearrangement in a P. falciparum histidine-rich protein gene is associated with the knobless phenotype. 1986 Jul 31-Aug 6Nature. 322(6078):474–477. doi: 10.1038/322474a0. [DOI] [PubMed] [Google Scholar]
- Vernick K. D., McCutchan T. F. Sequence and structure of a Plasmodium falciparum telomere. Mol Biochem Parasitol. 1988 Mar;28(2):85–94. doi: 10.1016/0166-6851(88)90055-2. [DOI] [PubMed] [Google Scholar]
- Walliker D., Quakyi I. A., Wellems T. E., McCutchan T. F., Szarfman A., London W. T., Corcoran L. M., Burkot T. R., Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987 Jun 26;236(4809):1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- Wellems T. E., Walliker D., Smith C. L., do Rosario V. E., Maloy W. L., Howard R. J., Carter R., McCutchan T. F. A histidine-rich protein gene marks a linkage group favored strongly in a genetic cross of Plasmodium falciparum. Cell. 1987 Jun 5;49(5):633–642. doi: 10.1016/0092-8674(87)90539-3. [DOI] [PubMed] [Google Scholar]
- de la Cruz V. F., Lal A. A., Welsh J. A., McCutchan T. F. Evolution of the immunodominant domain of the circumsporozoite protein gene from Plasmodium vivax. Implications for vaccines. J Biol Chem. 1987 May 15;262(14):6464–6467. [PubMed] [Google Scholar]