Abstract
Background
Activated monocytes/macrophages play a role in severe forms of HIV-associated neurocognitive disorders (HAND), but little is known about mechanisms driving milder forms that are prevalent despite combination antiretroviral therapy (cART). To examine relationships of monocyte activation markers to HAND of varying severity, we compared plasma and CSF biomarker levels to neurocognitive test scores in HIV+ subjects.
Methods
Plasma and CSF sCD14, CCL2, and IL-6 were measured by ELISA in 67 HIV+ subjects with nadir CD4 <300, and CSF inflammatory biomarkers were measured by multiplex assay in 14 subjects on suppressive cART.
Results
82% were on cART, with 31% having undetectable plasma VL. CSF sCD14 was increased in subjects with impaired neurocognitive testing (p=0.02), correlated inversely with global T scores in subjects with detectable but not undetectable plasma VL (p=0.02), and yielded higher AUROC values for predicting impaired T scores (0.659) than plasma or CSF VL and current or nadir CD4 counts in single-marker and multivariate models. CSF sCD14, IL-6, IL-8, CCL2, CCL3, CXCL10, and IFNγ were increased in subjects on suppressive cART regardless of cognitive status and predicted patient class in unsupervised analyses, with IL-8, CCL2, and IFNγ explaining most of the variance.
Conclusions
CSF sCD14 is associated with impaired neurocognitive testing in HIV patients on nonsuppressive cART, suggesting potential utility as a biomarker to monitor HAND progression. CSF sCD14, IL-6, IL-8, CCL2, CCL3, CXCL10, and IFNγ remain elevated in patients on suppressive cART regardless of cognitive status, implying ongoing intrathecal inflammation even in the absence of clinical manifestations.
Keywords: HIV, HIV-associated neurocognitive disorders, immune activation, cerebrospinal fluid, biomarkers
Introduction
HIV-associated neurocognitive disorders (HAND), consisting of asymptomatic neurocognitive impairment (ANI), minor cognitive-motor disorder (MCMD), and HIV-associated dementia (HAD), affect 20–50% of HIV-infected individuals despite widespread use of combination antiretroviral therapy (cART).1–4 HIV enters the central nervous system (CNS) via trafficking of infected monocytes and lymphocytes, and activation of monocytes/macrophages is associated with severe forms of HAND in patients with evidence of ongoing viral replication.5–10 However, little is known about mechanisms underlying milder forms of HAND in patients with cART-mediated virological suppression.
In the pre-cART era, CSF markers associated with more severe forms of HAND included CSF HIV RNA11–14 and markers indicating intrathecal immune activation (CCL2,15–19 neopterin,20–22 β-2 microglobulin,12, 23 TNF,23–25 IL-6,23, 24 and quinolinic acid23, 24). Since widespread use of cART, the prevalence of HAD has decreased but prevalence of milder forms of HAND has remained similar or increased.1–3, 26 Furthermore, several studies show evidence of ongoing intrathecal immune activation despite cART. High CSF neopterin and β-2-microglobulin, both markers of immune activation, have been found in subjects with suppressed plasma viral loads (VL).27–30 Elevated soluble CD14 (sCD14), a marker of monocyte activation, was detected in plasma6, 31 and CSF32 of HIV+ patients with neurocognitive impairment. High CSF levels of CCL2, a monocyte chemoattractant, have also been associated with cognitive impairment and altered metabolites in brain in cART-treated HIV+ patients.33, 34 These studies provide evidence that immune activation continues to contribute to HAND pathophysiology in the cART era.
Recently, we found that plasma sCD14 was associated with impaired neurocognitive testing, particularly in attention and learning domains, in a cART era cohort of HIV+ subjects with advanced disease.31 Here, we examined relationships between CSF monocyte activation markers (sCD14, CCL2, and IL-6) and neurocognitive test scores in HIV+ subjects from the same study cohort and used a multiplex assay to examine inflammatory cytokines/chemokines in plasma and CSF from subjects on suppressive cART.
Methods
Subjects
67 HIV+ subjects (98% with nadir CD4 counts < 300) with samples and data collected between 1999 and 2009 were from 4 sites (Manhattan HIV Brain Bank, National Neurological AIDS Bank, California NeuroAIDS Tissue Network, Texas NeuroAIDS Research Center) within the National NeuroAIDS Tissue Consortium (NNTC) (n=57) and from CNS Highly Activate Retroviral Therapy Effects Research (CHARTER) (n=10), a six-center observational cohort study. 82% were on cART, with 31% having undetectable plasma HIV RNA. 66 subjects were examined in our previous study,31 with available CSF samples from the same time as plasma samples and neurocognitive testing. All subjects provided written informed consent under local institutional IRB approval. HAND clinical diagnoses were determined using established criteria35 based on formal neurocognitive testing and neurological evaluation. Neuropsychological impairment due to other causes (NPI-O) was diagnosed when factors in addition to primary HIV could contribute to neurocognitive impairment (Table 1). Current substance abuse was determined by Psychiatric Research Interview for Substance and Mental Disorders (PRISM) or Composite International Diagnostic Interview (CIDI) and urine toxicology. Subjects with severe psychiatric disorders, a confounding neurological disorder, or active systemic infection were excluded. Plasma and CSF HIV RNA were log10 transformed for statistical analysis. Undetectable plasma and CSF VL values were assigned log10 values of 2.6 or 1.7, reflecting sensitivity cutoffs of the assay; values below these cutoffs reflect lower assay sensitivity for some sites. Fifteen plasma samples from healthy donors testing HIV/HCV seronegative and 20 CSF samples from non-diseased controls were from Bioreclamation LLC (NY). Nineteen HIV/HCV seronegative plasma samples were from healthy donors at Research Blood Components (Brighton, MA) with written informed consent and IRB approval. Because there was no clinical information available for CSF samples from Bioreclamation, CSF samples were pre-screened for sCD14 and CCL2 levels by ELISA to identify samples with values outside normal ranges reported in the literature (<0.25 µg/ml and <1,000 pg/ml, respectively), which excluded 6 male and 10 female CSF samples with high CCL2 or sCD14 levels, respectively.
Table 1.
Demographic and clinical characteristics of HIV+ subjects in the study cohort (n=67).
| Age (years) | |
| Mean ± SD | 46 ± 7 |
| Median (range) | 44 (32– 63) |
| Gender | |
| Male | 55 (82%) |
| Female | 12 (18%) |
| Race | |
| African American | 31 (46%) |
| Caucasian | 27 (40%) |
| Hispanic | 5 (7%) |
| Other | 4 (6%) |
| Risk factor | |
| Sexual transmission | 27 (40%) |
| Intravenous drug abuse | 40 (60%) |
| CD4 T cell count (cells/µl) | |
| Mean ± SD | 155 ± 162 |
| Median (range) | 85 (1– 688) |
| Nadir CD4 T cell count (cells/µl) | |
| Mean ± SD | 75 ± 93 |
| Median (range) | 52 (1 – 536) |
| Plasma HIV RNA (copies/ml) | |
| Mean ± SD | 127,975 ± 236,077 |
| Median (range) | 13,500 (undetectable – 843,720) |
| > 400 copies/ml | 45 (67%) |
| < 400 copies/ml | 21 (31%) |
| Unknown | 1 (1.5%) |
| CSF HIV RNA (copies/ml) | |
| Mean ± SD | 3,554 ± 13,049 |
| Median (range) | 50 (undetectable – 75,000) |
| < 50 copies/ml | 31 (46%) |
| cART | |
| Yes | 55 (82%) |
| No | 12 (18%) |
| ARV CPE Penetration rank 2008 | |
| ≥ 2 | 27 (49%) |
| < 2 | 28 (51%) |
| HCV co-infection | |
| Positive | 25 (37%) |
| Negative | 26 (39%) |
| Unknown | 16 (23%) |
| HAND Diagnosis | |
| No impairment | 19 (28%) |
| ANI | 10 (15%) |
| MCMD | 12 (18%) |
| HAD | 11 (16%) |
| NPI-O* | 12 (18%) |
| Unknown | 3 (4%) |
HAND, HIV-associated neurocognitive disorders; No NCI, no neurocognitive impairment; ANI, asymptomatic neurocognitive impairment; MCMD, minor cognitive-motor disorder; HAD, HIV-associated dementia; NPI-O, Neuropsychological impairment due to other causes.
Neuropsychological impairment due to other causes (NPI-O) was diagnosed in patients with factors in addition to primary HIV that could contribute to neurocognitive impairment (past traumatic head injury (n=2), remote cerebral vascular accident (n=3), remote CNS toxoplasmosis (n=2), chronic hepatic cirrhosis (n=1), old frontal lesion (n=1), depression (n=1), and unknown NPI-O diagnosis (n=2)).
Neurocognitive testing
All subjects were administered an identical comprehensive test battery designed to assess seven categories of neurocognitive function.36 Demographically corrected T global T scores were generated from individual T scores as described.31, 36 T scores correlate negatively with severity of neurocognitive impairment, with values below 40 signifying impairment (40 corresponds to one standard deviation of 10 from a normalized mean of 50).
Biomarker quantification
sCD14, IL-6, and CCL2 were quantified by ELISA (R & D systems). A multiplex immunoassay (Bio-source 25-plex Human Cytokine Assay; Invitrogen) was used to measure IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17, IFN-α, IFNγ, TNF, GM-CSF, CCL2/MCP-1, CCL3/MIP-1α, CCL4/MIP-1β, CXCL10/IP-10, CXCL9/MIG, CCL11/eotaxin, and CCL5/RANTES using a Bio-Plex Workstation (Bio-Rad).
Data analysis
Data was analyzed using the Mann-Whitney test and Spearman correlation. Receiver operating characteristic (ROC) curves were generated to measure the ability of markers to predict neurocognitive impairment (global T score <40) in univariate and multivariate models. Hierarchical clustering was performed with dChip software37, 38 using Euclidean distance and average linkage. Comparison criteria required the fold change (FC) between group means to exceed a specific threshold, with mean difference significant by unpaired t-test (p<0.05). Concentration values were log2-transformed and cytokines with >50% missing data were excluded from further analysis; 17 plasma and 7 CSF biomarkers met these criteria and were included for further analysis. The limit of detection (LOD) was provided by the manufacturer. In lowest-value mode,39 the lowest detected value (LDV) corresponded to the mode among detectable values below the LOD. Missing values were imputed using the LOD unless >15% of samples had detectable values below the LOD; in this case, missing values were imputed using the LDV. Principal component analysis (PCA) and partial-least squares discriminant analysis (PLS-DA) were performed on the Metaboanalyst web portal40 using normalized and auto-scaled expression values. The web portal utilizes the prcomp function of the stats package, and plsr function of the pls package of R. Class labels were permuted 2000 times to test whether differences between groups were significant.41
Results
Higher plasma and CSF sCD14 levels are associated with impaired neurocognitive test performance
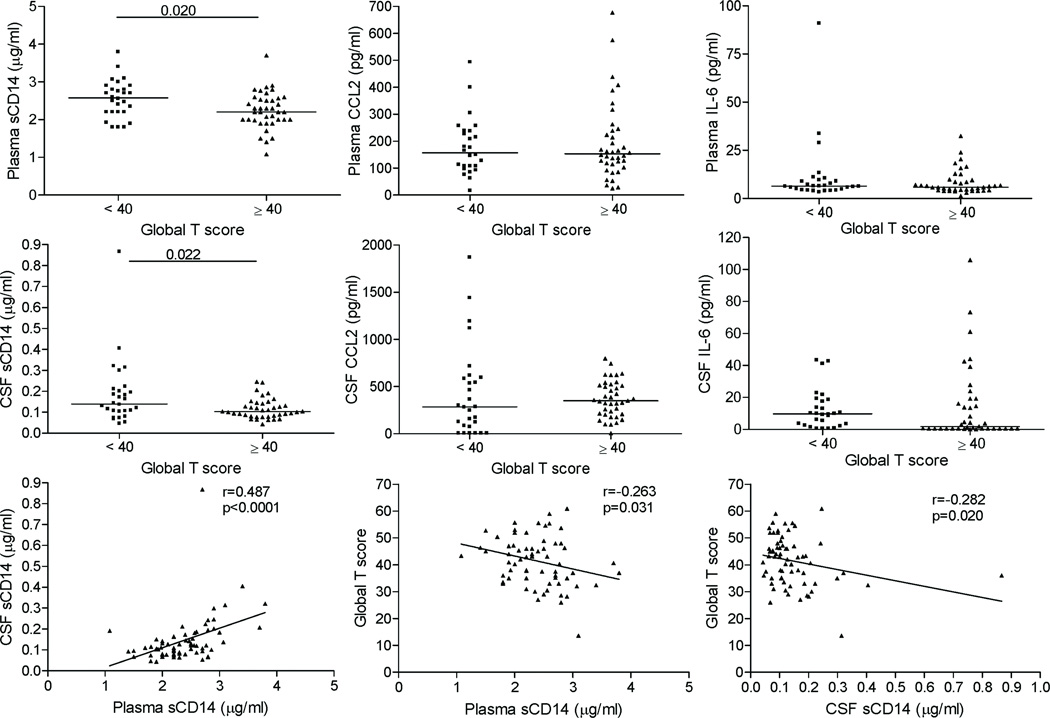
The study cohort consisted of 67 HIV+ subjects with advanced disease (median current and nadir CD4 counts of 85 and 52 cells/µl, respectively), and high frequency of drug abuse (49%) and HCV co-infection (36%) (Table 1). The majority (82%) were treated on cART, with 31% and 46% having undetectable plasma or CSF HIV RNA, respectively (<400 and < 50 copies/ml, respectively). First, we compared monocyte activation markers between HIV+ and uninfected subjects in plasma and CSF. Plasma and CSF levels of sCD14, CCL2, and IL-6 were higher in HIV+ compared to uninfected subjects (p<0.05) (Supplemental Digital Content 1). In HIV+ subjects, HIV RNA and sCD14 were 2–3 fold higher in plasma compared to CSF, while CCL2 levels were 2-fold higher in CSF than plasma (p<0.0001, Supplemental Digital Content 2). CD4 count correlated inversely with plasma and CSF VL, sCD14, and plasma CCL2, while plasma sCD14 correlated positively with IL-6 (r=0.248, p=0.04) (Supplemental Digital Content 3). Plasma and CSF sCD14 levels were strongly correlated (r=0.487, p<0.0001), while plasma and CSF CCL2 levels trended toward correlation (r=0.234, p=0.06). These results show that sCD14 levels are higher in plasma than in CSF, while CCL2 levels are higher in CSF than in plasma, and sCD14 levels in plasma and CSF are strongly correlated in HIV+ subjects.
To examine relationships between monocyte activation markers and HAND, we compared plasma and CSF levels of HIV RNA, sCD14, CCL2, and IL-6 to global T scores. Plasma and CSF sCD14 levels were higher in subjects with global T scores <40 (impaired) versus ≥40 (unimpaired) (p=0.02 and p=0.022, respectively) and correlated inversely with global T scores (r=−0.263, p=0.03 and r=−0.282, p=0.02, respectively) (Figure 1). In contrast, plasma and CSF CCL2 or IL-6 levels were similar between groups. ROC analysis for single markers demonstrated that sCD14 levels in plasma and CSF yielded higher Area Under the ROC Curve (AUROC) values for classification of global T scores <40 (0.657 and 0.659, respectively) compared to four conventional markers (plasma and CSF VL and current and nadir CD4 count; AUROC=0.541, 0.566, 0.607, and 0.598, respectively). In multivariate models for predicting T score <40, plasma and CSF sCD14 added incremental value to logistic regression models using age and cART, and improved AUROC values (by 0.12 and 0.14, respectively) when added to age and cART, whereas the four conventional markers did not improve the predictive ability of these models. Thus, elevated CSF sCD14 levels are associated with global T scores indicating neurocognitive impairment, and yield higher AUROC values for predicting impaired global T scores than four conventional markers in univariate and multivariate models.
Figure 1. Higher plasma and CSF sCD14 levels are associated with global T scores indicating neurocognitive impairment.
HIV+ subjects were grouped by global T score <40 or ≥40 and biomarker levels were compared. Plasma and CSF sCD14 levels were higher in subjects with global T scores <40 versus global T scores ≥40, and correlated inversely with global T scores, while no differences were seen for plasma or CSF CCL2 or IL-6 levels. Median values are indicated as horizontal lines. Statistical significance between groups was calculated using the Mann-Whitney test, and relationships between continuous variables were examined by Spearman correlation; significant differences (p<0.05) are indicated.
Plasma and CSF sCD14, CCL2, and IL-6 levels do not distinguish between groups defined by HAND clinical diagnoses
We compared global T scores between subjects stratified into two groups: no neurocognitive impairment (no NCI) and HAND or NPI-O clinical diagnosis grouped together. As expected, global T scores were higher in subjects with no NCI compared to those with a HAND or NPI-O clinical diagnosis (p<0.0001, Supplemental Digital Content 4). We compared levels of plasma and CSF sCD14, CCL2, and IL-6 between the two groups but found no differences (Supplemental Digital Content 5). Thus, plasma and CSF sCD14, CCL2, and IL-6 did not distinguish between subjects defined by HAND clinical diagnoses.
Plasma and CSF sCD14 levels are associated with global T scores indicating neurocognitive impairment in viremic but not aviremic HIV+ subjects
Next, we compared relationships between monocyte activation markers and global T scores in plasma and CSF for subjects grouped according to detectable (≥400 HIV RNA copies/ml) or undetectable (<400 HIV RNA copies/ml) plasma VL. These groups had similar median age, but median current and nadir CD4 counts were lower in viremic compared to aviremic subjects (54 and 41 versus 249 and 71 cells/µl, p<0.0001 and 0.05, respectively). sCD14, CCL2, and IL-6 remained elevated in plasma and CSF in both viremic and aviremic subjects compared to controls (Figure 2A). Conversely, CCL2 and IL-6 were higher in CSF than plasma of aviremic subjects. Plasma sCD14 correlated with CSF sCD14 (r=0.574, p <0.0001), while plasma and CSF sCD14 correlated inversely with global T scores (r=−0.354, p=0.017 and r=−0.297, p=0.047, respectively) in viremic but not aviremic subjects (Figure 2B and C). In aviremic subjects, CD4 count correlated inversely with CSF CCL2 levels (r=−0.598, p=0.003), while plasma CCL2 correlated with CSF CCL2 (r=0.520, p=0.018). Unexpectedly, aviremic subjects with global T scores <40 had lower CSF CCL2 compared to those with global T scores ≥40 (p=0.005). Because illicit drug use and HCV co-infection are comorbidities of HIV infection with immunomodulatory effects that may influence risk of HAND,42–44 we examined performed subgroup analysis. Neither CSF nor plasma biomarkers levels showed significant differences when subjects were classified according to patterns of substance abuse (within 6 months) (opiate or cocaine users compared to non-users, n=19, 21, and 27, respectively) or HCV co-infection (data not shown). Thus, plasma and CSF sCD14 levels correlated inversely with global T scores in subjects with detectable but not undetectable plasma VL.
Figure 2. Plasma and CSF sCD14 levels correlate inversely with global T scores in viremic but not aviremic HIV+ subjects.
A, sCD14, CCL2, and IL-6 levels in plasma and CSF of viremic and aviremic patients. B and C, Subgroup analysis of viremic (plasma VL >400 HIV RNA copies/ml) (B) and aviremic (plasma VL <400 HIV RNA copies/ml) (C) subjects showed positive correlation between CSF and plasma sCD14 levels and inverse correlation between plasma and CSF sCD14 levels and global T scores in viremic but not aviremic subjects. Statistical significance was determined by Mann-Whitney test (A) and Spearman correlation analysis (B and C); medians (A) and significant differences (p<0.05) are indicated.
CNS penetration effectiveness (CPE) score is not associated with differences in CSF biomarker levels
To examine relationships between CNS penetration of cART regimens and biomarker levels, we compared CSF sCD14, CCL2, and IL-6 level to CNS penetration effectiveness (CPE) score, which assigns each drug a value of 0 (low), 0.5 (intermediate), or 1.0 (good penetration); scores are summed to determine overall CPE rank of a regimen.45 The median value for 2008 CPE rank (2.0) was the cutoff for comparing biomarker levels in subjects with good (≥2) versus poor (<2) CPE; scores were then evaluated as continuous variables compared to CSF sCD14, CCL2, and IL-6 levels. Additionally, data was analyzed using the revised CPE 2010 scoring system.46 No associations were found between CPE rank and CSF sCD14, CCL2, and IL-6 levels, or global T scores, using either CPE scoring system. Subjects with suppressed plasma VL (<400 copies/ml) also showed no significant association between CPE rank and CSF biomarker levels or global T scores. Thus, higher CPE scores did not appear to influence CSF monocyte activation markers or global T scores in this cohort.
Increased CSF inflammatory biomarkers distinguish HIV+ subjects on suppressive cART from uninfected controls regardless of cognitive status
To examine inflammatory biomarker patterns in plasma and CSF of HIV+ subjects with cART-mediated virological suppression, we used a multiplex assay to measure 18 plasma and 7 CSF biomarkers in 14 aviremic HIV+ subjects and 14 healthy uninfected controls. Univariate analysis identified 8 cytokines/chemokines (sCD14, IL-6, IL-8, CCL2, CXCL9, CXCL10, IL-2R, CCL11, and CCL3) with higher levels in plasma from aviremic HIV+ subjects compared to healthy controls (FC 1.37–4.38, p<0.05) (Supplemental Digital Content 6), while CCL4 showed a trend towards significance (FC 2.6, p=0.054). Similar analysis of matched CSF samples detected 7 cytokines/chemokines (CXCL10, CCL3, CCL2, IL-8, IFNγ, IL-6, and sCD14) at higher levels in aviremic HIV+ subjects compared to healthy controls (FC 1.82 – 13.09, p<0.05) (Supplemental Digital Content 6). IL-8, IFN γ, and CCL2 were the top- ranked CSF biomarkers distinguishing aviremic HIV+ subjects from healthy controls (FC 2.89 – 13.09, p<0.0001).
Next we examined whether aviremic HIV+ patients clustered according to clinical subgroups and covariates defined by neurocognitive diagnosis, global T score (impaired or unimpaired, corresponding to <40 and ≥40, respectively), and HIV disease markers. Supervised hierarchical clustering for 18 plasma and 7 CSF biomarkers across aviremic HIV+ subjects and healthy controls showed that none of these biomarkers in plasma and CSF clustered aviremic HIV+ subjects according to neurocognitive diagnosis, global T score, or current or nadir CD4 counts. When analysis was applied to only 7 plasma biomarkers detected in CSF, we identified a major cluster of 11/14 controls with low rate of misclassification (14.2%), second cluster of 6 aviremic HIV+ subjects, and third cluster with 6 aviremic and 3 control subjects (Figure 3A). Unsupervised hierarchical clustering of 7 CSF biomarkers revealed that aviremic HIV+ subjects and healthy controls segregated with 100% accuracy into 2 major clusters (Figure 3B). The aviremic HIV+ subject cluster separated into 2 subclusters based on differential CSF CCL3 levels, each subcluster consisting of 7 aviremic subjects including various HAND diagnoses. IL-8 and IFNγ levels were higher (FC 3.58, p=0.001 and FC 1.45, p=0.023, respectively), while CCL2 trended towards significance (FC 1.99, p=0.07), in the subgroup with higher versus lower CSF CCL3 levels. These findings show sCD14, IL-6, CCL2, CCL3, CXCL10, IL-8, and IFNγ are increased in CSF from aviremic HIV+ subjects compared to controls in a small study regardless of neurocognitive status, and identify IL-8, CCL2, and IFNγ as a CSF biomarker cluster.
Figure 3. CSF inflammatory biomarkers distinguish HIV subjects on suppressive cART from healthy uninfected controls.
A and B, Unsupervised hierarchical clustering by Euclidean distance and average linkage of 7 biomarkers (sCD14, IL-6, IL-8, CCL2, CCL3, CXCL10, and IFNγ) detected in both plasma (A) and CSF (B) of 14 aviremic HIV+ subjects and healthy controls using dChip software. Clustering analysis was also run across the following clinical groups and covariates as indicated above each heatmap: clinical groups (A, aviremic; C, healthy control), neurocognitive diagnosis (H, HAD; M, MCMD; A, Asymptomatic neurocognitive impairment; P, Neurocognitive impairment due to other causes; N, Normal; and U, Unknown), global T score (N, not impaired; I, impaired, corresponding to ≥40 and < 40, respectively), and current and nadir CD4 count. In heatmaps, red and blue represent increased and decreased levels relative to the mean level of a biomarker, respectively. Each column and row defines individual patients and cytokines/chemokines, respectively. Unsupervised clustering of plasma biomarkers (A) predicts a major cluster with 11/14 healthy controls (highlighted in blue) and second major cluster consisting of 6 aviremic subjects (highlighted in red), with increased expression of 7 biomarkers in aviremic HIV+ subjects compared to controls. Unsupervised hierarchical clustering of 7 CSF biomarkers segregates aviremic HIV+ subjects (highlighted in red) from controls (highlighted in blue) with 100% accuracy (B). Within the major cluster consisting of HIV+ subjects are two subclusters with differential CCL3 levels, each with 7 aviremic HIV+ subjects. C, PCA represented as a three dimensional scatter plot showing the top 3 principal components of cytokine/chemokine level data measured in aviremic HIV+ subjects (red dots, n=14) and controls (green dots, n=14). D, PLS-DA represented as a three dimensional scatter plot on the left panel shows the top 3 components of cytokine/chemokine level data measured in aviremic HIV+ subjects (red dots, n=14) and controls (green dots, n=14). The plot shows that 83.9% variance is explained by the first 3 components. Plot of variables important in projection (VIP) (right panel) ranks IL-8, IFNγ, and CCL2 as the top 3 biomarkers accounting for separation between aviremic HIV+ subjects and uninfected controls.
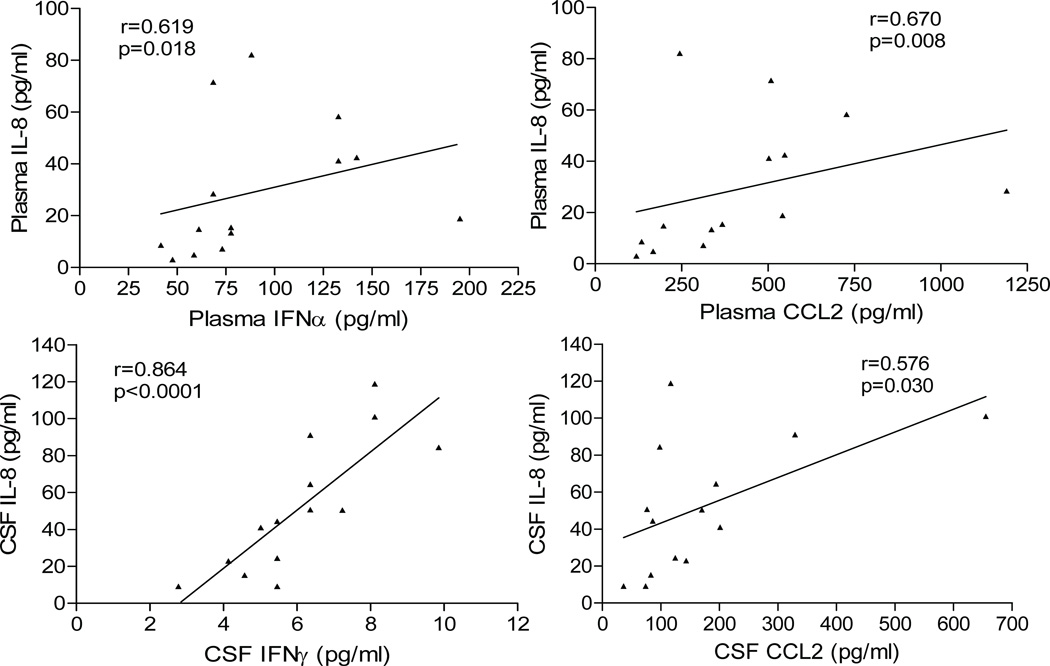
Next, we performed PCA and PLS-DA to examine variance in the CSF biomarker data. Unsupervised analysis of 7 CSF biomarkers by PCA revealed that the first 3 components explain 88.7% of the variance and discriminate aviremic HIV+ subjects from controls with 100% accuracy (Figure 3C). Supervised analysis by PLS-DA revealed that aviremic HIV+ subjects and controls could be separated along the axis defined by 3 components explaining 83.9% of the variance (Figure 3D); permutation tests confirmed significant separation (p<0.0005). Variables important in projection (VIP) scores plot ranked IL-8, IFNγ, and CCL2 as the top 3 biomarkers explaining variance between the two groups (Figure 3D). For plasma biomarkers, PCA did not reveal significant discrimination between aviremic HIV+ subjects and controls, while PLS-DA predicted 56% of the variance along the axis defined by 3 components with significant separation (p=0.019). We further examined IL-8, IFNγ, and CCL2 by Spearman correlation; in aviremic HIV+ subjects, IL-8 correlated positively with IFNα and CCL2 in plasma, and with IFNγ and CCL2 in CSF (Figure 4). Additionally, CCL3 levels correlated with IL-8 and IFNγ (r=0.815, p=0.0003 and r=0.728, p=0.003, respectively) in CSF. These findings suggest that increased CSF sCD14, IL-6, CCL2, CCL3, CXCL10, IL-8, and IFNγ distinguishes HIV+ patients on suppressive cART from uninfected controls.
Figure 4. Relationships between IL-8 and other inflammatory biomarkers in aviremic HIV+ subjects on cART.
Plots show positive correlations of inflammatory IL-8 with IFNα, and CCL2 in plasma (upper panel) and with IFNγ, and CCL2 in CSF (lower panel) of aviremic HIV+ subjects (Spearman correlation; p<0.05).
Discussion
In this study of HIV+ subjects with advanced disease, CSF sCD14 levels were higher in subjects with impaired neurocognitive test performance, and correlated inversely with global T scores. CSF CCL2 and IL-6 did not show these associations. AUROC values for predicting impaired global T score <40 were higher for plasma and CSF sCD14 than for current or nadir CD4 count or CSF or plasma VL, and remained significant after adjusting for age and cART. The predictive ability of CSF sCD14 in single-marker and multivariate models was at least as good as plasma sCD14 for predicting impaired global T scores. These findings extend our previous analysis of plasma sCD14 in the study cohort,31 and suggest that CSF sCD14 is a useful biomarker to monitor intrathecal inflammation during HAND progression and therapeutic responses.
Despite cART-mediated virological suppression, monocyte/macrophage activation markers (e.g., neopterin, sCD14, CCL2, CCL3, CCL4, CCL5, CXCL10) are frequently detected in CSF.6, 27, 29, 30, 33, 34, 47–50 In the present study, plasma and CSF sCD14 levels were associated with global T scores in subjects with detectable but not undetectable VL. Furthermore, increased CSF sCD14, IL-6, CCL2, CCL3, CXCL10, IL-8, and IFNγ robustly distinguished aviremic HIV+ subjects from controls regardless of cognitive status in a small study of 28 subjects. In unsupervised analyses, these CSF biomarkers discriminated aviremic HIV+ subjects from controls with 100% accuracy, with IL-8, CCL2, and IFNγ explaining most of the variance between groups. IL-8, produced mainly by activated monocytes and NK cells, is a chemoattractant for neutrophils, T cells, and NK cells expressing CXCR1 or CXCR2. In a recent study, increased CSF IL-8, CCL2, and CXCL10 were strongly associated with cerebral metabolites related to neuronal injury and neuroinflammation in subjects with HAND34. Subgroup analysis of untreated subjects or those failing cART indicated no correlation of IL-8, CCL2, and CXCL10 with inflammatory pattern scores related to neuronal injury, suggesting cART alters relationships between these chemokines and cerebral metabolites. Consistent with these findings, we found clustering of CSF IL-8, CCL2, and IFNγ in heatmaps and positive correlations of CSF IL-8 levels with IFNγ and CCL2 in subjects on suppressive cART. These findings are consistent with a model in which IFNγ-driven pathways contribute to ongoing intrathecal immune activation in HIV+ patients despite cART-mediated virological suppression.
Another interesting finding was the identification of 2 clusters of aviremic HIV+ subjects distinguished by differential CSF CCL3 levels: one with higher CCL3 levels consisting of 5/7 subjects with impaired global T scores along with higher CSF IL-8/IFN-γ levels, and a second cluster with lower CCL3 levels consisting of 5/7 subjects with unimpaired T scores. Although the small sample size limits conclusions that can be drawn, this preliminary finding, together with previous studies implicating increased CSF CCL3 in HAND pathogenesis,51, 52 suggests that CCL3 as a potential CSF biomarker that may distinguish clinical subgroups warrants further investigation. Further studies of larger cohorts of aviremic subjects followed longitudinally are needed to determine relationships of CSF IL-8, CCL2, and IFN-γ (and CCL3) to risk of developing HAND in HIV+ patients on suppressive cART and their utility as biomarkers to monitor intrathecal inflammation and therapeutic responses.
Although we found an association between CSF sCD14 levels and impaired neurocognitive test performance, we found no differences in plasma or CSF sCD14 levels between HIV+ subjects classified by HAND diagnosis. A previous study of CD14+/CD16+ monocytes in HAD patients32 found higher levels of sCD14 in CSF from HIV+ individuals with HAD compared to controls, but differences were significant only for those with a diagnosis of moderate to severe dementia and sample sizes were small. We cannot exclude the possibility that our inability to detect differences in plasma or CSF biomarkers between HAND subtypes reflected methodological problems related to patient selection or criteria used for assigning a clinical diagnosis. Nonetheless, our findings suggest that T scores are more sensitive indicators of neurocognitive impairment than clinical diagnoses in the cART era, and using continuous descriptors rather than categorical diagnoses is important for demonstrating associations between biomarker levels and impaired neurocognitive function.
The relationship of CPE score to improvement in clinical outcome is not linear and might be explained by a delicate balance between viral suppression in the CNS and potential neurotoxicity of certain antiretroviral drugs.46 We found no association between CPE score and CSF sCD14, CCL2, or IL-6 levels for the total cohort or aviremic subgroup. Similarly, a recent magnetic resonance spectroscopy study did not find an association between CPE score and commonly measured cerebral metabolites.53 Although interpretation of our findings is limited by the small sample size, they are indicative of ongoing intrathecal inflammation in HIV+ patients regardless of CPE score.
Limitations of our study include its cross-sectional design and small sample size, which may have limited the power to detect significant associations. Also, narrow selection criteria used to define the study cohort (CD4 nadir <300) limit our findings to those with advanced HIV disease. We included NPI-O subjects, as many likely exhibit neurocognitive deficits attributable to HIV, and there is site-to-site variation in assigning a diagnosis of NPI-O. Subgroup analysis by type of illicit drug use or HCV co-infection did not show significant differences in plasma or CSF sCD14, CCL2, or IL-6, consistent with other studies that failed to demonstrate associations between these comorbidities and elevated sCD14 or CCL2.5, 54 Thus, these comorbidities are unlikely to account for associations between elevated sCD14 and impaired global T scores. The study cohort was from NNTC, which specifically recruits individuals with advanced disease, and CHARTER, which includes a large population of well-controlled HIV+ subjects, to represent a diverse population of HIV-infected individuals with broad range of viral loads. As such, the study cohort reflects a bias of urban cohorts with large populations of non-suppressed patients and IV drug users and results cannot be generalized to all populations.
In conclusion, plasma and CSF sCD14 levels are associated with impaired neurocognitive test scores in HIV+ patients on nonsuppressive cART, providing evidence that monocyte activation continues to contribute to HAND pathogenesis in the cART era. CSF IL-8, CCL2, CCL3, CXCL10, IFN-γ, and IL-6 levels remain elevated in patients on suppressive cART, even in subjects without cognitive impairment. Plasma and CSF sCD14 may be particularly useful as biomarkers to monitor systemic and intrathecal monocyte activation, HAND progression, and therapeutic responses in HIV+ patients on cART.
Supplementary Material
Figure, Supplemental Digital Content 1: Plasma and CSF levels of sCD14, CCL2, and IL-6 are higher in HIV+ subjects than in uninfected healthy controls. Plasma and CSF sCD14, CCL2, and IL-6 levels were compared between cohort subjects (n=67) and healthy controls (n=34). HIV+ subjects had higher plasma and CSF levels of sCD14, CCL2, and IL-6 compared to healthy controls. Median values are indicated as horizontal lines. Statistical significance between groups was calculated using the Mann-Whitney test; significant differences (p<0.05) are indicated.
Figure, Supplemental Digital Content 2: sCD14 levels are higher in plasma than CSF, while CCL2 levels are higher in CSF than plasma, in HIV+ subjects with advanced disease. HIV RNA, sCD14, CCL2, and IL-6 levels were compared between plasma and CSF. HIV RNA and sCD14 levels were higher in plasma compared to CSF, whereas CCL2 levels were significantly lower in plasma compared to CSF. IL-6 levels were similar between plasma and CSF. Median values are indicated as horizontal lines and significant differences were determined using the Mann-Whitney test; significant differences (p<0.05) are indicated.
Figure, Supplemental Digital Content 4: Global T scores are lower in HIV+ subjects with HAND or NPI-O clinical diagnoses. Global T scores were compared between subjects with no neurocognitive impairment (no NCI) (n=19) and subjects with HAND or NPI-O clinical diagnoses (n=44). Scores were lower in the group with HAND or NPI-O diagnoses. Median values are indicated as horizontal lines. Statistical significance between groups was calculated using the Mann-Whitney test; significant differences (p<0.05) are indicated.
Figure, Supplementary Digital Content 5: Plasma and CSF sCD14, CCL2, and IL-6 levels do not distinguish between subjects with and without HAND or NPI-O clinical diagnoses. Plasma and CSF sCD14, CCL2, and IL-6 levels were compared between subjects with no neurocognitive impairment (no NCI, n=19) and subjects with HAND or NPI-O (n=44). No differences were found for any of the markers tested. Median values are indicated as horizontal lines. Statistical significance between groups was calculated using the Mann-Whitney test.
Acknowledgements
We thank NNTC and CHARTER sites for providing plasma samples and clinical data for AIDS patients. We also acknowledge support from the Harvard Center for AIDS Research Biostatistics Core and the Mount Sinai Institute for NeuroAIDS Disparities.
Financial support: Supported by National Institutes of Health Grant DA26322, DA28994, and MH083588 to D.G. and a Mount Sinai Institute for NeuroAIDS Disparities (MSINAD) Scholar Grant (funded through R25MH080663) to J.L.L. National NeuroAIDS Tissue Consortium (NNTC) sites were supported by National Institutes of Health Grants U01MH083501, R24MH59724, U01MH083506, R24MH59745, U01MH083507, R24 NS45491, 5U01MH083500, NS38841, U01MH083545, and N01MH32002. CNS HIV Antiretroviral Therapy Effects Research (CHARTER) was supported by N01MH22005. Core facilities were supported by the Harvard Center for AIDS Research and Dana-Farber Cancer Institute/Harvard Center for Cancer Research grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The study authors report no disclosures or competing interests.
References
- 1.McArthur JC, Brew BJ. HIV-associated neurocognitive disorders: is there a hidden epidemic? AIDS. Jun 1;24(9):1367–1370. doi: 10.1097/QAD.0b013e3283391d56. [DOI] [PubMed] [Google Scholar]
- 2.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010 Jun;67(6):699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 3.Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002 Apr;8(2):136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 4.Tozzi V, Balestra P, Lorenzini P, et al. Prevalence and risk factors for human immunodeficiency virus-associated neurocognitive impairment, 1996 to 2002: results from an urban observational cohort. J Neurovirol. 2005 Jul;11(3):265–273. doi: 10.1080/13550280590952790. [DOI] [PubMed] [Google Scholar]
- 5.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3(6):e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan LA, Zheng J, Brester M, et al. Plasma levels of soluble CD14 and tumor necrosis factor-alpha type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. J Infect Dis. 2001 Sep 15;184(6):699–706. doi: 10.1086/323036. [DOI] [PubMed] [Google Scholar]
- 7.Gartner S, Liu Y. Insights into the role of immune activation in HIV neuropathogenesis. J Neurovirol. 2002 Apr;8(2):69–75. doi: 10.1080/13550280290049525. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005 Jan;5(1):69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 9.Gras G, Kaul M. Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology. 7:30. doi: 10.1186/1742-4690-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005 Aug;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 11.Ellis RJ, Hsia K, Spector SA, et al. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol. 1997 Nov;42(5):679–688. doi: 10.1002/ana.410420503. [DOI] [PubMed] [Google Scholar]
- 12.Enting RH, Foudraine NA, Lange JM, et al. Cerebrospinal fluid beta2-microglobulin, monocyte chemotactic protein-1, and soluble tumour necrosis factor alpha receptors before and after treatment with lamivudine plus zidovudine or stavudine. J Neuroimmunol. 2000 Jan 24;102(2):216–221. doi: 10.1016/s0165-5728(99)00219-2. [DOI] [PubMed] [Google Scholar]
- 13.McArthur JC, McClernon DR, Cronin MF, et al. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997 Nov;42(5):689–698. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- 14.Brew BJ, Pemberton L, Cunningham P, Law MG. Levels of human immunodeficiency virus type 1 RNA in cerebrospinal fluid correlate with AIDS dementia stage. J Infect Dis. 1997 Apr;175(4):963–966. doi: 10.1086/514001. [DOI] [PubMed] [Google Scholar]
- 15.Cinque P, Vago L, Mengozzi M, et al. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS. 1998 Jul 30;12(11):1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Conant K, Garzino-Demo A, Nath A, et al. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998 Nov;44(5):831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- 18.Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. AIDS. 1998 Jun 18;12(9):1021–1026. [PubMed] [Google Scholar]
- 19.Sevigny JJ, Albert SM, McDermott MP, et al. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004 Dec 14;63(11):2084–2090. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- 20.Brew BJ, Bhalla RB, Paul M, et al. Cerebrospinal fluid neopterin in human immunodeficiency virus type 1 infection. Ann Neurol. 1990 Oct;28(4):556–560. doi: 10.1002/ana.410280413. [DOI] [PubMed] [Google Scholar]
- 21.Griffin DE, McArthur JC, Cornblath DR. Neopterin and interferon-gamma in serum and cerebrospinal fluid of patients with HIV-associated neurologic disease. Neurology. 1991 Jan;41(1):69–74. doi: 10.1212/wnl.41.1.69. [DOI] [PubMed] [Google Scholar]
- 22.Shaskan EG, Brew BJ, Rosenblum M, Thompson RM, Price RW. Increased neopterin levels in brains of patients with human immunodeficiency virus type 1 infection. J Neurochem. 1992 Oct;59(4):1541–1546. doi: 10.1111/j.1471-4159.1992.tb08471.x. [DOI] [PubMed] [Google Scholar]
- 23.Wiley CA, Achim CL, Schrier RD, Heyes MP, McCutchan JA, Grant I. Relationship of cerebrospinal fluid immune activation associated factors to HIV encephalitis. AIDS. 1992 Nov;6(11):1299–1307. doi: 10.1097/00002030-199211000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Achim CL, Heyes MP, Wiley CA. Quantitation of human immunodeficiency virus, immune activation factors, and quinolinic acid in AIDS brains. J Clin Invest. 1993 Jun;91(6):2769–2775. doi: 10.1172/JCI116518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mastroianni CM, Paoletti F, Valenti C, Vullo V, Jirillo E, Delia S. Tumour necrosis factor (TNF-alpha) and neurological disorders in HIV infection. J Neurol Neurosurg Psychiatry. 1992 Mar;55(3):219–221. doi: 10.1136/jnnp.55.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhaskaran K, Mussini C, Antinori A, et al. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol. 2008 Feb;63(2):213–221. doi: 10.1002/ana.21225. [DOI] [PubMed] [Google Scholar]
- 27.Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis. 2007 Dec 15;196(12):1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- 28.Cysique LA, Brew BJ, Halman M, et al. Undetectable cerebrospinal fluid HIV RNA and beta-2 microglobulin do not indicate inactive AIDS dementia complex in highly active antiretroviral therapy-treated patients. J Acquir Immune Defic Syndr. 2005 Aug 1;39(4):426–429. doi: 10.1097/01.qai.0000165799.59322.f5. [DOI] [PubMed] [Google Scholar]
- 29.Hagberg L, Cinque P, Gisslen M, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yilmaz A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr. 2008 Feb 1;47(2):168–173. doi: 10.1097/QAI.0b013e31815ace97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyons JL, Uno H, Ancuta P, et al. Plasma sCD14 Is a Biomarker Associated With Impaired Neurocognitive Test Performance in Attention and Learning Domains in HIV Infection. J Acquir Immune Defic Syndr. 2011 Aug 15;57(5):371–379. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer-Smith T, Croul S, Sverstiuk AE, et al. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001 Dec;7(6):528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 33.Chang L, Ernst T, St Hillaire C, Conant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir Ther. 2004 Jun;9(3):431–440. doi: 10.1177/135965350400900302. [DOI] [PubMed] [Google Scholar]
- 34.Letendre SL, Zheng JC, Kaul M, et al. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J Neurovirol. 2011 Feb;17(1):63–69. doi: 10.1007/s13365-010-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007 Oct 30;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004 Sep;26(6):759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- 37.Li C, Hung Wong W. Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol. 2001;2(8) doi: 10.1186/gb-2001-2-8-research0032. RESEARCH0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A. 2001 Jan 2;98(1):31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Domenici E, Wille DR, Tozzi F, et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS One. 2010;5(2):e9166. doi: 10.1371/journal.pone.0009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009 Jul 1;37(Web Server issue):W652–W660. doi: 10.1093/nar/gkp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bijlsma S, Bobeldijk I, Verheij ER, et al. Large-scale human metabolomics studies: a strategy for data (pre-) processing and validation. Anal Chem. 2006 Jan 15;78(2):567–574. doi: 10.1021/ac051495j. [DOI] [PubMed] [Google Scholar]
- 42.Burdo TH, Katner SN, Taffe MA, Fox HS. Neuroimmunity, drugs of abuse, and neuroAIDS. J Neuroimmune Pharmacol. 2006 Mar;1(1):41–49. doi: 10.1007/s11481-005-9001-3. [DOI] [PubMed] [Google Scholar]
- 43.Martin-Thormeyer EM, Paul RH. Drug abuse and hepatitis C infection as comorbid features of HIV associated neurocognitive disorder: neurocognitive and neuroimaging features. Neuropsychol Rev. 2009 Jun;19(2):215–231. doi: 10.1007/s11065-009-9101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR. Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol. 2001 Feb;7(1):66–71. doi: 10.1080/135502801300069737. [DOI] [PubMed] [Google Scholar]
- 45.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008 Jan;65(1):65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010 Apr-May;18(2):45–55. [PMC free article] [PubMed] [Google Scholar]
- 47.Gisolf EH, van Praag RM, Jurriaans S, et al. Increasing cerebrospinal fluid chemokine concentrations despite undetectable cerebrospinal fluid HIV RNA in HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2000 Dec 15;25(5):426–433. doi: 10.1097/00042560-200012150-00007. [DOI] [PubMed] [Google Scholar]
- 48.Cinque P, Bestetti A, Marenzi R, et al. Cerebrospinal fluid interferon-gamma-inducible protein 10 (IP-10, CXCL10) in HIV-1 infection. J Neuroimmunol. 2005 Nov;168(1–2):154–163. doi: 10.1016/j.jneuroim.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Brew BJ, Letendre SL. Biomarkers of HIV related central nervous system disease. Int Rev Psychiatry. 2008 Feb;20(1):73–88. doi: 10.1080/09540260701878082. [DOI] [PubMed] [Google Scholar]
- 50.Sinclair E, Ronquillo R, Lollo N, et al. Antiretroviral treatment effect on immune activation reduces cerebrospinal fluid HIV-1 infection. J Acquir Immune Defic Syndr. 2008 Apr 15;47(5):544–552. doi: 10.1097/QAI.0b013e318162754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidtmayerova H, Nottet HS, Nuovo G, et al. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Letendre SL, Lanier ER, McCutchan JA. Cerebrospinal fluid beta chemokine concentrations in neurocognitively impaired individuals infected with human immunodeficiency virus type 1. J Infect Dis. 1999 Aug;180(2):310–319. doi: 10.1086/314866. [DOI] [PubMed] [Google Scholar]
- 53.Harezlak J, Buchthal S, Taylor M, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011 Mar 13;25(5):625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Farag NH, Rashed HA, Hassan M, et al. Hepatitis C infection, Cognition, and inflammation in an Egyptian sample. J Med Virol. 2011 Feb;83(2):261–266. doi: 10.1002/jmv.21879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure, Supplemental Digital Content 1: Plasma and CSF levels of sCD14, CCL2, and IL-6 are higher in HIV+ subjects than in uninfected healthy controls. Plasma and CSF sCD14, CCL2, and IL-6 levels were compared between cohort subjects (n=67) and healthy controls (n=34). HIV+ subjects had higher plasma and CSF levels of sCD14, CCL2, and IL-6 compared to healthy controls. Median values are indicated as horizontal lines. Statistical significance between groups was calculated using the Mann-Whitney test; significant differences (p<0.05) are indicated.
Figure, Supplemental Digital Content 2: sCD14 levels are higher in plasma than CSF, while CCL2 levels are higher in CSF than plasma, in HIV+ subjects with advanced disease. HIV RNA, sCD14, CCL2, and IL-6 levels were compared between plasma and CSF. HIV RNA and sCD14 levels were higher in plasma compared to CSF, whereas CCL2 levels were significantly lower in plasma compared to CSF. IL-6 levels were similar between plasma and CSF. Median values are indicated as horizontal lines and significant differences were determined using the Mann-Whitney test; significant differences (p<0.05) are indicated.
Figure, Supplemental Digital Content 4: Global T scores are lower in HIV+ subjects with HAND or NPI-O clinical diagnoses. Global T scores were compared between subjects with no neurocognitive impairment (no NCI) (n=19) and subjects with HAND or NPI-O clinical diagnoses (n=44). Scores were lower in the group with HAND or NPI-O diagnoses. Median values are indicated as horizontal lines. Statistical significance between groups was calculated using the Mann-Whitney test; significant differences (p<0.05) are indicated.
Figure, Supplementary Digital Content 5: Plasma and CSF sCD14, CCL2, and IL-6 levels do not distinguish between subjects with and without HAND or NPI-O clinical diagnoses. Plasma and CSF sCD14, CCL2, and IL-6 levels were compared between subjects with no neurocognitive impairment (no NCI, n=19) and subjects with HAND or NPI-O (n=44). No differences were found for any of the markers tested. Median values are indicated as horizontal lines. Statistical significance between groups was calculated using the Mann-Whitney test.