Abstract
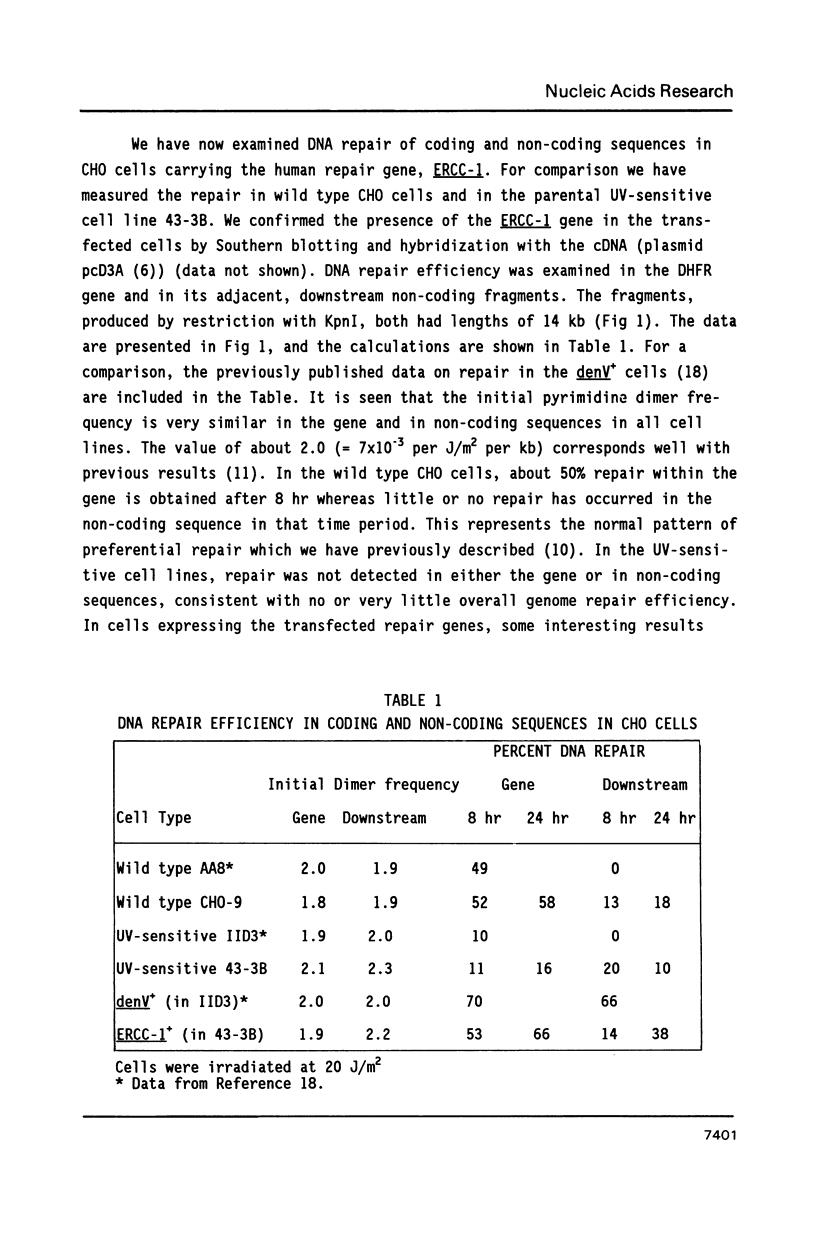
The pattern of preferential DNA repair of UV-induced pyrimidine dimers was studied in repair-deficient Chinese hamster ovary (CHO) cells transfected with the human excision repair gene, ERCC-1. Repair efficiency was measured in the active dihydrofolate reductase (DHFR) gene and in its flanking, non-transcribed sequences in three cell lines: Wild type CHO cells, a UV-sensitive excision deficient CHO mutant, and the transfected line of the mutant carrying the expressed ERCC-1 gene. The CHO cells transformed with the human ERCC-1 gene repaired the active DHFR gene much more efficiently than the non-transcribed sequences, a pattern similar to that seen in wild type CHO cells. This pattern differs from that previously reported in CHO cells transfected with the denV gene of bacteriophage T4, in which both active and non-transcribed DNA sequences were efficiently repaired (Bohr and Hanawalt, Carcinogenesis 8: 1333-1336, 1987). The ERCC-1 gene product may specifically substitute for the repair enzyme present in normal hamster cells while the denV product, T4 endonuclease V, does not be appear to be constrained in its access to inactive chromatin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohr V. A., Hanawalt P. C. Enhanced repair of pyrimidine dimers in coding and non-coding genomic sequences in CHO cells expressing a prokaryotic DNA repair gene. Carcinogenesis. 1987 Sep;8(9):1333–1336. doi: 10.1093/carcin/8.9.1333. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Okumoto D. S., Hanawalt P. C. Survival of UV-irradiated mammalian cells correlates with efficient DNA repair in an essential gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3830–3833. doi: 10.1073/pnas.83.11.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Okumoto D. S., Ho L., Hanawalt P. C. Characterization of a DNA repair domain containing the dihydrofolate reductase gene in Chinese hamster ovary cells. J Biol Chem. 1986 Dec 15;261(35):16666–16672. [PubMed] [Google Scholar]
- Bohr V. A., Phillips D. H., Hanawalt P. C. Heterogeneous DNA damage and repair in the mammalian genome. Cancer Res. 1987 Dec 15;47(24 Pt 1):6426–6436. [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Inactivation of ultraviolet repair in normal and xeroderma pigmentosum cells by methyl methanesulfonate. Cancer Res. 1982 Mar;42(3):860–863. [PubMed] [Google Scholar]
- Hori T., Shiomi T., Sato K. Human chromosome 13 compensates a DNA repair defect in UV-sensitive mouse cells by mouse--human cell hybridization. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5655–5659. doi: 10.1073/pnas.80.18.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leadon S. A. Differential repair of DNA damage in specific nucleotide sequences in monkey cells. Nucleic Acids Res. 1986 Nov 25;14(22):8979–8995. doi: 10.1093/nar/14.22.8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Bohr V. A., Smith C. A., Hanawalt P. C. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Rubin J. S., Joyner A. L., Bernstein A., Whitmore G. F. Molecular identification of a human DNA repair gene following DNA-mediated gene transfer. Nature. 1983 Nov 10;306(5939):206–208. doi: 10.1038/306206a0. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Mooney C. L., Burkhart-Schultz K., Carrano A. V., Siciliano M. J. Correction of a nucleotide-excision-repair mutation by human chromosome 19 in hamster-human hybrid cells. Somat Cell Mol Genet. 1985 Jan;11(1):87–92. doi: 10.1007/BF01534738. [DOI] [PubMed] [Google Scholar]
- Valerie K., de Riel J. K., Henderson E. E. Genetic complementation of UV-induced DNA repair in Chinese hamster ovary cells by the denV gene of phage T4. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7656–7660. doi: 10.1073/pnas.82.22.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. M., Hanawalt P. C. Processing of psoralen adducts in an active human gene: repair and replication of DNA containing monoadducts and interstrand cross-links. Cell. 1987 Aug 28;50(5):789–799. doi: 10.1016/0092-8674(87)90337-0. [DOI] [PubMed] [Google Scholar]
- Westerveld A., Hoeijmakers J. H., van Duin M., de Wit J., Odijk H., Pastink A., Wood R. D., Bootsma D. Molecular cloning of a human DNA repair gene. Nature. 1984 Aug 2;310(5976):425–429. doi: 10.1038/310425a0. [DOI] [PubMed] [Google Scholar]
- Zdzienicka M. Z., Roza L., Westerveld A., Bootsma D., Simons J. W. Biological and biochemical consequences of the human ERCC-1 repair gene after transfection into a repair-deficient CHO cell line. Mutat Res. 1987 Jan;183(1):69–74. doi: 10.1016/0167-8817(87)90047-2. [DOI] [PubMed] [Google Scholar]
- Zdzienicka M. Z., Simons J. W. Analysis of repair processes by the determination of the induction of cell killing and mutations in two repair-deficient Chinese hamster ovary cell lines. Mutat Res. 1986 Jul;166(1):59–69. doi: 10.1016/0167-8817(86)90041-6. [DOI] [PubMed] [Google Scholar]
- van Duin M., de Wit J., Odijk H., Westerveld A., Yasui A., Koken M. H., Hoeijmakers J. H., Bootsma D. Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10. Cell. 1986 Mar 28;44(6):913–923. doi: 10.1016/0092-8674(86)90014-0. [DOI] [PubMed] [Google Scholar]



