Abstract
Centromeres of eukaryotic chromosomes mark the site for kinetochore formation and microtubule attachment and are essential for accurate chromosome segregation. Although centromere identity is defined by the presence of the histone H3 variant CenH3/centromere protein A (CENP-A), little is known about how epigenetic modifications on CenH3 might regulate kinetochore assembly and centromere function. Here we show that CENP-A from Saccharomyces cerevisiae, termed Cse4, is methylated on arginine 37 (R37) and that this methylation regulates the recruitment of kinetochore components to centromeric sequences. The absence of Cse4 R37 methylation caused a growth defect in cells lacking the centromere binding factor Cbf1 and synthetic lethality when combined with mutations in components of the Ctf19 linker complex that connects the inner kinetochore to microtubule-binding proteins. The cells showed a cell-cycle arrest in G2/M phase and defects in plasmid and chromosome segregation. Furthermore, the levels of Mtw1/MIND (Mtw1 including Nnf1-Nsl1-Dsn1) and Ctf19 components at the centromere, but not of Cse4 itself, were reduced in the absence of Cse4 R37 methylation, thus showing that this modification regulates the recruitment of linker components to the centromere. Altogether, our data identify a unique regulatory principle on centromeric chromatin by posttranslational modification of the amino terminus of CenH3.
In eukaryotic cells, the accurate segregation of chromosomes to the daughter cell during mitosis is mediated by the centromeres, which specify the site of assembly of the kinetochores and the attachment of the microtubuli. The underlying centromeric chromatin is distinct from chromatin in other genomic regions in that it contains the histone variant CenH3/centromere protein A (CENP-A) (1) that specifies centromere identity (2). In organisms with a regional centromere, blocks of CenH3 are interspersed with histone H3-containing nucleosomes in the centromeric chromatin (3). In the point centromere of the yeast Saccharomyces cerevisiae, CenH3 (termed Cse4) localizes to a single centromeric nucleosome that is wrapped around ∼125 bp of centromeric DNA, where it replaces canonical histone H3 (4–7). Cse4 consists of a C-terminal histone-fold domain with more than 60% identity to the histone-fold domain of histone H3. It furthermore carries a unique 135-aa N terminus that extends from the core nucleosome and undergoes contacts with kinetochore proteins that are essential for kinetochore assembly (8, 9).
Kinetochores are large modular structures that are built of three main classes of protein complexes that have extensive similarities between larger eukaryotes and S. cerevisiae (10, 11). First, the inner kinetochore plate is in direct contact with centromeric chromatin and provides an interface between the kinetochore and the centromeric nucleosomes. It comprises DNA-binding proteins like Cbf1 (12) and Mif2/CENP-C (13), as well as protein complexes like CBF3, which contains the proteins Cep3, Ctf13, Ndc10, and Skp1 (14). Second, protein complexes of the outer kinetochore plate interact with the microtubule ends. Foremost, the Ndc80 complex, consisting of Ndc80, Spc24, Spc25, and Nuf2 (15), forms a dumbbell-shaped structure that crosses the kinetochore vertically from the inner to the outer plate, and its outer end, together with the Dam1 complex, interacts directly with the microtubuli (11). Third, the linker layer provides a link between the inner and the outer kinetochore. The inner end of the Ndc80 complex interacts with the complexes Knl1/Spc105 (16) and Mtw1/MIND (Mtw1 including Nnf1-Nsl1-Dsn1) (17) of the linker layer. Furthermore, the Ctf19 complex (17) is a large constituent of the linker layer comprising at least 13 proteins [including the Ctf19-Okp1-Mcm21-Ame1 subcomplex, COMA (8)], and it is regarded as the yeast equivalent of the constitutive centromere-associated network complex (CCAN) that interacts with CENP-A (11).
In humans, large tandem arrays of AT-rich α-satellite DNA are found at centromeres and define the regional centromere (3). At the S. cerevisiae point centromere, the ∼125 bp of centromeric sequence consists of three conserved sequence elements—CDEI, CDEII, and CDEIII—that are required for centromere function (10). CDEI is bound by Cbf1 and enhances centromere function (12); CDEII folds around the centromeric nucleosome containing Cse4 (18); and CDEIII binds the CBF3 complex (14).
Canonical histones are abundantly modified by various posttranslational modifications (PTMs) that are critical for the regulation of chromatin function and the establishment of chromatin domains (19). In contrast, little is known about PTMs on CenH3. A role for phosphorylation of human CENP-A by Aurora B kinase has been described in the final stages of cytokinesis (20), and maize CenH3 is also phosphorylated (21). Additionally, Cse4 levels in the cell are controlled by ubiquitination of Cse4 that is mediated by the ubiquitin ligase Psh1. Ubiquitination-dependent proteolysis prevents Cse4 from being aberrantly localized to euchromatic sites (22, 23), although the exact site of ubiquitination on Cse4 remains to be determined.
Here we sought to identify PTMs on Cse4 and to determine their effect on kinetochore assembly and centromere function. Significantly, we found mono- and dimethylation of arginine 37 (R37) of Cse4. The absence of Cse4 R37 methylation caused defects in plasmid and chromosome segregation, synthetic growth defects/lethality in the absence of Cbf1 or components of the Ctf19 linker complex, and reduced levels of Mtw1/MIND and Ctf19 components at the centromere. Altogether, our data identify a unique mechanism of regulation on centromeric chromatin by controlling the recruitment of the inner kinetochore plate through a PTM on CenH3.
Results
Methylation of Cse4 on Arginine 37.
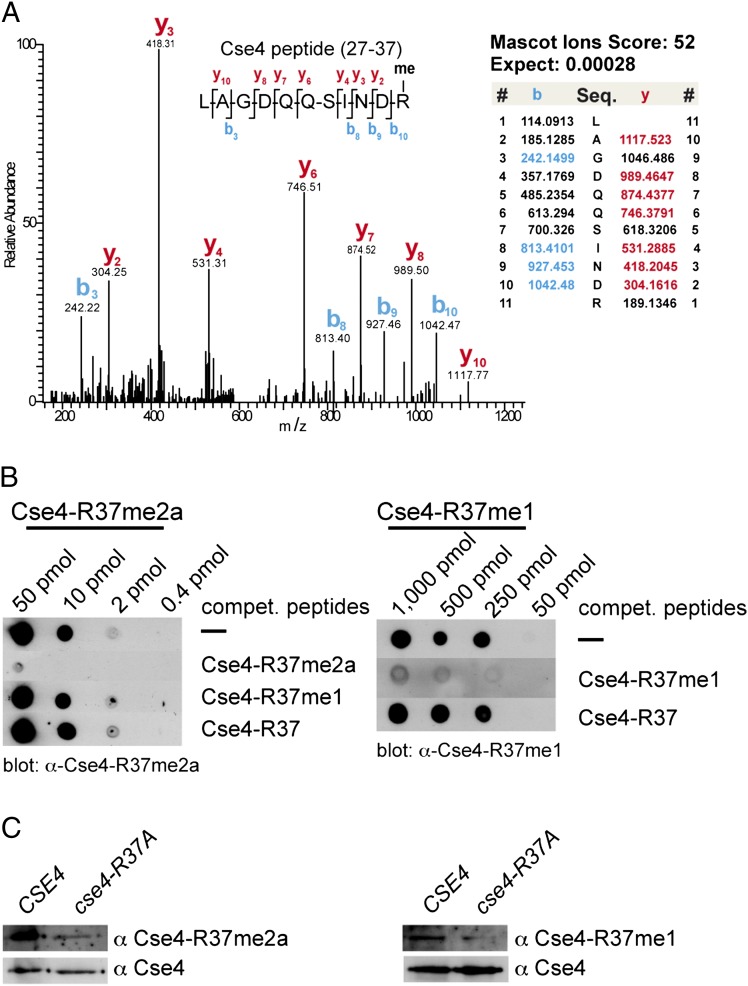
We sought to address the question whether CenH3 (Cse4) from yeast carries PTMs, as is the case for canonical histones, and whether such modifications on Cse4 regulate its function in kinetochore formation and chromosome segregation. To uncover such PTMs, we purified Cse4 from S. cerevisiae cells and analyzed it by gel-enhanced liquid chromatography mass spectrometry (GeLCMS) (24). In doing so, we were able to identify methylation of Cse4 on R37. Collision-induced dissociation fragmentation analysis of trypsin-digested Cse4 identified the peptide 27-R.LAGDQQSINDR.A-37 as unmodified [mass-to-charge ratio (m/z) = 608.799] and monomethylated (m/z = 615.807). Inspection of the MS/MS spectrum of the parent ion of m/z = 615.807 allowed us to uniquely assign the methylation site to R37 (Fig. 1A and Fig. S1). To further validate this, we generated antibodies that recognized Cse4 that is mono- or asymmetrically dimethylated at R37 (Cse4-R37me1, Cse4-R37me2a) (Fig. 1B and Fig. S2). In wild-type cells, these antibodies were able to detect methylated Cse4, and the signal was reduced or absent from cells in which Cse4-R37 was mutated to alanine (cse4-R37A) (Fig. 1C). This showed that Cse4 was both mono- and asymmetrically dimethylated on R37 in vivo. Whether Cse4 carries symmetrical dimethylation on R37 remains to be determined.
Fig. 1.
Cse4 is methylated at arginine 37. (A) Collision-induced dissociation analysis and full annotation of the parent ion (Cse4 27-LAGDQQSINDRme-37) with m/z = 615.81. Score and expected values as calculated by Mascot are reported, and fragments detected in the experiment are indicated in color (blue for b ions and red for y ions). (B) Specificity of antibodies generated against Cse4-R37me1 and R37me2a. The indicated amount of Cse4 peptide carrying R37me2a (Left) or R37me1 (Right) was spotted on nitrocellulose membrane. Antibodies were preincubated with the competing peptides indicated to the right of the blots. (C) Histone extracts from WT (AEY2781) and cse4-R37A (AEY5040) cells were probed with α-Cse4-R37me1 and R37me2a antibodies, as well as with α-HA to detect 3xHA-Cse4 as a loading control.
Cse4-R37 Methylation Is Essential in the Absence of the Centromere DNA Element I (CDEI)-Binding Factor Cbf1.
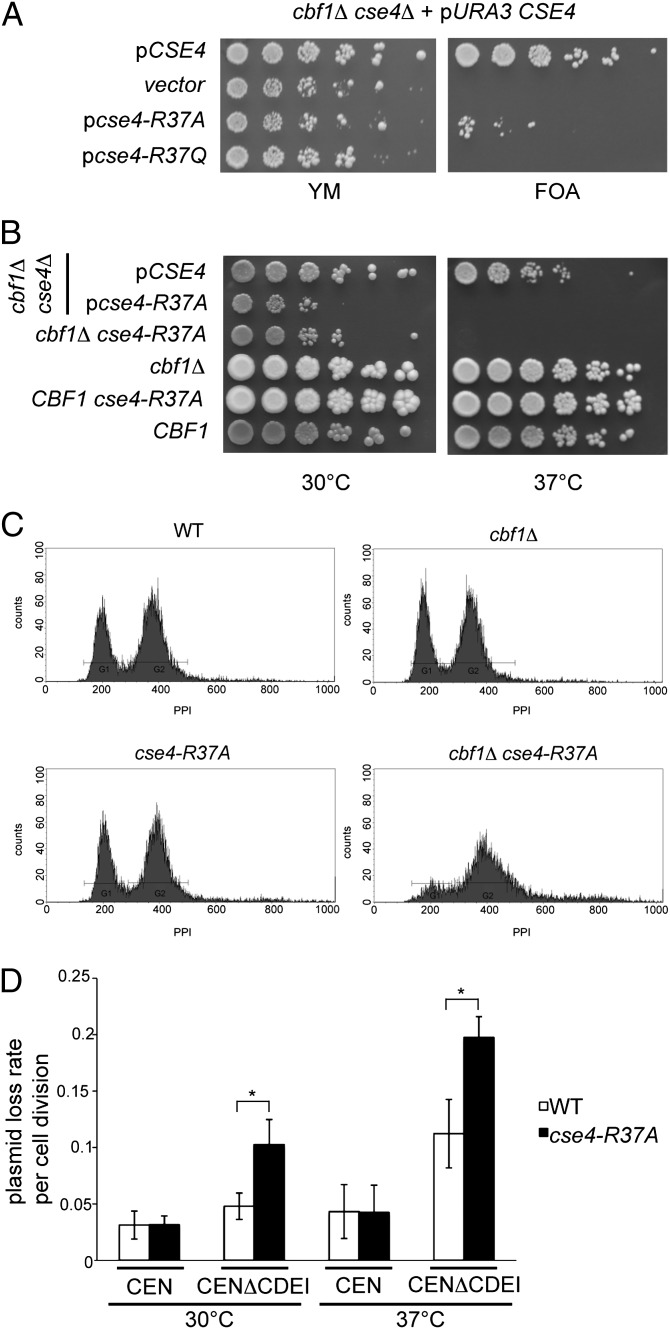
Arginine 37 is located within the essential N-terminal domain (END, amino acids 28–60) of Cse4 (25), which is part of the 135-aa N terminus of Cse4 that is necessary for kinetochore function. We next sought to determine how the absence of Cse4 R37 methylation affected centromere function. For this purpose, we generated an allele of CSE4 that encodes a Cse4 variant with R37 replaced by alanine to imitate the unmethylated state of Cse4 (cse4-R37A). Cells carrying cse4-R37A showed no appreciable growth defect or temperature sensitivity (see below; Fig. 2B). However, in cells lacking the CDEI-binding protein Cbf1, cse4-R37A caused a severe growth defect and temperature sensitivity. Specifically, CSE4 or cse4-R37A was introduced on a plasmid into a cbf1Δ cse4Δ strain carrying CSE4 on a URA3-marked plasmid, and the ability of the strain to grow in the absence of pURA3-CSE4 was tested on URA3-counterselective medium containing 5-fluoro-orotic acid (5-FOA) (Fig. 2A). Only few cells carrying cse4-R37A were able to survive on 5-FOA, and these cells showed slow growth at lower temperatures (30 °C) and were unable to grow at high temperatures (Fig. 2B), showing that this allele caused a strong synthetic growth defect in the absence of Cbf1. This defect was observed both when cse4-R37A was present on a centomere (CEN)-based plasmid (Fig. 2A) and when it was chromosomally integrated in the native CSE4 locus (Fig. 2B). This result indicated that methylation of Cse4-R37 becomes essential when centromere function is compromised by cbf1Δ. Notably, most mutations of modification sites on canonical histones cause no obvious growth defect (26), and some display a growth defect in the absence of a second factor (27). Thus, the synthetic growth defect of cse4-R37A observed here was at least as severe as that observed for canonical histones.
Fig. 2.
Cse4-R37 was required for cell-cycle progression and chromosome segregation. (A) Mutation of Cse4-R37 to alanine (cse4-R37A) or glutamine (cse4-R37Q) caused slow growth and inviability, respectively, in combination with cbf1Δ. Plasmid-borne cse4-R37A/-Q was introduced into a cbf1Δ cse4Δ strain carrying a URA3-marked CSE4 plasmid, and the ability of the cells to survive in the absence of CSE4 was tested on URA3-counterselective medium containing 5-FOA. (B) cse4-R37A on a plasmid (second row) as well as chromosomally integrated (third row) caused slow growth at 30 °C and lethality at 37 °C in the cbf1Δ background. Rows 3–6 show a tetratype tetrad from a genetic cross of cse4-R37A with cbf1Δ. (C) cbf1Δ cse4-R37A cells arrested at the G2/M phase of the cell cycle at the restrictive temperature. WT (AEY4), cbf1Δ (AEY4816), cse4-R37A (AEY4965), and cbf1Δ cse4-R37A (AEY4985) cells were grown to early logarithmic phase at 23 °C and shifted to 37 °C for 5 h. DNA content as measured by FACS analysis is shown. (D) cse4-R37A showed a maintenance defect for plasmids lacking the CDEI sequence of CEN6. Error bars give SD of three independent experiments. *Significant difference, P < 0.01.
Conceptually, the methylation of Cse4 could affect its function by influencing protein stability or by regulating its interaction with (a) kinetochore protein(s). However, protein levels of the mutant Cse4 protein were not altered, as measured by Western analysis of HA-tagged Cse4-R37A in cbf1Δ cells at the restrictive temperature (see below and Fig. 4A), suggesting that the absence of methylation regulated interactions of the Cse4 N terminus with the kinetochore, rather than protein stability. A growth defect/inviability was also seen when R37 was mutated to glutamine (cse4-R37Q; Fig. 2A), arguing that the phenotype was related to the absence of methylation rather than to the size difference of the substituted amino acid. Furthermore, mutation of another residue in the END domain in the vicinity of R37, cse4-S40A, did not show a growth defect in cbf1Δ or ctf19Δ (Fig. S3), indicating that the synthetic genetic effects of R37 mutation were not a general effect of all residues in the END domain.
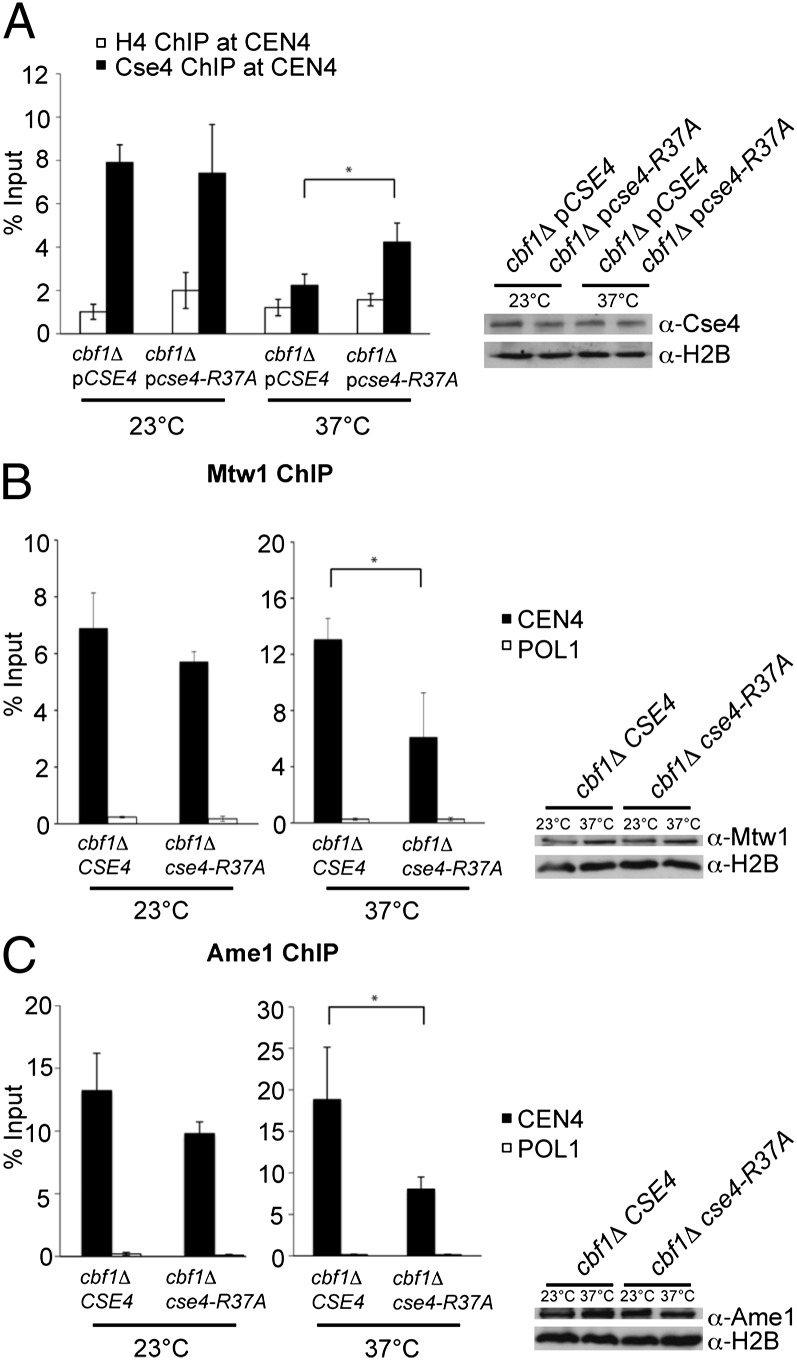
Fig. 4.
Mutation of Cse4-R37 caused a defect in the recruitment of Mtw1/MIND and Ctf19 complex components to the kinetochore. (A) The association of Cse4 at CEN4 as measured by ChIP analysis was not affected by cse4-R37A. cbf1Δ cse4Δ strains carrying either CSE4 or cse4-R37A on a plasmid were grown to early logarithmic phase at the permissive temperature (23 °C) and kept at this temperature or shifted to the restrictive temperature (37 °C) for 4 h. ChIP analysis was performed with α-HA (for Cse4) and, as a control, with α-H4 antibody. Values give the enrichment relative to input. Error bars give SD of three independent ChIP experiments. *Significant difference, P < 0.05. Right: Western blot analysis of the amounts of HA-Cse4 and histone H2B in whole cell extracts. (B) ChIP of 9xmyc-Mtw1 at CEN4 and an unrelated genomic region, POL1. ChIP was performed as in A. Right: Western blot analysis of the amounts of 9xmyc-Mtw1 and H2B in whole-cell extracts. (C) ChIP of 9xmyc-Ame1 at CEN4 and POL1. Analysis and representation are as in B.
The growth defect of cbf1Δ cse4-R37A cells suggested that centromere function might be disrupted in these cells, which may cause a synchronous cell cycle arrest. To test this, cbf1Δ cse4-R37A cells, WT cells, or single mutants were shifted to the restrictive temperature, and the DNA content of the cells was measured by FACS analysis. Importantly, cbf1Δ cse4-R37A cells, but not cbf1Δ or cse4-R37A single mutants, arrested with a 2n DNA content (Fig. 2C), indicating an arrest at the G2/M phase transition of the cell cycle. This suggested that in the absence of Cbf1, Cse4-R37A, which did not have R37 methylation, caused a defect in centromere function and chromosome segregation at the G2/M boundary.
cse4-R37A Causes Plasmid and Chromosome Segregation Defects.
Because the Cbf1 protein binds to the CDEI element of centromeric DNA sequences (12), the observation that cse4-R37A caused a cell-cycle arrest in cbf1Δ suggested that it affected the segregation of chromosomes lacking Cbf1 function. To directly test this, we examined the stability of plasmids lacking CDEI (CDEIΔ) in cells that were WT for Cbf1 but carried cse4-R37A and thus had no observable growth defect. As expected, CDEIΔ had a slight effect on plasmid stability in WT cells (Fig. 2D) (28). However, cse4-R37A cells showed a strongly increased plasmid loss rate with the CDEIΔ plasmid compared with WT, but not the equivalent plasmid carrying an intact CDEI, and this effect was exacerbated upon shift of the cells to a higher temperature (Fig. 2D). The effect of cse4-R37A was not due to the nature of the substituted amino acid, because cse4-R37Q showed a similar effect on plasmid loss of the CDEIΔ plasmid (Fig. S4). These results showed that centromere function and segregation of a plasmid compromised in CDEI was severely disrupted in the absence of Cse4-R37 methylation. Furthermore, cse4-R37A caused increased loss rates of a large linear, nonessential chromosome fragment with a deletion in CDEI of CEN6 (29), but not of the respective WT CEN6 fragment (Fig. S5), indicating that it caused not only minichromosome loss but also the loss of a large chromosome fragment. These results showed that cse4-R37A disrupted centromere stability and chromosome segregation in the absence of CDEI or the CDEI-binding factor Cbf1, suggesting a role for Cse4 R37 methylation in the assembly of a functional kinetochore.
Synthetic Lethality Between cse4-R37A and Mutations in Components of the Ctf19 Complex.
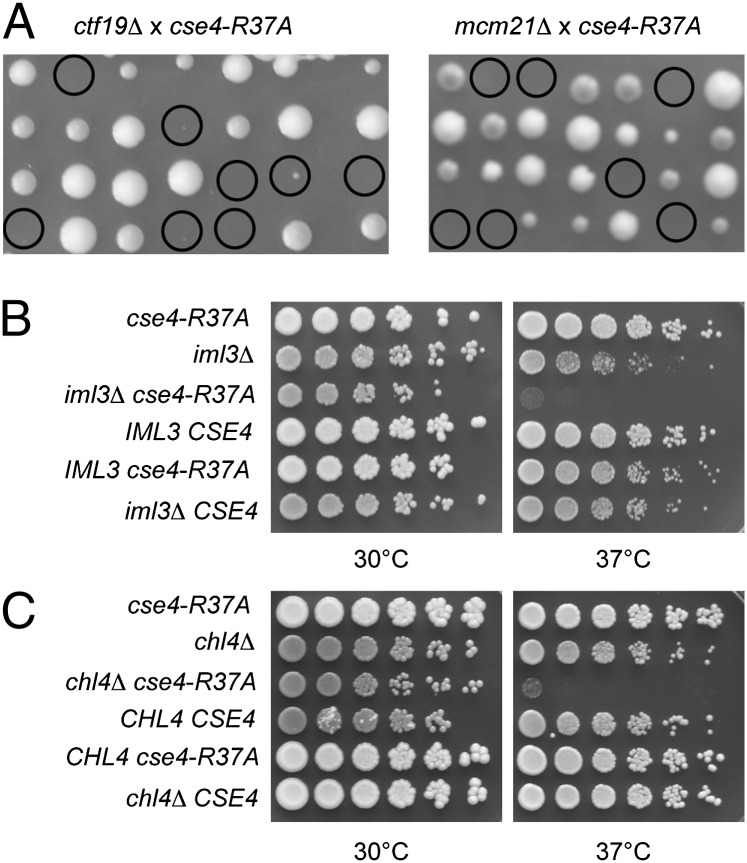
Because the absence of Cse4 R37 methylation showed a synthetic genetic interaction with cbf1Δ, we next asked whether this was a global genetic effect, or whether it was specific to the loss of certain kinetochore proteins. To test this, we performed genetic crosses between cse4-R37A and strains carrying mutations in genes encoding components of the inner and outer kinetochore as well as the linker layer. Interestingly, we found that cse4-R37A showed a strong specificity for synthetic genetic interactions in combination with defects in the Ctf19 complex. cse4-R37A was synthetically lethal with ctf19Δ, mcm21Δ, and ame1-4 and showed a strong synthetic growth defect with okp1-5 (Fig. 3A and Table 1). All four respective proteins are components of the COMA complex (8), a subcomplex of the Ctf19 complex (17).
Fig. 3.
Mutation of Cse4-R37 caused synthetic lethality and growth defects when combined with mutations in genes encoding Ctf19 complex components. (A) Tetrad dissection of genetic crosses of cse4-R37A with isogenic W303 strains carrying ctf19Δ (Left) or mcm21Δ (Right). The four spores from individual asci are aligned in vertical rows. Double mutants are marked with circles. (B) cse4-R37A caused a synthetic growth defect in the absence of the Ctf19 complex component Iml3/Mcm19. Serial dilutions of four segregants of a tetrad from a cross between cse4-R37A and iml3Δ were spotted on complete medium and grown at the indicated temperatures for 3 d. The top two rows show the parental strains. (C) cse4-R37A caused a synthetic growth defect with chl4Δ. Representation as in B.
Table 1.
Synthetic genetic interactions of cse4-R37A with mutations in genes encoding kinetochore components
| Kinetochore component/complex | Allele | Synthetic phenotype with cse4-R-37A* |
| CENP-C | mif2-3 | — |
| Cbf1 | cbf1Δ | Growth defect |
| CBF3 complex | cep3-1, cep3-2 | — |
| ndc10-1 | Slight growth defect | |
| ndc10-2 | — | |
| Ctf19 complex | ctf19Δ | Lethality |
| okp1-5 | Growth defect | |
| mcm21Δ | Lethality | |
| ame1-4 | Lethality | |
| iml3Δ/mcm19Δ | Growth defect | |
| chl4Δ | Growth defect | |
| ctf3Δ/chl3Δ | Growth defect | |
| Mtw1/MIND complex | mtw1-11 | Slight growth defect |
| Ndc80 complex | ndc80-1 | — |
| spc25-1 | — | |
| spc24-1 | — | |
| Knl1 complex | spc105-4 | Slight growth defect |
| Kinesin motor protein | cin8Δ | Lethality |
| Replication fork-associated factor | csm3Δ | — |
*Additional phenotype caused by cse4-R37A in combination with the indicated allele of the gene encoding the respective kinetochore component. —, no additional phenotype observed.
We furthermore found severe synthetic growth defects of cse4-R37A, with mutations in the genes encoding the Ctf19 complex components Iml3/Mcm19 (Fig. 3B), Chl4 (Fig. 3C), and Ctf3 (Table 1). A slight synthetic growth defect was observed in cse4-R37A cells, with a defect in Mtw1 (mtw1-11), a subunit of the Mtw1/MIND complex, as well as with Spc105 (spc105-4). Furthermore, cse4-R37A was synthetically lethal in the absence of the kinesin motor protein Cin8 (Table 1). In contrast, no defects were found in combination with mutations in components of the Ndc80 complex, nor with proteins and complexes acting at the DNA–kinetochore interface like Mif2/CENP-C and CBF3 (10) (Table 1), thus indicating that Cse4-R37 acted in the same pathway as these proteins or protein complexes. This is in agreement with earlier observations of a synthetic lethality between ctf19Δ and mutations in components of the inner kinetochore like Mif2, Cep3, and Ndc10 (30). These results showed a remarkable selectivity in the effect of Cse4 R37 methylation in that it specifically became essential when centromere function was compromised by mutations in Ctf19 components.
Absence of Cse4-R37 Methylation Causes a Defect in Kinetochore Assembly.
Our above results indicated that Cse4 R37 methylation was essential for full centromere and kinetochore function, raising the question of how this function was compromised in the absence of this modification. Two principle mechanisms can be envisioned: one in which the modification regulates protein proteolysis and restriction of Cse4 to the centromere (22, 23), and one in which it dictates the interaction with kinetochore proteins and thus regulates kinetochore assembly. We can exclude the first possibility, because we found equal amounts of cellular Cse4 and Cse4-R37A even at the restrictive temperature for cbf1Δ cse4-R37A cells (Fig. 4A). Additionally, cse4-R37A did not compromise the recruitment of Cse4 at CEN4 at the permissive or restrictive temperature, as measured by ChIP (Fig. 4A), nor did it lead to an enrichment of Cse4 at noncentromeric sites (Fig. S6). The level of Cse4 associated with CEN4 was reduced upon shift of the cells to the restrictive temperature, but this reduction (albeit to a lesser degree) was observed in cse4-R37A. This indicated that Cse4 deposition at centromeres was not affected by R37 methylation.
We next measured the association of the MIND complex component Mtw1 to CEN4 by ChIP analysis. Importantly, upon shift of cbf1Δ cse4-R37A to the restrictive temperature, the level of Mtw1 associated with CEN4 was significantly reduced compared with the cbf1Δ strain, but this reduction was not the result of a reduction in the cellular level of Mtw1 (Fig. 4B). This indicated that Cse4 R37 methylation was necessary for efficient recruitment of the MIND complex to the centromere.
We further asked whether the association of other kinetochore complexes was compromised by cse4-R37A. As for Mtw1, we observed a marked reduction in the association of the Ctf19/COMA component Ame1 to centromeric sequences upon shift of the cells to the restrictive temperature, whereas the cellular levels of Ame1 were unaffected (Fig. 4C), suggesting that Ame1 association to the centromere required Cse4-R37 methylation. Taken together, these results provided mechanistic insight into the role of Cse4 R37 methylation in centromere function and showed that it acts by regulating the recruitment of complexes of the linker layer of the kinetochore to the centromere.
Discussion
The modification of histones by PTMs is a mechanism that is central to the regulation of chromatin function in eukaryotic cells. Here, we have identified a unique PTM on CenH3/Cse4 in S. cerevisiae, methylation of arginine 37. Furthermore, we show that this modification has a defined regulatory role in centromere biology in that it controls the recruitment of kinetochore proteins to centromeric sequences. The absence of this modification has striking phenotypic consequences and causes synthetic lethality with several components of the Ctf19 linker complex, a severity of phenotype that rivals that of mutations in PTM sites of canonical histones. Furthermore, cse4-R37A causes defects in plasmid and chromosome segregation of centromeres lacking CDEI, and a cell cycle arrest at G2/M in the absence of the CDEI-binding protein Cbf1.
It is interesting to note that the phenotype of cse4-R37A shows such specificity for mutations in the Ctf19 complex but displayed no additional effect when combined with mutations in the other linker complexes MIND and Knl1, as well as in the outer kinetochore complex Ndc80. This suggests that potential interactions between the Cse4 N terminus and the MIND, Knl1, and Ndc80 complexes become essential in the absence of R37 methylation, either by direct contact with Cse4 or via an indirect association that is mediated by other kinetochore proteins. Therefore, in analogy to PTMs on other histones (31), we propose that R37 methylation regulates the interaction of the Cse4 N terminus with such (a) kinetochore protein(s), for instance by attracting a positive regulator with a methyl-arginine-binding module, or by repelling a negative regulator. One possibility is that, as cells go through S phase and initiate kinetochore assembly, this modification is directed to Cse4, perhaps because the chromatin structure is more accessible during DNA replication and chromatin assembly, which is in agreement with the fact that we do not find full Cse4-R37 methylation (Fig. S1). Subsequently, the methylated N terminus of Cse4 recruits kinetochore proteins to the centromeric chromatin, which ultimately leads to the formation of a functional kinetochore and to microtubuli attachment. Consequently, the absence of this modification impairs the ability of Cse4 to nucleate a kinetochore, which ultimately disrupts chromosome segregation and leads to chromosome loss. Notably, this regulation is markedly distinct from that of Cse4 ubiquitination by Psh1 (22, 23), because unlike ubiquitination, the methylation of Cse4 did not affect the stability of the Cse4 protein. Methylation of Cse4-R37 is likely to be catalyzed by a previously unknown or the combination of several methyltransferases, because the absence of the three known arginine methyltransferases (Hmt1, Rmt2, and Hsl7) did not decrease Cse4-R37 methylation, nor did they cause the same genetic defects as mutation of Cse4-R37 (Fig. S7). Thus, like for H3 R2 methylation in yeast (32), the responsible enzyme for Cse4-R37 methylation remains to be identified.
The Cse4 N terminus has previously been shown to interact with the Ctf19 protein (9). However, this interaction seems not to be affected by R37 methylation, because the absence of R37 methylation causes lethality in cells lacking Ctf19. Therefore, other interaction partners with the Cse4 N terminus must exist, perhaps another component of the Ctf19 linker complex, whose interaction is mediated by Cse4 methylation.
In summary, with this work, we have revealed a unique mechanism for the epigenetic regulation of centromeric chromatin in that we identified a PTM of the centromeric CenH3/Cse4 histone variant itself, rather than the canonical histones, that has a regulatory role in centromere function. The disruption of this process causes chromosome segregation defects, which in larger eukaryotes can lead to tumor formation by causing aneuploidy and genomic instability. Thus, by extension, the study of similar processes in larger eukaryotes will shed light on hitherto unknown regulatory mechanisms controlling genomic stability in those organisms.
Materials and Methods
Yeast Strains, Plasmids, and Methods.
Yeast strains and plasmids used in this study are described in Tables S1 and S2. Yeasts were grown and manipulated using standard genetic techniques (33). Plasmid-borne cse4 alleles were constructed using the gap repair method and verified by sequence analysis. For chromosomal integration of cse4-R37A, the cse4-R37A allele was transferred into a URA3-marked integrating vector (pAE1636) and introduced into a WT strain (AEY4) by integrative transformation followed by loop-out on 5-FOA medium. Candidate strains were tested for the presence of the cse4-R37A allele using an allele-specific PCR. In addition, the cse4 allele was amplified from such strains by PCR and verified by sequence analysis.
Plasmid loss was measured in a WT (AEY4) and a cse4-R37A strain (AEY4965) carrying a CEN6-TRP1 plasmid containing either a functional centromere sequence (pAE264) or a centromere sequence without the CDEI element (pAE1771) as previously described (34). FACS of yeast cells was performed as previously described (35).
Mass Spectrometry.
Partially purified histones (36) from cells containing 3xHA-tagged Cse4 were resolved on 10% (weight/vol) SDS-polyacrylamide gels. The Cse4 band was excised and digested in-gel with trypsin as previously described (37). The peptide mixture was separated by nano-liquid chromatography (LC)-MS/MS using an Agilent 1100 Series nanoflow LC system and analyzed by MS/MS on a 7-Tesla LTQ-FT-Ultra mass spectrometer (ThermoFisher Scientific). Details on MS acquisition are described in SI Materials and Methods.
Antibody Generation and Western Blotting.
Rabbit polyclonal antibodies were raised against a synthetic Cse4 peptide containing R37me1 [SINDR(me)ALSLGGC] or R37me2a [QSINDR(me2a)ALSLGGC]. The antibodies were isolated from the crude serum by two subsequent affinity purification steps with the synthetic modified peptides cross-linked to SulfoLink (Pierce). The serum was first applied to a column with the modified peptide and eluted with 0.1 M glycine (pH 2.5). After neutralization, the eluate was applied to a column with the unmodified peptide, and the supernatant was used for Western blotting (1:200) on histone extracts isolated as described above. For peptide competition, the antibodies were preincubated with 100 pmol (R37me2a) or 4,000 pmol (R37me1) of the indicated peptide for 60 min at 28 °C before Western blotting. HA-tagged Cse4 was detected using an α-HA antibody (1:10,000; Covance). Other antibodies used in this study were anti-H2B (39237; Active Motif), anti-H4 (31827; Abcam), and anti-myc (M4439; Sigma).
Additional details are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank S. Biggins, M. Fitzgerald-Hayes, J. Kilmartin, L. Hartwell, P. Hieter, J. Lechner, A. Marston, J. Rine, M. M. Smith, and J. Vogel for reagents; R. Schneider for technical advice with antibody generation; K. Nicklasch, M. Rübeling, and J. Wohlgemuth for technical support; S. Seitz for early work on Cse4 in the laboratory; and H. Meyer and A. Musacchio for comments on the manuscript. This work was supported by Deutsche Forschungsgemeinschaft Grant GRK1431/1 (to A.E.E.-M.) and the University of Duisburg-Essen (A.E.E.-M.). T.B. was supported by grants from the Giovanni Armenise-Harvard Foundation Career Development Program, the Association of International Cancer Research, the Italian Association for Cancer Research, the Cariplo Foundation, and the Italian Ministry of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120968109/-/DCSupplemental.
References
- 1.Smith MM. Centromeres and variant histones: What, where, when and why? Curr Opin Cell Biol. 2002;14:279–285. doi: 10.1016/s0955-0674(02)00331-9. [DOI] [PubMed] [Google Scholar]
- 2.Mendiburo MJ, Padeken J, Fülöp S, Schepers A, Heun P. Drosophila CENH3 is sufficient for centromere formation. Science. 2011;334:686–690. doi: 10.1126/science.1206880. [DOI] [PubMed] [Google Scholar]
- 3.Blower MD, Sullivan BA, Karpen GH. Conserved organization of centromeric chromatin in flies and humans. Dev Cell. 2002;2:319–330. doi: 10.1016/s1534-5807(02)00135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meluh PB, Yang P, Glowczewski L, Koshland D, Smith MM. Cse4p is a component of the core centromere of Saccharomyces cerevisiae. Cell. 1998;94:607–613. doi: 10.1016/s0092-8674(00)81602-5. [DOI] [PubMed] [Google Scholar]
- 5.Furuyama S, Biggins S. Centromere identity is specified by a single centromeric nucleosome in budding yeast. Proc Natl Acad Sci USA. 2007;104:14706–14711. doi: 10.1073/pnas.0706985104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camahort R, et al. Cse4 is part of an octameric nucleosome in budding yeast. Mol Cell. 2009;35:794–805. doi: 10.1016/j.molcel.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoler S, Keith KC, Curnick KE, Fitzgerald-Hayes M. A mutation in CSE4, an essential gene encoding a novel chromatin-associated protein in yeast, causes chromosome nondisjunction and cell cycle arrest at mitosis. Genes Dev. 1995;9:573–586. doi: 10.1101/gad.9.5.573. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz J, Stemmann O, Rank S, Lechner J. A putative protein complex consisting of Ctf19, Mcm21, and Okp1 represents a missing link in the budding yeast kinetochore. Genes Dev. 1999;13:1140–1155. doi: 10.1101/gad.13.9.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, et al. The N terminus of the centromere H3-like protein Cse4p performs an essential function distinct from that of the histone fold domain. Mol Cell Biol. 2000;20:7037–7048. doi: 10.1128/mcb.20.18.7037-7048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westermann S, Drubin DG, Barnes G. Structures and functions of yeast kinetochore complexes. Annu Rev Biochem. 2007;76:563–591. doi: 10.1146/annurev.biochem.76.052705.160607. [DOI] [PubMed] [Google Scholar]
- 11.Santaguida S, Musacchio A. The life and miracles of kinetochores. EMBO J. 2009;28:2511–2531. doi: 10.1038/emboj.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai M, Davis RW. Yeast centromere binding protein CBF1, of the helix-loop-helix protein family, is required for chromosome stability and methionine prototrophy. Cell. 1990;61:437–446. doi: 10.1016/0092-8674(90)90525-j. [DOI] [PubMed] [Google Scholar]
- 13.Meluh PB, Koshland D. Evidence that the MIF2 gene of Saccharomyces cerevisiae encodes a centromere protein with homology to the mammalian centromere protein CENP-C. Mol Biol Cell. 1995;6:793–807. doi: 10.1091/mbc.6.7.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lechner J, Carbon J. A 240 kd multisubunit protein complex, CBF3, is a major component of the budding yeast centromere. Cell. 1991;64:717–725. doi: 10.1016/0092-8674(91)90501-o. [DOI] [PubMed] [Google Scholar]
- 15.Wigge PA, Kilmartin JV. The Ndc80p complex from Saccharomyces cerevisiae contains conserved centromere components and has a function in chromosome segregation. J Cell Biol. 2001;152:349–360. doi: 10.1083/jcb.152.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nekrasov VS, Smith MA, Peak-Chew S, Kilmartin JV. Interactions between centromere complexes in Saccharomyces cerevisiae. Mol Biol Cell. 2003;14:4931–4946. doi: 10.1091/mbc.E03-06-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Wulf P, McAinsh AD, Sorger PK. Hierarchical assembly of the budding yeast kinetochore from multiple subcomplexes. Genes Dev. 2003;17:2902–2921. doi: 10.1101/gad.1144403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keith KC, Fitzgerald-Hayes M. CSE4 genetically interacts with the Saccharomyces cerevisiae centromere DNA elements CDE I and CDE II but not CDE III. Implications for the path of the centromere dna around a cse4p variant nucleosome. Genetics. 2000;156:973–981. doi: 10.1093/genetics/156.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeitlin SG, Shelby RD, Sullivan KF. CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J Cell Biol. 2001;155:1147–1157. doi: 10.1083/jcb.200108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Li X, Marshall JB, Zhong CX, Dawe RK. Phosphoserines on maize CENTROMERIC HISTONE H3 and histone H3 demarcate the centromere and pericentromere during chromosome segregation. Plant Cell. 2005;17:572–583. doi: 10.1105/tpc.104.028522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranjitkar P, et al. An E3 ubiquitin ligase prevents ectopic localization of the centromeric histone H3 variant via the centromere targeting domain. Mol Cell. 2010;40:455–464. doi: 10.1016/j.molcel.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hewawasam G, et al. Psh1 is an E3 ubiquitin ligase that targets the centromeric histone variant Cse4. Mol Cell. 2010;40:444–454. doi: 10.1016/j.molcel.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundby A, Olsen JV. GeLCMS for in-depth protein characterization and advanced analysis of proteomes. Methods Mol Biol. 2011;753:143–155. doi: 10.1007/978-1-61779-148-2_10. [DOI] [PubMed] [Google Scholar]
- 25.Keith KC, et al. Analysis of primary structural determinants that distinguish the centromere-specific function of histone variant Cse4p from histone H3. Mol Cell Biol. 1999;19:6130–6139. doi: 10.1128/mcb.19.9.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Megee PC, Morgan BA, Mittman BA, Smith MM. Genetic analysis of histone H4: Essential role of lysines subject to reversible acetylation. Science. 1990;247:841–845. doi: 10.1126/science.2106160. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Bone JR, Edmondson DG, Turner BM, Roth SY. Essential and redundant functions of histone acetylation revealed by mutation of target lysines and loss of the Gcn5p acetyltransferase. EMBO J. 1998;17:3155–3167. doi: 10.1093/emboj/17.11.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panzeri L, Landonio L, Stotz A, Philippsen P. Role of conserved sequence elements in yeast centromere DNA. EMBO J. 1985;4:1867–1874. doi: 10.1002/j.1460-2075.1985.tb03862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegemann JH, Shero JH, Cottarel G, Philippsen P, Hieter P. Mutational analysis of centromere DNA from chromosome VI of Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:2523–2535. doi: 10.1128/mcb.8.6.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyland KM, Kingsbury J, Koshland D, Hieter P. Ctf19p: A novel kinetochore protein in Saccharomyces cerevisiae and a potential link between the kinetochore and mitotic spindle. J Cell Biol. 1999;145:15–28. doi: 10.1083/jcb.145.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yap KL, Zhou MM. Keeping it in the family: Diverse histone recognition by conserved structural folds. Crit Rev Biochem Mol Biol. 2010;45:488–505. doi: 10.3109/10409238.2010.512001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirmizis A, et al. Arginine methylation at histone H3R2 controls deposition of H3K4 trimethylation. Nature. 2007;449:928–932. doi: 10.1038/nature06160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 34.McNally FJ, Rine J. A synthetic silencer mediates SIR-dependent functions in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:5648–5659. doi: 10.1128/mcb.11.11.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loo S, et al. The origin recognition complex in silencing, cell cycle progression, and DNA replication. Mol Biol Cell. 1995;6:741–756. doi: 10.1091/mbc.6.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waterborg JH. Steady-state levels of histone acetylation in Saccharomyces cerevisiae. J Biol Chem. 2000;275:13007–13011. doi: 10.1074/jbc.275.17.13007. [DOI] [PubMed] [Google Scholar]
- 37.Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.