Abstract
Chemotaxis is crucial for many physiological processes including the recruitment of leukocytes to sites of infection, trafficking of lymphocytes in the human body, and metastasis of cancer cells. A family of small proteins, chemokines, serves as the signals, and a family of G-protein coupled receptors (GPCRs) detects chemokines and direct cell migration. One of the basic questions in chemotaxis of eukaryotes is how a GPCR transduces signals to control the assembly of the actin network that generates directional force for cell migration. Over the past decade, a variety of signaling components have been implicated to transduce the GPCR signaling to the actin cytoskeleton. Studies in a lower eukaryotic organism, Dictyostelium discoideum, have allowed us to discover evolutionary conversed components involved in the GPCR-controlled actin network during chemotaxis. However, complete pathways linking GPCR to the actin network are still far from clear. Here we first summarize the previous studies on these components, and then update with our finding showing a new pathway, consisting of a GPCR, Gβγ, Elmo/Dock, Rac and Arp2/3 and actin. We suggest that this pathway serves as a direct linkage between the GPCR/G-protein, the chemoattractant sensing machinery, and the actin cytoskeleton, the machinery of cell movement during chemotaxis of eukaryotic cells.
Keywords: Dictyostelium, Dock, Elmo, GPCR, actin, chemotaxis, cytoskeleton, signaling
Introduction
Chemotaxis, the directional movement of cells toward chemoattractants, plays critical roles in diverse physiological processes, such as the recruitment of leukocytes to sites of invading pathogens, trafficking of lymphocytes throughout a human body, and metastasis of cancer cells.1 This behavior is achieved by integrating chemoattractant signals from receptors, such as chemokine receptors, a family of GPCR which sense gradients of chemokines, and regulating cell movement accordingly. The chemotactic response in eukaryotes such as Dictyostelium discoideum, immune cells and cancer cells is accomplished by signal transduction between two machineries—the GPCR/G-protein sensing machinery and the actin cytoskeleton moving machinery. In response to a chemokine, GPCRs signal through heterotrimeric G-proteins consisting of Gα and Gβγ subunits, which in turn regulates a variety of downstream signaling pathways involved in chemotaxis.2,3 Currently, many aspects that regulate chemokine gradient sensing and migration of human cells are still unknown. Fortunately, the molecular mechanisms that control the fundamental aspects of chemotaxis appear to be evolutionarily conserved, and studies in the lower eukaryotic model system, D. discoideum, have allowed us to uncover molecular components. Over the years, many of the core components of the signaling network have been elucidated, and basic questions have been addressed in the studies of chemotaxis using the model system D. discoideum.4 Here, we will mainly focus on the major signaling components and pathways that connect GPCR/G-protein, the chemoattractant sensing machinery to the actin cytoskeleton, the machinery of cell movement in D. discoideum. Because this is a mini review, the reference list is incomplete. Whenever possible, reference is made to reviews or papers that provide access to the original literature.
Signaling Components Involved in Chemotaxis in D. discoideum
Many of the core components of chemotaxis signaling have been elucidated. In D. discoideum, binding of cAMP, a chemoattractant, to the receptor, cAR1, induces the dissociation of heterotrimeric G-proteins into Gα and Gβγ subunits.5 Free Gβγ activates the small Ras-like G-proteins, leading to the activation of phosphoinsositide 3-kinase (PI3K), and the generation of phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) from phosphatidylinositol 4,5-bisphosphate (PIP2). Once generated, PIP3 prompts the membrane translocation of proteins containing pleckstrin homology (PH) domains, such as the cytosolic regulator of adenylyl cyclase (CRAC) and protein kinase B (PKB), which play roles in the regulation of actin polymerization during chemotaxis.6,7 Phosphatase and tensin homolog (PTEN), a well-known tumor suppressor, acts as an opposite regulator by converting PIP3 to PIP2. When PTEN is missing, cells lose the regulation of PIP3 production and localization, and as a consequence, cells are defective in chemotaxis by generating multiple protrusions in random directions due to the misregulation of the actin cytoskeleton.8,9 It has been therefore well established that PIP3 is essential for chemotaxis until it was faced with a challenge from the later studies by Hoeller and Kay. They found that D. discoideum cells lacking all five PI3K isoforms and PTEN are still able to migrate directionally suggesting that there must be parallel pathways that transduce signals from GPCR/G-protein to the actin network.10
Indeed, additional components that overlap with the PIP3 pathway have been revealed recently. For instance, Chen et al. reported that a patatin-like phospholipase A2 (PLA2) functions in parallel to PI3K signaling to mediate chemotaxis.11 By using a genetic screen in the D. discoideum cells treated with PI3K inhibitors, the authors identify the genes whose disruption causes more serious chemotaxis. They found that PLA2 mutation alone gives only mild defects of chemotaxis, however the mutant cells show dramatically impaired chemotaxis in the presence of PI3K inhibitors, indicating that both PLA2 and PIP3 function in pathways which redundantly regulate chemotaxis.
Following the finding that PI3Ks and PLA2 are involved in chemotaxis, Veltman and Van Haastert went further to screen mutants exhibiting normal chemotaxis with inhibited PI3K and PLA2 activity, and thus revealed another signaling mechanism that is PI3K and PLA2 independent. They found that 7 h starved pi3k/pla2 null cells have normal chemotaxis, but cells under typical 5 h starvation do not. By using genetic screening, they identified a soluble guanylyl cyclase (sGC) that can compensate for PI3Ks and PLA2 in later development. Interestingly sGC has two functions: as a protein that interacts with the actin filaments at the front, while as an enzyme which produces cyclic guanosine monophosphate (cGMP) to induce myosin filaments at the back of cells.12
Recent findings about TORC2 (target of rapamycin complex 2) provide more insights into elucidating the signaling mechanisms in chemotaxis. TORC2 was first reported to regulate polarized actin assembly in S. cerevisiae.13 Lee et al. then identified a TORC2 that plays a role in integrating cell movement during chemotaxis and signal relay in Dictyostelium.14 Additionally, Kamimura and colleagues found that PKB and PKBR1, which is tethered to the plasma membrane via N-terminal myristoylation, can be phosphorylated at their hydrophobic motifs mediated by TORC215 and at their activation loops mediated by phosphoinositide-dependent kinase.16 Besides, Cai et al. found a RasC-TORC2-PKB signaling pathway and demonstrated that activated RasC regulates TORC2 activity.17,18 Interestingly, this work was complemented by the discovery of the Sca1/RasGEF/PP2A complex. Charest and colleagues reported that this complex translocates to the plasma membrane upon cAMP stimulation, regulates RasC and the downstream TORC2-PKB pathway, and is the target of PKB-mediated negative feedback that terminates RasC activation.19
ElmoE Links GPCR Signaling to Actin Network in Chemotaxis
Despite much progress, there are still significant gaps in our understanding of the signaling pathways controlling chemotaxis. On one hand, as summarized above, the cAR1/GαGβγ machinery regulates four signaling enzymes, PI3K, PLA2, sGC and TORC2, all of which are involved in chemotaxis, but it remains unclear how these proteins associate with upstream G protein subunits; on the other hand, although these enzymes are implicated in regulating the actin cytoskeleton to drive cell migration, it is still not well understood by what mechanisms they link to downstream components to control the actin network.
Recently, we discovered a novel signaling pathway that directly links GPCR signaling to the actin cytoskeleton during eukaryotic chemotaxis. We demonstrated that a Gβγ effector, ElmoE, transduces GPCR signaling to the actin network during chemotaxis in D. discoideum.20 The ELMO (EnguLfment and cell Motility) protein family was first discovered as an essential component involved in engulfment of dead cells in C. elegans. ELMO and DOCK180, dedicator of cytokinesis, form a complex that functions as a bipartite guanine nucleotide exchange factor to optimally activate the small GTPase Rac and promote actin cytoskeleton rearrangement.21 It was reported that DOCK-ELMO complexes receive signals from GPCRs to activate Rac leading to cell migration.22,23 However, how GPCRs and other receptors link to and activate DOCK-ELMO has not been determined so far. We demonstrated that GPCRs regulates ELMO function through forming a complex containing Gβγ and an Elmo protein. In addition, we discovered that ElmoE associates with Dock-like proteins, small GTPase RacB, and actin nucleation Arp2/3 complex as well. We also found that activation of cAR1 promotes the association between ElmoE and Gβγ and induces RacB activation. Furthermore, we observed that ElmoE is associated with F-actin, and enriched at the leading pseudopod of chemotaxing cells. Taken together, these findings reveal a new paradigm including a GPCR, Gβγ, Elmo/Dock, Rac, Arp2/3 and F-actin as essential components, and represent the first direct linkage between Gβγ and the downstream effectors (Fig. 1).
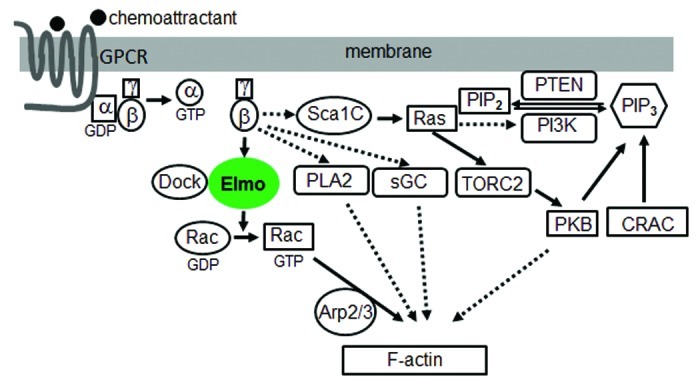
Figure 1. A model of the signaling network from GPCR to the actin cytoskeleton during chemotaxis. Solid lines indicate association and dashed lines indicate a lack of data for association.
Concluding Remarks
Although the chemotaxis signaling cascade in D. discoideum is one of the best-established signal transduction systems, many important questions are still open. A few examples follow. (1) Are there any additional signaling components and pathways which link GPCR and the actin cytoskeleton? (2) Why multiple signaling pathways are needed for chemotaxis? (3) Do these pathways contribute equally to regulate chemotaxis? (4) Do the multiple signaling pathways talk to each other? If so, when and where? (5) Do these pathways play an evolutionarily conserved role in mammalian cells? The knowledge obtained, together with the novel approaches developed in the genetically amendable model organism D. discoideum, will help us to further understanding the molecular mechanisms underlying chemokine GPCR-mediated chemotaxis of mammalian cells, which, in turn, will provide the foundation needed to rationally select the targets for medical interventions and open therapeutic opportunities for human diseases.
Acknowledgments
J.Y. and T.J. are supported by intramural fund from National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/19740
References
- 1.Jin T, Xu X, Hereld D. Chemotaxis, chemokine receptors and human disease. Cytokine. 2008;44:1–8. doi: 10.1016/j.cyto.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin T. GPCR-controlled chemotaxis in Dictyostelium discoideum. Wiley interdisciplinary reviews Systems biology and medicine 2011; 3:717-27. [DOI] [PubMed]
- 3.Wang Y, Chen CL, Iijima M. Signaling mechanisms for chemotaxis. Dev Growth Differ. 2011;53:495–502. doi: 10.1111/j.1440-169X.2011.01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin T, Xu X, Fang J, Isik N, Yan J, Brzostowski JA, et al. How human leukocytes track down and destroy pathogens: lessons learned from the model organism Dictyostelium discoideum. Immunol Res. 2009;43:118–27. doi: 10.1007/s12026-008-8056-7. [DOI] [PubMed] [Google Scholar]
- 5.Xu X, Meckel T, Brzostowski JA, Yan J, Meier-Schellersheim M, Jin T. Coupling mechanism of a GPCR and a heterotrimeric G protein during chemoattractant gradient sensing in Dictyostelium. Sci Signal. 2010;3:ra71. doi: 10.1126/scisignal.2000980. [DOI] [PubMed] [Google Scholar]
- 6.Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 1998;95:81–91. doi: 10.1016/S0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- 7.Meili R, Ellsworth C, Lee S, Reddy TB, Ma H, Firtel RA. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 1999;18:2092–105. doi: 10.1093/emboj/18.8.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/S0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- 9.Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–23. doi: 10.1016/S0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 10.Hoeller O, Kay RR. Chemotaxis in the absence of PIP3 gradients. Curr Biol. 2007;17:813–7. doi: 10.1016/j.cub.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Iijima M, Tang M, Landree MA, Huang YE, Xiong Y, et al. PLA2 and PI3K/PTEN pathways act in parallel to mediate chemotaxis. Dev Cell. 2007;12:603–14. doi: 10.1016/j.devcel.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veltman DM, Keizer-Gunnik I, Van Haastert PJ. Four key signaling pathways mediating chemotaxis in Dictyostelium discoideum. J Cell Biol. 2008;180:747–53. doi: 10.1083/jcb.200709180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–68. doi: 10.1016/S1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Comer FI, Sasaki A, McLeod IX, Duong Y, Okumura K, et al. TOR complex 2 integrates cell movement during chemotaxis and signal relay in Dictyostelium. Mol Biol Cell. 2005;16:4572–83. doi: 10.1091/mbc.E05-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamimura Y, Xiong Y, Iglesias PA, Hoeller O, Bolourani P, Devreotes PN. PIP3-independent activation of TorC2 and PKB at the cell’s leading edge mediates chemotaxis. Curr Biol. 2008;18:1034–43. doi: 10.1016/j.cub.2008.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamimura Y, Devreotes PN. Phosphoinositide-dependent protein kinase (PDK) activity regulates phosphatidylinositol 3,4,5-trisphosphate-dependent and -independent protein kinase B activation and chemotaxis. J Biol Chem. 2010;285:7938–46. doi: 10.1074/jbc.M109.089235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai H, Das S, Kamimura Y, Long Y, Parent CA, Devreotes PN. Ras-mediated activation of the TORC2-PKB pathway is critical for chemotaxis. J Cell Biol. 2010;190:233–45. doi: 10.1083/jcb.201001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan J, Hereld D, Jin T. Chemotaxis: new role for Ras revealed. Protein Cell. 2010;1:879–80. doi: 10.1007/s13238-010-0119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charest PG, Shen Z, Lakoduk A, Sasaki AT, Briggs SP, Firtel RA. A Ras signaling complex controls the RasC-TORC2 pathway and directed cell migration. Dev Cell. 2010;18:737–49. doi: 10.1016/j.devcel.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan J, Mihaylov V, Xu X, Brzostowski JA, Li H, Liu L, et al. A Gβγ Effector, ElmoE, Transduces GPCR Signaling to the Actin Network during Chemotaxis. Dev Cell. 2012;22:92–103. doi: 10.1016/j.devcel.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, et al. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol. 2002;4:574–82. doi: 10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- 22.Côté JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 2007;17:383–93. doi: 10.1016/j.tcb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddien PW, Horvitz HR. The engulfment process of programmed cell death in caenorhabditis elegans. Annu Rev Cell Dev Biol. 2004;20:193–221. doi: 10.1146/annurev.cellbio.20.022003.114619. [DOI] [PubMed] [Google Scholar]


