Summary
Selective ligands are lacking for many neuronal signaling proteins. Photoswitched tethered ligands (PTLs) have enabled fast and reversible control of specific proteins containing a PTL anchoring site and have been used to remote control over-expressed proteins. We report here a novel scheme for optical remote control of native proteins using a “photoswitchable conditional subunit” (PCS), which contains the PTL anchoring site as well as a mutation that prevents it from reaching the plasma membrane. In cells lacking native subunits for the protein, the PCS remains non-functional internally. However, in cells expressing native subunits, the native subunit and PCS co-assemble, traffic to the plasma membrane and place the native protein under optical control provided by the co-assembled PCS. We apply this approach to the TREK1 potassium channel, which lacks selective, reversible blockers. We find that TREK1, typically considered to be a leak channel, contributes to the hippocampal GABAB response.
Introduction
While the production of pharmacological reagents targeted to membrane signaling proteins has been a major objective for both academic laboratories and the pharmaceutical industry, many important membrane proteins are still without specific blockers. Moreover, where specific blockers exist, they often have high affinity and are selective only at low concentrations, so that the onset of their effect upon exposure takes a long time to develop and they bind so tightly that they are difficult to remove. The development of Photoswitched Tethered Ligands (PTLs) that are targeted to an introduced cysteine near ligand binding sites of membrane proteins opened the door to the reversible control of membrane signaling, by using two wavelengths to photoisomerize the tether between one state that permits ligand binding and a second state which prevents binding (Szobota and Isacoff, 2010). Because specificity derives from the unique geometric relationship between the ligand binding site and the engineered anchoring site, rather than from tight binding, photo-isomerization to the non-binding state rapidly removes the ligand. Moreover, the high effective concentration of the ligand near its binding site in the permissive state leads to rapid binding upon photoisomerization, itself a very rapid transition (Szobota and Isacoff, 2010). Together, these properties enable highly selective optical control of binding and unbinding on the millisecond time-scale and micron space-scale (Szobota and Isacoff, 2010).
So far, optical control with PTLs has been applied to ion channels and receptors that are over-expressed in cells. Because the introduction of the anchoring site can usually be done with minimal perturbation to protein function (Szobota and Isacoff, 2010), it should be possible to introduce the mutation into the native protein via genetic knock-in. Still, generation of a knock-in animal is laborious and expensive, making sense only when one is directly interested in the signaling by the targeted protein, but not for exploring the function of several candidate proteins as in typical pharmacological experiments. To address this, we developed a scheme for targeting optical control via a PTL to native proteins without the requirement for genetic knock-in. Our approach is to express a “photoswitchable conditional subunit” (PCS) that contains a PTL anchoring site and a mutation that retains the subunit inside the cell. This engineered subunit will not function in cells where native subunits are missing. However, in cells that express the native subunits that are required to form the functional protein complex, the native and engineered subunits will assemble inside the cell and the complex will be trafficked to the plasma membrane, thereby placing the native protein under optical control provided by the co-assembled engineered subunit.
GABAB receptors set the inhibitory tone, provide the critical feed-forward and feedback inhibition that shapes the spread of neural activity, regulates the filtering properties of neural circuits and prevents hyper-excitation and seizure (Kohl and Paulsen, 2010; Semyanov et al., 2004). In the hippocampus, the postsynaptic GABAB response was long thought to be mediated exclusively through Kir3 potassium channels (Luscher et al., 1997; Padgett and Slesinger, 2010; Ulrich and Bettler, 2007), but the genetic knockout of Kir3 subunits has suggested that another channel might also contribute to GABAB inhibition (Koyrakh et al., 2005). The identity of this additional channel has not been revealed and its function in tissue from wild-type animals remains to be determined. Using the PCS approach we show that TREK1, a 2P-potassium channel typically thought of as a leak channel, is an additional target of GABAB receptors in the hippocampus.
Results
One interesting class of channels to consider for participation in hippocampal GABAB signaling is the large family of 2P-potassium channels. These channels are typically thought of as leak channels, whose function is to set the resting potential (Noel et al., 2011). However, some of them can be regulated by GPCRs (Deng et al., 2009; Noel et al., 2011). The physiological function of these channels has remained elusive due to a lack of specific blockers. One of the 2P-potassium channels, TREK2, was found recently to be involved in the GABAB control of spatial learning in the entorhinal cortex (Deng et al., 2009). However, the entorhinal GABAB current deactivates more than ten times more slowly than the hippocampal GABAB current, suggesting that TREK2 is not the missing hippocampal channel. In the absence of specific pharmacological blockers of most 2P-potassium-channels, and because knock-out of specific genes can lead to compensatory expression of related genes, we searched for an alternative approach for selective pharmacology. We turned to the strategy of PTLs, which obtain their target selectivity not from the specificity of the ligand but from their selective attachment to the protein of interest and the precise geometric relation of the attachment site to the ligand binding site (Banghart et al., 2004; Fehrentz et al., 2011; Szobota and Isacoff, 2010; Volgraf et al., 2006). Because the PTLs are photo-isomerized between two conformations by distinct wavelengths of light and because only one of the conformations permits the ligand to bind, they can activate or block the target protein rapidly and reversibly. Thus, in principle, photo-block should provide a clear assay for when the channel is activated.
PTL for the TREK1 channel
We developed a light-blocked version of the 2P-Potassium Channel TREK1, using the PTL MAQ, which contains a maleimide (M) that tethers the molecule to a genetically engineered cysteine, a photoisomerizable azobenzene (A) linker and a pore-blocking quatenary ammonium group (Q) (Figure 1A, top). In its relaxed state, MAQ is in the trans configuration (Figure 1A and Figure 1B, left). It is rapidly photo-isomerized to the cis configuration by 380 nm light and rapidly photo-isomerized back to the trans form by 500 nm light (Figure 1B). MAQ was previously employed to photo-control the voltage-gated Shaker potassium channel (Banghart et al., 2004).
Figure 1. Light-gated TREK1.
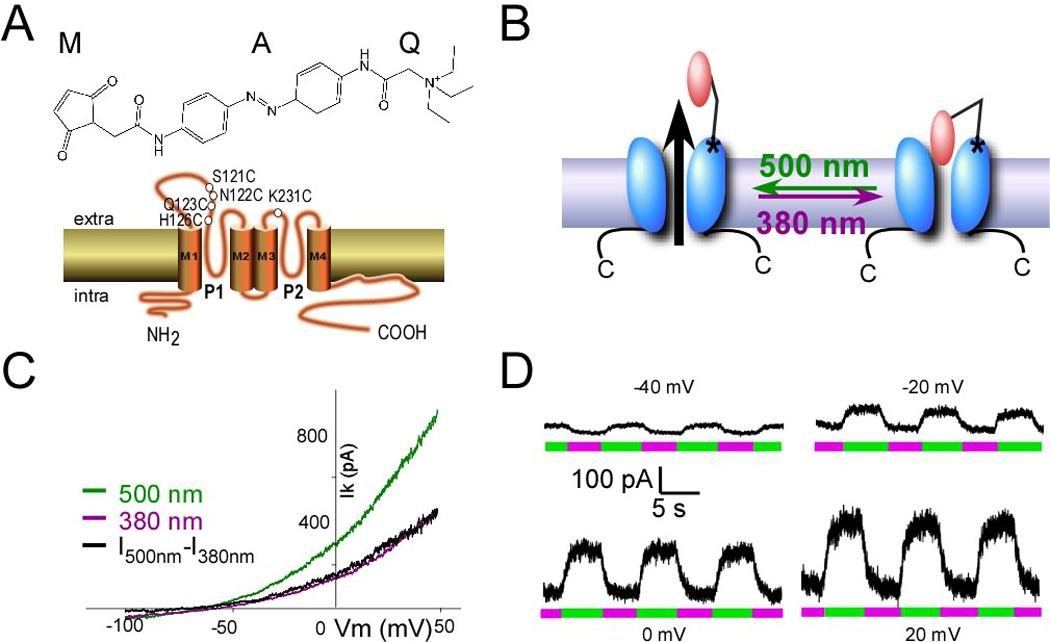
(A) (Top) MAQ consists of a maleimide (M), which tethers the photoswitch to a cysteine introduced into the outer portion of the first P-loop of the channel (bottom), a photoisomerable azobenzene (A) linker and a quaternary ammonium (Q) pore blocker. (Bottom) Cartoon showing the membrane topology of TREK1 channel and the different position tested. (B) Schematic representation of light-gated TREK1. MAQ is covalently attached to cysteine (S121C). MAQ blocks the pore in the cis configuration (380 nm light). Exposure to 500 nm light places MAQ in the trans state where the pore is unblocked. (C, D) Whole-cell recording from HEK293T cell expressing TREK1(S121C) and labeled with MAQ. Current was elicited by voltage-ramps (from −100 to 50 mV, 1s in duration) (C). Alternating illumination at 500 nm (green) and 380 nm (magenta) reversibly blocks and unblocks constant outward current, as seen at different holding potentials (D).
We introduced single cysteine mutations as attachment sites at a series of different positions in portions of the first and second pore loops (P1 and P2) of TREK1 (Figure 1A) and expressed the channel in HEK293 cells. MAQ was applied in the external solution to each of these cysteine-substituted mutants and photoswitching was tested by measuring the modulation of the current when illumination was switched back and forth from 500 nm to 380 nm. We first examined cysteine substitutions at residue N122 in P1 and K231 in P2 of TREK1, since these are homologous to the optimal site for photo-block by MAQ in the Shaker channel (Shaker 422) (Banghart et al., 2004). While both sites showed photo-modulation, they had a different dependence on light, i.e. on the configuration of the MAQ photoswitch. TREK1(K231C-MAQ) produced photo-block in the trans state (500 nm illumination), as found in Shaker (Banghart et al., 2004), but TREK1(N122C-MAQ) produced photo-block in the cis state (380 nm illumination) (Table 1). The opposite photoswitching at the two attachment positions indicates P1 and P2 differ structurally and that P2 more closely resembles the P loop of Shaker. This is interesting in view of the levels of homology of the conserved C-terminal portion of the P regions, where TREK1’s P1 and P2 have 17% and 23% identity (55% and 57% similarity), respectively, to the P of Shaker, and a unique long loop precedes TREK’s P1 (Figure S1).
Table 1. Effect of mutation on MAQ block under 380 nm light.
Values are reported as mean ± SEM with the number of determination in parenthesis. The block percentages are reported for 0 mV holding potential. To compare the blocking effects, the cells were recorded the same day with the same MAQ aliquot.
| Position And mutation |
Photoswitch | 380 nm | Light gated current amplitude (pA) |
|---|---|---|---|
| S121C | Yes | Block | 124 ± 9 (6) |
| N122C | Yes | Block | 59 ± 12 (7) |
| Q123C | No | No block | 0 (4) |
| H126C | Yes | Block | 31 ± 4 (4) |
| K231C | Yes | Unblock | − 98 ±13 (4) |
Photo-modulation was also seen at two other MAQ attachment sites in P1 (Table 1). The strongest photo-modulation was at S121C (Table 1), which displayed 64 ± 3% (n = 14) block under 380 nm light and was unblocked by isomerization to trans under illumination at 500 nm (Figure 1C). Since MAQ thermally relaxes into the trans state, TREK1(S121C-MAQ) has the advantage that the channel is unblocked and can function normally in the dark.
Subunit replacement strategy
Cysteine-substituted versions of TREK1 that can be photo-blocked by MAQ could be introduced into neurons by transfection, but this would add the heterologous protein to the native protein and result in over-expression. One way around this would be to generate a genetic knock-in that replaces the native TREK1 with a version of TREK1 that is identical except for the single cysteine substitution. However, knock-in production is lengthy and costly. We therefore sought an alternative easier strategy for introducing the MAQ photoswitch into native channels.
We developed a subunit replacement strategy to obtain optical control over a neuron’s native TREK1 channels (Figure 2A). As shown earlier, deletion of the TREK1 carboxy-terminal tail (TREK1ΔC) results in retention of the channel in the endoplasmic reticulum (Chemin et al., 2005). In agreement with this, TREK1ΔC(S121C-MAQ) in HEK293 cells produced no detectable current above background: current amplitude at 0mV was 71 ± 37 pA (n=11) for TREK1ΔC-S121C-transfected HEK293 cells versus 68 ± 40 pA (n=7) for non-transfected cells. Moreover, following exposure to MAQ, alternating illumination between 380 nm and 500 nm produced no change in the basal current in TREK1ΔC-S121C-transfected cells (Figure 2B).
Figure 2. Development of a subunit replacement strategy.
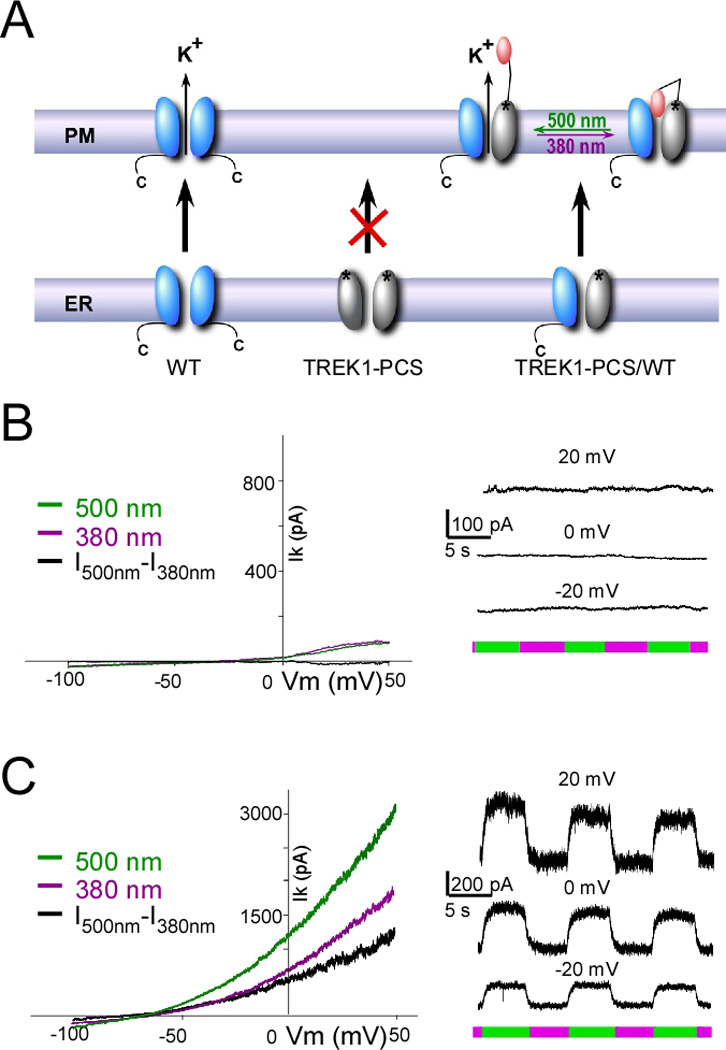
(A) Schematic representation of subunit replacement strategy. Deletion of the TREK1 carboxy-terminal tail (TREK1-PCS, grey) results in retention of the homomeric mutant channel in the endoplasmic reticulum. In contrast, the wildtype homomeric channel (WT, blue) traffics to the plasma membrane. Co-expression of TREK1-PCS with WT produces a heteromeric channel that traffics to the membrane because of the WT subunit and which can be light-gated because of MAQ attachment to the TREK1-PCS. (B–C) Whole-cell recording from HEK293T cell expressing either TREK1-PCS alone (B) or co-expressed with WT (C) and labeled with MAQ. Current was elicited by voltage-ramps (from - 100 to 50 mV, 1s in duration) (left). Alternating illumination at 500 nm (green) and 380 nm (magenta) reversibly blocks and unblocks constant outward current, as seen at different holding potentials (right).
Because the cytoplasmic N-terminal domain and the first transmembrane segment (M1) of TREK1 are sufficient to dimerize with the full-length TREK1 channel (Veale et al., 2010), we hypothesized that TREK1ΔC would dimerize with the wildtype TREK1 channel (WT) and produce a functional channel (Figure 2A). In contrast with the lack of photo-modulation of current in MAQ-labeled cells expressing TREK1ΔC(S121C) alone (Figure 2B), co-expression of TREK1ΔC(S121C) with WT in HEK293 cells yielded a TREK1 current that was strongly photo-modulated (Figure 2C). This indicates that TREK1ΔC(S121C) assembles with the WT subunits and that the heteromeric channel goes to the cell surface, where the TREK1ΔC(S121C) subunit is labeled by the charged, membrane impermeant MAQ endowing the channel with regulation by light via photoisomerization of MAQ. From here on, we refer to the TREK1ΔC(S121C) subunit that contains the cysteine photoswitch attachment site and that is retained internally unless co-assembled with a WT native subunit as the TREK1 Photoswitchable Conditional Subunit (TREK1-PCS).
Heteromeric TREK1-PCS/TREK1 channel functions normally
For the approach to work as intended, the heteromeric TREK1-PCS/WT channel would need to retain normal functions of the TREK1 channel. We tested the TREK1-PCS/WT heteromeric channel to determine whether it was regulated by external and internal stimuli in the same way as WT. To do this, we examined the sensitivity to stimuli of total WT current in cells expressing WT alone and compared this to the photo-blocked current component from cells co-expressing the TREK1-PCS along with WT, where the light-sensitive current is attributed solely to the heteromeric TREK1-PCS/WT channel labeled with MAQ on the TREK1-PCS.
TREK1 channels are inhibited by external acidification, due, it has been proposed, to titration of a histidine residue in P1 (Cohen et al., 2008; Sandoz et al., 2009), an effect which has been attributed to C-type inactivation (Bagriantsev et al., 2011; Cohen et al., 2008; Sandoz et al., 2009). We found that the light-gated current obtained from MAQ-labeled HEK293T cells co-expressing the TREK1-PCS and WT subunit is also inhibited by external acidification (Figure 3A). This inhibition of the photo-gated current in the TREK1-PCS/WT heterodimer was 53.6 ± 8 % (n=8), similar to the 60.6 ± 5 % (n=8) inhibition of total current in WT alone (p > 0.7, t-test).
Figure 3. Heteromeric channels formed by TREK1-PCS and WT retain normal regulation by external acidification.
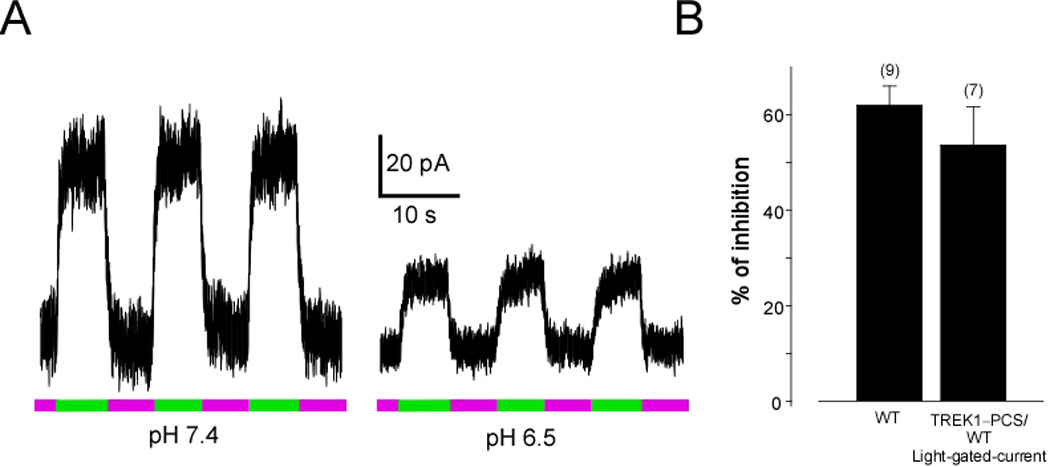
(A) Representative example of the effect of acidic pH on the light-gated current of TREK1-PCS/WT. Alternating illumination at 500 nm (green) and 380 nm (magenta) reversibly blocks and unblocks, respectively, the constant outward current, both at pH7.4 and pH6.5, but the amplitude of the photo-modulation is smaller at pH6.5. (B) Inhibition by external acidification from pH7.4 to pH 6.5 of the photo-blocked current component of TREK1-PCS/WT heteromeric channels is similar to the inhibition of total WT current under the same condition. Numbers in parentheses above bars indicate number of cells tested.
We next investigated the regulation of the TREK1-PCS/WT heterodimer channel by internal modification induced by GPCR activation. Gi-coupled receptors have been shown to enhance TREK1 current (Cain et al., 2008). We therefore tested the ability of the Gi-coupled GABAB receptor (GABABR) to regulate the light-gated current of the TREK1-PCS/WT heterodimer. Activation of GABABR induced a 2.6 ± 0.3 (n=8) fold increase in total current in cells expressing WT alone. This value was similar (p > 0.8, t-test) to the 2.7 ± 0.3 (n=9) fold increase observed in the light-gated current from the heterodimeric channel in MAQ-labeled cells co-expressing the TREK1-PCS and WT subunits (Figure 4A, C).
Figure 4. Mutation of Serine 333 to Aspartate reduces the photoswitchable current and prevents its up-regulation by GiPCR activation.
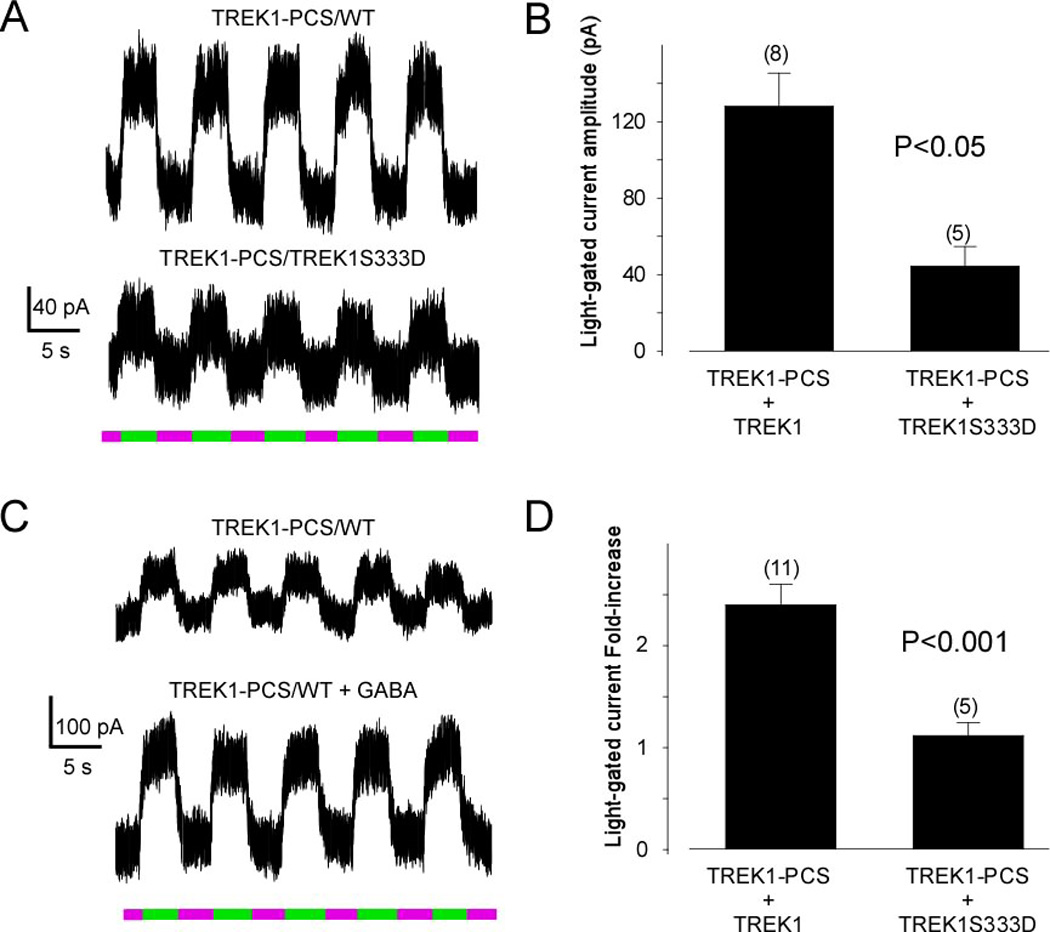
The residue S333 has been reported to be a PKA phosphorylation site and mutation to aspartic acid (to mimic the phosphorylated state) has been shown to decrease TREK1 current. (A) Alternating illumination between 380 nm (magenta) and 500 nm (green) reversibly blocks and unblocks constant outward current from TREK1-PCS/WT heterodimer (top) and from TREK1-PCS/TREK1S333D dimer (bottom). (B) Bar graph representing the light-gated current recorded from HEK cells transfected by either TREK1-PCS + TREK1 or TREK1-PCS + TREK1S333D. The S333D mutation decreased the photocurrent. (C) Alternating illumination between 380 nm (magenta) and 500 nm (green) reversibly blocks and unblocks constant outward current from TREK1-PCS/WT heterodimer before (top) and after GABA application (bottom). (D) Bar graph representing the effect of activation of GABAB receptor in HEK cells transfected with GABAB1 and GABAB2 in combination with either TREK1-PCS + TREK1 or TREK1-PCS + TREK1S333D. The S333D mutation prevents regulation of photocurrent by GABAB activation.
Residue S333 of TREK1 is a phosphorylation site that has been shown to be involved in inhibition of current by PKA (Patel et al., 1998). Moreover, it is the dephosphorylation of S333 that appears to underlie the enhancement of TREK1 current by Gi-coupled GPCRs (Cain et al., 2008; Deng et al., 2009). Part of the evidence for this is that mutation of S333 reduces or eliminates the enhancement of current by Gi-coupled GPCRs (Cain et al., 2008; Deng et al., 2009). We therefore examined the effect of the mutation S333D, which mimics the phosphorylated state of S333 and reduces current in homomeric WT channels (Lauritzen et al., 2005). Co-expression of the TREK1-PCS with TREK1(S333D) yielded a small light-gated current (44 ± 8 pA at 0 mV, n=5), approximately 3-fold smaller than the light-gated current of TREK1-PCS co-expressed with the WT subunit (128 ± 8 pA at 0 mV, n=8, p < 0.05; Figure 4A, B). In addition, as observed for total current from WT channels (Cain et al., 2008; Deng et al., 2009), the enhancement of the light-gated current by activation of GABABR was considerably reduced by the S333D mutation (Figure 4C).
Taken together, these results indicate that not only does the heteromeric TREK1-PCS/WT channel retain the typical TREK1 rectification (Figure 2), but it also retains TREK1’s normal internal and external regulation (Figures 3 and 4). In other words, the TREK1-PCS approach endows the native channel with sensitivity to light while maintaining its normal function.
TREK1-PCS approach reveals a role of TREK1 in the hippocampal GABAB response
To investigate the role of TREK1 in neurons, we transfected TREK1-PCS into dissociated cultured hippocampal neurons, labeled with MAQ and examined the effect of light. While untransfected neurons labeled with MAQ were not responsive to light (Figure S2), light could be used to control the resting membrane potential of TREK1-PCS transfected neurons that were labeled with MAQ (Figure 5A, top). Photo-block by illumination with 380 nm light induced a small but reproducible depolarization of 4.0 ± 0.8 mV (n=29) (Figure 5B). This depolarization was sufficient to increase the rate of action potential firing in response to spontaneous excitatory synaptic potentials (EPSPs) (Figure 5C–D). A similar light-induced modulation of membrane potential was seen in TREK1-PCS transfected CA1 and CA3 pyramidal neurons of hippocampal slices, indicating that PCS expression, its assembly with native TREK1 subunits and labeling with MAQ can be achieved in tissue with intact circuitry (Figure 5A, bottom).
Figure 5. TREK1 in hippocampal neurons.
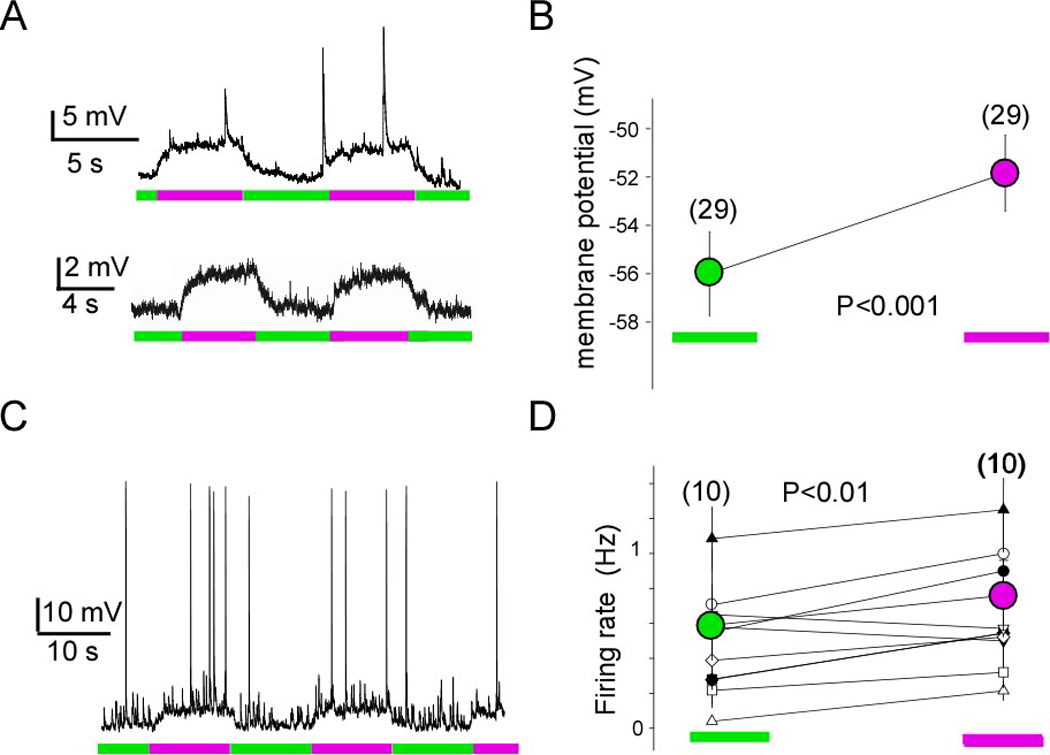
(A) Whole-cell recording from hippocampal neurons expressing TREK1-PCS in dissociated neuronal culture (top) and cultured hippocampal slice (bottom). Representative example of light modulation of the resting membrane potential, hyperpolarizing under 500 nm light (green), when TREK1 channels are unblocked, and depolarizing under 380 nm light (magenta) when the channels are blocked. (B) Average of resting membrane potential measured under 500 nm light (green, unblocked) and 380 nm light (magenta, blocked). (C) Photo-modulation of spontaneous firing of hippocampal neuron expressing TREK1-PCS and labeled with MAQ. (D) Average of the spontaneous firing rate over several minutes (2–4 minutes while alternating 500 nm and 380 nm light for 5 s each). Statistical significance determined with paired t-test.
The postsynaptic GABAB response, described by Newberry and Nicoll in 1984 (Newberry and Nicoll, 1984) which is involved in GABA-induced slow inhibitory postsynaptic potential (Dutar and Nicoll, 1988), has long been thought to be mediated by Kir3 potassium channels via activation of the Gi/o pathway (Luscher et al., 1997; Padgett and Slesinger, 2010; Ulrich and Bettler, 2007). However, not all of the current induced by the GABAB agonist baclofen is blocked by external Ba2+, a signature of Kir3 channels, and, moreover, there is a residual potassium current in Kir3.2 and Kir3.3 double knockout mice, suggesting that an additional, unidentified, K+ channel may contribute to the GABAB response (Koyrakh et al., 2005). Since the TREK1 channe1 is expressed in hippocampal neurons (Sandoz et al., 2008) and is only weakly sensitive to Ba2+ (Zhou et al., 2009), and, moreover, since it is enhanced by Gi-coupled receptors (Cain et al., 2008), we hypothesized that the TREK1 channel could be this unknown channel.
We found that TREK1-PCS transfected hippocampal neurons have no detectable photoswitched TREK1 current at rest (Figure 6C). However, the outward current induced by the GABAB receptor agonist balcofen included a component that was blocked by 380 nm light and unblocked by 500 nm light and represented 18.3 ± 3% (n = 6) of the total GABAB induced current (Figure 6B). The photoswitched component of the GABAB response could also be seen in organotypic hippocampal slice (Figure 6D; n=3 CA1 cells).
Figure 6. TREK1 is activated by GABAB in hippocampal neurons.
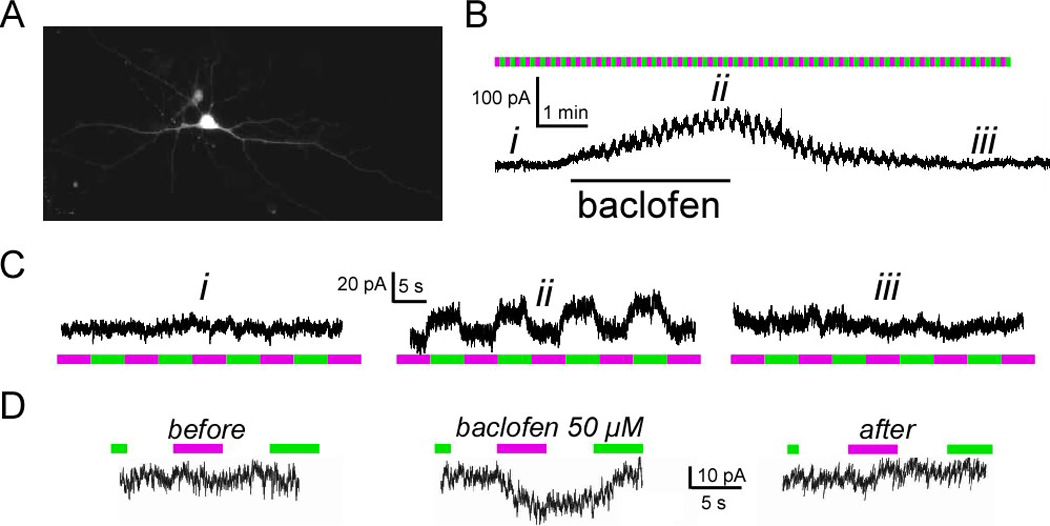
(A) Image of a cultured hippocampal neuron expressing TREK1-PCS and soluble Tomato. (B) Representative current obtained from hippocampal neuron expressing TREK1-PCS. Application of baclofen (60 µM) induces an outward current, which can be reversed upon washout. During the baclofen response the current is modulated by light (ii), indicating that TREK1 contributes to the GABAB receptor triggered current. (C) Zoom-in of the recorded current in top right during periods marked as (i), (ii) and (iii). Illumination at 500 nm (green) and 380 nm (magenta). (D) Representative current obtained from hippocampal neuron in organotypic slice expressing TREK1-PCS. During the baclofen response the current is modulated by light, indicating that TREK1 contributes to the GABAB receptor triggered current under conditions when the hippocampal circuit is intact.
To isolate the photoswitched component of the baclofen response, we blocked Kir3. Addition of 1 mM external barium, which completely blocks Kir3 current (Hibino H et al., 2010) and only partially blocks TREK1 current (Zhou et al., 2009), blocked a large component of the current and left a residual photoswitchable current (Figure S3). Finally, to address the specificity of GABAB activation, we used the competitive GABAB antagonist CGP55845. CGP55845 prevented induction of the photoswitched current by baclofen and stopped it once it had been already induced (Figure S4A, B). In addition, as expected for its ability to block signaling by GABAB receptors, pertussis toxin prevented induction of the photoswitched current by baclofen (n=5) (Figure S4C). Together, these results indicate that activation of hippocampal GABAB receptors activates not only Kir3 channels but also TREK1 channels, which are made light sensitive by the expression of the TREK1-PCS.
As neurons where recorded after 3–6 days expression of the TREK1-PCS and its expression was driven by a strong promoter (CMV), it is likely that the PCS outnumbers the native (WT) TREK1 subunit and that most newly assembled channels plasma membrane targeted channels will be PCS/WT (light-blocked) heterodimers. Assuming that the light-block of PCS/WT heterodimers will be the same in neurons as it is in HEK293 cells and taking into account that the MAQ block of TREK1 channels is approximately 64%, this suggests that approximately 30% of the GABAB induced current is carried by TREK1 channels. This value is in agreement with the amount of residual baclofen-induced current in the Kir3.1 knockout mouse and in Kir3.2 knockout mouse (26%), as well as in the Kir3.2/Kir3.3 double knockout mice (15%) (Koyrakh et al., 2005). The relative contribution of Kir3 and TREK1 channels depends, in part, on membrane voltage. Since Kir3 is an inward rectifier and TREK1 is an outward rectifier, a holding potential of −55 mV favors the TREK1 channel component, while at more negative potentials, such as −70 mV, the Kir3 component will be favored.
Discussion
Photoswitched tethered ligands have opened the door to the selective, rapid and reversible optical control of membrane signaling through proteins that are normally insensitive to light (Szobota and Isacoff, 2010, Fehrentz et al., 2011). We have developed a scheme for targeting optical control via a PTL to native proteins without the requirement for genetic knock-in. The approach is to express a PCS that contains an anchoring site for the photoswitch as well as a mutation that retains the subunit inside the cell. The engineered subunit does not traffic and so has no function unless native subunits are present, co-assemble with it and carry it to the cell surface. The PCS/WT complex is rendered light-sensitive once an externally applied membrane impermeant photoswitch is attached to the PCS, something possible only at the plasma membrane.
PCS strategy
To generate a PCS requires the fulfillment of three conditions. First, it is necessary to identify an appropriate site for cysteine modification on the extracellular face of the protein complex where the PTL will be covalently anchored so that its photoisomerization between trans and cis states results in liganding, and hence modulation of signaling, in one isomer state but not the other. Second, this cysteine-substituted subunit must be mutated in a manner that eliminates its cell-surface trafficking as a homomultimer, but which allows for its surface targeting when it is co-assembled with the wildtype subunit. Finally, the function of the heteromeric complex between the cysteine-substituted, trafficking deficient (PCS) subunit and the wildtype subunit must be efficiently gated by PTL(s) on the PCS subunit(s). We describe here how these conditions can be met.
The first step in developing a PCS—that of anchoring a PTL for photocontrol—has been successfully accomplished in a variety of proteins with a variety of ligands. The two main approaches have been steric block of an active site (either a pore of an ion channel or a catalytic site of an enzyme) or allosteric regulation by the PTL attached to a receptor’s ligand binding domain (Gorostiza and Isacoff, 2008; Szobota and Isacoff, 2010, Fehrentz et al., 2011). The method that has been generalized the most so far is pore block of potassium channels using MAQ as a photoswitchable tethered quaternary ammonium that is attached via its maleimide to a cysteine that has been introduced near the outer mouth of the pore. This was initially demonstrated for the Shaker channel (Banghart et al., 2004). Because of the high degree of conservation of the pore region of potassium channels, photo-block by MAQ was readily generalized to a diverse set of additional potassium channels, including members of two subfamilies of classical voltage-gated channels (Kv1.3 and Kv3.1), the M-current channel (Kv7.2) and one of the Ca++-activated K+ channels that generates the long-lasting action potential afterhyperpolarization (SK2) (Fortin et al., 2011). A major reason for the ease of transferring the strategy to other channels is that the high effective concentration of the quaternary ammonium ligand near the pore in the blocking state assures efficient block, even if the affinity for the blocker is low. Moreover, the energy of the azobenzene isomerization is so large that it ensures efficient dissociation in the non-blocking state even if the affinity for block by quaternary ammonium ions is high. In the present study, photo-block with MAQ was successfully applied for the first time to a 2P potassium channel, the TREK1 channel, despite its low affinity for the most broadly used quaternary ammonium blocker, tetraethylammonium (Noel et al., 2011). As further evidence of the generalizability of the approach, we also adapted MAQ photo-block to an additional 2P potassium channel target: TASK3. Based on the success of MAQ so far, it seems likely that it will work on the majority of potassium channels.
Since the PCS approach requires that the photo-control work when only a subset of subunits (the PCS) carry the PTL, but the wildtype subunits do not, the approach is particularly well suited to photo-block of an enzyme active site or channel pore, because block can usually be accomplished by a single ligand, as is the case for quaternary ammonium block of potassium channels. However, the system should also work in cases where the protein complex is composed of more than one type of subunit, such as in the NMDA receptor. In this case the subunit that controls function, the NR2 subunit, would serve as the PCS and be controlled allosterically by a PTL attached to a cysteine introduced into the ligand binding domain.
The second condition that must be fulfilled for the PCS strategy to work is that the only PCS subunits to arrive at the plasma membrane are ones that have co-assembled with native subunits. To achieve this either the subunit must naturally require co-assembly with a distinct partner to traffic to the surface or mutation(s) need to be introduced into the PCS that result in its intracellular retention except in cells that express the wild-type subunit. In addition to the C-terminal deletion of TREK1 that we employed here, several other methods have been reported that provide for this kind of control. A variety of forward trafficking signals that drive localization to the plasma membrane have been identified and these can be disabled by mutation. In addition, it is possible to introduce retention signals that hold proteins in the endoplasmic reticulum. For the PCS approach to work, the effect of the mutation(s) must be rescued by co-assembly with a wild-type subunit. Of course, the mutation(s) must also not affect the function or regulation of the protein of interest.
An example of such a strategy is found in Kir2.1 mutation of the di-acidic forward trafficking site, which leads to retention in the endoplasmic reticulum, but where co-assembly of the diacidic mutant with wildtype subunits traffics the heteromeric channel to the plasma membrane (Ma et al., 2001; 2002). A summary of characterized retention and export signals is presented in Supplemental Table 1.
In some protein complexes there is no need to introduce a mutation in order to induce endoplasmic reticulum retention since one or more of the subunits naturally use this strategy to ensure that only heteromeric assemblies of a particular kind reach the cell surface. This is what is seen in Kir6 channels, where the channel forming subunit is retained inside the cell unless co-assembled with SUR (Sakura et al., 1995; Zerangue et al, 1999). It is also what is seen with the GABAB receptor, a GPCR that is composed of GB1 and GB2 subunits, in which RXR endoplasmic reticulum retention motifs, which prevent trafficking to the cell surface, are masked in a complementary manner to allow for surface trafficking in the GB1/GB2 heteromer (Margeta-Mitrovic, et al., 2000). A functionally analogous scheme operates in NMDA receptors, which are composed of two NR1 and two NR2 subunits, with neither subtype arriving on the cell surface on its own (Okabe et al., 1999; Standley et al. 2000; Xia et al., 2001).
As long as the PTL is anchored to an introduced cysteine, one is limited to the use of charged PTLs that will not cross the plasma membrane, which are targeted to either secreted proteins or the extracellular face of membrane proteins. In this way one avoids conjugating the PTL to functionally important lone cysteines on cytoplasmic proteins, such as enzymes that have cysteine at their active sites. However, with several new strategies now available for the orthogonal attachment of probes to proteins (Boyce and Bertozzi, 2011; Liu et al., 2012; Yao et al., 2012; Cohen et al., 2012) it should be possible to expand the PCS strategy to intracellular domains of multimeric membrane proteins. Moreover, orthogonal labeling inside the cell should make it possible to apply the approach to soluble intracellular proteins as long as they are obligate heteromultimers where the PCS cannot function without the wildtype endogenous partner. It should be noted that if the PCS can heteromerize with more than one native subunit then the analysis becomes more complex, analogous to the complexity of interpreting subunit-specific pharmacological agents, knockout and dominant negative effects.
TREK1-PCS development
We generated a PCS of the TREK1 potassium channel. This is a member of the large 2P potassium channel family. Since their first isolation, the 2P potassium channels have posed a fascinating conundrum. On one hand, they are always open, leading to the impression that they are leak channels that “merely” set up the resting membrane potential. On the other hand, they are regulated by a very large number of signaling systems (including polyunsaturated fatty acids, phosphoinositides, pH, GPCRs, protein kinases, temperature and mechanical force), giving the impression that they are a vital hub of neuronal control. Adding to the mystery, their genetic knock-out often has only subtle effects, although in some cases intriguing specificity has emerged for different family members, for example in poly-unsaturated-fatty-acid-mediated neuroprotection, anesthesia, pain perception, and for a possible role in the treatment of depression (Heurteaux et al., 2004; Heurteaux et al., 2006; Mazella et al., 2010; Noel et al., 2009). Attempts at definitive determination of function have been hampered by a lack of specific, reversible pharmacological agents. Our TREK1-PCS paves the way for solving this pharmacological problem, since the 2P potassium channels show similar block by external quaternary ammonium moieties and this is the blocking ligand of the MAQ photoswitch.
In the present case of TREK1, the Shaker channel served as a successful guide for where to introduce the MAQ attachment site, even though, outside of the pore region, the 2P potassium channels have strongly diverged from the Shaker-type Kv channels. Our screen for MAQ attachment sites in the P regions of TREK1 provided one preferred position, at which block is relieved in the dark, conferred under 380 nm illumination (cis state) and relieved under 500 nm illumination (trans state). As with other azobenzene PTLs, on and off gating can be repeated many times without loss of efficacy and the switch is bistable, persisting for long periods without illumination in the higher energy cis-blocked state, but available for a rapid return to trans with light. Interestingly, in TREK1 we found differences in photo-block by MAQ when it was attached to homologous positions in the first (P1) pore region versus the second (P2) pore region. Recently obtained structures of the pore of 2P-potassium channels, TRAAK (Brohawn et al., 2012) and TWIK1 (Miller et al., 2012), have shown that the two-fold symmetry converges to an essential four-fold symmetric pore helix and selectivity filter. However, the regions homologous to our cysteine attachment sites in TREK1 are not seen in these crystal structures. Our finding that MAQ attachment to homologous positions in the P1 and P2 of TREK1 yield different blocking characteristics suggests that these portions of the pore region are not four-fold symmetric. The tandem coupling of pairs of subunits that characterizes 2P channels may serve to constrain this asymmetry.
TREK1-PCS reveals a role of TREK1 in hippocampal GABAB response
While TREK1 is highly expressed in the hippocampus, no hippocampal function had yet been identified. We took interest in the regulation of TREK1 by Gi-coupled GPCRs, since several transmitter-gated versions of these are found in the hippocampus (Padgett and Slesinger, 2010). Postsynaptically, hippocampal GABAB receptors can inhibit calcium channels (Mintz and Bean, 1993), but they are primarily known to enhance the potassium channels that underlie the slow inhibitory postsynaptic potential (IPSP). The slow IPSP is known to involve G-protein-coupled inwardly-rectifying potassium (Kir3) channels (Luscher et al., 1997). Baclofen is generally used to study the GABAB response (Dutar and Nicoll, 1988). Using baclofen, Koyrakh and colleagues showed evidences for an additional unidentified GABAB channel target (Koyrakh et al., 2005). Our PCS approach enables us to identify this channel as TREK1. As with Kir3 channels, TREK1 is also post-synaptic (Sandoz et al., 2008), where it is complexed with the post-synaptic machinery via interaction with AKAP150 (Sandoz et al., 2006). This is the second case where a 2P potassium channel has been implicated in GABAergic signaling, since TREK2 appears to mediate a different and much slower IPSP in entorhinal cortex (Deng et al., 2009).
These findings suggest that 2P potassium channels may have a broad role in synaptic signaling in the brain. It breaks with the traditional notions that Kir3 channels are the sole targets of postsynaptic GABAB receptors and that 2P-potassium channels serve simply as leak channels in the hippocampus. Our PCS approach offers an affordable and powerful strategy for identifying the molecular basis of unknown ionic currents and for obtaining a pharmacological foothold in multi-subunit signaling proteins.
Methods
Molecular Biology and Gene Expression
Cysteine mutations were introduced into mTREK1 cDNA in the pIRES2EGFP expression vector using the QuickChange mutagenesis kit (Agilent). The PCR protocol used was 1 cycle (95°, 30 s), 16 cycles (95°, 30 s; 55°, 1 min; 68°, 12 min). TREK1-PCS has been made by PCR and introduced in pIRES2EGFP expression vector. HEK293 Cells were transiently cotransfected using Lipofectamine 2000 (Invitrogen) with TREK1 mutants or TREK1-PCS. For co-expression, TREK1 or TREK1-PCS are cotransfected with a ratio of 1:3 to 1:5 with 1.6 µg of DNA total per 18 mm diameter cover slip. Hippocampal neurons were transfected using the calcium phosphate method. Each 12 mm coverslip received 1.1 µg of TREK1-PCS DNA and 0.2 µg of Tomato DNA.
Cell Culture
HEK293 cells were maintained in DMEM with 5% FBS on poly-L-lysine-coated glass coverslips. Dissociated hippocampal neurons were obtained from postnatal rats (P0-1) and plated at 75,000 cells/coverslip on poly-L-lysine-coated glass coverslips (12 mM). Neurons were maintained in media containing MEM supplemented with 5% fetal bovine serum, B27 (Invitrogen), and GlutaMAX (Invitrogen).
Electrophysiology
HEK293 cell electrophysiology was performed 24–72 h after transfection solution containing (in mM): 145 mM NaCl, 4 mM KCl, 1 mM MgCl2, 2 mM CaCl2 and 10 mM HEPES. Glass pipettes of resistance between 3 and 6 MΩ were filled with intracellular solution containing (in mM): 140 KCl, 10 Hepes, 3 Na2ATP, 0.2 Na2GTP, 5 EGTA, 3 MgCl2, pH 7.4. Cells were voltage clamped using an Axopatch 200A (Molecular Devices) amplifier in the whole cell mode.
Hippocampal neuron whole cell patch clamp electrophysiology was performed 3–6 days after transfection (DIV 12–15 for cultured neurons; DIV 6–8 for slices). For voltage and current clamp experiments in cultured neurons, extracellular solution contained (in mM): 138 NaCl, 1.5 KCl, 1.2 MgCl2, 2.5 CaCl2, 10 glucose, 5 Hepes, (plus 10 CNQX, 10 Bicucculine only for voltage clamp experiments), pH 7.4. In slices ACSF contained (in mM) 19 NaCl, 2.5 KCl, 1.3 MgSO4, 1 NaH2PO4-H2O, 26.2 NaHCO3, 11 glucose and 2.5 CaCl2 and was continuously perfused and bubbled with 95% O2/5% CO2. For all experiments, intracellular solution contained (in mM): 140 K-Gluconate, 10 NaCl, 5 EGTA, 2 MgCl2, 1 CaCl2, 10 Hepes, 2 MgATP, 0.3 Na2GTP, pH 7.2. For slice experiments MAQ was diluted in NMDG-labeling solution containing (in mM): 150 NMDG-HCl, 3 KCl, 0.5 CaCl2, 5 MgCl2, 10 HEPES and 5 glucose, pH 7.4. Only cells with a resting potential <−45 mV were analyzed. All pharmacological compounds for voltage clamp recording were dissolved in appropriate extracellular buffers before application using a gravity-driven perfusion system.
Illumination was controlled using a Polychrome V monochromator (TILL Photonics) through a 20× objective or with a Lambda DG4 high speed wavelength switcher (Sutter) with 380 nm and 500 nm filters through a 40× objective. pClamp software was used for both data acquisition and control of illumination. To conjugate MAQ, cells were incubated in 50–100 µM MAQ for 60 minutes in the dark at room temperature in standard extracellular cell buffer for either HEK293 cells or hippocampal neurons. The percentage of block was calculated from the current induced by a voltage-ramp at −20 mV as (I500-I380/I500)*100.
Preparation of cultured hippocampal slices
Hippocampi were obtained from postnatal Sprague-Dawley rats (postnatal days 6 and 7), cut into 400-µm slices and cultured on 0.4-µm Millicell culture inserts (Millipore) in Neurobasal-A medium (Gibco) supplemented with 20% horse serum (vol/vol), insulin, ascorbic acid, GlutaMAX (Gibco), penicillin/streptomycin, HEPES and Ara-C. Slices were transfected 2–3 d after isolation by Biolistic gene transfer using a BioRad Helios Gene Gun and gold microcarriers coated with both DNA encoding TREK1-PCS in Pires2EGFP and cytosolic tdTomato (to aid in the visualization of the transfected cells).
Supplementary Material
Acknowledgments
We thank Mu-Ming Poo, Andreas Reiner, Thomas Berger and Sylvain Feliciangeli for helpful discussion, Amanda Patel and Michel Lazdunski for the TREK1 construct in pIRES2EGFP, Jean-Philippe Pin for GABABR constructs, Dirk Trauner for MAQ and Alexandre Mourot for guidance in its use, and Sandra Wiese, Zhu Fu and Wayland Chu for technical assistance. The work was supported by a grant to E.Y.I. and R.H.K from the National Institutes of Health (PN2EY018241), as well as grants to E.Y.I from the National Science Foundation (FIBR 0623527) and the National Institutes of Health (R01 NS35549) and by support to G.S. from the Philippe Foundation and the French National Center for Scientific Research (CNRS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagriantsev SN, Peyronnet R, Clark KA, Honore E, Minor DL., Jr Multiple modalities converge on a common gate to control K2P channel function. EMBO J. 2011;30:3594–3606. doi: 10.1038/emboj.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7:1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Bertozzi CR. Bringing chemistry to life. Nat Methods. 2011;8:638–642. doi: 10.1038/nmeth.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain SM, Meadows HJ, Dunlop J, Bushell TJ. mGlu4 potentiation of K(2P)2.1 is dependant on C-terminal dephosphorylation. Mol Cell Neurosci. 2008;37:32–39. doi: 10.1016/j.mcn.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Chemin J, Patel AJ, Duprat F, Lauritzen I, Lazdunski M, Honore E. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 2005;24:44–53. doi: 10.1038/sj.emboj.7600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Ben-Abu Y, Hen S, Zilberberg N. A Novel Mechanism for Human K2P2.1 Channel Gating: Facilitation of C-type gating by protonation of extracellular histidine residues. J Biol Chem. 2008;283:19448–19455. doi: 10.1074/jbc.M801273200. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Zou P, Ting AY. Site-specific protein modification using lipoic Acid ligase and bis-aryl hydrazone formation. Chembiochem. 2012;13:888–894. doi: 10.1002/cbic.201100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Xiao Z, Yang C, Rojanathammanee L, Grisanti L, Watt J, Geiger JD, Liu R, Porter JE, Lei S. GABA(B) receptor activation inhibits neuronal excitability and spatial learning in the entorhinal cortex by activating TREK-2 K+ channels. Neuron. 2009;63:230–243. doi: 10.1016/j.neuron.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P, Nicoll RA. A physiological role for GABAB receptors in the central nervous system. Nature. 1988;332:156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Fehrentz T, Schonberger M, Trauner D. Optochemical genetics. Angew Chem Int Ed Engl. 2011;50:12156–12182. doi: 10.1002/anie.201103236. [DOI] [PubMed] [Google Scholar]
- Fortin DL, Dunn TW, Fedorchak A, Allen D, Montpetit R, Banghart MR, Trauner D, Adelman JP, Kramer RH. Optogenetic photochemical control of designer K+ channels in mammalian neurons. J Neurophysiol. 2011;106:488–496. doi: 10.1152/jn.00251.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorostiza P, Isacoff EY. Optical switches for remote and noninvasive control of cell signaling. Science. 2008;322:395–399. doi: 10.1126/science.1166022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, Lang-Lazdunski L, Widmann C, Zanzouri M, Romey G, Lazdunski M. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. Embo J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD, Noble F, Blondeau N, Widmann C, Borsotto M, et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Kohl MM, Paulsen O. The roles of GABAB receptors in cortical network activity. Adv Pharmacol. 2010;58:205–229. doi: 10.1016/S1054-3589(10)58009-8. [DOI] [PubMed] [Google Scholar]
- Koyrakh L, Lujan R, Colon J, Karschin C, Kurachi Y, Karschin A, Wickman K. Molecular and cellular diversity of neuronal G-protein-gated potassium channels. J. Neurosci. 2005;25:11468–11478. doi: 10.1523/JNEUROSCI.3484-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen I, Chemin J, Honore E, Jodar M, Guy N, Lazdunski M, Jane Patel A. Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 2005;6:642–648. doi: 10.1038/sj.embor.7400449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DS, Tangpeerachaikul A, Selvaraj R, Taylor MT, Fox JM, Ting AY. Diels-Alder cycloaddition for fluorophore targeting to specific proteins inside living cells. J Am Chem Soc. 2012;134:792–795. doi: 10.1021/ja209325n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Ma D, Zerangue N, Lin YF, Collins A, Yu M, Jan YN, Jan LY. Role of ER export signals in controlling surface potassium channel numbers. Science. 2001;291:316–319. doi: 10.1126/science.291.5502.316. [DOI] [PubMed] [Google Scholar]
- Ma D, Zerangue N, Raab-Graham K, Fried SR, Jan YN, Jan LY. Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron. 2002;33:715–729. doi: 10.1016/s0896-6273(02)00614-1. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000 Jul 27;(1):97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- Mazella J, Petrault O, Lucas G, Deval E, Beraud-Dufour S, Gandin C, El-Yacoubi M, Widmann C, Guyon A, Chevet E, et al. Spadin, a sortilin-derived peptide, targeting rodent TREK-1 channels: a new concept in the antidepressant drug design. PLoS Biol. 2010;8:e1000355. doi: 10.1371/journal.pbio.1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AN, Long SB. Crystal structure of the human two-pore domain potassium channel K2P1. Science. 2012;335:432–436. doi: 10.1126/science.1213274. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Bean BP. GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron. 1993;10:889–898. doi: 10.1016/0896-6273(93)90204-5. [DOI] [PubMed] [Google Scholar]
- Newberry NR, Nicoll RA. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. Nature. 1984;308:450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Noel J, Sandoz G, Lesage F. Molecular regulations governing TREK and TRAAK channel functions. Channels (Austin) 2011;5 doi: 10.4161/chan.5.5.16469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009;28:1308–1318. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Miwa A, Okado H. Alternative Splicing of the C-Terminal Domain Regulates Cell Surface Expression of the NMDA Receptor NR1 Subunit. J Neurosci. 1999;19:7781–7792. doi: 10.1523/JNEUROSCI.19-18-07781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakura H, Ammälä C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- Padgett CL, Slesinger PA. GABAB receptor coupling to G-proteins and ion channels. Adv Pharmacol. 2010;58:123–147. doi: 10.1016/S1054-3589(10)58006-2. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz G, Douguet D, Chatelain F, Lazdunski M, Lesage F. Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proc Natl Acad Sci U S A. 2009;106:14628–14633. doi: 10.1073/pnas.0906267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz G, Tardy MP, Thummler S, Feliciangeli S, Lazdunski M, Lesage F. Mtap2 is a constituent of the protein network that regulates twik-related K+ channel expression and trafficking. J Neurosci. 2008;28:8545–8552. doi: 10.1523/JNEUROSCI.1962-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz G, Thummler S, Duprat F, Feliciangeli S, Vinh J, Escoubas P, Guy N, Lazdunski M, Lesage F. AKAP150, a switch to convert mechano-, pH- and arachidonic acid-sensitive TREK K(+) channels into open leak channels. Embo J. 2006;25:5864–5872. doi: 10.1038/sj.emboj.7601437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakura H, Ammälä C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci. 2001;21:3063–3072. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Standley S, Roche KW, McCallum J, Sans N, Wenthold RJ. PDZ domain suppression of an ER retention signal in NMDA receptor NR1 splice variants, Neuron. 2000;28:887–898. doi: 10.1016/s0896-6273(00)00161-6. [DOI] [PubMed] [Google Scholar]
- Szobota S, Isacoff EY. Optical control of neuronal activity. Annu Rev Biophys. 2010;39:329–348. doi: 10.1146/annurev.biophys.093008.131400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich D, Bettler B. GABA(B) receptors: synaptic functions and mechanisms of diversity. Curr Opin Neurobiol. 2007;17:298–303. doi: 10.1016/j.conb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Veale EL, Rees KA, Mathie A, Trapp S. Dominant negative effects of a non-conducting TREK1 splice variant expressed in brain. J Biol Chem. 2010;285:29295–29304. doi: 10.1074/jbc.M110.108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgraf M, Gorostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. Allosteric control of an ionotropic glutamate receptor with an optical switch. Nat Chem Biol. 2006;2:47–52. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Reddy MM, Quinton PM. Effects of a new cystic fibrosis transmembrane conductance regulator inhibitor on Cl- conductance in human sweat ducts. Exp Physiol. 2004;89:417–425. doi: 10.1113/expphysiol.2003.027003. [DOI] [PubMed] [Google Scholar]
- Xia H, Hornby ZD, Malenka RC. An ER retention signal explains differences in surface expression of NMDA and AMPA receptor subunits. Neuropharm. 2001;41:714–723. doi: 10.1016/s0028-3908(01)00103-4. [DOI] [PubMed] [Google Scholar]
- Yao JZ, Uttamapinant C, Poloukhtine A, Baskin JM, Codelli JA, Sletten EM, Bertozzi CR, Popik VV, Ting AY. Fluorophore targeting to cellular proteins via enzyme-mediated azide ligation and strain-promoted cycloaddition. J Am Chem Soc. 2012;134:3720–3728. doi: 10.1021/ja208090p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channel. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- Zuzarte M, Rinne S, Schlichthorl G, Schubert A, Daut J, Preisig-Muller R. A di-acidic sequence motif enhances the surface expression of the potassium channel TASK-3. Traffic. 2007;8:1093–1100. doi: 10.1111/j.1600-0854.2007.00593.x. [DOI] [PubMed] [Google Scholar]
- Zhou M, Xu G, Xie M, Zhang X, Schools GP, Ma L, Kimelberg HK, Chen H. TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J Neurosci. 2009;29:8551–8564. doi: 10.1523/JNEUROSCI.5784-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


