Abstract
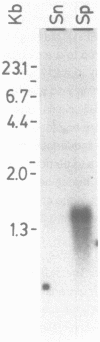
Because of the lack of de novo purine biosynthesis, hypoxanthine-guanine phosphoribosyltransferase (HGPRTase) is a critical enzyme in the purine metabolic pathway of the human parasite, Schistosoma mansoni. Using a cDNA clone encoding mouse HGPRTase and subsequently a synthetic oligonucleotide derived from sequencing a clone of genomic DNA, two clones were isolated from an adult schistosome cDNA library. One clone is 1.374 Kilobases (Kb) long and has an open reading frame of 693 bases. The deduced 231 amino acid sequence has 47.9% identity in a 217 amino acid overlap with human HGPRTase. Northern blot analysis indicates that the full length of mRNA for the S. mansoni HGPRTase is 1.45-1.6 Kb. Analysis of the primary structures of the putative active site for human and parasite enzymes reveal specific differences which may eventually be exploitable in the design of drugs for the treatment of schistosomiasis.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Hanei M., Wilson J. M., Kelley W. N. A possible nucleotide-binding domain in the tertiary fold of phosphoribosyltransferases. J Biol Chem. 1983 May 25;258(10):6450–6457. [PubMed] [Google Scholar]
- Barnes W. M., Bevan M., Son P. H. Kilo-sequencing: creation of an ordered nest of asymmetric deletions across a large target sequence carried on phage M13. Methods Enzymol. 1983;101:98–122. doi: 10.1016/0076-6879(83)01008-3. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Chang K. P. Phosphorylation and anti-leishmanial activity of formycin B. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1377–1383. doi: 10.1016/0006-291x(81)91976-8. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Dovey H. F., McKerrow J. H., Aldritt S. M., Wang C. C. Purification and characterization of hypoxanthine-guanine phosphoribosyltransferase from Schistosoma mansoni. A potential target for chemotherapy. J Biol Chem. 1986 Jan 15;261(2):944–948. [PubMed] [Google Scholar]
- Dovey H. F., McKerrow J. H., Wang C. C. Purine salvage in Schistosoma mansoni schistosomules. Mol Biochem Parasitol. 1984 Apr;11:157–167. doi: 10.1016/0166-6851(84)90062-8. [DOI] [PubMed] [Google Scholar]
- Dush M. K., Sikela J. M., Khan S. A., Tischfield J. A., Stambrook P. J. Nucleotide sequence and organization of the mouse adenine phosphoribosyltransferase gene: presence of a coding region common to animal and bacterial phosphoribosyltransferases that has a variable intron/exon arrangement. Proc Natl Acad Sci U S A. 1985 May;82(9):2731–2735. doi: 10.1073/pnas.82.9.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough J. A., Murray N. E. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983 May 5;166(1):1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge W. E., Gaborak M. A re-examination of purine and pyrimidine synthesis in the three main forms of Trypanosoma cruzi. Int J Biochem. 1979;10(5):415–422. doi: 10.1016/0020-711x(79)90065-x. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Hershey H. V., Taylor M. W. Nucleotide sequence and deduced amino acid sequence of Escherichia coli adenine phosphoribosyltransferase and comparison with other analogous enzymes. Gene. 1986;43(3):287–293. doi: 10.1016/0378-1119(86)90218-0. [DOI] [PubMed] [Google Scholar]
- Heyworth P. G., Gutteridge W. E., Ginger C. D. Purine metabolism in Trichomonas vaginalis. FEBS Lett. 1982 May 3;141(1):106–110. doi: 10.1016/0014-5793(82)80026-4. [DOI] [PubMed] [Google Scholar]
- Holden J. A., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Evidence for tetrameric structure. J Biol Chem. 1978 Jun 25;253(12):4459–4463. [PubMed] [Google Scholar]
- Johnson G. G., Eisenberg L. R., Migeon B. R. Human and mouse hypoxanthine-guanine phosphoribosyltransferase: dimers and tetramers. Science. 1979 Jan 12;203(4376):174–176. doi: 10.1126/science.569362. [DOI] [PubMed] [Google Scholar]
- Jolly D. J., Okayama H., Berg P., Esty A. C., Filpula D., Bohlen P., Johnson G. G., Shively J. E., Hunkapillar T., Friedmann T. Isolation and characterization of a full-length expressible cDNA for human hypoxanthine phosphoribosyl transferase. Proc Natl Acad Sci U S A. 1983 Jan;80(2):477–481. doi: 10.1073/pnas.80.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W. N., Greene M. L., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. Hypoxanthine-guanine phosphoribosyltransferase deficiency in gout. Ann Intern Med. 1969 Jan;70(1):155–206. doi: 10.7326/0003-4819-70-1-155. [DOI] [PubMed] [Google Scholar]
- King A., Melton D. W. Characterisation of cDNA clones for hypoxanthine-guanine phosphoribosyltransferase from the human malarial parasite, Plasmodium falciparum: comparisons to the mammalian gene and protein. Nucleic Acids Res. 1987 Dec 23;15(24):10469–10481. doi: 10.1093/nar/15.24.10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecki D. S., Brennand J., Fuscoe J. C., Caskey C. T., Chinault A. C. Hypoxanthine-guanine phosphoribosyltransferase genes of mouse and Chinese hamster: construction and sequence analysis of cDNA recombinants. Nucleic Acids Res. 1982 Nov 11;10(21):6763–6775. doi: 10.1093/nar/10.21.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Bifunctional messenger RNAs in eukaryotes. Cell. 1986 Nov 21;47(4):481–483. doi: 10.1016/0092-8674(86)90609-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Marr J. J., Berens R. L., Nelson D. J. Antitrypanosomal effect of allopurinol: conversion in vivo to aminopyrazolopyrimidine nucleotides by Trypanosoma curzi. Science. 1978 Sep 15;201(4360):1018–1020. doi: 10.1126/science.356267. [DOI] [PubMed] [Google Scholar]
- Marr J. J., Berens R. L., Nelson D. J. Purine metabolism in Leishmania donovani and Leishmania braziliensis. Biochim Biophys Acta. 1978 Dec 1;544(2):360–371. doi: 10.1016/0304-4165(78)90104-6. [DOI] [PubMed] [Google Scholar]
- Melton D. W., Konecki D. S., Brennand J., Caskey C. T. Structure, expression, and mutation of the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2147–2151. doi: 10.1073/pnas.81.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa S., Nishimura S., Seela F. Improvement of the dideoxy chain termination method of DNA sequencing by use of deoxy-7-deazaguanosine triphosphate in place of dGTP. Nucleic Acids Res. 1986 Feb 11;14(3):1319–1324. doi: 10.1093/nar/14.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. J., LaFon S. W., Tuttle J. V., Miller W. H., Miller R. L., Krenitsky T. A., Elion G. B., Berens R. L., Marr J. J. Allopurinol ribonucleoside as an antileishmanial agent. Biological effects, metabolism, and enzymatic phosphorylation. J Biol Chem. 1979 Nov 25;254(22):11544–11549. [PubMed] [Google Scholar]
- Poulsen P., Jensen K. F., Valentin-Hansen P., Carlsson P., Lundberg L. G. Nucleotide sequence of the Escherichia coli pyrE gene and of the DNA in front of the protein-coding region. Eur J Biochem. 1983 Sep 15;135(2):223–229. doi: 10.1111/j.1432-1033.1983.tb07641.x. [DOI] [PubMed] [Google Scholar]
- Pratt D., Subramani S. Nucleotide sequence of the Escherichia coli xanthine-guanine phosphoribosyl transferase gene. Nucleic Acids Res. 1983 Dec 20;11(24):8817–8823. doi: 10.1093/nar/11.24.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey P., Garrett C. E., Santi D. V. The metabolism and cytotoxic effects of Formycin B in Trypanosoma cruzi. Biochem Pharmacol. 1983 Feb 15;32(4):749–752. doi: 10.1016/0006-2952(83)90511-7. [DOI] [PubMed] [Google Scholar]
- Rainey P., Santi D. V. Metabolism and mechanism of action of formycin B in Leishmania. Proc Natl Acad Sci U S A. 1983 Jan;80(1):288–292. doi: 10.1073/pnas.80.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes P., Rathod P. K., Sanchez D. J., Mrema J. E., Rieckmann K. H., Heidrich H. G. Enzymes of purine and pyrimidine metabolism from the human malaria parasite, Plasmodium falciparum. Mol Biochem Parasitol. 1982 May;5(5):275–290. doi: 10.1016/0166-6851(82)90035-4. [DOI] [PubMed] [Google Scholar]
- Richardson K. K., Fostel J., Skopek T. R. Nucleotide sequence of the xanthine guanine phosphoribosyl transferase gene of E. coli. Nucleic Acids Res. 1983 Dec 20;11(24):8809–8816. doi: 10.1093/nar/11.24.8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Senft A. W., Miech R. P., Brown P. R., Senft D. G. Purine metabolism in Schistosoma mansoni. Int J Parasitol. 1972 Jun;2(2):249–260. doi: 10.1016/0020-7519(72)90013-6. [DOI] [PubMed] [Google Scholar]
- Tso J. Y., Zalkin H., van Cleemput M., Yanofsky C., Smith J. M. Nucleotide sequence of Escherichia coli purF and deduced amino acid sequence of glutamine phosphoribosylpyrophosphate amidotransferase. J Biol Chem. 1982 Apr 10;257(7):3525–3531. [PubMed] [Google Scholar]
- Wang C. C., Aldritt S. Purine salvage networks in Giardia lamblia. J Exp Med. 1983 Nov 1;158(5):1703–1712. doi: 10.1084/jem.158.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Simashkevich P. M. Purine metabolism in the protozoan parasite Eimeria tenella. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6618–6622. doi: 10.1073/pnas.78.11.6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Verham R., Cheng H. W., Rice A., Wang A. L. Differential effects of inhibitors of purine metabolism on two trichomonad species. Biochem Pharmacol. 1984 Apr 15;33(8):1323–1329. doi: 10.1016/0006-2952(84)90187-4. [DOI] [PubMed] [Google Scholar]
- Wang C. C., Verham R., Rice A., Tzeng S. Purine salvage by Tritrichomonas foetus. Mol Biochem Parasitol. 1983 Aug;8(4):325–337. doi: 10.1016/0166-6851(83)90079-8. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Structural alteration in a dysfunctional enzyme variant (HPRTMunich) isolated from a patient with gout. J Biol Chem. 1984 Jan 10;259(1):27–30. [PubMed] [Google Scholar]
- Wilson J. M., Kelley W. N. Molecular basis of hypoxanthine-guanine phosphoribosyltransferase deficiency in a patient with the Lesch-Nyhan syndrome. J Clin Invest. 1983 May;71(5):1331–1335. doi: 10.1172/JCI110884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Tarr G. E., Kelley W. N. Human hypoxanthine (guanine) phosphoribosyltransferase: an amino acid substitution in a mutant form of the enzyme isolated from a patient with gout. Proc Natl Acad Sci U S A. 1983 Feb;80(3):870–873. doi: 10.1073/pnas.80.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Tarr G. E., Mahoney W. C., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Complete amino acid sequence of the erythrocyte enzyme. J Biol Chem. 1982 Sep 25;257(18):10978–10985. [PubMed] [Google Scholar]