SUMMARY
The primary cause of Huntington’s disease (HD) is expression of huntingtin with a polyglutamine expansion. Despite an absence of consensus on the mechanism(s) of toxicity, diminishing the synthesis of mutant huntingtin will abate toxicity if delivered to the key affected cells. With antisense oligonucleotides (ASOs) that catalyze RNase H-mediated degradation of huntingtin mRNA, we demonstrate that transient infusion into the cerebral spinal fluid of symptomatic HD mouse models not only delays disease progression, but mediates a sustained reversal of disease phenotype that persists longer than the huntingtin knockdown. Reduction of wild type huntingtin, along with mutant huntingtin, produces the same sustained disease reversal. Similar ASO infusion into non-human primates is shown to effectively lower huntingtin in many brain regions targeted by HD pathology. Rather than requiring continuous treatment, our findings establish a therapeutic strategy for sustained HD disease reversal produced by transient ASO-mediated diminution of huntingtin synthesis.
INTRODUCTION
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disease caused by a CAG expansion in exon 1 of the huntingtin gene (Huntington’s Disease Collaborative Research Group, 1993). This mutation translates into an elongated glutamine tract in the N-terminus of the huntingtin protein. Patients with HD display progressive movement dysfunction, including hyperkinetic involuntary movements, chorea, and dystonia, as well as cognitive impairments. Presently, there is no effective treatment for HD. The majority of potential therapies now under development are aimed at ameliorating symptoms of one of several proposed molecular consequences of mutant huntingtin, i.e., at down-stream targets in one of the many potential pathways possibly involved in HD pathogenesis (Melone et al., 2005).
Irrespective of the many mechanistically divergent proposals for the underlying toxicity of expanded huntingtin, a therapy aimed at diminishing the synthesis of the toxic mutant protein is an approach that will directly target the primary disease mechanism(s), as long as it is effective in the key HD-affected cells and any coincident suppression of wild type huntingtin is tolerated. Gene silencing strategies that suppress the synthesis of huntingtin that could be deployed as potential therapeutics include virally encoded short-hairpin RNAs (shRNAs) or microRNAs (miRNAs) (Franich et al., 2008; Harper et al., 2005; Machida et al., 2006; McBride et al., 2008; Rodriguez-Lebron et al., 2005), as well as direct infusion of synthetic siRNAs (DiFiglia et al., 2007; Wang et al., 2005). In their current forms, each of these agents needs to be delivered by direct intraparenchymal injections, and therapeutic correction is limited to only a small portion of the striatum immediately adjacent to the sites of injection (Boudreau et al., 2009; DiFiglia et al., 2007; Drouet et al., 2009; Harper et al., 2005; McBride et al., 2008).
While the striatum is particularly vulnerable to mutant huntingtin-mediated toxicity, huntingtin is ubiquitously expressed (Hoogeveen et al., 1993), and selective expression of mutant huntingtin in striatal neurons is not sufficient to cause locomotor deficits or neuropathology in rodents (Gu et al., 2007). To date, the collective evidence strongly supports a disease mechanism in which mutant huntingtin expression in multiple cell-types within at least the striatum and cortex is likely required for disease development and progression. Indeed, cortical thinning is observed in human patients prior to the onset of symptoms (Rosas et al., 2002; Rosas et al., 2006), and by endstage, typically more than 30% of an HD patient’s brain mass is lost (de la Monte et al., 1988). Finally, the human striatum accounts for only ~1% of the total brain volume, indicating the disease is affecting other areas of the brain. All of this evidence suggests that a fully effective treatment of HD will likely require targeting multiple brain regions.
An alternative approach to preceding efforts for achieving reduction in huntingtin synthesis is infusion of single stranded antisense oligonucleotides (ASOs). ASOs base pair with target mRNAs and direct their catalytic degradation through the action of RNase H, an endogenous enzyme present in most mammalian cells (Cerritelli and Crouch, 2009; Crooke, 1999). Phosphorothioate-modified chimeric ASOs with 2′-O-methoxyethyl (MOE) and deoxynucleotide (DNA) sugar modifications are water soluble and resistant to exonucleases (Bennett and Swayze, 2010; Henry et al., 2001; Yu et al., 2004), and RNAs paired with them are efficiently degraded by RNase H. We describe here development of a therapeutic strategy for HD using transient ASO infusion into the central nervous system that effectively suppresses huntingtin accumulation, and generates sustained phenotypic reversal in HD-like disease after transient huntingtin reduction in rodents.
RESULTS
ASOs facilitate suppression of huntingtin that is sustained for three months post-infusion into the nervous system
To determine the extent and duration of suppression of mutant huntingtin synthesis achievable with ASO infusion into the nervous system, a 20-mer phosphorothioate modified oligonucleotide complementary to human huntingtin mRNA (HuASO) and containing 2′-O-(-2-methoxy) ethyl modifications on the five nucleotides on the 3′ and 5′ ends to increase its stability, tolerability and potency (Bennett and Swayze, 2010; Henry et al., 2001; Yu et al., 2004) was infused continuously (10, 25 or 50μg/day) for two weeks into the right lateral ventricle of the BACHD mouse model of HD. BACHD mice harbor a full-length mutant human huntingtin gene with an expansion of 97 CAG/CAA repeats and express the mutant protein at approximately 1.5 times the level of the endogenous mouse huntingtin (Gray et al., 2008).
Infusion of the HuASO significantly decreased the levels of human huntingtin mRNA in a dose dependent manner (Figure 1A) (25μg/day, to 42% of the level of vehicle alone [p=0.007]; 50μg/day, to 28% vehicle [p=0.005]). For all subsequent studies a dose of 50μg/day of HuASO was used. At the end of infusion, the ASO had accumulated to significant levels (170 ± 16 μg/g brain tissue) that then decreased in abundance with approximately first order kinetics over a subsequent 16-week period (Figure 1B). This pharmacokinetic profile is similar to that observed in peripheral tissues following systemic administration of similarly modified ASOs (Yu et al., 2001). At all times post infusion, more than 90% of the remaining ASO was full length, as judged by capillary gel electrophoresis, indicating the ASO was chemically stable within cells of the nervous system.
Figure 1. Sustained reduction in human and mouse huntingtin mRNA and protein by transient ASO infusion into the CNS.
(A) Dose response for HuASO in the brain of BACHD mice immediately following continuous two-week infusion of a human huntingtin targeting ASO (HuASO) at 10, 25 or 50 μg/day. Human huntingtin mRNA levels determined by qPCR and are expressed as mean ± SEM percent (%) of human huntingtin mRNA relative to saline treated controls (n=3 per dose).
(B–E) Animals treated with 50 μg/day HuASO euthanized at 0, 4, 8, 12 and 16 weeks post-treatment termination (n=4 per time point per dose). (B) Concentrations of accumulated HuASO in brain tissue measured by capillary gel electrophoresis. (C) Levels of human mRNAs as measured by qPCR. (D) Soluble mutant huntingtin levels quantified with time resolved forester resonance energy transfer (TR-FRET) assay. (E) Immunoblot of total huntingtin levels in brains of HuASO infused mice. The more slowly migrating band is human huntingtin protein (BACHD Tg); the more rapidly migrating band is endogenous mouse huntingtin (Mouse Htt).
(F–H) Animals treated with 75 μg/day MoHuASO euthanized at 0, 2, 4, 6, 8, 11 and 16 weeks post-treatment termination (n=4 per time point per dose). Levels of (F) human and mouse (G) huntingtin mRNAs as measured by qPCR. (H) Immunoblot of total huntingtin levels in brains of infused mice. Asterisks (*p<0.05, **p<0.01, and ***p<0.001) denote statistically significant changes relative to saline infused animals (Two-way ANOVA and Bonferroni’s post hoc tests). See also Figure S1.
A significant reduction in human huntingtin mRNA levels (reduced to 38 ± 3% [p<0.001] of the vehicle-infused animals) was observed at the earliest time point (after 2 weeks of continuous infusion). This reduction persisted for 12 weeks, rising back to untreated levels only 16 weeks after the termination of treatment (Figure 1C). At 12 weeks post-treatment only 13μg/g of ASO was present in the brain (Figure 1B), yet huntingtin reduction persisted, indicating that low doses of ASO in the correct cellular compartments are sufficient to be effective and are maintained with long in vivo half lives, particularly in the brain where many of the cells are non-dividing. A similar pattern of reduction was observed for the accumulated mutant human huntingtin protein; however, the reduction was delayed relative to the mRNA (Figure 1D), reflecting a longer half-life of the protein than the mRNA. Nevertheless, by 4 weeks post-termination of ASO infusion, mutant human huntingtin protein levels were reduced by two thirds and gradually returned to untreated levels 16 weeks after the end of infusion (Figure 1D). As expected, mouse huntingtin protein (Figure 1E) and mRNA (Figure S1A) levels remained unaltered at all time points, as expected from the 8 base difference in complementarity of the mRNA with the HuASO.
The durable suppression achieved with the human huntingtin selective ASO (HuASO) was replicated with a second ASO complementary to a sequence that is identical in mouse and human huntingtin (MoHuASO). A 75μg 2-week infusion of MoHuASO into the right lateral ventricle of BACHD animals significantly reduced both human (Figure 1F) and mouse (Figure 1G) huntingtin mRNA (human reduced to 31 ± 4% [p<0.001] and mouse reduced to 17 ± 4% [p<0.001] of the vehicle-infused animals). Mouse and human huntingtin mRNA and protein remained suppressed for 3 months and did not return to vehicle treated levels until 16 weeks after the end of treatment. Accumulated protein levels were similarly reduced beginning 2 weeks after the reduction in RNA, and remaining suppressed until 16 weeks post-treatment termination (Figure 1H). As expected, control ASOs (Cnt1 and Cnt2), without complementarity in the mouse genome or human huntingtin, did not suppress mouse or human huntingtin mRNA (Figure S1B, C).
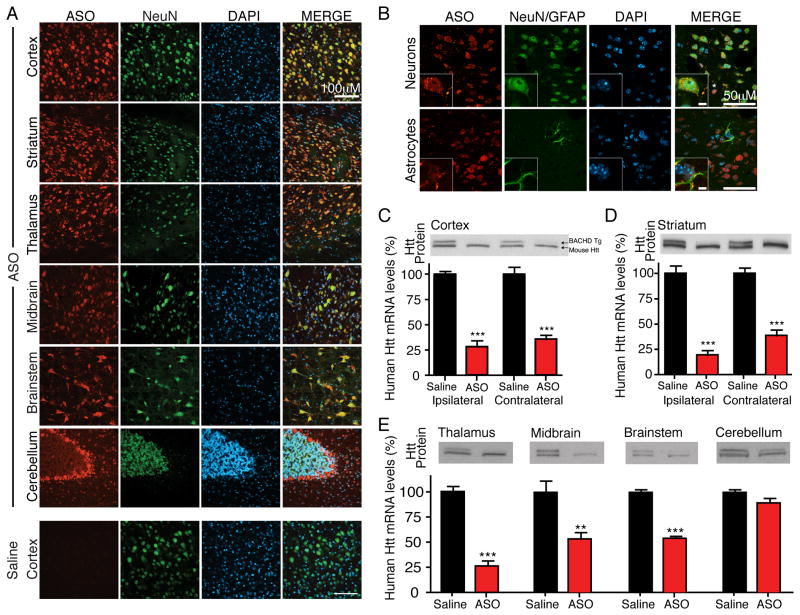
To determine the distribution and cellular uptake of antisense oligonucleotides (ASOs) delivered by infusion into the CNS, an antibody that selectively recognizes the phosphorthioate backbone of the ASOs (see Figure S2A for additional saline controls from the various brain regions) was used to probe 30 μm coronal sections from the olfactory bulb to the cerebellum (see Figure S2B for schematic of sectioning and dissections). Following a two week infusion of the HuASO into non-transgenic animals, ASO accumulation was detected in the neurons of most brain regions, including the frontal cortex, striatum, thalamus, midbrain, brainstem and cerebellum, with the exception of dense regions of white matter and cerebellar granule cells (Figure 2A). ASOs were also present in neuronal nuclei, cell bodies and neurites, as determined by colocalization of accumulated ASOs with the neuronal marker NeuN (Figure 2B). ASOs also accumulated in non-neuronal cells, including glial fibrillary acidic protein (GFAP) expressing astrocytes (Figure 2B).
Figure 2. ASOs infused into the cerebral spinal fluid distribute and are active throughout the CNS.
(A and B) An ASO targeting human huntingtin (HuASO) was infused continuously for two weeks into the right lateral ventricle of 2-month-old non-transgenic mice. Immunohistochemical staining of antisense oligonucleotides (red), neurons (NeuN, green), astrocytes (GFAP, green) and nuclei (DAPI, Blue). Antisense oligonucleotides are present in most neuronal nuclei (B, top inset), astrocytes (B, bottom inset) and other non-neuronal cells (examples denoted with *). Scale bar: (A) 100μm, (B) 50μm and inset 5μm. Representative example from two independent experiments, n=4 per treatment.
(C–E) HuASO was infused continuously for two weeks into the right lateral ventricle of 2-month old BACHD mice. Immunoblot of human (upper band) and mouse (lower band) huntingtin, and quantification of human huntingtin mRNA levels (mean % ± SEM relative to saline controls) 8 weeks post-treatment termination in BACHD (C) cortex ipsilateral and contralateral to the injection site, (D) ipsilateral and contralateral striatum (E) thalamus, midbrain, brainstem and cerebellum. Asterisks (*p<0.05, **p<0.01, and ***p<0.001) mark statistically significant changes relative to saline infused animals (n=5 per treatment, two-tailed unpaired t-tests). See also Figure S2.
In BACHD mice, the HuASO significantly suppressed production of human huntingtin mRNA in the cortex and striatum both ipsilateral (cortex to 28 ± 6% and striatum to 19 ± 4% of vehicle [p<0.001]) and contralateral to the injection site (cortex to 36 ± 4% and striatum to 39 ± 6% of vehicle [p<0.001]) (Figure 2C, D), as well more caudal regions including the thalamus (to 25 ± 5% of vehicle [p<0.001]), midbrain (to 53 ± 7% of vehicle [p=0.0096]), and brainstem (to 54 ± 3% of vehicle [p<0.001]) (Figure 2E).
Suppression of mutant huntingtin synthesis reverses disease course in YAC128 mice
To determine if suppression of mutant huntingtin, by ASO infusion into the CNS, could affect HD-like disease development, we initially used YAC128 mice that express a full-length mutant human huntingtin transgene (including the human huntingtin promoter) to about 75% the level of normal mouse huntingtin. These mice develop a progressive phenotype with many of the hallmarks of human HD, including motor and cognitive dysfunction, as well as brain atrophy (Hodgson et al., 1999). The HuASO, complementary to human huntingtin mRNA, was infused for two weeks into the right lateral ventricle of YAC128 mice beginning at 3 months of age, after which the osmotic pumps used to deliver the ASOs were removed and the animals were allowed to recover for two weeks prior to being assessed for motor coordination and anxiety (Figure 3A). As in BACHD mice, treatment with the human huntingtin specific ASO (HuASO) led to a significant reduction in huntingtin mRNA (p=0.0012) and protein levels (to 16 ± 3% of vehicle [p<0.001]) at 6 weeks after the termination of treatment (Figure 3C).
Figure 3. Motor coordination reverts to normal levels after suppression of mutant huntingtin with ASO therapy.
(A) Schematic of experimental design (B–C). At 3 months of age YAC128 mice were infused into the right lateral ventricle with an ASO that targets human huntingtin (HuASO) or saline for two weeks at 50 μg/day.
(B) Motor performance on a rotarod of mouse cohorts before (3 months old) and 1 and 2 months after ASO infusion (4 and 5 months of age) (n=7–16).
(C) Human huntingtin protein levels in brains of YAC128 mice 6 weeks after treatment termination. Data are expressed as mean percentage ± SEM relative to saline treated controls (All animals in B were analyzed, two-tailed unpaired t-test).
(D) Schematic of experimental design (E–F). Six-month-old YAC128 mice were intraventricularly infused for two weeks with 50 μg/day of HuASO or a vehicle control (saline).
(E) Human huntingtin protein levels in brains of YAC128 mice sacrifice at 9 months of age, 3 months after treatment. Data are expressed as mean percentage ± SEM relative to saline treated controls (All animals in F were analyzed, two-tailed unpaired t-test).
(F) Motor performance on a rotarod of mouse cohorts at ages between 6 and 9 months (n=8–12). Asterisks denote (*p<0.05, **p<0.01, and ***p<0.001) statistically significant differences of YAC128 HuASO treated mice (using one-way ANOVA and Tukey’s post hoc test for all behavioral tests). See also Figure S3.
Motor deficits, which develop in the YAC128 animals as early as two months of age, improved within 1 month of initiating HuASO treatment (4 months of age), and were significantly different from saline (p=0.024) and restored to non-transgenic control levels after two months (5 months of age) (Figure 3B). Behavioral assays directed at measuring anxiety (elevated plus maze, Figure S3A) and ambient motor activity (open-field, Figure S3B) revealed that deficits in ASO-treated mice were restored to non-transgenic performance levels within two months of ASO infusion, although improvements in these two behaviors failed to reach significance. Thus, transient ASO-mediated treatment after disease initiation leads to a sustained reduction in expanded huntingtin accumulation that in turn is reflected in a progressive restoration of initial motor deficits to normal over a two-month period.
To test for a therapeutic benefit from reducing expanded huntingtin in older animals, the HuASO was infused for two-weeks into 6 month old (Figure 3D), more phenotypic YAC128 mice. This yielded a sustained reduction in mutant huntingtin mRNA and protein (which remained suppressed to 42% [p<0.001] and 44% [p=0.0057], respectively) when measured 2.5 months after discontinuing treatment (Figure 3E). Phenotypic reversal was again achieved. After a two-month lag, motor function improved and treated animals were no longer significantly different from non-transgenic animals (Figure 3F). Some behavioral characteristics improved sufficiently to reach nearly normal levels by 9 months of age (Figures S3C and S3D), albeit these did not reach a p<0.05 level of confidence. Thus, while mice transiently treated at this older (6 month) age never reached the improvement achieved in younger animals, therapy initiated in these more phenotypic mice provided sustained suppression of mutant huntingtin synthesis and partial reversal of disease characteristics 3 months after stopping treatment (Figure 3A–C).
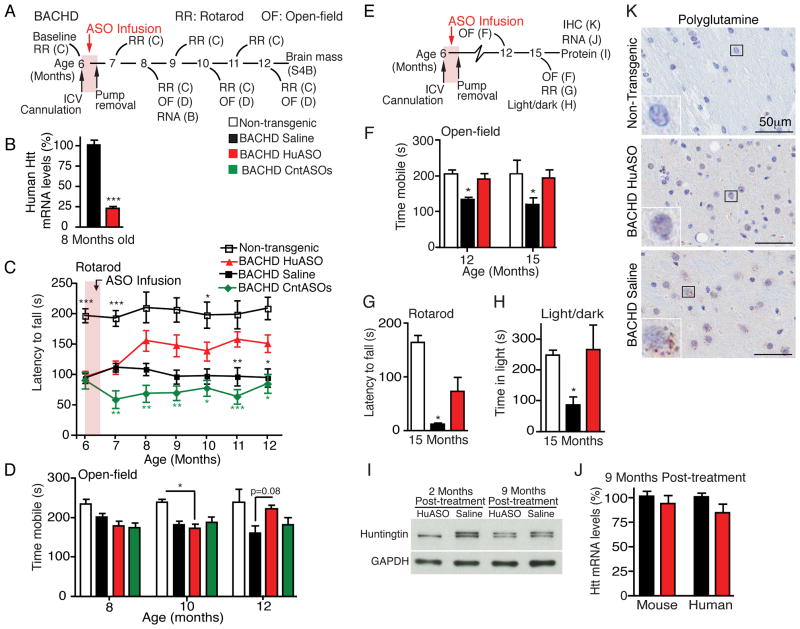
Long term sustained reversal of disease in BACHD mice after transient ASO-mediated suppression of huntingtin synthesis
Efficacy of huntingtin ASO-mediated disease attenuation was tested in a second mouse model, the BACHD animals which, like the YAC128 animals, recapitulate characteristics of human HD from expression of the full-length expanded human huntingtin gene under the control of the endogenous huntingtin promoter. BACHD mice develop progressive motor incoordination, hypokinetic motor activity and brain atrophy. Six-month old BACHD mice were infused for two weeks with an ASO specific to human huntingtin (HuASO at 50 μg/day) or vehicle and then followed for 6 months (Figure 4A), 3 months longer than the YAC128 mice were followed (Figures 3D–F, S3C,D). The degree of human huntingtin mRNA suppression (to 25% of vehicle) was the same in aged 8-month-old BACHD mice (Figure 4B) as it was in younger BACHD animals (Figure 2C).
Figure 4. Sustained phenotypic reversal from transient ASO infusion into the CNS of BACHD mice for 8 months after treatment termination.
(A) Schematic of treatment paradigms (B-D). At 6 months of age, BACHD mice were infused for two weeks with 50μg/day of an ASO that targets human huntingtin (HuASO), ASOs that have no target in the mouse genome (CntASO) or vehicle (Saline). Non-transgenic littermates were infused with vehicle (Saline).
(B) Levels of human huntingtin mRNAs 2 months post-treatment, as measured by qPCR in BACHD mice treated at 6 months of age (n=5 per treatment, data are expressed as mean % ± SEM, two-tailed unpaired t-test, p<0.0001).
(C) Motor performance on a rotarod between ages 6 and 12 months, and measured every 4 weeks. Asterisks denote statistically significant changes compared to BACHD HuASO treated animals (n=8–13).
(D) Open-field performance 2 (8 months old, p=0.111), 4 (10 months old, p=0.027) and 6 (12 months old, p=0.034) months post-treatment in BACHD mice (non-transgenic, n=3–6; BACHD, n=12–16).
(E) Schematic of treatment paradigm (F–K). At 6 months of age, BACHD mice were infused for two weeks with 50μg/day of HuASO (red) or saline (black). Non-transgenic mice are included as behavioral controls (white).
(F) Performance of mice 6 and 9 months after ASO infusion determined by time mobile in an open-field test, p=0.002 and p=0.016, respectively (n=6–8).
(G) Rotarod performance after two consecutive days of training at 9 months post-treatment (15 months of age), p<0.0001 (n=6–8).
(H) Light/dark choice at 15 months of age, p=0.016 (n=6–8). In all instances (F–H), saline treated BACHD mice performed significantly worse than non-transgenic and HuASO treated BACHD mice (One-way ANOVAs and Tukey’s post hoc tests).
(I) Immunoblot of huntingtin protein 2 and 9 months post-treatment, GAPDH is used as a loading control.
(J) 9 months post-treatment qPCR was used to quantify mouse and human huntingin mRNA levels. qPCR are expressed as mean ± SEM percent (%) of huntingtin mRNA relative to saline treated controls (n=6 per treatment).
(K) Immunohistochemical (IHC) staining for mutant huntingtin aggregates with anti-polyglutamine antibody (3B5H10) (brown) and nuclear counterstain hematoxylin (blue), 9 months post-treatment. Scale bar: 50μm. Representative images, n=6 BACHD per treatment. See also Figure S4.
BACHD mice develop significant symptoms by 6 months of age (Figure 4C; with a latency to fall in a rotarod task of 94 ± 6 seconds versus 197 ± 12 in normal, non-transgenic animals). Eight weeks after the initiation of treatment, the motor skills of the HuASO-treated BACHD mice were improved compared to their initial performance before treatment (one-way repeated measures ANOVA followed by Tukey’s post hoc test, 6 month old compared to 8 month old BACHD HuASO, p=0.0002) and to their BACHD littermates treated with control ASOs (CntASO) (Figure 4C). This improvement in performance persisted through 12 months of age (the oldest age assessed), a time 6 months after treatment had ended and more than 2 months after restoration of mutant huntingtin synthesis to untreated levels (Figure 1C, E). Similarly, a sustained, phenotypic reversal in behavior was seen in an open-field assay (Figure 4D). This latter phenotypic improvement in ASO-treated animals was not seen until 6 months after initiating ASO infusion, during which time the saline treated BACHD animals had become progressively more hypoactive.
Reversal of motor phenotype was likely due to suppression of mutant huntingtin, as ASOs that do not target huntingtin (CntASOs) did not improve motor coordination (Figure 4C) or hypoactivity (Figure 4D). Amelioration of motor phenotype was not the result of a change in body mass, as transient suppression of mutant huntingtin levels did not ameliorate transgene-mediated gain in body weight (Van Raamsdonk et al., 2006) (Figure S4A). Treatment with the HuASO also did not alter brain mass in BACHD mice (Figure S4B), consistent with improvement of function arising from recovery of damaged neurons, rather than prevention of degeneration.
To verify the longevity of the beneficial effect, a second cohort of BACHD animals was treated for two weeks at 6 months of age with the human huntingtin ASO (HuASO) or vehicle, and behavior was assessed at 12 and 15 months of age (Figure 4E). Immediately following behavior assessment at 15 months of age, huntingtin levels were determined. Hypoactivity was improved in ASO-treated BACHD animals at 6 (p=0.019) and 9 (p=0.047) months post-treatment (12 and 15 months of age, respectively) (Figure 4F). Motor coordination (rotarod) was similarly improved 9 months after treatment with the human huntingtin ASO (p=0.05) (Figure 4G). At 15 months of age, BACHD animals are anxious, as measured by failure to explore a lit arena (light/dark choice, time in light 88 ± 27 seconds for saline treated BACHD and 248 ± 20 seconds for non-transgenic animals, p=0.036). Anxiety was significantly ameliorated in HuASO treated BACHD animals (compared to saline treated BACHD, p=0.027) and was similar to non-transgenic levels (Figure 4H).
Nine months after treatment, human huntingtin levels in ASO-treated animals, measured immediately after the improvement in motor activity, anxiety, and motor coordination was recorded, were comparable to vehicle levels (Figures 4I, J). Thus, the improvement in behavior at 15 months came after mutant huntingtin had been restored to its initial level, demonstrating that the beneficial effects of ASO treatment persist for longer than target suppression.
Using an antibody directed against the expanded polyglutamine tract of mutant huntingtin (3B5H10 whose immunogen was a human huntingtin fragment containing 65 glutamines) (Brooks et al., 2004; Peters-Libeu et al., 2012), a diffuse cytoplasmic staining and pronounced puncta were visible in most striatal cells (including medium spiny neurons) of 15 month old BACHD mice treated with saline at 6 months of age (Figure 4K, bottom). In contrast, striatum from 15 month-old BACHD, treated at 6 months with HuASO, exhibited only a diffuse staining pattern, similar to that seen in vehicle treated BACHD brains, but contained very few aggregates (Figure 4K, middle). No aggregates or diffused staining were observed in non-transgenic brains (Figure 4K, top). Thus, despite the restoration of soluble mutant protein levels 9 months post-treatment (Figure 4J), transient suppression of mutant huntingtin was sufficient to delay the formation of polyglutamine aggregates, and the delay lasted longer than the reduction of the soluble mutant protein.
Suppression of mutant huntingtin reverses disease independent of wild type huntingtin level
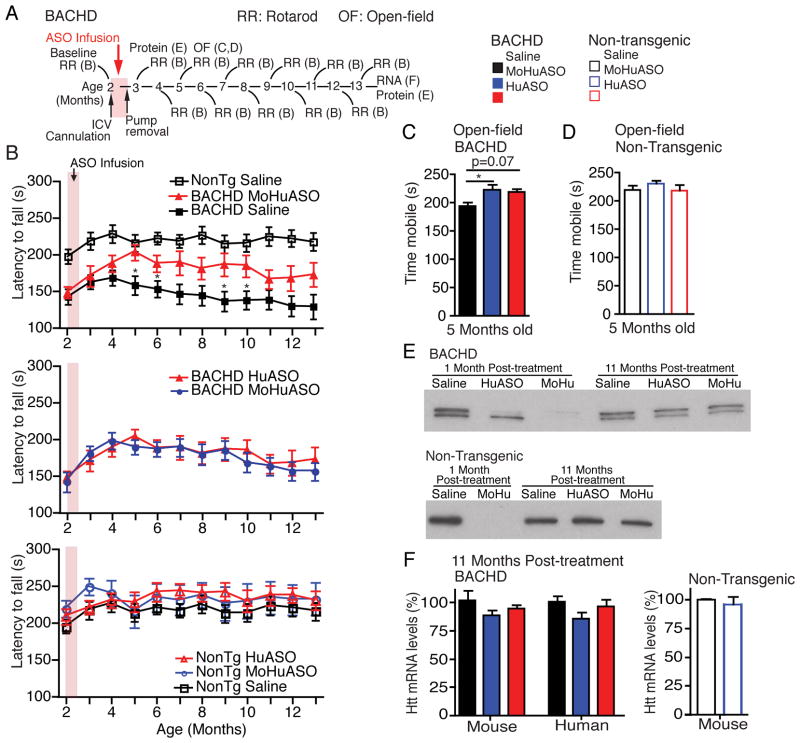
To determine if suppression of endogenous, wild type huntingtin attenuates the benefits of lowering mutant huntingtin and to determine if normal huntingtin can safely be lowered in adult animals, BACHD and non-transgenic littermates were treated at 2 months of age (Figure 5A) with vehicle, the mutant human huntingtin selective ASO that does not alter normal mouse huntingtin (HuASO) (Figure S1A), or an ASO that reduces mutant huntingtin to the same level as the HuASO while simultaneously lowering normal mouse huntingtin to 75% normal levels (MoHuASO) (Figures 1F–H). At treatment, 2-month-old BACHD animals already exhibit impaired motor coordination (before treatment the latency to fall of saline treated BACHD mice is 142 ± 11 seconds and non-transgenic animals is 197 ± 10 seconds, p=0.013) (Figure 5B, top; see also Figure S5 for all p-values).
Figure 5. Simultaneous suppression of mutant and normal huntingtin does not attenuate sustained phenotypic reversal.
(A) Schematic of experimental design. At 2 months of age BACHD mice and non-transgenic littermates were infused for two weeks with 50μg/day HuASO (red), 75μg/day of an ASO that targets mouse and human huntingtin (MoHuASO) (Blue) or saline (black) (B–D, non-transgenic, n=11–15; BACHD, n=15–20).
(B) Motor coordination (rotarod) immediately before treatment (2 months old) and monthly for 11 months after treatment. (B, Top) HuASO treated BACHD animals, saline treated BACHD animals and saline treated non-transgenic littermates, asterisks denote significance relative to HuASO treated BACHD animals. (B, Middle) MoHu treated BACHD animals and HuASO treated BACHD animals, no significant differences were found. (B, Bottom) Non-transgenic animals treated with saline, HuASO or MoHuASO, no significant differences were found.
(C–D) Time mobile in the open-field 3 months post-treatment in 5-month old (C) BACHD (p=0.025) and (D) non-transgenic littermates (p=0.515).
(E) Immunoblot of huntingtin from (top) BACHD mice and (bottom) non-transgenic littermates, 1 month or 11 months post-treatment.
(F) 11 months post-treatment qPCR was used to quantify mouse and human huntingin mRNA levels. qPCR are expressed as mean ± SEM percent (%) of huntingtin mRNA relative to saline controls (BACHD, ASO n=4 and saline n=3; Non-transgenic, ASO n=3 and saline n=2). Asterisks (*p<0.5, **p<0.01, ***p<0.001) denote statistically significant differences (using one-way ANOVA and Tukey’s post hoc test for all behavioral assays). See also Figure S5.
Selective suppression of mutant huntingtin (HuASO) improved motor coordination 3 months after treatment (5 months of age) (p=0.016) and this remained significantly improved compared to saline treated animals for 5 additional months (p=0.04) (Figure 5B, top). Remarkably, simultaneous suppression of mouse (normal) and human (mutant) huntingtin (MoHuASO) improved motor coordination to a similar magnitude and duration as selective suppression of mutant huntingtin (HuASO) (Figure 5B, middle). Hypoactivity was also returned to normal levels in BACHD animals treated with the human and mouse huntingtin-targeting ASO (MoHuASO), and had a similar effect as the human selective ASO (HuASO) (Figure 5C). Thus, transient co-suppression of normal huntingtin does not attenuate the long-term beneficial effect of ASO-mediated mutant huntingtin suppression.
Treatment of non-transgenic animals with the human huntingtin targeting ASO (HuASO), which does not target any sequence in a normal mouse, did not affect performance, consistent with the beneficial effect in BACHD animals being a direct consequence of lowered mutant huntingtin (Figure 5B, bottom). Moreover, a 75% reduction in mouse huntingtin in the normal (non-transgenic) adult brain for up to 4 months (by infusion of an ASO targeting both human and mouse RNAs (MoHuASO) (Figure 1G) did not alter motor coordination (Figure 5B, bottom) or activity (Figure 5D), indicating that this level of ASO-directed suppression of normal huntingtin is within a window for therapeutic benefit that is well tolerated. As expected, 11 months post-treatment, normal and mutant huntingtin levels in these animals was comparable to vehicle treated controls (Figures 5E, F).
ASO therapy prevents brain loss and promotes survival in an acute, fatal model of HD
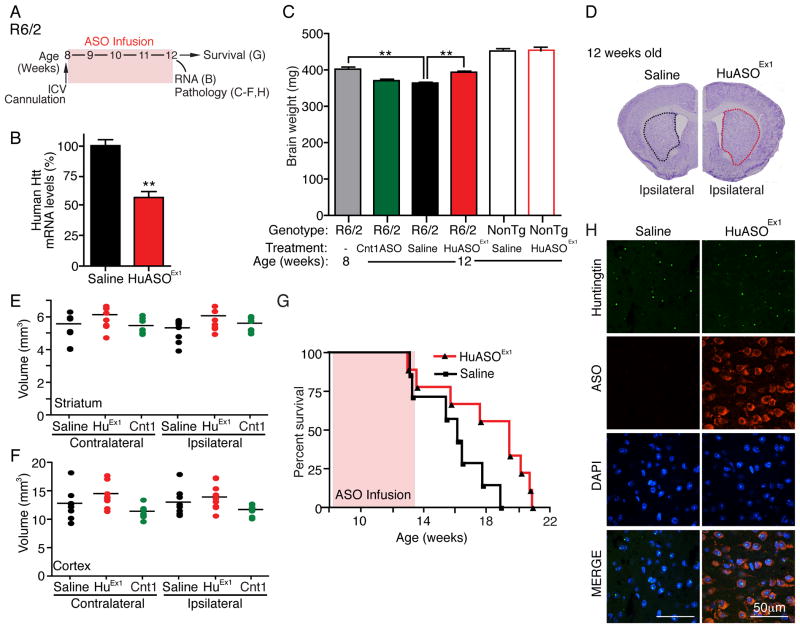
To assess the efficacy of ASO treatment in an HD mouse model that develops a very rapidly progressing fatal disease, we utilized R6/2 mice that express a fragment of the human huntingtin gene with an expanded CAG repeat and exhibit a progressive motor phenotype, a dramatic loss of brain mass, and a lifespan of approximately 16 weeks (Mangiarini et al., 1996). Infusion of an ASO designed to target the mutant R6/2 transgene (HuASOEx1) into the right lateral ventricle of R6/2 animals (50μg/day for 4 weeks) (Figure 6A) selectively suppressed production of human huntingtin mRNA (by 43 ± 5% compared to vehicle treated littermates [p=0.002]) (Figure 6B). At treatment initiation (8 weeks old), R6/2 mice had already developed obvious symptoms and had sustained gross loss of brain mass (Figure 6C, R6/2 untreated baseline). This loss in brain mass was continuous with an additional 10% of initial total brain mass lost by week 12 (Figure 6C, R6/2 vehicle treated) and further loss continuing until endstage.
Figure 6. Delayed disease progression and prolonged survival in R6/2 mice after CNS infusion of an ASO to mutant human huntingtin.
(A) Schematic of treatment paradigm. R6/2 mice and non-transgenic littermates (NonTg) received intraventricular infusion of 50μg/day of human huntingtin ASO targeting the R6/2 transgene (HuASOEx1), a control ASO (Cnt1ASO) or saline. Treatment began at 8 weeks of age and animals were euthanized at 12 weeks of age for RNA analysis, pathology, or aged for survival.
(B) Human huntingtin mRNA levels in brain of R6/2 mice treated with ASO targeting human huntingtin compared to saline treated mice (n=4). qPCR data expressed as mean ± SEM; percent (%) of RNA is relative to saline treated controls (two-tailed unpaired t test, **p<0.01).
(C) Total brain weight (mg) immediately following the 4-week ASO infusion. Untreated 8-week-old R6/2 mice are included for comparison of the disease state at the time of treatment initiation (HuASOEx1 treated R6/2, n=10; saline treated R6/2, n=11; control ASO treated R6/2, n=8; saline treated Non-tg, n=10; HuASOEx1 treated Non-tg, n=5; and untreated 8-week-old R6/2, n=4, p<0.0001). Asterisks (**p<0.01) denote statistically significant differences using one-way ANOVA, Tukey’s post hoc test.
(D) Representative image of HuASOEx1 treated R6/2 brain (left) or saline treated R6/2 brain (right) stained with cresyl violet. The striatum is outlined (Red: HuASOEx1 treated, Black: saline treated).
(E–F) Quantification of (E) striatal and (F) cortical volume in R6/2 both ipsilateral and contralateral to injection site using the cavaleri method (saline, n=9; HuASOEx1, n=10; CntASO, n=7).
(G) Plots of ages of R6/2 mice at time of death (saline, n=7; HuASOEx1, n=9). Data are expressed as Kaplan-Meyer survival curves (p=0.049). See also Figure S6.
HuASOEx1 infusion at 8 weeks of age blocked further brain loss. Brain mass of 12-week-old HuASOEx1 treated animals (394±14 mg) was comparable to the brain mass of 8-week-old untreated animals (402±14 mg) and was significantly larger than the 12-week old animals that received vehicle (364±10 mg [p=0.004]) (Figure 6C). The retention in brain mass could not be attributed to ASO-induced inflammation, as infusion of HuASOEx1 attenuated the presence of astrocytosis and microgliosis in R6/2 brains, as determined by immunohistochemical staining for glial fibrillary acidic protein (GFAP) and ionized calcium binding adaptor molecule 1 (Iba1) (Figures S6A–C). Indeed, the volumes of both the right (ipsilateral to the infusion site) and left (contralateral) sides of the striatum and cortex trended toward larger in HuASOEx1 human huntingtin ASO treated mice than in vehicle-treated and control ASO- treated animals (Figures 6D–F).
ASO-mediated suppression of mutant huntingtin mRNA initiated mid-disease (8 weeks) also significantly increased lifespan of R6/2 mice (to a median of 136 days) compared with vehicle- treated littermates (median survival of 113 days [p=0.0498]) (Figure 6G). Despite the prevention of brain loss and improvement in survival and suppression of new huntingtin synthesis, mutant huntingtin aggregates were not substantially altered in the time course of this experiment (Figure 6H). Thus, once formed the large mutant huntingtin-containing aggregates are cleared very slowly. More importantly, disease mechanism underlying mutant huntingtin-derived brain loss and disease progression must be independent of these mutant protein aggregates in this very aggressive disease model.
Suppression of huntingtin levels in the CNS after ASO infusion into non-human primates
To determine the effectiveness of ASO delivery into a larger, more complex brain whose anatomy more closely resembles the human brain, we used continuous infusion into the cerebral spinal fluid of Rhesus monkeys (brain size 90g, 75cm3, that is, 180 times larger than the mouse brain and about 1/15th the volume of a human brain). Intrathecal infusion was chosen as several devices have already been approved for infusion of drugs by this route of administration into human patients, and another antisense drug is currently in clinical trials for the treatment of familial ALS (Smith et al., 2006). Moreover, it is considerably safer to surgically implant and chronically maintain a catheter in the intrathecal space than in the lateral ventricle or the brain parenchyma.
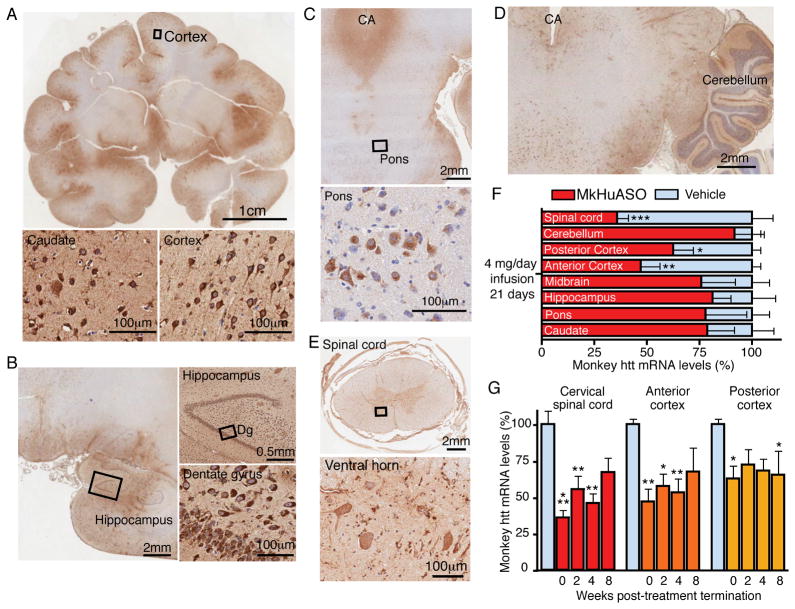
An ASO completely complementary to both Rhesus monkey and human huntingtin mRNA (MkHuASO) was infused into the cerebral spinal fluid of Rhesus monkeys at a dose of 4 mg/day for 21 days. Analysis of a series of rostral to caudal sections was used to determine that ASOs were distributed to neurons of most periventricular and lateral brain regions, as determined by immunohistochemistry with an antibody (anti-pan ASO) that recognizes the backbone of the phosphorothioate containing ASO (see Figure S7 for saline controls). ASOs accumulated in most regions of the cortex (Figures 7A and S7), distributing to pyramidal neurons as well as the surrounding tissue (Figure 7A, bottom right). Immediately after infusion, huntingtin mRNA levels in the anterior (frontal) and posterior (occipital) cortex of ASO-infused animals were significantly reduced to 47% (p=0.005) and 63% (p=0.015) of the normal levels, respectively (Figure 7F).
Figure 7. Broad ASO distribution and suppression of huntingtin mRNA in a non-human primate brain after ASO infusion into the cerebral spinal fluid.
(A) Distribution of ASOs in the Rhesus monkey brain visualized with immunostaining (anti-pan ASO) and counterstained for nuclei with hematoxylin following a 21 day intrathecal infusion of 4 mg/day ASO targeting monkey huntingtin (MkHuASO), including (A, bottom right) pyramidal neurons of the cortex and (A, bottom left) medium spiny neurons in the caudate.
(B) ASO distribution in the hippocampus and (B, bottom right) neurons in the dentate gyrus.
(C) ASO accumulation in a coronal section including the pons and cerebral aqueduct, CA; ASO is present in pontine cell bodies despite low levels of ASO accumulation in the surrounding tissue (lower panel).
(D) ASO accumulation in the cerebellum and surrounding brain regions.
(E) ASO accumulation in motor neurons in the spinal cord.
(F) mRNA levels of monkey huntingtin from various brain regions immediately following infusion, as determined by qPCR (n=3 per treatment, two-tailed unpaired t-tests).
(G) Monkey huntingtin mRNA levels in the anterior (frontal) cortex (red), posterior (occipital) cortex (orange), and cervical spinal cord (yellow), 0, 2, 4 and 8 weeks post-treatment termination (n=3 per treatment, two-way ANOVA, Bonferroni post hoc test). Data expressed as mean ± SEM, percent (%) of RNA relative to saline treated controls (blue). Asterisk (*p<0.5, **p<0.01, ***p<0.001) denotes statistically significant differences. See also Figure S7.
The infused MkHuASO could also be detected in the caudate nucleus of the striatum (Figure 7A, bottom left), particularly in periventricular medium spiny neurons, and levels of huntingtin mRNA trended toward a 25% reduction (p=0.23) (Figure 7F). ASOs were also distributed to neurons in the hippocampus (Figure 7B), pons (Figure 7C), cerebellum (Figure 7D) and spinal cord (Figure 7E). Huntingtin mRNA levels remained reduced in the anterior (frontal) cortex (to 53%), posterior (occipital) cortex (to 68%) and spinal cord (to 46%), for 4 weeks after the termination of treatment, and only began to rise toward normal levels 8 weeks after the termination of treatment (Figure 7G), similar to the duration of target-reduction observed in the rodent brain (Figure 1C). Thus, ASOs infused transiently into the cerebral spinal fluid of non-human primates produced sustained reduction in huntingtin mRNA in most brain and spinal cord regions, including those heavily implicated in HD pathology.
DISCUSSION
Our efforts have established what we believe is now a clinically feasible, dose dependent approach for providing long term disease mitigation and partial phenotypic reversal of Huntington’s disease, as well as establishing the utility of sustained benefit from a transient reduction of mutant huntingtin synthesis and accumulation. We have obtained significantly suppressed production of huntingtin mRNA and protein in a regulatable, dose-dependent manner throughout most regions of the nervous system of rodents and non-human primates by exploiting the natural flow of cerebral spinal fluid to widely deliver ASOs after focal infusion. When used in each of three mouse models of HD, short term therapy with ASOs produced sustained phenotypic disease reversal or extended survival while stopping loss of brain mass. ASO suppression of huntingtin mRNA levels was surprisingly long lived (2 or 3 months) in mice and non-human primates. Most surprisingly, and of high impact for therapy design, partial disease reversal after transient therapy was demonstrated to persist for at least 4 months after mutant huntingtin RNA and protein levels had returned to their initial levels and was unaffected by simultaneous reduction of normal huntingtin.
Our results extend, with a clinically viable strategy, earlier efforts demonstrating delayed development of motor impairments in transgenic mouse models of HD using either intraventricularly delivered siRNAs (delivered at birth) (Wang et al., 2005), or focal viral delivery of shRNAs presymptomatically into the striatum (Denovan-Wright et al., 2008; Harper et al., 2005; Rodriguez-Lebron et al., 2005). Other efforts with siRNA (DiFiglia et al., 2007) and virally delivered shRNAs (Drouet et al., 2009; Franich et al., 2008) injected into the striatum with focal expression of mutant huntingtin (also injected into the striatum, and encoded by virus) have prevented motor impairments, striatal atrophy and cell loss. To this, our efforts have established slowed disease progression, extension of survival and sustained phenotypic reversal from transient ASO infusion into symptomatic adult animals.
Furthermore, in contrast to prior approaches, we have established that the ASO infusion approach is effective and achieves a broad distribution in the non-human primate brain. This bodes well for use of an ASO approach in human therapy. It is well established that huntingtin is not simply a disease of the striatum (Gu et al., 2007). Atrophy in cortical regions is linked to patients with phenotypes manifesting primarily as emotional and cognitive impairment (Rosas et al., 2008), suggesting that a treatment selectively targeting the striatum is not likely to ameliorate these symptoms. However, a treatment with a technology like ASOs capable of targeting many regions of the brain has the potential to treat more of the symptoms of this complex disease.
Phenotypic reversal after a therapeutically feasible, transient ASO infusion initiated after symptom onset in an adult animal is consistent with phenotypic reversal in a conditional mouse model (after doxycycline administration to suppress transcription of mutant huntingtin driven by a tet-promoted transgene (Yamamoto et al., 2000)). Remarkably, the time scales for phenotype reversal are virtually identical among the two mouse models presented here (YAC128 and BACHD) and the previously characterized conditional model (Diaz-Hernandez et al., 2005). In all cases, suppression of mutant huntingtin for 8-weeks is required before reversal in phenotype is apparent. This suggests that, at least in the rodent brain, mutant huntingtin mediated dysfunction, regardless of whether it is caused by expression of an expanded full-length transgene or a fragment, shares similar mechanism and timing. More importantly, our evidence establishes that a considerable proportion of the confirmed phenotype reflects reversible dysfunction, even in aged animals. Moreover, by comparing therapeutic intervention in multiple models at various disease stages, it is clear that earlier treatment produces a quicker and more robust reversal of disease.
Regarding mechanism of mutant huntingtin toxicity, the retention of brain mass following suppression of mutant huntingtin synthesis in the R6/2 mouse without reduction in mutant huntingtin aggregates indicates that those aggregates are not the primary toxic species responsible for the remarkable loss in brain mass in this aggressive model. Conversely, a delay in aggregate formation in the ASO-treated BACHD mice is consistent with huntingtin suppression allowing clearance of toxic oligomers that seed the large aggregates or toxicity derived from the large aggregates.
A key previously unresolved question of relevance for all gene silencing approaches is how essential is normal huntingtin encoded by the unmutated allele in the adult nervous system. Huntingtin is essential for one or more early developmental steps (Nasir et al., 1995; White et al., 1997; Zeitlin et al., 1995). A continued need for huntingtin in the CNS has been proposed from efforts in mouse models with deletion of both huntingtin alleles at embryonic day 15.5 or postnatal day 5 (Dragatsis et al., 2000) and in cultured neuronal cells (Gauthier et al., 2004; Zuccato et al., 2003). However, no evidence has demonstrated toxicity following suppression of huntingtin in the adult brain. In fact, simultaneous suppression of mutant and normal huntingtin by 60% in the adult rodent striatum, and suppression of normal huntingtin by 45% in the non-human primate striatum were both well tolerated (Boudreau et al., 2009; Drouet et al., 2009; McBride et al., 2011).
Our ASO approach has extended these earlier efforts: reducing huntingtin levels by 75% throughout the CNS neither exacerbates disease nor lessens the therapeutic benefit from suppression of mutant huntingtin. Moreover, suppression of normal huntingtin for up to 3 months (the latest time assessed) in healthy primates was well tolerated. These findings provide experimental support for the existence of a therapeutic window for safe, yet efficacious, transient suppression with a non-allele selective ASO approach. They also lay the foundation for sustained phenotypic reversal from allele selective reduction of mutant huntingtin with mutant CAG targeting ASOs (Gagnon et al., 2010; Hu et al., 2009) or ASOs that target single nucleotide polymorphisms present in the mutant allele (Carroll et al., 2011; Liu et al., 2008; Pfister et al., 2009).
Finally, our evidence has provided an initial demonstration that transient suppression of huntingtin can be sufficient to ameliorate disease for an extended period of time. For diseases like Huntington’s where a mutant protein product is tolerated for decades prior to disease onset, this finding opens up the provocative possibility that transient suppression of huntingtin can lead to a prolonged effect in patients. Indeed, this raises the prospect that a transient decrease in huntingtin synthesis may allow for clearance of disease causing species that form only very slowly and may then take weeks or months to reform. If so, then a single transient application of ASOs may ‘reset the disease clock’, providing a benefit long after huntingtin suppression has ended. Of obvious interest in this regard is to use the rodent examples to determine how long the beneficial effect can persist after a single ASO injection.
EXPERIMENTAL PROCEDURES
Animals
BACHD animals were acquired from William Yang (Gray et al., 2008). BACHD mice were maintained on the congenic FVB/N background, and only female mice were used. YAC 128 mice (Hodgson et al., 1999) were obtained from the Genzyme colony at Charles River Laboratories and maintained on the congenic FVB/NJ background. R6/2 animals (Mangiarini et al., 1996) were obtained from Jackson laboratories and maintained by crossing transgene positive males with F1 (CBA × C57BL6) females (CAG repeats were maintained between 110–135). All procedures were accomplished using a protocol approved by the Institutional Animal Care and Use Committee (Department of Health and Human Services, NIH Publication 86–23). See Supplemental Experimental Procedures for detailed surgical procedures.
Oligonucleotides
ASOs used in this study were 20 nucleotides in length with five 2′-O-methoxyethyl-modified nucleosides on the 5′ and 3′-termini and 10 oligodeoxynucleotides in the central region to support RNase H. All of the internucleosidic bonds were phosphorothioate to improve nuclease resistance and enhance cellular uptake (Bennett and Swayze, 2010). Oligonucleotides were synthesized as described previously (Cheruvallath et al., 2003; McKay et al., 1999). ASOs were solubilized in 0.9% sterile saline solution or PBS. See Supplemental Experimental Procedures for ASO sequences.
Pathology and immunostaining
Mice: Anesthetized animals were subject to transcardial perfusion with ice-cold Sorenson’s phosphate buffer (SPB), and fixed with 4% paraformaldyhyde in phosphate buffer. Brains are removed, and trimmed with coronal cuts immediately rostral to the forebrain (removing the olfactory bulbs) and immediately caudal to the cerebellum (removing the spinal cord). The remaining brain was weighed in mg. Immunofluorescence was performed on 30mm coronally cut fixed frozen free-floating sections as described previously (Boillee et al., 2006). Monkey: Immunostaining was performed as described previously (Smith et al., 2006). See Supplemental Experimental Procedures for detailed methods.
Quantitative real-time PCR (TaqMan)
RNA levels were measured by quantitative real-time RT-PCR method. YAC128: total RNA was extracted using Ambion MagMAX-96 RNA isolation kit (Applied Biosystems). The RNA was reverse transcribed and amplified using TaqMan One-Step RT-PCR Master Mix Kit (Applied Biosystems). Quantitative RT-PCR reactions were conducted and analyzed on an ABI PRISM® 7500 Real Time PCR System (Applied Biosystems). Expression levels for huntingtin mRNA were normalized to Ppia (peptidylprolyl isomerase A) mRNA levels. R6/2: cDNA is made from 1ug of RNA (RNAeasy Mini Kit, Qiagen) by reverse transcription with random hexamers (SuperScript III, RT-PCR). Quantitative real-time PCR was performed on the iQ cycler (Biorad) using the TaqMan system. Huntingtin mRNA levels were normalized to Atp5b and Eif4a2. BACHD: qPCR was performed as previously described (Winer et al., 1999). Total RNA was prepared from tissue lysate utilizing Qiagen RNeasy 96 (Qiagen USA). The prepared RNA was assayed for huntingtin and cyclophilin A levels utilizing an ABI Prism 7700 (Applied Biosystems) and the resulting data analyzed by ABI Sequence Detector v1.7a software. Monkey: huntingtin mRNA levels were performed with the same protocol as the BACHD samples. See Supplemental Experimental Procedures for primers sequences.
Immunoblotting
YAC128: Tissues were homogenized in T-Per lysis buffer (Pierce). 20–30 μg of total protein was resolved on a 3–8% Novex tris-acetate gel. Blots were probed with anti-Htt MAB2166 (1:2,000 Millipore) and anti-β-tubulin (1:750, Santa Cruz Biotechnology). Proteins were visualized by quantitative fluorescence (LI-COR Biosciences). Huntingtin protein levels were normalized to β-tubulin and expressed as % vehicle treated controls.
BACHD: Tissues were homogenized in RIPA lysis buffer. 15μg of total protein lysate was resolved on a 4–12% bis-tris gel (Invitrogen). Proteins were transferred to a nitrocellulose membrane and probed with MAB2166 and anti-GAPDH (1:5,000 Abcam).
Behavioral analysis
All behavioral tests with the YAC128 mice were conducted independently at Genzyme, and tests in BACHD and R6/2 animals were conducted independently at UCSD. The range of numbers of animals used in the various behavioral assays is listed in the figure legends. The exact animals numbers in each treatment group, in each of the various behavioral assays is included in the Supplemental Experimental Procedures. Also see Supplemental Experimental Procedures for detailed procedures for accelerating rotarod, elevated-plus maze, open-field test, light-dark analysis, body mass and survival.
Statistics
Mean values were used for statistical analyses. Data are expressed as mean ± SEM. For two groups unpaired two-tailed t-tests were used; for more than two group comparisons, one-way ANOVAs were used followed by the post hoc Tukey’s multiple comparison test; for more than two comparisons of two or more groups, two-way ANOVAs followed by Bonferroni’s post hoc tests were used (Prism GraphPad and Kalidagraph). P-values for the ANOVAs are reported in the figure legends, and p-values from the post hoc tests are included in the text when making paired comparisons. A chart listing the post hoc comparisons of all rotarod analyses is provided (Figure S5). Significance of survival was determined using the Kaplan-Meyer method. p < 0.05 was considered a statistically significant difference.
Please see Supplemental Experimental Procedures for the following experimental procedures: Time resolved foerster resonance energy transfer (TR-FRET) and capillary gel electrophoresis.
Supplementary Material
Acknowledgments
We thank W. Yang (University of California, Los Angeles) for the BACHD animal model and his technical support. We also thank G. Bates (Kings College London) for the R6/2 model and her technical support. We would like to thank S. Freier, A. Watt and A. Salim (Isis Pharmaceuticals) for identification of the huntingtin ASOs and J. Matson for bioanalytical support on the mouse and monkey tissues. We thank J. Boubaker for her technical support. We thank R. Smith and D. Macdonald for their thoughtful discussions.
Footnotes
Supplemental information includes Supplemental Experimental Procedures, references and seven figures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 2010;50:259–293. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Boudreau RL, McBride JL, Martins I, Shen S, Xing Y, Carter BJ, Davidson BL. Nonallele-specific silencing of mutant and wild-type huntingtin demonstrates therapeutic efficacy in Huntington’s disease mice. Mol Ther. 2009;17:1053–1063. doi: 10.1038/mt.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks E, Arrasate M, Cheung K, Finkbeiner SM. Using antibodies to analyze polyglutamine stretches. Methods Mol Biol. 2004;277:103–128. doi: 10.1385/1-59259-804-8:103. [DOI] [PubMed] [Google Scholar]
- Carroll JB, Warby SC, Southwell AL, Doty CN, Greenlee S, Skotte N, Hung G, Bennett CF, Freier SM, Hayden MR. Potent and selective antisense oligonucleotides targeting single-nucleotide polymorphisms in the huntington disease gene/allele-specific silencing of mutant huntingtin. Mol Ther. 2011;19:2178–2185. doi: 10.1038/mt.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerritelli SM, Crouch RJ. Ribonuclease H: the enzymes in eukaryotes. FEBS J. 2009;276:1494–1505. doi: 10.1111/j.1742-4658.2009.06908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheruvallath ZS, Kumar RK, Rentel C, Cole DL, Ravikumar VT. Solid phase synthesis of phosphorothioate oligonucleotides utilizing diethyldithiocarbonate disulfide (DDD) as an efficient sulfur transfer reagent. Nucleosides Nucleotides Nucleic Acids. 2003;22:461–468. doi: 10.1081/NCN-120022050. [DOI] [PubMed] [Google Scholar]
- Crooke ST. Molecular mechanisms of action of antisense drugs. Biochim Biophys Acta. 1999;1489:31–44. doi: 10.1016/s0167-4781(99)00148-7. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Vonsattel JP, Richardson EP., Jr Morphometric demonstration of atrophic changes in the cerebral cortex, white matter, and neostriatum in Huntington’s disease. J Neuropathol Exp Neurol. 1988;47:516–525. doi: 10.1097/00005072-198809000-00003. [DOI] [PubMed] [Google Scholar]
- Denovan-Wright EM, Rodriguez-Lebron E, Lewin AS, Mandel RJ. Unexpected off-targeting effects of anti-huntingtin ribozymes and siRNA in vivo. Neurobiol Dis. 2008;29:446–455. doi: 10.1016/j.nbd.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Hernandez M, Torres-Peraza J, Salvatori-Abarca A, Moran MA, Gomez-Ramos P, Alberch J, Lucas JJ. Full motor recovery despite striatal neuron loss and formation of irreversible amyloid-like inclusions in a conditional mouse model of Huntington’s disease. J Neurosci. 2005;25:9773–9781. doi: 10.1523/JNEUROSCI.3183-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M, Sena-Esteves M, Chase K, Sapp E, Pfister E, Sass M, Yoder J, Reeves P, Pandey RK, Rajeev KG, et al. Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proc Natl Acad Sci U S A. 2007;104:17204–17209. doi: 10.1073/pnas.0708285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragatsis I, Levine MS, Zeitlin S. Inactivation of Hdh in the brain and testis results in progressive neurodegeneration and sterility in mice. Nat Genet. 2000;26:300–306. doi: 10.1038/81593. [DOI] [PubMed] [Google Scholar]
- Drouet V, Perrin V, Hassig R, Dufour N, Auregan G, Alves S, Bonvento G, Brouillet E, Luthi-Carter R, Hantraye P, Deglon N. Sustained effects of nonallele-specific Huntingtin silencing. Ann Neurol. 2009;65:276–285. doi: 10.1002/ana.21569. [DOI] [PubMed] [Google Scholar]
- Franich NR, Fitzsimons HL, Fong DM, Klugmann M, During MJ, Young D. AAV vector-mediated RNAi of mutant huntingtin expression is neuroprotective in a novel genetic rat model of Huntington’s disease. Mol Ther. 2008;16:947–956. doi: 10.1038/mt.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon KT, Pendergraff HM, Deleavey GF, Swayze EE, Potier P, Randolph J, Roesch EB, Chattopadhyaya J, Damha MJ, Bennett CF, et al. Allele-Selective Inhibition of Mutant Huntingtin Expression with Antisense Oligonucleotides Targeting the Expanded CAG Repeat. Biochemistry. 2010 doi: 10.1021/bi101208k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, Rangone H, Cordelieres FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, Saudou F. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Gray M, Shirasaki DI, Cepeda C, Andre VM, Wilburn B, Lu XH, Tao J, Yamazaki I, Li SH, Sun YE, et al. Full-length human mutant huntingtin with a stable polyglutamine repeat can elicit progressive and selective neuropathogenesis in BACHD mice. J Neurosci. 2008;28:6182–6195. doi: 10.1523/JNEUROSCI.0857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Andre VM, Cepeda C, Li SH, Li XJ, Levine MS, Yang XW. Pathological cell-cell interactions are necessary for striatal pathogenesis in a conditional mouse model of Huntington’s disease. Mol Neurodegener. 2007;2:8. doi: 10.1186/1750-1326-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper SQ, Staber PD, He X, Eliason SL, Martins IH, Mao Q, Yang L, Kotin RM, Paulson HL, Davidson BL. RNA interference improves motor and neuropathological abnormalities in a Huntington’s disease mouse model. Proc Natl Acad Sci U S A. 2005;102:5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry SP, Geary RS, Yu R, Levin AA. Drug properties of second-generation antisense oligonucleotides: how do they measure up to their predecessors? Curr Opin Investig Drugs. 2001;2:1444–1449. [PubMed] [Google Scholar]
- Hodgson JG, Agopyan N, Gutekunst CA, Leavitt BR, LePiane F, Singaraja R, Smith DJ, Bissada N, McCutcheon K, Nasir J, et al. A YAC mouse model for Huntington’s disease with full-length mutant huntingtin, cytoplasmic toxicity, and selective striatal neurodegeneration. Neuron. 1999;23:181–192. doi: 10.1016/s0896-6273(00)80764-3. [DOI] [PubMed] [Google Scholar]
- Hoogeveen AT, Willemsen R, Meyer N, de Rooij KE, Roos RA, van Ommen GJ, Galjaard H. Characterization and localization of the Huntington disease gene product. Hum Mol Genet. 1993;2:2069–2073. doi: 10.1093/hmg/2.12.2069. [DOI] [PubMed] [Google Scholar]
- Hu J, Matsui M, Gagnon KT, Schwartz JC, Gabillet S, Arar K, Wu J, Bezprozvanny I, Corey DR. Allele-specific silencing of mutant huntingtin and ataxin-3 genes by targeting expanded CAG repeats in mRNAs. Nat Biotechnol. 2009;27:478–484. doi: 10.1038/nbt.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington’s disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Liu W, Kennington LA, Rosas HD, Hersch S, Cha JH, Zamore PD, Aronin N. Linking SNPs to CAG repeat length in Huntington’s disease patients. Nat Methods. 2008;5:951–953. doi: 10.1038/nmeth.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida Y, Okada T, Kurosawa M, Oyama F, Ozawa K, Nukina N. rAAV-mediated shRNA ameliorated neuropathology in Huntington disease model mouse. Biochem Biophys Res Commun. 2006;343:190–197. doi: 10.1016/j.bbrc.2006.02.141. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- McBride JL, Boudreau RL, Harper SQ, Staber PD, Monteys AM, Martins I, Gilmore BL, Burstein H, Peluso RW, Polisky B, et al. Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: implications for the therapeutic development of RNAi. Proc Natl Acad Sci U S A. 2008;105:5868–5873. doi: 10.1073/pnas.0801775105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride JL, Pitzer MR, Boudreau RL, Dufour B, Hobbs T, Ojeda SR, Davidson BL. Preclinical safety of RNAi-mediated HTT suppression in the rhesus macaque as a potential therapy for Huntington’s disease. Mol Ther. 2011;19:2152–2162. doi: 10.1038/mt.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay RA, Miraglia LJ, Cummins LL, Owens SR, Sasmor H, Dean NM. Characterization of a potent and specific class of antisense oligonucleotide inhibitor of human protein kinase C-alpha expression. J Biol Chem. 1999;274:1715–1722. doi: 10.1074/jbc.274.3.1715. [DOI] [PubMed] [Google Scholar]
- Melone MA, Jori FP, Peluso G. Huntington’s disease: new frontiers for molecular and cell therapy. Curr Drug Targets. 2005;6:43–56. doi: 10.2174/1389450053344975. [DOI] [PubMed] [Google Scholar]
- Nasir J, Floresco SB, O’Kusky JR, Diewert VM, Richman JM, Zeisler J, Borowski A, Marth JD, Phillips AG, Hayden MR. Targeted disruption of the Huntington’s disease gene results in embryonic lethality and behavioral and morphological changes in heterozygotes. Cell. 1995;81:811–823. doi: 10.1016/0092-8674(95)90542-1. [DOI] [PubMed] [Google Scholar]
- Peters-Libeu C, Miller J, Rutenber E, Newhouse Y, Krishnan P, Cheung K, Hatters D, Brooks E, Widjaja K, Tran T, et al. Disease-Associated Polyglutamine Stretches in Monomeric Huntingtin Adopt a Compact Structure. J Mol Biol. 2012 doi: 10.1016/j.jmb.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister EL, Kennington L, Straubhaar J, Wagh S, Liu W, DiFiglia M, Landwehrmeyer B, Vonsattel JP, Zamore PD, Aronin N. Five siRNAs targeting three SNPs may provide therapy for three-quarters of Huntington’s disease patients. Curr Biol. 2009;19:774–778. doi: 10.1016/j.cub.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Lebron E, Denovan-Wright EM, Nash K, Lewin AS, Mandel RJ. Intrastriatal rAAV-mediated delivery of anti-huntingtin shRNAs induces partial reversal of disease progression in R6/1 Huntington’s disease transgenic mice. Mol Ther. 2005;12:618–633. doi: 10.1016/j.ymthe.2005.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Salat DH, Lee SY, Zaleta AK, Pappu V, Fischl B, Greve D, Hevelone N, Hersch SM. Cerebral cortex and the clinical expression of Huntington’s disease: complexity and heterogeneity. Brain. 2008;131:1057–1068. doi: 10.1093/brain/awn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Tuch DS, Hevelone ND, Zaleta AK, Vangel M, Hersch SM, Salat DH. Diffusion tensor imaging in presymptomatic and early Huntington’s disease: Selective white matter pathology and its relationship to clinical measures. Mov Disord. 2006;21:1317–1325. doi: 10.1002/mds.20979. [DOI] [PubMed] [Google Scholar]
- Smith RA, Miller TM, Yamanaka K, Monia BP, Condon TP, Hung G, Lobsiger CS, Ward CM, McAlonis-Downes M, Wei H, et al. Antisense oligonucleotide therapy for neurodegenerative disease. J Clin Invest. 2006;116:2290–2296. doi: 10.1172/JCI25424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Gibson WT, Pearson J, Murphy Z, Lu G, Leavitt BR, Hayden MR. Body weight is modulated by levels of full-length huntingtin. Hum Mol Genet. 2006;15:1513–1523. doi: 10.1093/hmg/ddl072. [DOI] [PubMed] [Google Scholar]
- Wang YL, Liu W, Wada E, Murata M, Wada K, Kanazawa I. Clinico-pathological rescue of a model mouse of Huntington’s disease by siRNA. Neurosci Res. 2005;53:241–249. doi: 10.1016/j.neures.2005.06.021. [DOI] [PubMed] [Google Scholar]
- White JK, Auerbach W, Duyao MP, Vonsattel JP, Gusella JF, Joyner AL, MacDonald ME. Huntingtin is required for neurogenesis and is not impaired by the Huntington’s disease CAG expansion. Nat Genet. 1997;17:404–410. doi: 10.1038/ng1297-404. [DOI] [PubMed] [Google Scholar]
- Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- Yu RZ, Geary RS, Monteith DK, Matson J, Truong L, Fitchett J, Levin AA. Tissue disposition of 2′-O-(2-methoxy) ethyl modified antisense oligonucleotides in monkeys. J Pharm Sci. 2004;93:48–59. doi: 10.1002/jps.10473. [DOI] [PubMed] [Google Scholar]
- Yu RZ, Zhang H, Geary RS, Graham M, Masarjian L, Lemonidis K, Crooke R, Dean NM, Levin AA. Pharmacokinetics and pharmacodynamics of an antisense phosphorothioate oligonucleotide targeting Fas mRNA in mice. J Pharmacol Exp Ther. 2001;296:388–395. [PubMed] [Google Scholar]
- Zeitlin S, Liu JP, Chapman DL, Papaioannou VE, Efstratiadis A. Increased apoptosis and early embryonic lethality in mice nullizygous for the Huntington’s disease gene homologue. Nat Genet. 1995;11:155–163. doi: 10.1038/ng1095-155. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Tartari M, Crotti A, Goffredo D, Valenza M, Conti L, Cataudella T, Leavitt BR, Hayden MR, Timmusk T, et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003;35:76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.