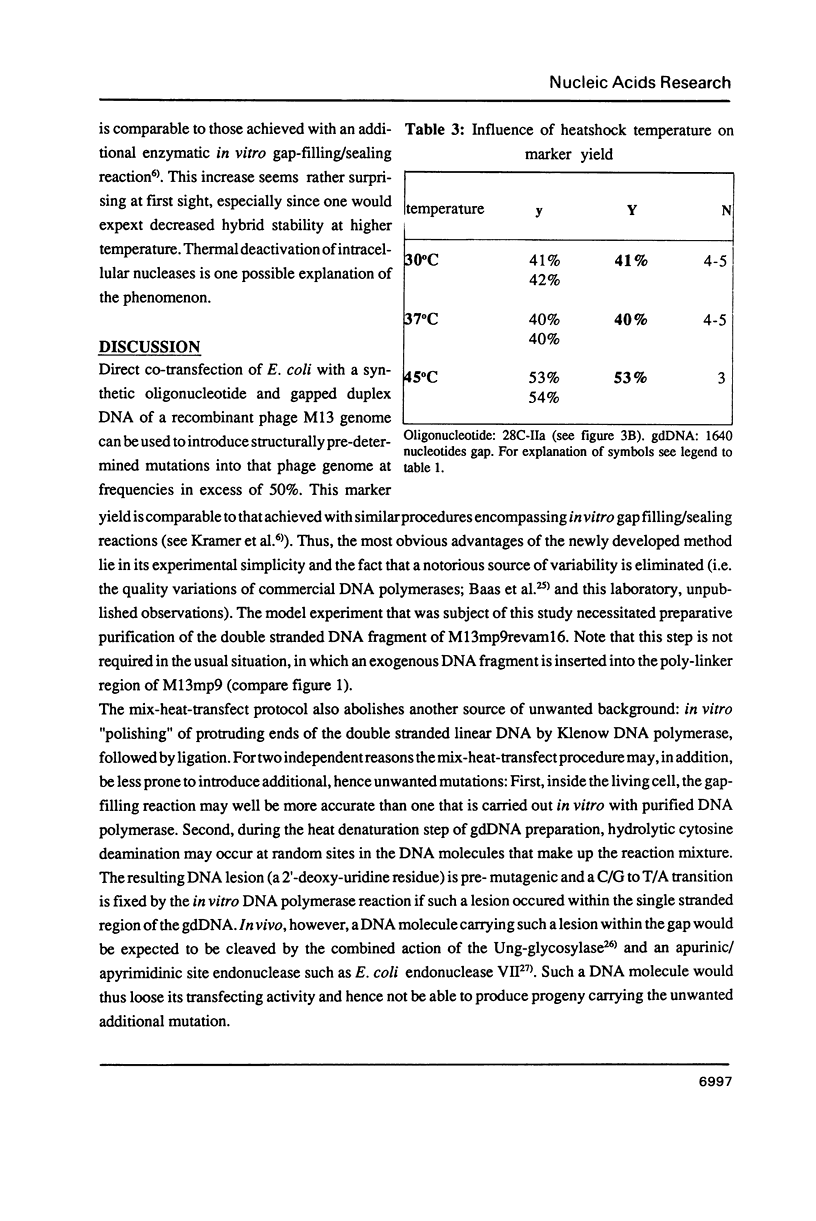
Abstract
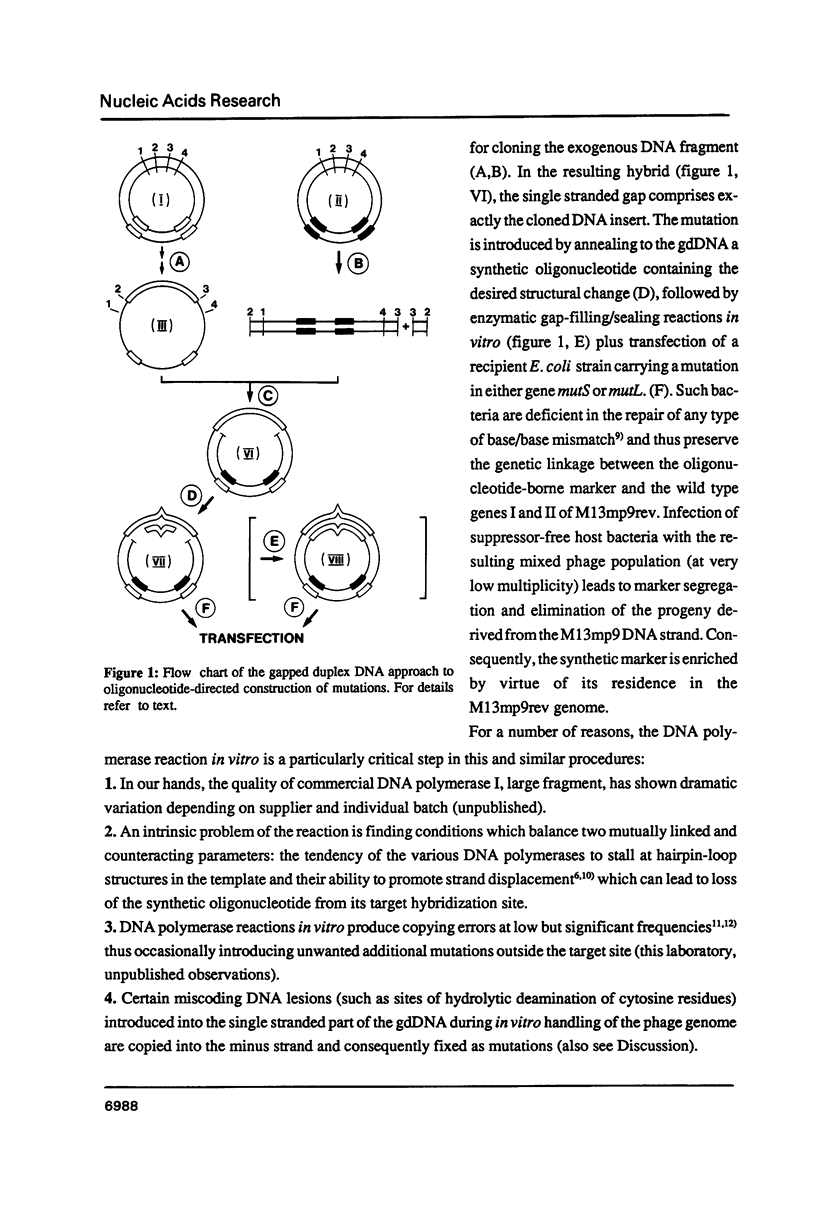
The gapped duplex DNA approach to oligonucleotide-directed construction of mutations (Kramer et al. 1984, Nucl. Acids Res. 12, 9441-9456) has been developed further. A procedure is described that makes in vitro DNA polymerase/DNA ligase reactions dispensable. Direct transfection of host bacteria with gdDNA molecules of recombinant phage M13 plus mutagenic oligonucleotide results in marker yields in excess of 50% (gap size 1640 nucleotides). An important feature incorporated into the mutagenic oligonucleotide is the presence of one or two internucleotidic phosphorothioate linkages immediately adjacent to the 5'-terminus. Automated preparation and biochemical properties of such compounds are described as well as their performance in oligonucleotide-directed mutagenesis. A systematic study of the following parameters influencing marker yield is reported: Gap size, length of oligonucleotide, chemical nature of oligonucleotide termini and heatshock temperature during transformation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas P. D., Teertstra W. R., van Mansfeld A. D., Jansz H. S., van der Marel G. A., Veeneman G. H., van Boom J. H. Construction of viable and lethal mutations in the origin of bacteriophage 'phi' X174 using synthetic oligodeoxyribonucleotides. J Mol Biol. 1981 Nov 15;152(4):615–639. doi: 10.1016/0022-2836(81)90120-0. [DOI] [PubMed] [Google Scholar]
- Bonura T., Schultz R., Friedberg E. C. An enzyme activity from Escherichia coli that attacks single-stranded deoxyribopolymers and single-stranded deoxyribonucleic acid containing apyrimidinic sites. Biochemistry. 1982 May 11;21(10):2548–2556. doi: 10.1021/bi00539a039. [DOI] [PubMed] [Google Scholar]
- Bryant F. R., Benkovic S. J. Stereochemical course of the reaction catalyzed by 5'-nucleotide phosphodiesterase from snake venom. Biochemistry. 1979 Jun 26;18(13):2825–2828. doi: 10.1021/bi00580a022. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Eckstein F., Hunneman D. H. Stereochemistry of hydrolysis by snake venom phosphodiesterase. J Biol Chem. 1979 Aug 25;254(16):7476–7478. [PubMed] [Google Scholar]
- Burgers P. M., Sathyanarayana B. K., Saenger W., Eckstein F. Crystal and molecular structure of adenosine 5'-O-phosphorothioate O-p-nitrophenyl ester (Sp diastereomer). Substrate stereospecificity of snake venom phosphodiesterase. Eur J Biochem. 1979 Oct 15;100(2):585–591. doi: 10.1111/j.1432-1033.1979.tb04205.x. [DOI] [PubMed] [Google Scholar]
- Burke D. T., Olson M. V. Oligodeoxynucleotide-directed mutagenesis of Escherichia coli and yeast by simple cotransformation of the primer and template. DNA. 1986 Aug;5(4):325–332. doi: 10.1089/dna.1986.5.325. [DOI] [PubMed] [Google Scholar]
- Carter P. Site-directed mutagenesis. Biochem J. 1986 Jul 1;237(1):1–7. doi: 10.1042/bj2370001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B. A., Potter B. V., Eckstein F., Pingoud A., Grotjahn L. Synthesis and characterization of an octanucleotide containing the EcoRI recognition sequence with a phosphorothioate group at the cleavage site. Biochemistry. 1984 Jul 17;23(15):3443–3453. doi: 10.1021/bi00310a010. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Fritz H. J., Belagaje R., Brown E. L., Fritz R. H., Jones R. A., Lees R. G., Khorana H. G. High-pressure liquid chromatography in polynucleotide synthesis. Biochemistry. 1978 Apr 4;17(7):1257–1267. doi: 10.1021/bi00600a020. [DOI] [PubMed] [Google Scholar]
- Kramer B., Kramer W., Fritz H. J. Different base/base mismatches are corrected with different efficiencies by the methyl-directed DNA mismatch-repair system of E. coli. Cell. 1984 Oct;38(3):879–887. doi: 10.1016/0092-8674(84)90283-6. [DOI] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Fritz H. J. Oligonucleotide-directed construction of mutations via gapped duplex DNA. Methods Enzymol. 1987;154:350–367. doi: 10.1016/0076-6879(87)54084-8. [DOI] [PubMed] [Google Scholar]
- Kramer W., Schughart K., Fritz H. J. Directed mutagenesis of DNA cloned in filamentous phage: influence of hemimethylated GATC sites on marker recovery from restriction fragments. Nucleic Acids Res. 1982 Oct 25;10(20):6475–6485. doi: 10.1093/nar/10.20.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A., Goodman M. F. On the fidelity of DNA replication. The accuracy of T4 DNA polymerases in copying phi X174 DNA in vitro. J Biol Chem. 1984 Feb 10;259(3):1539–1545. [PubMed] [Google Scholar]
- Leatherbarrow R. J., Fersht A. R. Protein engineering. Protein Eng. 1986 Oct-Nov;1(1):7–16. doi: 10.1093/protein/1.1.7. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Ljungquist S., Siegert W., Nyberg B., Sperens B. DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem. 1977 May 25;252(10):3286–3294. [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nossal N. G. DNA synthesis on a double-stranded DNA template by the T4 bacteriophage DNA polymerase and the T4 gene 32 DNA unwinding protein. J Biol Chem. 1974 Sep 10;249(17):5668–5676. [PubMed] [Google Scholar]
- Ott J., Eckstein F. Protection of oligonucleotide primers against degradation by DNA polymerase I. Biochemistry. 1987 Dec 15;26(25):8237–8241. doi: 10.1021/bi00399a032. [DOI] [PubMed] [Google Scholar]
- Potter B. V., Connolly B. A., Eckstein F. Synthesis and configurational analysis of a dinucleoside phosphate isotopically chiral at phosphorus. Stereochemical course of Penicillium citrum nuclease P1 reaction. Biochemistry. 1983 Mar 15;22(6):1369–1377. doi: 10.1021/bi00275a008. [DOI] [PubMed] [Google Scholar]
- Shi J. P., Fersht A. R. Fidelity of DNA replication under conditions used for oligodeoxynucleotide-directed mutagenesis. J Mol Biol. 1984 Aug 5;177(2):269–278. doi: 10.1016/0022-2836(84)90456-x. [DOI] [PubMed] [Google Scholar]
- Smith M. In vitro mutagenesis. Annu Rev Genet. 1985;19:423–462. doi: 10.1146/annurev.ge.19.120185.002231. [DOI] [PubMed] [Google Scholar]
- Stec W. J., Zon G., Uznanski B. Reversed-phase high-performance liquid chromatographic separation of diastereomeric phosphorothioate analogues of oligodeoxyribonucleotides and other backbone-modified congeners of DNA. J Chromatogr. 1985 Jun 19;326:263–280. doi: 10.1016/s0021-9673(01)87452-5. [DOI] [PubMed] [Google Scholar]



