Abstract
Rationale
Anhedonia—diminished capacity to experience pleasure—is associated with tobacco dependence and smoking cessation failure. However, the mechanisms linking anhedonia and smoking are unclear.
Objectives
This study examined whether trait anhedonia predicted cognitive processing of emotional faces during experimentally-manipulated acute tobacco deprivation in smokers. Because nicotine may offset reward processing deficits in anhedonia and these deficits may become expressed during abstinence, we hypothesized that anhedonia would predict diminished cognitive processing of happy (vs. neutral) facial expressions in nicotine deprived but not nondeprived states.
Methods
Smokers not attempting to quit (n=75; 10+cig/day) completed anhedonia questionnaires in a baseline session. Participants then attended two counterbalanced experimental sessions: one following 18-hours of tobacco abstinence and one after unrestricted smoking. At both sessions, they completed a computer-based measure of attentional interference induced by emotional facial expressions.
Results
The extent to which anhedonia predicted Happiness interference differed as a function of deprivation status (ps ≤ .04, ηp 2s > .06). Anhedonia predicted lower interference by happy (vs. neutral) faces in the deprived condition (r=−.28, p=.02) but not in the nondeprived condition (r=.08, p=.51). Analyses of a secondary measure of anhedonia found marginally-significant effects in the same direction.
Conclusions
These findings indicate that disrupted processing of positively-valenced social cues occurs upon abstinence in high-anhedonia individuals. This alteration may motivate reinstatement of smoking in order to remediate these deficits. More broadly, these results suggest that the neuropharmacological pathways affected by nicotine may underlie disrupted emotional processing in anhedonia—a prominent feature in several psychiatric disorders.
Keywords: Anhedonia, Smoking, Nicotine Dependence, Nicotine Withdrawal, Emotional Processing, Facial expressions
Introduction
Anhedonia—the inability to experience pleasure in response to rewarding stimuli—is a key depression phenotype that is implicated in addiction (Hatzigiakoumis et al. 2011). Though levels of anhedonia can acutely fluctuate (e.g., onset to offset of depressive episodes), anhedonia is typically stable (Loas et al. 2009). Trait anhedonia lies on a continuum, varies widely in the population, and is psychometrically distinct from other constructs such as affective flattening, sadness, and amotivation (Leventhal et al. 2006; Loas et al. 2009; Loas et al. 1994). Anhedonia’s neuropathology likely involves attenuated mesolimbic activity and reduced sensitivity to the effects of non-drug rewards on phasic mesolimbic dopamine release (Nutt et al. 2007; Stein 2008).
Emerging data demonstrates an association between anhedonia and various addictive disorders (Hatzigiakoumis et al. 2011). In the case of tobacco use, trait anhedonia prospectively predicts persistence of nicotine dependence and risk of relapse following cessation (Cook et al. 2010; Niaura et al. 2001), even after adjusting for other affective symptoms (Leventhal et al. 2008b; Zvolensky et al. 2009). A promising theoretical model of the mechanisms underlying this phenomenon is that individuals with high anhedonia are motivated to smoke, in part, because of nicotine’s reward-enhancing effects (Cook et al. 2007). Indeed, nicotine stimulates mesolimbic dopaminergic release, which amplifies the reinforcing (and potentially hedonic) properties of other rewards (Paterson 2009). Thus, nicotine may briefly counteract deficient mesolimbic activity and offset diminished reward processing in high-anhedonia individuals. Once habitual tobacco use is established, smoking discontinuation may lead to the expression and exacerbation of pre-existing reward processing deficits in anhedonic smokers (Watkins et al. 2000). Such changes could lead to the resumption of smoking either following brief periods of abstinence (e.g., overnight) or during an intentional cessation attempt, which could ultimately explain anhedonia’s relation with persistent nicotine dependence.
In support of this model, Cook et al. (2007) found that anhedonia predicted larger improvements in subjective affect during an experimental positive mood induction when participants concurrently smoked a nicotinized (vs. denicotinized) cigarette. In studies of acute nicotine deprivation, anhedonia predicts diminished acute positive affect and greater urge to smoke for pleasure enhancement when smokers are acutely abstinent (Cook et al. 2004; Leventhal et al. 2009). By contrast, anhedonia is not associated with negative affect or desire to smoke to reduce negative affect during nicotine deprivation (Cook et al. 2004; Leventhal et al. 2009), suggesting that this pathway is specific to appetitive processes rather than a general effect on any type of affective process.
Prior work examining emotional processes involved in anhedonia-smoking co-occurrence has utilized self-report measures of subjective appetitive states. However, emotional processing may be better characterized by indirect, objective measures that implicitly assess cognitive processing of appetitive stimuli (Van der Gucht et al. 2009). Indeed, a body of research illustrates that individuals with psychiatric disorders, including depression, exhibited altered attentional responses to emotional stimuli, particularly human facial expressions (Mathews and MacLeod 2005). Thus, it is important to examine the interactive effects of anhedonia and nicotine on cognitive processing of appetitive social stimuli, such as happy facial expressions. Examining anhedonia’s influence on processing emotional faces is also of interest because social stimuli are very powerful reinforcers and human faces have high ecological validity.
This study examined between-person variation in anhedonia as a predictor of cognitive processing of emotional faces in smokers as a function experimentally manipulated acute nicotine deprivation. Based on the notion that nicotine offsets appetitive processing deficits linked with anhedonia and that these deficits become expressed during abstinence, we hypothesized that anhedonia would predict diminished cognitive processing of happy faces in nicotine deprived but not nondeprived states.
Methods
Participants
Participants were 136 smokers recruited via community advertisements. Inclusion criteria were: (1) ≥ 18 years old; (2) regular cigarette smoking for 2+ years; (3) currently smoking 10+ cig/day; (4) normal or corrected-to-normal vision; and (5) fluent in English. Exclusion criteria were: (1) current DSM-IV non-nicotine substance dependence; (2) current DSM-IV mood disorder or psychotic symptoms to minimize cognition-impairing effects of acute psychiatric dysfunction; (3) breath carbon monoxide (CO) levels < 10ppm at intake; (4) use of non-cigarette forms of tobacco or nicotine products; (5) use of psychiatric medications; and (6) currently pregnant. Participants were compensated $200 for completing the study. Participants who were ineligible (n = 34), dropped out (n = 15), twice failed to meet abstinence criteria at the deprived session (n = 2; see below), or had outlying data on the study task (n = 10) were excluded, leaving a final sample of 75 for analyses. The University of Southern California Internal Review Board approved the protocol.
Procedure
Following a telephone screen, participants attended an in-person baseline session involving informed consent, breath CO analysis, psychiatric interview, and other measures of mood and smoking.
Participants then attended two counterbalanced (deprived and nondeprived) experimental sessions that commenced at 12pm. For deprived sessions, participants were instructed not to smoke after 8pm the night before the session. For nondeprived sessions, they were instructed to smoke normally.
The procedures were identical across the two sessions except that participants smoked a cigarette of their preferred brand (to standardize deprivation level) at the outset of the nondeprived session prior to providing an exhaled CO, whereas the deprived session began with CO assessment. Participants’ with CO indicating non-abstinence (> 9ppm) at their deprived session could return later that week for a second attempt (n = 12). Those with CO > 9ppm on their second attempt were dropped from further participation (n = 2). Participants were then administered measures of nicotine withdrawal, followed by a modified Stroop task and visual probe task assessing attentional bias toward smoking-related and emotional cues (not reported here), and then the face processing task, which was completed 30–40 minutes into the visit.1
Measures
Baseline Session
Structured Clinical Interview for DSM-IV Non Patient Edition (First et al. 2002) was used to assess psychiatric diagnoses for eligibility purposes.
Fagerström Test of Nicotine Dependence (FTND; Heatherton et al, 1991). The FTND is a well-validated six-item measure of nicotine dependence severity.
Snaith Hamilton Pleasure Scale (SHAPS; Snaith et al. 1995). The SHAPS is a 14-item anhedonia questionnaire. Participants rate the degree of pleasure (0 = Definitely agree to 3 = definitely disagree) they would hypothetically experience in response to various interest/pastimes, social activities, and sensory experiences that are typically pleasant (e.g., “I would enjoy being with family or close friends”). A mean score across the 14 items is generated. The construct validity, internal consistency, and test-retest reliability of the SHAPS are excellent (Franken et al. 2007; Leventhal et al. 2006).
Tripartite Pleasure Inventory (TPI; Leventhal 2010). The TPI is a self-report measure of trait anhedonia, for which participants rate 12 commonly pleasant experiences that span interest/pastimes, social interaction, sensory, and goals/mastery (e.g., “Accomplishing things, such as work, taking care of family, or housework”). For the Responsivity (TPI-R) subscale, participants rate how much pleasure/happiness/enjoyment they usually feel from each experience (4 = “No Pleasure” to “Extreme Pleasure” = 0). A mean score across the 12 items is calculated. Although the psychometric properties of the TPI-R have not yet been published, in this sample the TPI-R had good internal consistency (Cronbach’s α = .87) and excellent convergent validity with the SHAPS (r = .70). The TPI-R served as a secondary anhedonia measure in this study to examine if effects were consistent across multiple measures.
The 20-item Center for Epidemiologic Studies Depression Scale (CESD; Radloff 1977) was used to assess depressive symptom severity.
Experimental Sessions
The Minnesota Nicotine Withdrawal Scale (MNWS; Hughes and Hatsukami 1986), a well-validated measure of eleven symptoms and signs of tobacco withdrawal was used to assess robustness of the deprivation manipulation. The MNWS composite was the mean of each symptom rating (range 0–5).
Emotional Interference Gender Identification Task (EIGIT; Kolassa and Miltner 2006; Leventhal and Kahler 2010). The EIGIT is a measure of implicit cognitive processing of socioemotional stimuli. In this task, participants categorize the gender of pictures of human faces expressing varying emotions. Greater attentional capture by a stimulus’ emotional content will induce more interference away from the target response (i.e., gender categorization) and result in slower reaction times (RTs). EIGIT interference scores exhibit moderate internal consistency and associate with relevant personality traits (Leventhal and Kahler 2010).
Task procedure
The task was administered via computer, with stimuli and instructions presented on a 17″ monitor. Participants were instructed that the task examined detection speed and accuracy. They were informed that faces would be presented on the screen and they were to identify the gender of each face by pressing a corresponding keyboard button as quickly and as accurately as possible. Following eight practice trials with corrective feedback, participants completed 192 experimental trials with no feedback.
On each trial, a target was presented and remained on the screen until a response was made. An inter-stimulus-interval varying from 1000 to 1500 ms elapsed between trials to prevent anticipation of target onset.
Stimuli
Pictures were selected from the Japanese and Caucasian Facial Expressions of Emotion system (Matsumoto and Ekman 2004). As part of the development of these stimuli, pictures were coded by the Facial Action Coding System (Ekman and Friesen 1978) to ensure the validity for intended emotion and comparability of expression intensity across stimuli. Stimuli were 9″ by 6.25″ and presented at screen center. Eight different pictures were used for each affective category (Happiness, Anger, Fear, Surprise). Each affective category had a matching neutral control set of 8 pictures including the same actor displaying a neutral expression. Within each category, there were two Caucasian females, two Caucasian males, two Japanese females, and two Japanese males.
Task Structure
The 192 experimental trials included two meta-blocks of 96 trials. One meta-block contained emotional stimuli and the other contained the matched neutral stimuli, with block order counterbalanced across experimental sessions. Within each meta-block, four separate blocks (one for each affect category) were presented in random order. Within each 24-trial block, the 8 stimuli in that category were each presented 3 times in random order with a 15-second inter-block interval. The blocked structure was used to prevent carry-over of interference effects across stimulus categories (Waters et al. 2005).
Scoring
Outliers in RT data can have substantial impact on data interpretation, resulting in both false positive and false negative results (Ratcliff 1993). In addition, error responses on emotional interference tasks can be generated by a variety of factors outside of those central to the process being studied (e.g., fast guesses, lapses in attention) and are typically dealt with by eliminating them from analyses (Leventhal and Kahler 2010; Waters et al. 2003). Thus, task data from participants with mean RTs 3 SD above the mean for one or more of the study conditions (deprived-emotional, deprived-neutral, nondeprived-emotional, nondeprived-neutral) were discarded (n = 10) as were RTs for individual trials with incorrect responses (mean error rate = 3.6%) or responses that were > 3 SD of each participant’s mean RT to remove outliers (Leventhal and Kahler 2010).2 The remaining trials were used to calculate each participant’s mean RT for the 24 trials within each category. Outcomes were interference scores for each affect category (mean of emotional trials – mean of matched neutral trials). Interference scores for Happiness was the primary outcome. To examine the discriminant validity of findings (i.e., whether associations with anhedonia were specific to positively-valenced versus any affectively-valenced stimulus) we analyzed the other interference scores (i.e., Anger, Surprise, Fear) as a secondary outcomes.
Analytic Plan
Following calculation of descriptive statistics and examination of intercorrelations, all variables were checked for normality and transformations to approximate normality were applied when appropriate. To assess deprivation effects, MNWS and CO were compared across deprivation conditions using paired samples t-tests. Single-sample t-tests were conducted for each interference score to test departures from zero. To address the study’s primary aim, mixed general linear model analysis was used with interference score serving as the dependent variable. Each model included between-subjects continuous anhedonia score, within-subjects deprivation status (deprived vs. nondeprived), and their interaction as predictors. Separate models were tested for Happiness, Anger, Fear, and Surprise interference outcomes. Each model was tested twice—once using SHAPS score as the between-subjects predictor and once substituting the TPI-R score as the predictor. Each model was re-tested after adjusting for CESD scores and the CESD × Deprivation interaction term to examine whether anhedonia predicted variation in emotional processing over and above shared variance with depression. Simple effect analyses examined the correlation between anhedonia and interference scores separately in each deprivation condition (with and without adjusting for the effects of CESD). Additional models including order (deprived-first/nondeprived-second vs. nondeprived-first/deprived-second) yielded no significant main or interaction effects involving order, and the primary results for anhedonia were unchanged. Therefore, analyses reported do not include order as a factor.
Results
Sample Characteristics
The average age was 42.3 (SD = 10.0) years, 72% were male, self-identified racial composition was 61% black and 39% white, and 13% reported being Hispanic/Latino. On average, participants smoked 16.8 (SD = 7.2) cigarettes per day and started smoking regularly at 18.1 (SD = 3.6) years of age. The average FTND score was 5.3 (SD = 2.2), with 8% exhibiting very low dependence (score of 0 to 2), 35% low (3–4), 15% medium (5), 23% high (6–7), and 20% very high (8–10). None of the above demographic and smoking variables were significantly associated with EIGIT interference scores. Scores on the affect measures were SHAPS (M = 0.63; SD = 0.38), TPI-R (M = 1.05, SD = 0.59), and CESD (M = 9.04, SD = 7.38), and 17.3% scored above cutoffs on the SHAPS indicating clinically significant anhedonia (Snaith et al. 1995). Analyses of correlations among baseline variables showed that the SHAPS and TPI-R were not significantly associated with CESD, FTND, or age, but were associated with each other.
Manipulation Check
Deprivation effects were large for both CO (Deprived: M= 5.79, SD = 2.10; Nondeprived: M= 29.29, SD = 12.20; Contrast: t = −16.95, p < .0001, Cohen’s d = −2.00) and MNWS scores (Deprived: M= 1.83, SD = 1.01; Nondeprived: M= 0.98, SD = 0.93; Contrast: t = 7.31, p < .0001, d = 0.86).2
Analyses of Interference Scores
The mean interference scores for Happiness [Nondeprived, M(SD) = −6.9 (92.9); Deprived, M(SD) = −9.7 (125.2)], Anger [Nondeprived = −4.7 (86.2); Deprived = −22.2 (161.3)], Fear [Nondeprived = 3.6 (78.5); Deprived = −43.2 (167.4)], and Surprise [Nondeprived = −5.1 (65.3); Deprived = −1.9 (112.7)] stimuli were not significantly different from zero for any of the conditions, with the exception of a significant negative interference score for Fear faces in the deprived condition (p = .03).
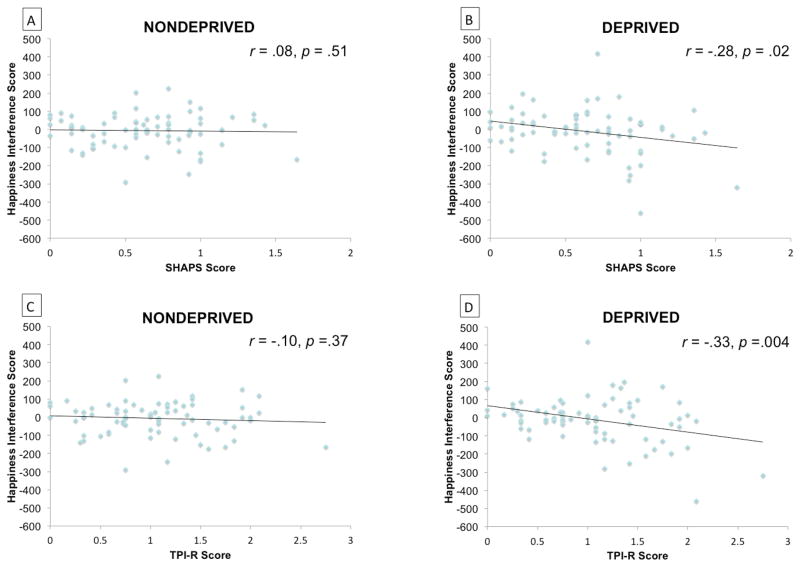
The extent to which anhedonia predicted Happiness interference scores significantly (SHAPS) or marginally (TPI-R) differed as a function of deprivation status (Table 1). Greater anhedonia was associated with significantly less interference from happy (vs. neutral) faces in the deprived condition and was not associated with interference scores in the nondeprived condition (Figure 1). These results remained after controlling for CESD score, which did not significantly predict happiness interferences scores in both deprivation conditions.
Table 1.
Results of General Linear Model Analyses Examining Anhedonia and Deprivation Status as Predictors of Happiness Interference Scores
| Predictors | Unadjusteda
|
Adjustedb
|
||||
|---|---|---|---|---|---|---|
| F(1, 72) | p | ηp2 | F(1, 71) | p | ηp2 | |
| Model with SHAPS | ||||||
| SHAPS | 2.80 | .10 | .04 | 2.60 | .11 | .04 |
| Deprivation | 0.82 | .89 | <.01 | 0.82 | .89 | <.01 |
| SHAPS × Deprivation | 4.45 | .04 | .06 | 4.50 | .04 | .06 |
| Model with TPI-R | ||||||
| TPI-R | 11.89 | .0009 | .14 | 12.07 | .0009 | .15 |
| Deprivation | 0.82 | .89 | <.01 | 0.82 | .89 | <.01 |
| TPI-R × Deprivation | 2.75 | .10 | .04 | 2.76 | .10 | .04 |
Note. N = 75.
Included primary predictors in model only;
Adjusted for CESD and CESD Deprivation interaction term.
Deprivation (Deprived vs. Nondeprived); SHAPS = Snaith Hamilton Pleasure Scale continuous score; TPI-R = Tripartite Pleasure Inventory-Responsiveness subscale continuous score; CESD = Center for Epidemiologic Studies Depression Scale; Happiness Interference Scores = Reaction Time (RT) on Happiness trials – RT on Neutral Trials.
Figure 1. Scatterplot of anhedonia level and Happiness Interference scores across conditions.
N = 75. Relation between SHAPS and Happiness Interferences scores in nondeprived (Part A) and (Part B) deprived conditions, and respective correlation coefficients. Relation between TPI-R and Happiness Interferences scores in nondeprived (Part C) and (Part D) deprived conditions, and respective correlation coefficients. SHAPS = Snaith Hamilton Pleasure Scale; TPI-R = Tripartite Pleasure Inventory-Responsiveness subscale score; Happiness Interference Scores = Reaction Time (RT) on Happiness trials – RT on Neutral Trials.
Analyses predicting Anger, Surprise, and Fear interference scores in separate models yielded no significant Anhedonia Deprivation Status interactions when either the SHAPS or TPI-R was incorporated as the measure of anhedonia.
Discussion
This study found that trait anhedonia predicted diminished cognitive processing of happy relative to neutral faces, but only under conditions of acute nicotine deprivation. The null association between anhedonia and happy face processing under nondeprived conditions may reflect that acute nicotine temporarily offsets emotion-processing disturbances due to anhedonia (Cook et al. 2007). By contrast, the inverse association between anhedonia and happy face processing following tobacco deprivation may reflect the exacerbation of anhedonia-related emotion processing disturbances by acute nicotine withdrawal (Cook et al. 2004). Anhedonia did not predict processing of angry, surprised, or fearful faces as a function of deprivation, and the primary results were not altered by statistically controlling for level of overall depressive symptoms.
It is important to consider that happiness interference scores did not differ significantly from zero in the overall sample in each condition. As illustrated in Figure 1, the trendline for the scatterplot regressing happiness interference scores on anhedonia in the nondeprived condition level hovered around zero. Though there was scatter above and below the trendline, this variation did not depend on level of anhedonia. By contrast, deprivation resulted in a separation of low and high anhedonia individuals—low anhedonia smokers demonstrated interference from happy faces, yet high anhedonia individuals demonstrated facilitation from happy faces or perhaps a greater interference from neutral relative to happy faces. Depressed individuals are more likely to inaccurately perceive neutral faces as negatively-valenced (Gollan et al. 2008; Leppanen et al. 2004). If this phenomenon extends to acutely deprived anhedonic smokers, high-anhedonia participants may have been more likely to incorrectly perceive negative affect in neutral faces while in nicotine withdrawal, which could have heightened the salience of neutral faces. At the same time, nicotine withdrawal may have exacerbated anhedonia-related reward processing deficits thereby diminishing the attentional salience of happy faces. Collectively, these two factors may have combined to generate happy interference scores that were in the negative direction for abstinent high-anhedonia smokers.
The findings were not entirely consistent across the two measures of anhedonia, as statistical evidence constituted only a trend for the TPI-R. The SHAPS primarily assesses low-arousal rewarding experiences, whereas the TPI assesses both low and high arousal experiences (e.g., physical activity, sexual interaction). Perhaps, variability in pleasure response across high versus low arousal experiences may influence interactions between nicotine and socioemotional processing. Alternatively, the disparate findings across the SHPAS and TPI-R may simply reflect measurement error in the instruments used.
These results shed light on individual differences in the underlying processes that maintain daily smoking behavior and underlie post-cessation relapse. For instance, the expression of disrupted emotional processing in high-anhedonia individuals following cessation may lead to increased risk of relapse in order to remediate these deficits. This supposition is supported by two lines of evidence: (a) data illustrating that diminished cognitive processing of appetitive words during acute nicotine withdrawal marginally predicts relapse following cessation (Powell et al. 2004); and (b) data showing that higher levels of anhedonia predicts shorter time to lapse and relapse (Cook et al. 2010; Niaura et al. 2001), increased odds of relapse (Leventhal et al. 2008b; Zvolensky et al. 2009), increased number of past failed quit attempts(Leventhal et al. 2009), and a greater proportion of prior quit attempts that end in early relapse (Leventhal et al. 2009).
The present findings may also be useful for considering the potential utility of social support in smoking cessation for smokers with high anhedonia. Data illustrate that social support may be a powerful aid to smoking cessation (Westmaas et al. 2010). Yet, those with higher levels of anhedonia may benefit less from social support, due to abstinence-induced disturbances in processing of socioemotional stimuli, and corresponding biases in social cognition. Thus, behavioral interventions designed to enhance engagement with and connection to social rewards may be a useful adjunctive smoking cessation treatment for individuals with high trait anhedonia (MacPherson et al. 2010).
These results may potentially generalize to other psychomotor stimulant drugs. Anhedonia is associated with amphetamine and cocaine use disorders (Leventhal et al. 2010; Leventhal et al. 2008a). Similar to nicotine, these drugs are known to promote sustained mesolimbic dopamine release and have reward-enhancing effects (Phillips and Fibiger 1990; Robbins 1977). Furthermore, trait anhedonia predicts enhanced sensitivity to the acute subjective mood-enhancing effects of d-amphetamine (Tremblay et al. 2002; Tremblay et al. 2005). Thus, other psychostimulant drugs may also offset emotion-processing disturbances associated with anhedonia, which may ultimately increase risk for cocaine and amphetamine dependence in high-anhedonia individuals.
The current results also are relevant to understanding the underpinnings of the anhedonia phenotype, which is prominent in a variety of psychiatric disorders that are comorbid with smoking, including psychosis (Cohen et al. 2011), mood disorder (Vrieze and Claes 2010), substance dependence (Hatzigiakoumis et al. 2011), borderline personality disorder (Bandelow et al. 2010), social phobia (Watson and Naragon-Gainey 2010), and posttraumatic stress disorder (Kashdan et al. 2006). This study suggests that the neural substrates affected by nicotine and nicotine deprivation, such as the mesolimbic dopamine system, may also mediate the emotional processing disturbances that are prominent in anhedonia and may potentially occur in a variety of psychiatric disorders.
There are some limitations to this study that should be considered when interpreting these results. First, by the nature of the tobacco deprivation manipulation that was used, the extent to which pharmacological (e.g., disruption of biological homeostasis caused by nicotine removal) versus non-pharmacological (e.g., beliefs about the effects of tobacco abstinence, loss of sensorimotor stimulation) factors affected the findings are not clear and should be addressed in future work. Second, we included smokers not interested in quitting, which leaves unclear whether the findings may generalize to individuals undergoing an actual cessation attempt. Also, individuals with current psychiatric disorders were excluded, thus, it is unknown if individuals with extreme anhedonia levels show similar patterns of alteration in emotional processing. Third, we did not compare effects between social and non-social emotional stimuli. Thus, while these findings suggest that anhedonia’s relation to altered emotional processing extends to social stimuli, it is unclear if these results reflect a general disruption of processing any type of positively-valenced stimulus or are specific to social cues. Finally, we included only self-report unidimensional measures of anhedonia. Anhedonia can be parsed into social versus physical (Fonseca-Pedrero et al. 2009) as well as anticipatory versus consummatory (Gard et al. 2007) subdimensions. Anhedonia can also be measured objectively via indirect methods (Pizzagalli et al. 2005). Accordingly, it will be of interest to examine whether altered emotional processing following tobacco abstinence is predicted by each of these subfacets of the anhedonia construct.
Limitations notwithstanding, to our knowledge this is the first study to demonstrate that trait anhedonia predicts altered emotional processing in acutely abstinent cigarette smokers. These findings provide insight into putative psychobiological mechanisms underlying anhedonia, nicotine dependence, and their comorbidity. Given these results, it is expected that mesolimbic dopamine system and emotional processing of social stimuli may be fruitful targets for research and treatment of anhedonia, particularly within the context of smoking cessation.
Acknowledgments
This research was supported by National Institute on Drug Abuse grants R01-DA026831 and K08-DA025041.
Footnotes
It is possible that very early withdrawal symptoms may have been emerging at the time of testing in the nondeprived condition (30–40 min post-cigarette), which could have impacted the results and reduced power to detect anhedonia by deprivation interactions. However, it is unlikely that early withdrawal had a substantial effect on the current findings, given that interactions between anhedonia and deprivation were found. Furthermore, previous research suggests that withdrawal-related changes in affective withdrawal symptoms do not emerge until at least 60 minutes of abstinence (Hendricks et al. 2006).
The number of outliers in this study may be considered high. Therefore, we re-ran all analyses without excluding outliers. Results of analyses that did not remove outliers were similar to the primary analyses, SHAPS × Deprivation interaction effect for predicting Happy face interference scores, F = 3.92, p = .051, ηp2 = .05 (deprived: r = .22, p = .04; nondeprived: r = −.02, p = .85); TPI-R × Deprivation interaction effect for predicting Happy face interference scores, F = 4.15, p = .045, ηp2 = .05 (deprived: r = .24, p = .03; nondeprived: r = .03, p = .81). Thus, primary results reported utilize data with outliers removed.
The authors report no disclosures or conflicts of interests related to this study.
References
- Bandelow B, Schmahl C, Falkai P, Wedekind D. Borderline personality disorder: a dysregulation of the endogenous opioid system? Psychological review. 2010;117:623–636. doi: 10.1037/a0018095. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Najolia GM, Brown LA, Minor KS. The state-trait disjunction of anhedonia in schizophrenia: potential affective, cognitive and social-based mechanisms. Clinical psychology review. 2011;31:440–448. doi: 10.1016/j.cpr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Archives of general psychiatry. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Cook J, Spring B, McChargue D, Doran N. Effects of anhedonia on days to relapse among smokers with a history of depression: a brief report. Nicotine Tob Res. 2010;12:978–982. doi: 10.1093/ntr/ntq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology. 2007;192:87–95. doi: 10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2004;6:39–47. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen W. Facial action coding system: A technique for the measurement of facial movement. Palo Alto, CA: Consulting Psychologists Press; 1978. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fonseca-Pedrero E, Paino M, Lemos-Giraldez S, et al. Psychometric properties of the Revised Physical and Social Anhedonia Scales in non-clinical young adults. The Spanish journal of psychology. 2009;12:815–822. doi: 10.1017/s1138741600002183. [DOI] [PubMed] [Google Scholar]
- Franken IH, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton Pleasure Scale (SHAPS) Journal of affective disorders. 2007;99:83–89. doi: 10.1016/j.jad.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophrenia research. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan JK, Pane HT, McCloskey MS, Coccaro EF. Identifying differences in biased affective information processing in major depression. Psychiatry research. 2008;159:18–24. doi: 10.1016/j.psychres.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Archives of general psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Gibb BE, Abela JR, Flory K. Selective attention to affective stimuli and clinical depression among youths: role of anxiety and specificity of emotion. Journal of abnormal psychology. 2010;119:491–501. doi: 10.1037/a0019609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of general psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. 1300506 [pii] [DOI] [PubMed] [Google Scholar]
- Hatzigiakoumis DS, Martinotti G, Giannantonio MD, Janiri L. Anhedonia and substance dependence: clinical correlates and treatment options. Frontiers in psychiatry/Frontiers Research Foundation. 2011;2:10. doi: 10.3389/fpsyt.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology. 2006;187:385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Elhai JD, Frueh BC. Anhedonia and emotional numbing in combat veterans with PTSD. Behaviour research and therapy. 2006;44:457–467. doi: 10.1016/j.brat.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biological psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kolassa IT, Miltner WH. Psychophysiological correlates of face processing in social phobia. Brain research. 2006;1118:130–141. doi: 10.1016/j.brainres.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Leppanen JM, Milders M, Bell JS, Terriere E, Hietanen JK. Depression biases the recognition of emotionally neutral faces. Psychiatry research. 2004;128:123–133. doi: 10.1016/j.psychres.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Leventhal AM. The Tripartite Pleasure Inventory: A Mulidimensional Measure of Anhedonia. Departments of Preventive Medicine and Psychology. University of Southern California; Los Angeles, CA, USA: 2010. [Google Scholar]
- Leventhal AM, Brightman M, Ameringer KJ, et al. Anhedonia associated with stimulant use and dependence in a population-based sample of American adults. Experimental and clinical psychopharmacology. 2010;18:562–569. doi: 10.1037/a0021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. Journal of Clinical Psychology. 2006;62:1545–1558. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW. Examining socioaffective processing biases in cigarette smokers with high versus low trait hostility. Behavioral medicine. 2010;36:63–69. doi: 10.1080/08964281003774927. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW, Ray LA, et al. Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. The American journal on addictions/American Academy of Psychiatrists in Alcoholism and Addictions. 2008a;17:218–223. doi: 10.1080/10550490802019774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2008b;10:507–517. doi: 10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Kahler CW, Ray LA, Sussman S. Relations between anhedonia and smoking motivation. Nicotine Tob Res. 2009;11:1047–1054. doi: 10.1093/ntr/ntp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loas G, Monestes JL, Ingelaere A, Noisette C, Herbener ES. Stability and relationships between trait or state anhedonia and schizophrenic symptoms in schizophrenia: a 13-year follow-up study. Psychiatry research. 2009;166:132–140. doi: 10.1016/j.psychres.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Loas G, Salinas E, Pierson A, Guelfi JD, Samuel-Lajeunesse B. Anhedonia and blunted affect in major depressive disorder. Comprehensive psychiatry. 1994;35:366–372. doi: 10.1016/0010-440x(94)90277-1. [DOI] [PubMed] [Google Scholar]
- MacPherson L, Tull MT, Matusiewicz AK, et al. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. Journal of consulting and clinical psychology. 2010;78:55–61. doi: 10.1037/a0017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual review of clinical psychology. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Matsumoto D, Ekman P. Japanese and Caucasian Facial Expressions of Emotion (JACFEE) and Neutral Faces (JACNeuF) Berkeley, CA: Paul Ekman; 2004. [Google Scholar]
- Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychology of addictive behaviors: journal of the Society of Psychologists in Addictive Behaviors. 2001;15:13–17. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- Nutt D, Demyttenaere K, Janka Z, et al. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol. 2007;21:461–471. doi: 10.1177/0269881106069938. [DOI] [PubMed] [Google Scholar]
- Paterson NE. The neuropharmacological substrates of nicotine reward: reinforcing versus reinforcement-enhancing effects of nicotine. Behavioural pharmacology. 2009;20:211–225. doi: 10.1097/FBP.0b013e32832c7083. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Fibiger HC. Role of reward and enhancement of conditioned reward in persistence of responding for cocaine. Behavioural Pharmacology. 1990;1:269–282. doi: 10.1097/00008877-199000140-00002. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biological psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addictive behaviors. 2004;29:1407–1426. doi: 10.1016/j.addbeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ratcliff R. Methods for dealing with reaction time outliers. Psychological bulletin. 1993;114:510–532. doi: 10.1037/0033-2909.114.3.510. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Reward enhancement by psychomotor stimulant drugs [proceedings] Neuropharmacology. 1977;16:529–530. doi: 10.1016/0028-3908(77)90015-6. [DOI] [PubMed] [Google Scholar]
- Schrader GD. Does anhedonia correlate with depression severity in chronic depression? Comprehensive psychiatry. 1997;38:260–263. doi: 10.1016/s0010-440x(97)90057-2. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. The British journal of psychiatry: the journal of mental science. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Stein DJ. Depression, anhedonia, and psychomotor symptoms: the role of dopaminergic neurocircuitry. CNS Spectr. 2008;13:561–565. doi: 10.1017/s1092852900016837. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Cardenas L, Herrmann N, Busto UE. Probing brain reward system function in major depressive disorder: altered response to dextroamphetamine. Archives of general psychiatry. 2002;59:409–416. doi: 10.1001/archpsyc.59.5.409. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, et al. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Archives of general psychiatry. 2005;62:1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- Van der Gucht E, Morriss R, Lancaster G, Kinderman P, Bentall RP. Psychological processes in bipolar affective disorder: negative cognitive style and reward processing. The British journal of psychiatry: the journal of mental science. 2009;194:146–151. doi: 10.1192/bjp.bp.107.047894. [DOI] [PubMed] [Google Scholar]
- Vrieze E, Claes SJ. Anhedonia and Increased Stress Sensitivity: Two Promising Endophenotypes for Major Depression. Current Psychiatry Reviews. 2010;5:143–152. [Google Scholar]
- Waters AJ, Sayette MA, Franken IH, Schwartz JE. Generalizability of carry-over effects in the emotional Stroop task. Behaviour research and therapy. 2005;43:715–732. doi: 10.1016/j.brat.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 2003;22:378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Watson D, Naragon-Gainey K. On the specificity of positive emotional dysfunction in psychopathology: evidence from the mood and anxiety disorders and schizophrenia/schizotypy. Clinical psychology review. 2010;30:839–848. doi: 10.1016/j.cpr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmaas JL, Bontemps-Jones J, Bauer JE. Social support in smoking cessation: reconciling theory and evidence. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2010;12:695–707. doi: 10.1093/ntr/ntq077. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Stewart SH, Vujanovic AA, Gavric D, Steeves D. Anxiety sensitivity and anxiety and depressive symptoms in the prediction of early smoking lapse and relapse during smoking cessation treatment. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2009;11:323–331. doi: 10.1093/ntr/ntn037. [DOI] [PMC free article] [PubMed] [Google Scholar]