Abstract
Aging is unmistakable and undeniable in mammals. Interestingly, mice develop cataracts, muscle atrophy, osteoporosis, obesity, diabetes and cognitive deficits after just 2–3 postnatal years, while it takes seven or more decades for the same age-specific phenotypes to develop in humans. Thus, chronological age corresponds differently with biological age in metazoan species and although many theories exist, we do not understand what controls the rate of mammalian aging. One interesting idea is that species-specific rate of aging represents a ratio of tissue attrition to tissue regeneration. Furthermore, current findings suggest that the age-imposed biochemical changes in the niches of tissue stem cells inhibit performance of this regenerative pool, which leads to the decline of tissue maintenance and repair. If true, slowing down stem cell and niche aging, thereby promoting tissue regeneration, could slow down the process of tissue and organismal aging. In this regard, recent studies of heterochronic parabiosis provide important clues as to the mechanisms of stem cell aging and suggest novel strategies for enhancing tissue repair in the old. Here we review current literature on the relationship between the vigor of tissue stem cells and the process of aging, with an emphasis on the rejuvenation of old tissues by the extrinsic modifications of stem cell niches.
Keywords: aging, muscle, niche, parabiosis, stem cells
The Puzzles of Stem Cell Aging
Organ systems are interconnected anatomically and physiologically, perhaps explaining why there is a general concordance of the rate of tissue aging in an individual. An emerging unified theme suggests that the aging of a tissue is generally caused by decline in the regenerative capacity of its resident stem cells. Moreover, recent data suggests that biochemical changes in the niches of tissue stem cells are responsible for such regenerative declines in old mammals. Consequentially, experimental “youthful” modifications of stem cell niches have been shown to boost the regenerative performance of tissue stem cells in the old, leading to healthier tissues. The youthful modifications of stem cell niches have been successfully achieved through heterochronic tissue transplants,1,2 where old stem cells are exposed to young tissue niches by being transplanted there and through heterochronic parabiosis, where old stem cells are exposed to a youthful environment by virtue of the effects of the young circulation.3-5 Interestingly, for many tissues (muscle, liver, brain, bone), the regenerative potential of the aged stem cells was determined by the age of the niche or environment rather than by the age of the stem cells themselves, such that young local and/or systemic environments promoted effective regeneration by the old stem cells.3 The same studies have also shown that aged niches inhibit the regenerative capacity of young stem cells.4,5 Together, these data reveal that in an old mammal, tissue stem cells retain youthful potential and are capable of effective tissue repair, but that their performance can be acutely and reversibly inhibited by molecular changes in local and systemic niches.
In addition to the proof of principle, these studies have also helped to identify key molecular and cellular mechanisms that account for the good performance of tissue stem cells in the young, but not in the old, organism. In skeletal muscle, such improved molecular understanding has been convincingly used for experimental rejuvenation of regenerative capacity of satellite cells for both old mice (in vitro and in vivo) and humans (in vitro).5,6 The ways to rejuvenate muscle regeneration are numerous and include physiological approaches, such as heterochronic parabiosis, and more molecular approaches, such as the exposure of aged muscle cells to the soluble proteins, including those secreted by embryonic stem cells.4,6,7 Molecularly defined conditions, such as forced activation of Notch, inhibition of TGFβ/pSmad3 and inhibition of Wnt were all shown to boost the regenerative performance of aged muscle stem cells and lead to tissue rejuvenation.4,8-10
Age-specific changes in the crosstalk between tissue-specific stem cells and their niches are certainly not unique to skeletal muscle and have been also demonstrated for liver and brain in studies on heterochronic parabiosis that have paved the way to molecular dissection of the key age-dependent signaling pathways regulating regeneration of these tissues.3,5 In this regard, recent work on the heterochronic intervention into the age-specific decline in neurogenesis identified several molecules that accumulate with age in the old circulation of mice and humans and are capable of inhibiting regenerative capacity of neural stem cells.5
Conceptual Challenges for the Study of Stem Cell Aging
In general, studies of cellular aging in vivo are challenging because of the difficulty of distinguishing intrinsic age-related changes in the stem cell themselves vs. functional changes imposed on the stem cells by the aged environment in which they reside.11,12 Clearly, isolation of tissue stem cells and analysis of their characteristics either biochemically or functionally can reveal cell-intrinsic changes that accompany the aging process but would include those imposed by the aged environment. Therefore, when considering in vivo heterochronic studies that result in apparent stem cell rejuvenation, the molecular mechanisms that underlie those changes may be directly transmitted to the stem cells by circulating factors or may be indirectly transmitted to stem cells by cells in their local environment.
Importantly, our ability to re-create the correct stem cell niches/environments in vitro is critically lacking, which precludes studying adult stem cells or expanding them for therapeutic purposes or harnessing their regenerative potential. This particular challenge results from the fact that the molecular determinants of the local and systemic niches of organ stem cells that regulate the regenerative responses are poorly understood; hence, stem cells cannot yet be provided with the optimal niches in vitro or upon transplantation into tissues in need of repair. Additionally, until technology allows the use of resident stem cells in a particular tissue for regenerative medicine, we are constrained to using stem cells obtained from other sources (e.g., embryonic, reprogrammed, donor-derived). Even gene-corrected or engineered tissue-derived cells have typically been grown for many cell divisions in culture. This means that most therapeutic cells go from one environment to another to which they are unaccustomed. In cell therapy experiments, by far the most common response of a given cell to its new environment is rapid death (typically by apoptosis), even in an immunosuppressed host. It is this “shock” of a different and temporarily unsupportive niche into which the therapeutic cells are placed which is thought to be the primary reason why the vast majority of donor cells fail in tissue regeneration and die. Therefore, a major current limitation of stem cell therapeutics is the inability to coordinate the therapeutic cell with a functional stem cell niche.
Unique Angles of Studies of Somatic Stem Cell Aging
One unique aspect of the study of somatic stem cell aging is related to a property that defines a stem cell, namely, the ability of that cell to self-renew during cell division. One consequence of this property is the fact that a stem cell in an aged individual represents a kind of “cellular continuum” with the parent stem cell that was initially born during the process of development. This reflects that fact that stem cells experience both replicative aging, by which dividing cells change based on their replicative history, and also chronological aging, which is more easily understood as age-related changes that occur in non-dividing, postmitotic cells, such as neurons, cardiomyocytes and skeletal myofibers.11,13 Both as part of the cellular continuum of the self-renewing stem cell lineage and also because so many stem cells persist for prolonged periods in non-dividing quiescent states, the chronological aspects of cellular aging are germane to stem cell aging. Therefore, the rejuvenation of stem cells would, in theory, require restoration of youthful status, both in terms of replicative processes (such as the restoration of telomere length) and in terms of chronological processes (such as the clearance of protein aggregates) in addition to changes that could accompany either (such as the acquisition of mitochondrial and genomic DNA mutations).
A related unique aspect of stem cell aging is linked to the known phenomenon of asymmetry of cell divisions, which is, in fact, a key undisputed property of both embryonic and adult stem cells.14 During the asymmetric cell division, one daughter cell self-renews with the properties of stemness, while the other daughter cell becomes more differentiated.15 Notably, macromolecules (proteins, DNA and mRNA) as well as organelles may localize asymmetrically during the asymmetric stem cell divisions, and, moreover, it has been suggested that the more differentiated daughter cell inherits “older” organelles and misfolded proteins, thus the self-renewed stem cell becomes “younger”/healthier after such asymmetric divisions.16,17 Such a conclusion fits well with the relative ease of “rejuvenation” of organ stem cells in heterochronic parabiosis: if these cells remain intrinsically younger than their niches, they should be prone to the productive regenerative responses in the biochemical milieu that is typical of a young organism.
With respect to DNA, the idea of the asymmetric segregation of DNA strands in the asymmetrically dividing stem cells remains controversial. This hypothesis, originally called the “immortal DNA strand hypothesis,” was proposed by Cairns as a mechanism to ensure genome stability in stem cells that frequently and rapidly replicate their DNA in tissues with high turnover, for example, skin and intestine.18 Several earlier studies had provided evidence of non-random segregation of chromosomes in vastly phylogenetically divergent organisms.19-21 In recent times, several laboratories have addressed the validity of the Cairns hypothesis in such diverse experimental systems as mouse intestinal epithelium, skeletal muscle, hematopoietic system, hair follicle, mammary tissue as well as fly male germline cells.22-28 Although, some of these studies suggested that there is indeed preferential segregation of older DNA template strands of all chromosomes to the self-renewing stem cells (muscle, mammary, intestinal and colon), other studies have observed random segregation of sister chromatids during the asymmetric divisions of organ stem cells (blood, hair follicle, intestinal, germline). While the phenomenon of the non-random segregation of DNA strands manifests in some tissue stem cells, the reason for such asymmetry is not well understood, and the age-related details are entirely unknown.
Aging of Muscle: A Stem Cell Paradigm
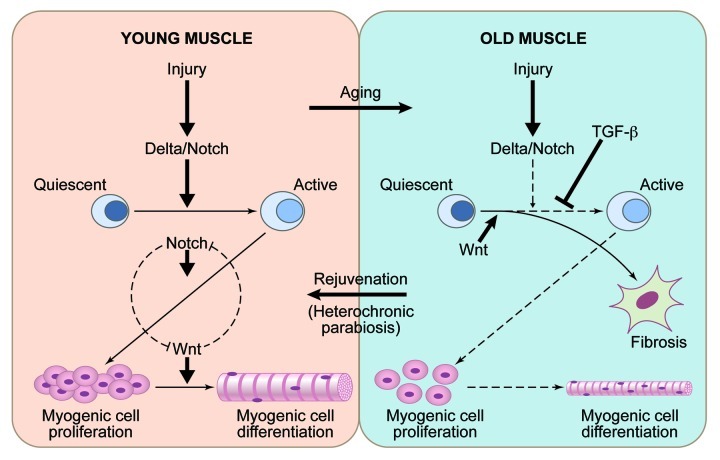
A significant part of our molecular understanding of stem cell aging has come from the studies in skeletal muscle (Fig. 1). In young and old tissue, muscle stem cells are quiescent and associate with post-mitotic multinucleated muscle fibers.29 When young muscle fibers are injured, they upregulate the Notch ligand Delta, inducing the proliferation of muscle stem cells at the vicinity of muscle injury.8,30,31 Overexpression of Notch rescues the defect in satellite cell proliferation in Syndecan 3-knockout mice, thus further supporting the pivotal role this myogenic regulator.32 By contrast, in old mice or humans, injured muscle fibers fail to activate the Delta/Notch pathway, and, consequentially, satellite cells fail to engage in tissue regeneration.8,10 Interestingly, TNFα inhibits activation of Notch-1 and, hence, causes a defect in satellite cell proliferation, which has implications for chronic inflammation that frequently accompanies tissue aging.33 In young muscle, Notch continues to be active in the expanding myogenic progenitor cells, where it prevents their precocious differentiation into fusion-competent myoblasts and myotubes by counteracting the Wnt signaling pathway.9,34,35 In old muscle, Wnt pathway becomes prematurely hyperactive, causing the lack of myogenic responses and promoting scarring and inflammation.4 Additionally, muscle fibers and serum from aged mice and humans have excessive TGFβ1, which signals through pSmad3 to induce CDK inhibitors in old muscle stem cells, exacerbating the lack of Notch and thwarting cell proliferation.9,10 Recent work on transcriptional profiling of young vs. aged muscle provided yet another confirmation to the role of Notch, Wnt and TGFβ pathways in regulation and aging of myogenesis.36
Figure 1. Aging and rejuvenation of adult myogenesis. In young muscle, injury upregulates the Notch ligand, Delta-like 1, which activates Notch signaling. Active Notch promotes a G0 to G1 transition in muscle stem cells after an injury; interplay between Notch and Wnt controls myogenic cell proliferation and differentiation. In old muscle, which regenerates poorly, in addition to a suppression of activation of Notch pathway following injury, there is also an excess of signaling via the TGFβ and Wnt pathways, leading to a suppression of myogenesis and a promotion of fibrosis. Notch, Wnt and likely other signaling pathways are regulated by systemic niche / circulatory milieu, such that in the aged environment, systemic signals contribute to the activities that inhibit myogenesis and promote fibrosis. By contrast, in the setting of heterochronic parabiosis, young systemic factors restore more youthful states and are able to rejuvenate the aged stem cells and stem cell niches to promote enhanced muscle regeneration. .
Youthful calibration of Notch, Wnt and TGFβ/pSmad rescues the deficiency of repair of old muscle, while experimental “aging” of these pathways hinders repair of young muscle, firmly establishing that the regenerative capacity of muscle stem cells is determined not so much by their intrinsic age, but rather by the extrinsically induced intensities of these signal transduction pathways.3,4,8-10,37 Expression of Delta, activation of Notch and correct timing of Wnt activation are all restored to aged muscle niche when old mice are connected in heterochronic parabiosis with young mice.3,4 These findings suggest a paradigm that biochemical and functional aging of the niches of tissue stem cells is regulated systemically, and, hence, the niches and consequentially the tissue regenerative responses can be rejuvenated by the systemic administration of specific molecular cues that are present in the young circulation. In theory, similar niche rejuvenation would be required for productive regenerative responses of young cells and tissues that are transplanted into older individuals.
Is Aging of Other Adult Tissues a Stem Cell Paradigm?
Experimental evidence suggests that in several other tissues (brain, blood, bone) the decline of regenerative potential of adult stem cells accounts for tissue aging and, similar to the situation in muscle, such declines are caused (at least partly) by the aging of the niche. New neurons are generated in mammalian hippocampus throughout adult life and integrate into neuronal circuitry, contributing to certain forms of learning and memory. Indeed, an age-specific decline in cognition is thought to be due to the diminished activity of the hippocampus, which, in turn, is thought to be caused by impaired neurogenesis.38,39 Adult neurogenesis can be enhanced by the external cues, e.g., environmental enrichment and physical exercise.40,41 Moreover, a number of studies have suggested that the experimental changes in local and systemic niches of neural stem cells are capable of reversing the age-related decline in hippocampal neurogenesis.42,43
Poor immune responses, anemia and an increased incidence of certain leukemias in the old are clearly associated with the aging of hematopoietic stem cells (HSC), which become skewed toward formation of myeloid and away from lymphoid lineages.44 Interestingly, HSCs have been experimentally rejuvenated by the down-modulation of mTOR, demonstrating that their age-specific alterations are not irreversible.45 Furthermore, while numerous intrinsic age-imposed changes have been identified in HSCs at genetic and epigenetic levels,44,46 it has been also shown that it is the HSC niche that regulates the performance and aging of these stem cells, including the state of quiescence, the accumulation and repair of DNA damage, the protection from oxidative damage and the modification of signal transduction.47,48
The aging of bone morphogenesis that leads to osteoporosis and poor fracture repair is caused by an imbalance between the bone formation and bone resorption, which, in turn, results from the age-specific changes in the properties of mesenchymal stem cells, MSCs.49,50 Recently, one of the key age-dependent alterations that causes the defect in bone lineage formation by MSCs was shown to be the downregulation of c-Maf, which, interestingly, has been linked to a prominent feature of old cells, e.g., oxidative stress.51 Similar to what was reported for aging of skeletal muscle,8 it was also shown that while the frequency of MSCs derived from the bone marrow of old mice is similar to that of young mice, the regenerative bone-forming capacity of the old MSCs is markedly lower than that of young cells. Notably, a young extracellular matrix rejuvenated the bone forming properties of the old MSCs.52
Heterochronic Transplantation
The rationale for heterochronic transplantation studies is to rigorously test the idea of intrinsic vs. extrinsic mode of stem cell aging by assaying the regenerative performance of young and old stem/progenitor cells in chronologically mismatched tissues. For example, young muscle fibers or minced muscle tissue containing satellite cells were isolated from young rodents and are placed into muscle defects of aged hosts; conversely, muscle cells derived from old donors are transplanted into muscle of young hosts.2 These seminal experiments demonstrated that the age of the host, rather than the age of the donor, dictated the success in tissue regeneration, which, in those early studies, was correlated with the efficiency of immune response and wound clearance.2 Such findings prompted future work on deciphering the age-dependent molecular cues by which muscle environment regulates satellite cell responses in young vs. aged mammals and were extrapolated further in studies of heterochronic parabiosis.
Heterochronic cell transplantation has been also performed with bone marrow cells, which has an inherent caveat of a mandatory niche irradiation. Since recipient mice die, if transplanted HSCs fail to engraft and produce blood lineages, young and old bone marrow cells and HSCs undergo positive selection in both young and old hosts, where only the best outcomes leading to host survival can be analyzed in detail. Additionally, irradiated bone marrow niches are most definitely altered in their physiology and biochemistry,53 and, hence, are quite different from natural young and old niches. Nevertheless, experiments where competitive reconstitution of blood lineages was examined by transplanting young and old bone marrow cells into young vs. old hosts suggested that the aged HSCs are nearly as efficient as young HSCs when transplanted into a youthful environment.1 In a more recent study, one of the key age-dependent phenotypes, e.g., a lack of functional B cells, was rescued in old mice in vivo by the transplantation of young bone marrow, demonstrating that young bone marrow niche complete with young HSCs is functional in the aged mammalian host.54 The rejuvenating effects of young bone marrow are not limited just to hematopoiesis, as recent work has shown that transplanted young bone marrow enhanced bone morphogenesis in old mice, which was due to the activity of the young inflammatory cells.55 Agreeing with and extrapolating these studies, young and old mice that were reconstituted with old bone marrow manifested diminished recovery from the transverse aortic constriction as compared with the animals transplanted with the young bone marrow.56
Heterochronic Parabiosis
The rationale for heterochronic parabiosis is to provide a proof of principle to the idea that there is a systemic regulation of tissue aging. To establish parabiosis, two animals (typically, mice or rats) are surgically connected through a large flap of skin, thereby establishing functional vascular anastomoses (joined circulation) in approximately one week. Two animals of the same age (young-to-young and old-to-old) are the control isochronic pairs, while young-to-old pairs are the experimental heterochronic parabionts. Additionally, a mock parabiosis can be set up, where animals are connected by restraints but do not have common blood circulation. One parabiont can be transgenic for a reporter gene (e.g., GFP), which enables the confirmation of blood chimerism and tests whether heterochronic blood cells or alternatively endogenous tissue stem cells contributed to tissue regeneration.3,5 Parabiosis can be maintained for variable time (weeks to years) and has been historically used to answer a number of such diverse questions, such as, what is the etiology of tooth decay, and what is the role of the immune system in fighting cancer.57
In 1864 Paul Bert published the first description of the surgical joining of two rats in parabiosis, which he compared with “Siamese twins,” in his “Sur la Greffe Animale.”58 In the first heterochronic parabiosis research that was published much later, approximately 50 y ago, McKay and colleagues examined the hypothesis that aging has a systemic component and tested whether the blood of young rodents can prolong lives of old rodents.59 In these studies, the number of joined animals was small, but data suggested that collagen and bones were rejuvenated in heterchronically parabiosed old rats, while those of the young partner were unchanged or slightly older. This work was followed in 1972, when Ludwig and colleagues published a study in which old rats that were joined in heterochronic parabiosis with young rats lived four to five months longer, which is ~20% increase in longevity.60 In many of these early experiments, the old animals shared circulation with a young animals for years; thus, they both continued to age, and at times, several young rats were connected to one old rat in an attempt to have the prevalence of young blood, but complicating the answer as to whether aged circulation has a negative effect on young organism.59,60
Heterochronic Parabiotic Studies of Stem Cell Aging
Recently, our improved understanding of stem cell aging prompted a new wave of studies that revisited the effect of heterochronic parabiosis on the aging phenotypes but with a new focus on tissue regenerative capacity and the key regulatory molecules, which are responsible for the parabiotic effects. Beginning about 10 years ago and first published in 2005, we resurrected the use of heterochronic parabiosis for the study of cellular aging and applied this specifically to the study of stem and progenitor aging. The body of this work has been instrumental in establishing that extrinsic age-specific factors present in blood serum regulate the regenerative responses of tissue stem cells in muscle, liver and brain.3-5 In these studies, in heterochronic parabionts, the old muscle tissue regenerated after an injury as well as young tissue, while young muscle tissue experienced somewhat diminished regenerative capacity.3,4 Similar age-specific effects of systemic milieu were observed in livers of heterochronically connected mice, where old liver regeneration was improved, while young liver regeneration was diminished.3 Recent work demonstrated that neurogenesis in brains of heterochronic parabionts is similarly improved for the old mouse and is decreased for the young mouse.5 These studies allowed further evaluation of the parabiotic effect by the demonstration of direct injection of serum from old mice intravenously into young mice mimicked the effects of aging on neurogenesis. Not only was stem cell proliferative activity modulated by heterochronic parabiosis and heterochronic serum administration, but physiological correlates of memory that are subserved by neurogenesis were likewise modified.
In all these studies, isochronic parabionts were not significantly different in their tissue regeneration as compared with the non-parabiotic young and old mice and the rejuvenation of old tissues was achieved through the much-improved regenerative performance of the aged tissue stem cells and not by the blood cells from the young partner. Similarly, there was no evidence of blood cell contribution from old partner to the regeneration of young tissues. These studies demonstrated that a broad improvement in tissue maintenance and repair can be promoted in an old mammal by “young” systemic factors through boosting the regenerative capacity of tissue stem cells and suggested that aged circulation might have “negative” molecular regulators of stem cell responses.
Remarkably, these heterochronic parabiosis studies also revealed that the biochemical pathways, which are known to regulate tissue regeneration and to become altered by the aging process, are calibrated to their “youthful” productive signaling strength in the aged stem cells of multiple lineages, including muscle, liver and brain, that are exposed to young circulation.3-5 Importantly, systemic intervention into Wnt signaling was shown to mimic the effects of heterochronic parabiosis and rejuvenate muscle repair.4 In proteomic analysis of blood from young and old mice and from heterochronic parabiotically paired mice, a limited set of serum chemokines and cytokines were identified that correlated with the age-related changes in neurogenesis.5 Further analysis showed that the injection of individual proteins, specifically CCL11, could either phenocopy the effect of the aging environment on neural stem cell function, an effect that could be blocked by the systemic or local injection of a CCL11-neutralizing antibody. While these studies implicate several molecules in systemic regulation and aging of tissue regeneration, the identification of the factors that are responsible for the rejuvenation of the old stem cells in heterochronically paired animals is still ongoing, and work is currently underway to identify those therapeutically relevant endocrine factors. The number of tissues examined in heterochronic parabiosis studies is certainly growing, and an interesting recent study reported that when male and female mice of different ages were parabiotically connected, surprisingly, factors circulating in blood of aged males enhanced the ovarian reserve (number of primordial follicles) in young females by inducing ovarian expression of the germ cell-specific meiosis gene, Stra8.61 Additionally, a recent work in telomerase-knockout mice suggests that the age-specific changes in the systemic environment might contribute to the HSC aging.62
Broader Implications of Mechanisms of Aging: Future Perspectives
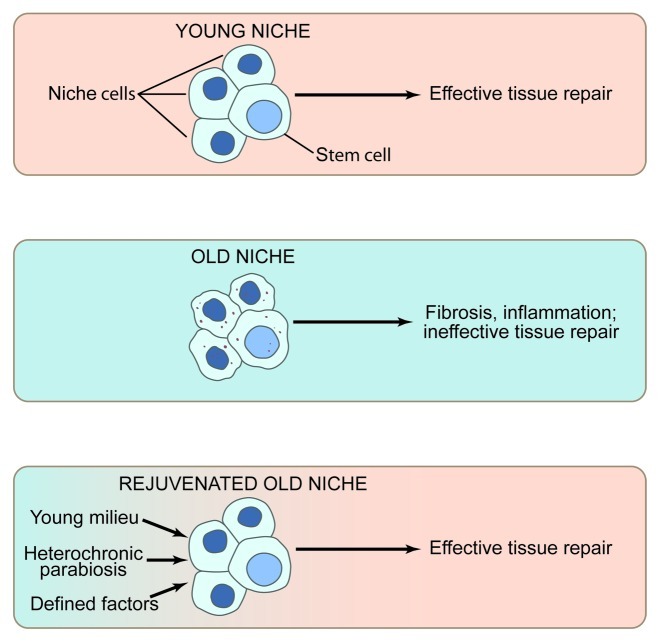
Altogether, current literature strongly suggests that aging in general (and not just in skeletal muscle) results from the abandonment of tissue maintenance by resident stem cells, which become inhibited by their aged organismal niches. With time, unrepaired tissues become progressively more damaged, and the inhibition on the resident stem cells ironically increases, ultimately leading to the downward spiral of organ dysfunction. Promisingly, experimental activation of tissue stem cells in the old is predicted to gradually rejuvenate the niche. Hence, eventually, old tissues are predicted to become younger, and experimental intervention into stem cell performance will be less needed. Studies of heterochronic parabiosis illuminated the role of systemic factors in broad mechanisms of stem cell aging and suggested several promising therapeutic molecules that can be delivered through circulation and can rejuvenate tissue maintenance and repair. Future work on comprehensive molecular dissection of the age-specific changes in local and systemic niches of tissue stem cells would enable tunable niche rejuvenation ,which is predicted to delay or reverse the onset of tissue aging (Fig. 2).
Figure 2. The role of the stem cell niche in stem cell performance (A) The young niche induces productive stem cell responses and, as a result, young tissues regenerate efficiently. (B) By contrast, the old niche inhibits stem cell responses. Rather than effective tissue regeneration, there is sustained inflammation and resulting fibrosis, perhaps further suppressing stem cell function and leading to apoptosis, senescence, or aberrant cell fate. (C) Promisingly, exogenous molecules that boost the regenerative responses of tissue stem cells, perhaps in part by rejuvenating the aged niche, promote effective repair of aged tissues, overriding the inhibitory influence of the old niches.
Acknowledgments
This work was supported by grants from the NIH (R01 AG 0277252), CIRM (RN1–00532) and the Keck Foundation to I.M.C. and by grants from the Glenn Foundation for Medical Research, the NIH [P01 AG036695, R01 AG023806 (R37MERIT Award), R01 AR056849, R01 AR062185 and DP1 OD000392 (an NIH Director's Pioneer Award)] and the Department of Veterans Affairs (Merit Review) to T.A.R.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/20437
References
- 1.Harrison DE. Long-term erythropoietic repopulating ability of old, young, and fetal stem cells. J Exp Med. 1983;157:1496–504. doi: 10.1084/jem.157.5.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256:C1262–6. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- 3.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–4. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 4.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–10. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 5.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–4. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conboy IM, Yousef H, Conboy MJ. Embryonic anti-aging niche. Aging (Albany NY) 2011;3:555–63. doi: 10.18632/aging.100333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4:407–10. doi: 10.4161/cc.4.3.1518. [DOI] [PubMed] [Google Scholar]
- 8.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–7. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 9.Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454:528–32. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson ME, Conboy MJ, Hsu M, Barchas L, Jeong J, Agrawal A, et al. Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell. 2009;8:676–89. doi: 10.1111/j.1474-9726.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Rando TA. Manifestations and mechanisms of stem cell aging. J Cell Biol. 2011;193:257–66. doi: 10.1083/jcb.201010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–8. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–6. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- 14.He S, Nakada D, Morrison SJ. Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol. 2009;25:377–406. doi: 10.1146/annurev.cellbio.042308.113248. [DOI] [PubMed] [Google Scholar]
- 15.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–97. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Rujano MA, Bosveld F, Salomons FA, Dijk F, van Waarde MA, van der Want JJ, et al. Polarised asymmetric inheritance of accumulated protein damage in higher eukaryotes. PLoS Biol. 2006;4:e417. doi: 10.1371/journal.pbio.0040417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–21. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 19.Lark KG, Consigli RA, Minocha HC. Segregation of sister chromatids in mammalian cells. Science. 1966;154:1202–5. doi: 10.1126/science.154.3753.1202. [DOI] [PubMed] [Google Scholar]
- 20.Lark KG. Regulation of chromosome replication and segregation in bacteria. Bacteriol Rev. 1966;30:3–32. doi: 10.1128/br.30.1.3-32.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lark KG. Nonrandom segregation of sister chromatids in Vicia faba and Triticum boeoticum. Proc Natl Acad Sci U S A. 1967;58:352–9. doi: 10.1073/pnas.58.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potten CS, Hume WJ, Reid P, Cairns J. The segregation of DNA in epithelial stem cells. Cell. 1978;15:899–906. doi: 10.1016/0092-8674(78)90274-X. [DOI] [PubMed] [Google Scholar]
- 23.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci. 2002;115:2381–8. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- 24.Conboy MJ, Karasov AO, Rando TA. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol. 2007;5:e102. doi: 10.1371/journal.pbio.0050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinin V, Gayraud-Morel B, Gomès D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol. 2006;8:677–87. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- 26.Karpowicz P, Morshead C, Kam A, Jervis E, Ramunas J, Cheng V, et al. Support for the immortal strand hypothesis: neural stem cells partition DNA asymmetrically in vitro. J Cell Biol. 2005;170:721–32. doi: 10.1083/jcb.200502073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith GH. Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development. 2005;132:681–7. doi: 10.1242/dev.01609. [DOI] [PubMed] [Google Scholar]
- 28.Karpowicz P, Pellikka M, Chea E, Godt D, Tepass U, van der Kooy D. The germline stem cells of Drosophila melanogaster partition DNA non-randomly. Eur J Cell Biol. 2009;88:397–408. doi: 10.1016/j.ejcb.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Schultz E, Gibson MC, Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. J Exp Zool. 1978;206:451–6. doi: 10.1002/jez.1402060314. [DOI] [PubMed] [Google Scholar]
- 30.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/S1534-5807(02)00254-X. [DOI] [PubMed] [Google Scholar]
- 31.Gnocchi VF, White RB, Ono Y, Ellis JA, Zammit PS. Further characterisation of the molecular signature of quiescent and activated mouse muscle satellite cells. PLoS One. 2009;4:e5205. doi: 10.1371/journal.pone.0005205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pisconti A, Cornelison DD, Olguín HC, Antwine TL, Olwin BB. Syndecan-3 and Notch cooperate in regulating adult myogenesis. J Cell Biol. 2010;190:427–41. doi: 10.1083/jcb.201003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acharyya S, Sharma SM, Cheng AS, Ladner KJ, He W, Kline W, et al. TNF inhibits Notch-1 in skeletal muscle cells by Ezh2 and DNA methylation mediated repression: implications in duchenne muscular dystrophy. PLoS One. 2010;5:e12479. doi: 10.1371/journal.pone.0012479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brack AS, Murphy-Seiler F, Hanifi J, Deka J, Eyckerman S, Keller C, et al. BCL9 is an essential component of canonical Wnt signaling that mediates the differentiation of myogenic progenitors during muscle regeneration. Dev Biol. 2009;335:93–105. doi: 10.1016/j.ydbio.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–9. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Scimè A, Desrosiers J, Trensz F, Palidwor GA, Caron AZ, Andrade-Navarro MA, et al. Transcriptional profiling of skeletal muscle reveals factors that are necessary to maintain satellite cell integrity during ageing. Mech Ageing Dev. 2010;131:9–20. doi: 10.1016/j.mad.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Carlson ME, Conboy IM. Regulating the Notch pathway in embryonic, adult and old stem cells. Curr Opin Pharmacol. 2007;7:303–9. doi: 10.1016/j.coph.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 2004;24:1726–33. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kempermann G, Brandon EP, Gage FH. Environmental stimulation of 129/SvJ mice causes increased cell proliferation and neurogenesis in the adult dentate gyrus. Curr Biol. 1998;8:939–42. doi: 10.1016/S0960-9822(07)00377-6. [DOI] [PubMed] [Google Scholar]
- 41.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 42.Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–7. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 43.Montaron MF, Drapeau E, Dupret D, Kitchener P, Aurousseau C, Le Moal M, et al. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol Aging. 2006;27:645–54. doi: 10.1016/j.neurobiolaging.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 44.Ergen AV, Goodell MA. Mechanisms of hematopoietic stem cell aging. Exp Gerontol. 2010;45:286–90. doi: 10.1016/j.exger.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konuma T, Oguro H, Iwama A. Role of the polycomb group proteins in hematopoietic stem cells. Dev Growth Differ. 2010;52:505–16. doi: 10.1111/j.1440-169X.2010.01191.x. [DOI] [PubMed] [Google Scholar]
- 47.Wagner W, Horn P, Bork S, Ho AD. Aging of hematopoietic stem cells is regulated by the stem cell niche. Exp Gerontol. 2008;43:974–80. doi: 10.1016/j.exger.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7:391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 49.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–26. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Lu C, Miclau T, Hu D, Hansen E, Tsui K, Puttlitz C, et al. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23:1300–7. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishikawa K, Nakashima T, Takeda S, Isogai M, Hamada M, Kimura A, et al. Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J Clin Invest. 2010;120:3455–65. doi: 10.1172/JCI42528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Y, Li W, Lu Z, Chen R, Ling J, Ran Q, et al. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J. 2011;25:1474–85. doi: 10.1096/fj.10-161497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–6. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hida D, Ishiguro N, Haneda M, Ishida Y, Suzuki H, Isobe K. Intra-bone marrow bone marrow transplantation rejuvenates the B-cell lineage in aged mice. Immunol Cell Biol. 2010;88:87–94. doi: 10.1038/icb.2009.69. [DOI] [PubMed] [Google Scholar]
- 55.Xing Z, Lu C, Hu D, Miclau T, 3rd, Marcucio RS. Rejuvenation of the inflammatory system stimulates fracture repair in aged mice. J Orthop Res. 2010;28:1000–6. doi: 10.1002/jor.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sopko NA, Turturice BA, Becker ME, Brown CR, Dong F, Popović ZB, et al. Bone marrow support of the heart in pressure overload is lost with aging. PLoS One. 2010;5:e15187. doi: 10.1371/journal.pone.0015187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Finerty JC. Parabiosis in physiological studies. Physiol Rev. 1952;32:277–302. doi: 10.1152/physrev.1952.32.3.277. [DOI] [PubMed] [Google Scholar]
- 58.Bert P. Expériences et Considérations Sur la Greffe Animale. Journal del al Anatomie et de la Physiologie. 1864;1:69–87. [Google Scholar]
- 59.McCay CM, Pope F, Lunsford W, Sperling G, Sambhavaphol P. Parabiosis between old and young rats. Gerontologia. 1957;1:7–17. doi: 10.1159/000210677. [DOI] [PubMed] [Google Scholar]
- 60.Ludwig FC, Elashoff RM. Mortality in syngeneic rat parabionts of different chronological age. Trans N Y Acad Sci. 1972;34:582–7. doi: 10.1111/j.2164-0947.1972.tb02712.x. [DOI] [PubMed] [Google Scholar]
- 61.Niikura Y, Niikura T, Wang N, Satirapod C, Tilly JL. Systemic signals in aged males exert potent rejuvenating effects on the ovarian follicle reserve in mammalian females. Aging (Albany NY) 2010;2:999–1003. doi: 10.18632/aging.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song Z, Wang J, Guachalla LM, Terszowski G, Rodewald HR, Ju Z, et al. Alterations of the systemic environment are the primary cause of impaired B and T lymphopoiesis in telomere-dysfunctional mice. Blood. 2010;115:1481–9. doi: 10.1182/blood-2009-08-237230. [DOI] [PubMed] [Google Scholar]