Abstract
Oxidation sensing and quorum sensing significantly affect bacterial physiology and host–pathogen interactions. However, little attention has been paid to the cross-talk between these two seemingly orthogonal signaling pathways. Here we show that the quorum-sensing agr system has a built-in oxidation-sensing mechanism through an intramolecular disulfide switch possessed by the DNA-binding domain of the response regulator AgrA. Biochemical and mass spectrometric analysis revealed that oxidation induces the intracellular disulfide bond formation between Cys-199 and Cys-228, thus leading to dissociation of AgrA from DNA. Molecular dynamics (MD) simulations suggest that the disulfide bond formation generates a steric clash responsible for the abolished DNA binding of the oxidized AgrA. Mutagenesis studies further established that Cys-199 is crucial for oxidation sensing. The oxidation-sensing role of Cys-199 is further supported by the observation that the mutant Staphylococcus aureus strain expressing AgrAC199S is more susceptible to H2O2 owing to repression of the antioxidant bsaA gene under oxidative stress. Together, our results show that oxidation sensing is a component of the quorum-sensing agr signaling system, which serves as an intrinsic checkpoint to ameliorate the oxidation burden caused by intense metabolic activity and potential host immune response.
Many pathogenic bacteria are dependent on their ability to swiftly sense and respond to surrounding population density and changing host microenvironments. Bacterial physiological rearrangements can be controlled by quorum-sensing systems in response to increasing population density (1–3). Meanwhile, in the context of host–pathogen interactions, host immune systems such as macrophages and neutrophils generate a burst of oxidants (O2−, HO•, H2O2, HClO, NO, etc.) to kill invading pathogens. Oxidation sensing, on the other hand, is exploited by pathogenic bacteria as a key signaling strategy to adapt and evade the hostile immune system (4–7). Although both quorum sensing and oxidation sensing have been extensively studied in bacterial pathogenesis and related virulence regulation (3, 8), these two distinct signaling processes are often regarded as independent events in gene regulation, and little is known about the interplay between them.
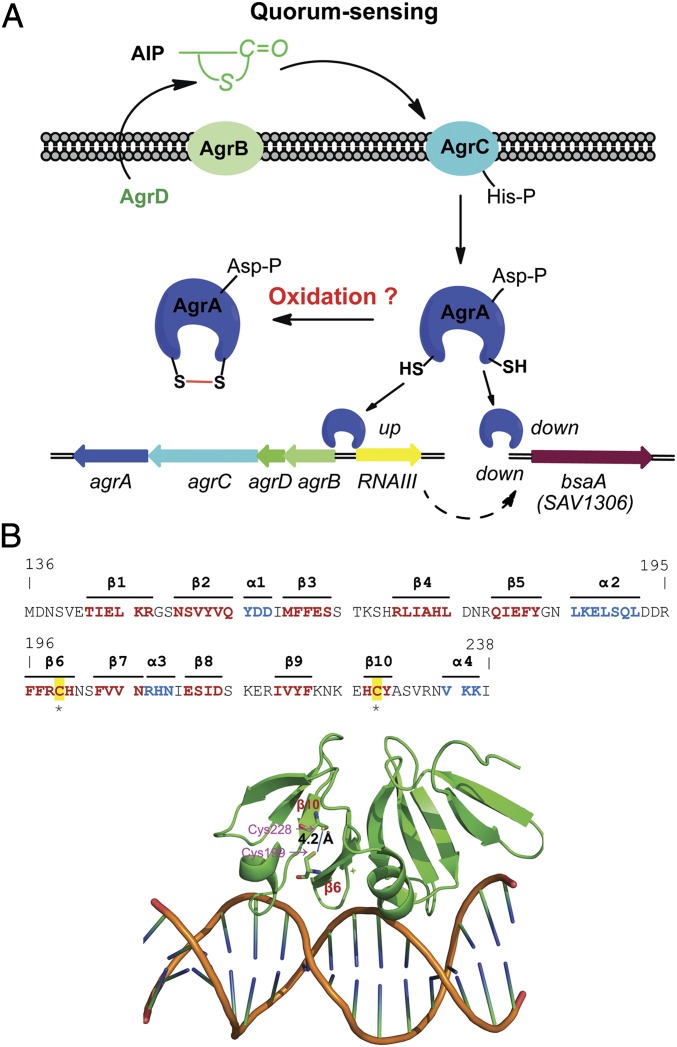
Staphylococcus aureus, a major human pathogen that is the most common source of nosocomial and community-acquired infections, causes a variety of diseases, ranging from minor skin infections to life-threatening blood infections (9). The success of this bacterium in pathogenesis is largely owing to the sophisticated regulatory network composed of several global transcriptional regulators (e.g., SigB, Rot, MgrA, SarA, and SarA homologs) and 16 two-component systems (TCSs) (e.g., agr, srrAB, arlRS, vraRS, hssRS, and saeRS) (10–12), which enable the bacterium to rapidly sense and adapt to changing environment. Central to this regulatory network is the quorum-sensing agr system, which controls the expression of bacterial virulence in response to changes in cell density (10). Transcriptional expression of the agr system generates two adjacent mRNAs corresponding to RNAII and RNAIII in opposite directions (Fig. 1A): RNAIII not only encodes a virulence factor δ-toxin (Hld) but also functions as a small regulatory RNA (sRNA) per se to modulate target gene expression; RNAII comprises a typical bacterial TCS consisting of the sensor kinase AgrC and the response regulator AgrA. In addition, it encodes AgrD, the precursor of the quorum signal that can further be processed and exported as a thiolactone-containing oligopeptide autoinducer (autoinducing peptide, AIP) by the cotranscribed AgrB. Upon binding to the extracellular sensory domain of AgrC, AIP activates the kinase activity of AgrC, subsequently leading to phosphorylation of the response regulator AgrA (13, 14). Phosphorylated AgrA regulates transcription of genes encoding metabolic factors and phenol-soluble modulin (PSM) peptides (15) and, more importantly, triggers the expression of the agr operon by binding to the promoter regions P2 (for RNAII) and P3 (for RNAIII), thereby forming an autoinduction genetic circuit to ensure a timely rearrangement of target gene expression at a certain threshold level of population density.
Fig. 1.
S. aureus AgrA. (A) Model of gene regulation by the quorum-sensing agr. AIP, with its thiolactone structure, is encoded by agrD, processed, and exported by AgrB. AIP serves as the quorum signal to activate the TCS consisting of the sensor kinase AgrC and the response regulator AgrA. The reported crystal structure of the DNA-binding domain of AgrA complexed with DNA (27) revealed two spatially proximate Cys residues (Cys-199 and Cys-228) with two sulfur atoms 4.2 Å away from each other (PDB entry: 3BS1), suggesting a potential oxidation-sensing mechanism that impacts the AgrA regulon, such as RNAIII, or the bsaA gene encoding the antioxidant, glutathione peroxidase. (B) Sequence and structure of the C-terminal DNA-binding domain of AgrA (residues 136–238) with two Cys residues highlighted. Strands are colored red, and helices are colored cyan.
In addition to this prominent agr-mediated quorum sensing in S. aureus, a number of studies have demonstrated that S. aureus uses oxidation-sensing global transcriptional regulators, including MgrA, SarZ, and SarA, to control global gene expression via the redox active Cys residue (16–19). Additionally, there are two S. aureus TCSs found to be capable of sensing oxidation. One is the [4Fe-4S]-containing TCS NreABC (20). NreABC is a specialized TCS that regulates a set of genes involved in anaerobic nitrate/nitrite uptake but with negligible effects on virulence gene expression (20). We also identified a [2Fe-2S]-containing redox-responsive TCS, AirSR, that globally impacts gene expression under oxygen-limited conditions (21). So far specialized regulatory systems have been shown to execute the oxidation-sensing process in S. aureus independently of quorum sensing.
In this work we discover that the S. aureus quorum-sensing agr system has integrated another level of the signaling pathway of oxidation sensing into its predominant quorum-sensing mode to counter oxidative stress. We demonstrate that the DNA-binding domain of the response regulator AgrA contains a redox-active Cys-199, which forms an intramolecular disulfide bond with the spatially proximate Cys-228 under oxidative stress. The oxidized AgrA dissociates from its cognate DNA, leading to down-regulation of the expression of RNAIII and up-regulation of that of the glutathione peroxidase gene (bsaA), which is shown to be essential for bacterial resistance to oxidative stress. We further show that abolishing the oxidation-sensing ability of AgrA through mutation of Cys-199 to Ser significantly impairs staphylococcal resistance toward oxidative stress. This discovery showcases a quorum-sensing system that has an intrinsic oxidation-sensing mechanism to coordinate its gene regulation. Moreover, the interplay between two seemingly independent signal transduction pathways—quorum sensing and oxidation sensing—has broad implications for understanding bacterial gene regulation.
Results
Potential Disulfide Redox Switch in AgrA.
Previous studies have demonstrated that transcription of the quorum-sensing agr system is significantly repressed under the oxidative stress exerted by oxidants such as Cu(II), H2O2, HClO, ONOO−, etc. (22, 23). Moreover, a detailed study revealed that oxidation of C-terminal methionine in the AIP by strong oxidants including HClO and ONOO, but not mild ones such as H2O2, represses the agr regulon (23). We confirmed that millimolar levels of H2O2 could down-regulate the transcription of the agr operon (Fig. S1A) in strain Newman independently of the redox-responsive SarA (24, 25), another global transcriptional regulator required for agr expression (26). Repression of RNAIII upon H2O2 treatment was observed in S. aureus strain Newman as well as in USA300 (Fig. S2A). Clearly, the model of AIP inactivation by oxidation, which is mainly responsible for extracellular factors that influence the agr regulation, accounts for the response of the agr system to certain types of reactive oxygen/nitrogen species (ROS/RNS), which prompted us to identify an alternative mechanism underlying the redox response of agr toward the most common H2O2 stress that S. aureus encounters.
The solved crystal structure of the DNA-binding domain of the response regulator AgrA complexed with DNA (27) shows a unique topology with 10 β-strands arranged into three antiparallel β-sheets and three small α-helices (Fig. 1B). Intriguingly, a close view of the crystal structure reveals two spatially proximate Cys residues, Cys-199 and Cys-228, residing in strands β6 and β10, respectively (Fig. 1B). The two sulfur atoms are approximately separated by 4.2 Å (Fig. 1B), an arrangement suggesting the presence of an intracellular disulfide switch similar to the OxyR type regulators (28, 29). However, the corresponding sulfur atoms in the reduced OxyR (Cys-199 and Cys-208) are ∼17 Å away from each other, far more separated than that of AgrA (30). The much shorter distance between Cys-199 and Cys-228 in AgrA makes the oxidation-induced formation of the disulfide bond highly conceivable. Given that the disulfide bond formation has been observed in many oxidation-sensing/defense proteins in addition to OxyR (31–34), we envisioned that this prospective disulfide switch might also play critical roles in redox regulation (Fig. 1A).
Oxidation Leads to Dissociation of AgrA from DNA.
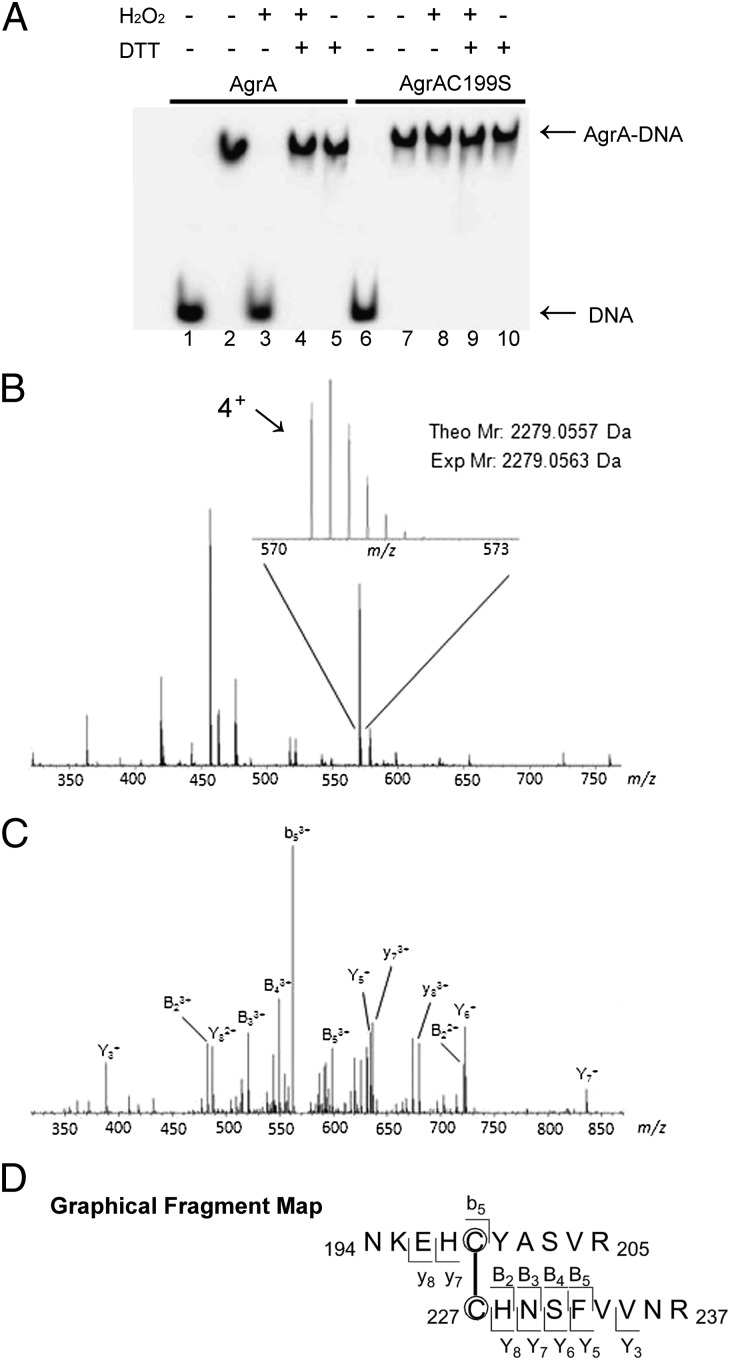
To substantiate that AgrA is responsive to oxidation, we cloned, expressed, and purified the DNA-binding domain of AgrA (Fig. S3) and examined its DNA-binding activity in the absence or presence of H2O2 (unless mentioned otherwise, we use AgrA to refer to the truncated version of this protein containing residues 137–238 for in vitro assays throughout this article). We performed EMSA with AgrA and the promoter region of the agr operon. As shown in Fig. 2A, treating the wild-type AgrA with H2O2 fully abolished its DNA binding (lane 3), which was restored by the addition of excessive DTT (lane 4), indicating that oxidation modification of AgrA disrupts its DNA-binding activity. To determine which of the two Cys residues contribute to the observed dissociation of AgrA from DNA, we sought to overexpress and obtain both mutant proteins AgrAC199S and AgrAC228S. We successfully expressed and purified recombinant AgrAC199S in Escherichia coli. However, the recombinant AgrAC228S expressed in E. coli was found to be insoluble as inclusion bodies (Fig. S3A), suggesting that Cys-228 might be structurally important for the folding of the AgrA protein. AgrAC228A and AgrAC228F also form inclusion bodies when overexpressed in E. coli (Fig. S3B), further indicating that Cys-228 is critical for the stability of the protein. With the purified AgrAC199S in hand, we tested its DNA-binding activity and found it was not affected by 1 mM H2O2 (Fig. 2A, lane 8), indicating that Cys-199 in AgrA is involved in the response to oxidation.
Fig. 2.
Dissociation of oxidized AgrA from DNA and mass spectrometric mapping of the disulfide bond in the oxidized AgrA. (A) EMSA with different forms of AgrA (1.0 μM). Both wild-type AgrA and AgrAC199S shifted with DNA in the absence of H2O2 (lanes 2 and 7). The presence of 1 mM H2O2 led to dissociation of the wild type (lane 3) but not AgrAC199S (lane 8) from DNA, indicating that Cys-199 is responsible for oxidation sensing. The DNA binding of H2O2-treated AgrA was restored by addition of 10 mM DTT (lane 4). Lanes 1 and 6 are controls with DNA alone. The intergenic region of RNAII and RNAIII was used as DNA probe. (B) ESI-Q-TOF mass spectrum (m/z 350–750) of an unfractionated tryptic peptide mixture. Inset: The 4+ charged peak (m/z 570–573) corresponding to the disulfide-containing peptide of interest (theoretical molecular mass, 2,279.0557 Da). (C) MS/MS fragmentation of the 4+ charged peptide (m/z 570). (D) Graphical fragment map correlating the fragmentation ions to the peptide sequence. The disulfide-linked cysteines are circled.
Disulfide Bond Formation Between Cys-199 and Cys-228 upon H2O2 Treatment.
As aforementioned, EMSA revealed that H2O2 treatment impairs the DNA-binding activity of AgrA in a Cys-199 dependent manner. Moreover, the proximity (4.2 Å) between Cys-199 and Cys-228 in AgrA is highly indicative of the existence of an intracellular disulfide switch. To determine the genuine oxidation modification occurring to AgrA after H2O2 treatment, we performed mass spectrometric mapping analysis. Specifically, purified AgrA (20 μM) was oxidized by 1 mM H2O2 at room temperature for 1 h, followed by excessive iodoacetamide treatment to block all unreacted cysteine residues. After trypsin digestion, the resulting tryptic peptide mixture was analyzed by electrospray ionization (ESI)–quadrupole (Q)-TOF mass spectrometry. In the resulting spectrum a prominent 4+ charged peak (m/z 570) corresponding to the disulfide-containing peptide of interest (cross-link between Cys-199 and Cys-228; theoretical molecular mass, 2,279.0557 Da) was identified (Fig. 2B). MS/MS fragmentation of this 4+ charged peptide gave rise to a variety of cross-linked fragment peaks, including b5, y7, y8, B2, B3, B4, and B5, further confirming the presence of a disulfide bond between Cys-199 and Cys-228 (Fig. 2 C and D). We failed to detect any intermonomer “homo-crosslink” between Cys-199 and Cys’-199, or Cys-228 and Cys’-228, which rules out the possibility that the disulfide-containing peptide captured by MS resulted from random oxidation reactions. Overall, this mass spectrometry characterization strongly indicates the formation of a specific intramolecular disulfide bond between Cys-199 and Cys-228 under oxidative stress.
Molecular Dynamics (MD) Simulations of the Reduced and Oxidized AgrA.
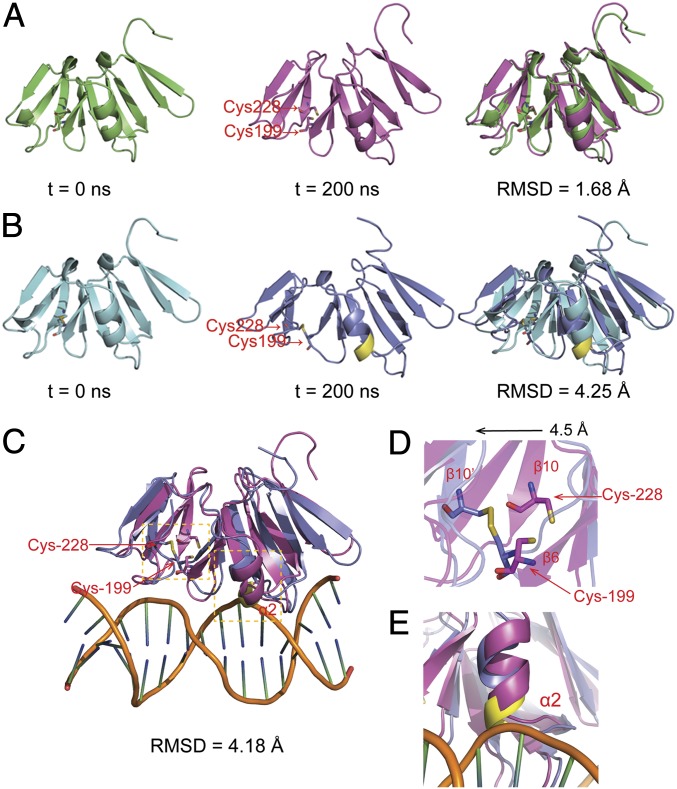
The aforementioned EMSA and MS analysis suggests that the attenuated DNA binding of AgrA is caused by the formation of an intramolecular disulfide bond. To unveil how this disulfide bond could impact DNA binding, we constructed an initial structural model of the oxidized AgrA based on the crystal structure of the reduced AgrA in the Protein Data Bank (PDB entry: 3BS1) as described in SI Experimental Procedures. To investigate its relative stability and obtain a stable conformation of oxidized AgrA, 200-ns MD simulations were performed on both forms of AgrA. The time evolutions of weighted rmsd for the backbone atoms of AgrA from their initial positions (t = 0) were monitored. As illustrated in Fig. 3, the reduced AgrA displayed very little conformational change after MD simulation, and steady rmsd for the backbone atoms in the reduced AgrA was 1.68 Å (Fig. 3A), indicating that the conformation of the reduced AgrA is relatively stable. However, a dramatic conformational change was observed in the oxidized AgrA during MD simulation, and the corresponding rmsd for the backbone atoms in the oxidized AgrA after 200-ns MD simulation was up to 4.25 Å (Fig. 3B), suggesting that the disulfide bond formation between Cys-199 and Cys-228 introduces a significant structural disturbance to AgrA. A close examination of structures after MD simulation revealed that the elongated β-β-β sandwich featured in the DNA-binding domain of AgrA (27) is more open in the oxidized form than in the reduced form owing to the formation of the disulfide bond (Fig. 3C, rmsd = 4.18 Å). Particularly, the β10 strand where Cys-228 resides is displaced approximately by 4.5 Å as a result of the cross-link with β6 (Fig. 3D). Moreover, the two-turn α2 helix in the oxidized AgrA after MD simulation is shifted allosterically and exerts an apparent steric clash with the DNA backbone (Fig. 3E). These results explain the dissociation of the oxidized AgrA from DNA.
Fig. 3.
Structural comparison of the reduced and oxidized AgrA during MD simulations. (A) Snapshot structure of the reduced AgrA at t = 0 ns (Left) and t = 200 ns (Center), along with the optimal backbone alignment of the two structures (Right). (B) Snapshot structure of the oxidized AgrA at t = 0 ns (Left) and t = 200 ns (Center) along with the optimal backbone alignment of the two structures (Right). For clarity, the residues involved in clash with DNA are colored in yellow. (C) Structural alignment of the reduced and oxidized AgrA in the presence of DNA at t = 200 ns. (D) Close view of Cys-199 and Cys-228 in the reduced and oxidized AgrA. (E) Close view of the steric clash between α2 and DNA backbone.
Cys-199 and Cys-228 Play Different Roles in AgrA Function.
Although Cys-199 and Cys-228 form the disulfide bond in the oxidized AgrA, the insolubility of AgrAC228S expressed in E. coli (Fig. S3) implies that Cys-228 is structurally essential for the folding of AgrA. To investigate the functional difference between Cys-199 and Cys-228, we constructed the initial models of AgrAC199S and AgrAC228S as described in SI Experimental Procedures. Subsequently, 200-ns MD simulations were performed on wild-type AgrA, AgrAC199S, and AgrAC228S. As illustrated in Fig. S4, after MD simulation, both wild-type AgrA and AgrAC199S exhibited minimal conformational changes, as reflected by their small rmds values (1.68 Å for wild-type and 1.70 Å for C199S) (Fig. S4 A and B). By contrast, after MD simulation the β10 strand, where Cys-228 locates, and the adjacent α4 helix completely unfolded in AgrAC228S (Fig. S4C). The large rmsd value (as high as 5.75 Å) of AgrAC228S before and after MD simulation reflects this drastic conformational change (Fig. S4C). The in silico result coincides with the experimental observation that AgrAC228S forms inclusion bodies upon expression. Thus, the MD simulation results confirm that Cys-228 is structurally important for the proper folding of AgrA.
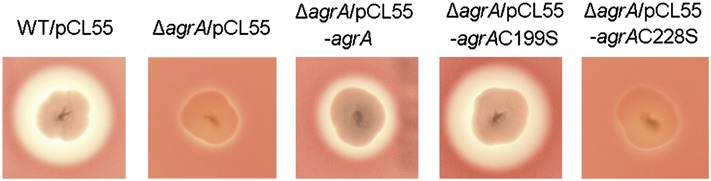
The agr system is essential for expression of a number of S. aureus virulence factors, including α-hemolysin, β-hemolysin, δ-hemolysin, and PSMs (15, 35–37). Consistent with previous results, we observed that the ΔagrA in-frame deletion mutant strain displayed almost no hemolytic activity compared with the wild-type Newman strain, as indicated by zones of clearance on 5% (vol/vol) sheep blood agar (Fig. 4). Introduction of the integration single-copy pCL55-agrA into the ΔagrA mutant only partially restored hemolysis (Fig. 4), which might be attributed to the slightly altered expression level of AgrA due to changed genetic context (note that the intergenic region of RNAII and RNAIII is directly fused with the ORF corresponding to the agrA gene in the complementation strain in which agrBDC was excluded). However, introduction of pCL55-agrAC199S into ΔagrA fully restored the hemolytic activity comparable to that of wild-type Newman (Fig. 4), showing that AgrAC199S is a hyperactive transcriptional regulator compared with the wild-type AgrA, most likely because of the resistance of AgrAC199S toward oxidation. Although immuno-detection of the whole-cell extracts showed that AgrAC228S can be detected in ΔagrA (Fig. S5), introduction of pCL55-agrAC228S into ΔagrA completely failed to restore hemolysis (Fig. 4), indicating that AgrAC228S, although expressed, is inactive inside S. aureus, which is in line with the fact that substitution of Cys-228 with Ser destabilizes and unfolds the protein. Taken together, we suspect that Cys-199 is the primary redox-active site that initiates the disulfide bond formation, whereas Cys-228 is structurally essential for the folding of AgrA.
Fig. 4.
Effects of Cys-199 and Cys-228 in AgrA on hemolysis. The strains tested were spotted on 5% sheep blood agar plate. Zones of clearance indicate hemolysis. WT/pCL55, wild-type Newman carrying an empty chromosome-integrated vector pCL55; ΔagrA/pCL55, agrA in-frame deletion mutant carrying an empty pCL55; ΔagrA/pCL55, agrA deletion mutant carrying pCL55-agrA; ΔagrA/pCL55-agrAC199S, agrA deletion mutant carrying pCL55-agrAC199S; ΔagrA/pCL55-agrAC228S, agrA deletion mutant carrying pCL55-agrAC228S.
AgrA Modulates Target Gene Expression in Response to Oxidative Stress.
As mentioned above, millimolar levels of H2O2 repress the transcription of RNAIII in strain Newman in an AgrA-dependent manner (Fig. S1A). However, expression of RNAIII in the ΔagrA mutant complemented with pCL55-agrAC199S was hardly affected by H2O2 (Fig. S6A), indicating that mutation of Cys-199 to Ser renders the agr regulon unresponsive to oxidation, consistent with the EMSA result that the binding of AgrAC199S to the promoter region of RNAIII is inert to oxidation (Fig. 2A). A previous microarray study of the agr system showed that AgrA down-regulates transcription of S. aureus glutathione peroxidase (bsaA, SAV1306) (38), a putative enzyme responsible for defense against oxidative stress. Our transcriptional analysis confirmed this observation. As shown in Figs. S1B and S6, the ΔagrA mutant displayed the increased expression of bsaA compared with wild-type Newman in the absence of H2O2. Transcription of bsaA in wild-type Newman (Fig. S1B) and the complementation strain ΔagrA/pCL55-agrA (Fig. S6 A and B) can be triggered by H2O2 treatment. However, in ΔagrA/pCL55-agrAC199S, transcription of bsaA was unaffected by the presence of H2O2 (Fig. S6 A and B), supporting the redox-sensing role of Cys-199 in regulating bsaA expression. In addition, EMSAs established that AgrA is capable of binding to the promoter region of bsaA and that the binding is significantly weakened by H2O2 treatment, indicating that AgrA might control the expression of bsaA via a direct DNA-binding mechanism (Fig. S7).
The redox-sensing mechanism of AgrA impacts not only the bsaA gene but also the AgrA-activated psmα operon. As shown in Fig. S6C, a treatment of H2O2 repressed the expression of psmα in wild-type Newman but had negligible effects on the expression of psmα in ΔagrA/pCL55-agrAC199S. Collectively, these results provide strong evidence that oxidative stress modulates transcription of RNAIII, bsaA, and psmα through AgrA, of which Cys-199 is required for its oxidation sensing. These results are also consistent with the observation that AgrAC199S triggers expression of hemolysins (α-hemolysin and PSMs) responsible for sheep erythrocyte lysis (Fig. 4).
AgrA Mediates S. aureus Defense Against Oxidative Stress via Cys-199.
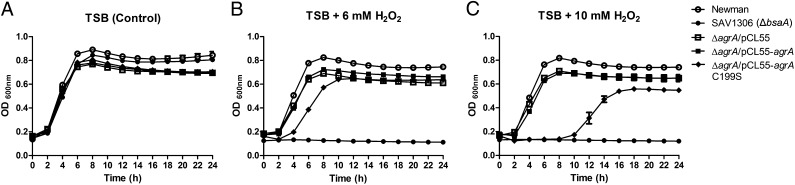
S. aureus has developed an intricate regulatory system to counter oxidative stress. Our growth assays in the absence and presence of H2O2 revealed that the ΔbsaA mutant is extremely susceptible to H2O2, indicating that S. aureus glutathione peroxidase (BsaA) is essential for its survival under oxidative stress (Fig. 5). Given that repression of the bsaA gene by AgrA can be eradicated by H2O2 in a Cys-199 dependent manner (Fig. S6), we envisioned that AgrA, with the redox-sensing capability, might protect S. aureus against oxidative stress via derepressing antioxidant genes such as bsaA (Fig. 6). To test this hypothesis, we compared the growth of various S. aureus strains in the absence and presence of H2O2 over 24 h. In the absence of H2O2, all strains (wild-type Newman, ΔagrA/pCL55, ΔagrA/pCL55-agrA, and ΔagrA/pCL55-agrAC199S) exhibited similar growth patterns (Fig. 5A). In the presence of H2O2 (6 mM or 10 mM), although no significant growth defect was observed in wild-type Newman, ΔagrA/pCL55, or ΔagrA/pCL55-agrA, the growth of ΔagrA/pCL55-agrAC199S was significantly inhibited, especially during the first 10 h (Fig. 5C), confirming the redox switch role of Cys-199 in the wild-type AgrA. With this defense mechanism, both wild-type Newman and the complementation strain ΔagrA/pCL55-agrA were able to survive a high dose of H2O2 (10 mM) as well as ΔagrA/pCL55, the strain that constantly maintains a high expression level of bsaA because of the absence of AgrA (Fig. S1B). In ΔagrA/pCL55-agrAC199S, mutation of Cys-199 to Ser abolishes the oxidation-sensing ability of AgrA, which leads to repression of the bsaA gene under oxidative stress (Fig. 6 and Fig. S6) and an increased susceptibility of ΔagrA/pCL55-agrAC199S to H2O2 (Fig. 5). It is noteworthy that the growth inhibition of ΔagrA/pCL55-agrAC199S by H2O2 is temporary and that the bacterium can eventually overcome this growth delay after 10 h (Fig. 5C), indicating the presence of other unknown factors contributing to this redox regulation in S. aureus. Taken together, our results demonstrate that the oxidation sensing of AgrA is essential for S. aureus defense against oxidative stress and that mutation of Cys-199 to Ser renders the bacterium more susceptible to H2O2.
Fig. 5.
Mutation of Cys-199 to Ser renders S. aureus more susceptible to H2O2. Strains of wild-type Newman, ΔbsaA (SAV1306), ΔagrA/pCL55, ΔagrA/pCL55-agrA, and ΔagrA/pCL55-agrAC199S were inoculated and grown in the presence of (A) 0 mM, (B) 6 mM H2O2, or (C) 10 mM H2O2. The effects of H2O2 on the growth pattern of these strains were examined at 37 °C for 24 h. All measurements were done in sextuplicate. Mean and SD are presented. ΔbsaA, the bursa transposon insertion mutant of S. aureus glutathione peroxidase gene (SAV1306) which is vulnerable to H2O2, served as a positive control.
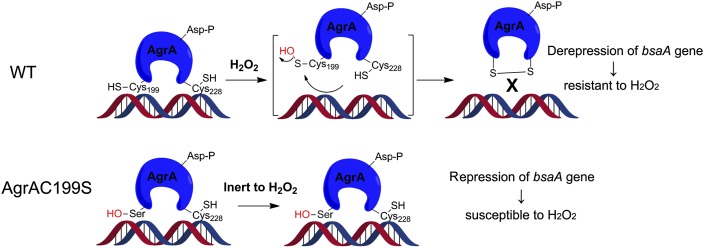
Fig. 6.
Model of oxidation sensing for AgrA. For wild-type AgrA (Upper), Cys-199 plays a redox-sensing role, which can be converted to a sulfenic acid upon H2O2 treatment and subsequently reacts with its spatially proximate residue Cys-228 to yield an intramolecular disulfide bond. Oxidation of AgrA leads to its dissociation from DNA, thereby resulting in derepression of the bsaA gene and resistance to H2O2. For AgrAC199S (Lower), mutation of Cys-199 to Ser abolishes the oxidation-sensing ability of AgrA, causing repression of bsaA gene under oxidative stress, which renders the bacterium more susceptible to H2O2.
Discussion
The S. aureus agr system is one of the most thoroughly studied quorum-sensing systems in Gram-positive bacteria, which uses AIP-based signals to coordinate bacterial behaviors with the local cell density (13, 14). Here we show that the DNA-binding domain of the response regulator AgrA possesses an intrinsic intracellular disulfide switch responsive to oxidative stress, which reveals the presence of an additional layer of the redox signaling in this paradigmatic quorum-sensing system. The cross-talk between oxidation sensing and quorum sensing within a single regulatory system fully demonstrates the complexity of bacterial signal transduction. It is conceivable that other quorum-sensing systems, such as N-acyl homoserine lactones-based systems in Gram-negative bacteria, might also have subordinate built-in signaling pathways to compensate or modulate the cell density-dependent regulatory network. This study provides an example of additional oxidation signals that affect the cell density-dependent quorum-sensing system.
The S. aurues agr system, like most quorum-sensing systems, constitutes an autoinduction genetic circuit, resulting in a rapid burst of activity once a certain threshold of cell density has been reached (10). Previous studies have demonstrated that the activation of this system is inevitably accompanied by an intense metabolic burden, which could be detrimental and presumably responsible for frequent spontaneous agr mutations in the laboratory (10, 39). One severe consequence of the intense metabolic activity is the generation of a heavy oxidation burden concomitant with increased concentrations of ROS/RNS (40). This metabolic burden and the concomitant oxidative stress necessitate a delicate sensing system to enable the bacterium to avoid the detrimental effects caused by agr activation. Indeed, S. aureus, as a successful human pathogen, has developed a rapid adaptive response to a sublethal dose of oxidants (4, 6), thereby reorienting gene expression for antioxidation. Our discovery suggests that the agr system serves to trigger bacterial response (through activation of bsaA gene in particular) to oxidative stress through an intracellular disulfide switch contained in the response regulator AgrA. In fact, the two Cys residues, Cys-199 and Cys-228, are only 4.2 Å away from each other in the reduced AgrA, which are poised to form a disulfide bond upon oxidation. This additional built-in oxidation-sensing ability of the primary quorum-sensing agr, which seems to be conserved in many Gram-positive bacteria (Fig. S8), enables the bacterium to timely adjust gene expression with the metabolic activity and oxidation burden.
Cys-199 and Cys-228 are the only two Cys residues conserved in the DNA-binding domain of AgrA. AgrA has three additional cysteines, Cys-6, Cys-55, and Cys-123 (Fig. S9A). Although no structure of this domain is available, we built a virtual structural model (residue range: 1–127) using SWISS-MODEL (http://swissmodel.expasy.org/). As shown in Fig. S9B, this model suggests that these three Cys residues are all located far apart. They are also less likely to form disulfide bonds with Cys-199 and Cys-228, which are buried in the elongated β-β-β sandwich in the DNA-binding domain. It would be interesting to see whether these other Cys residues play any regulatory role in the agr system in the future.
In summary, our study shows that S. aureus has integrated an additional oxidation-sensing mechanism into the primary quorum-sensing agr system through an intrinsic intracellular disulfide switch in the response regulator AgrA. The oxidation-sensing ability of AgrA is shown to be critical for bacterial defense against oxidative stress. This rare demonstration of the cross-talk between oxidation sensing and quorum sensing in human pathogen provides a unique perspective for understanding the bacterial cell density control and gene regulation during oxidative stress and host–pathogen interactions in general.
Experimental Procedures
Bacterial Strains, Plasmids, and Culture Conditions.
The bacterial strains and plasmids used in this study are listed in Table S1. Unless otherwise mentioned, S. aureus strain Newman was used in the study. S. aureus cells were grown in either tryptic soy broth or brain–heart infusion broth. E. coli cultures were grown in LB medium. Whenever required, antibiotics were added to the culture medium (for E. coli, 100 μg/mL ampicillin; for S. aureus, 10 μg/mL nalidixic acid or 5 μg/mL chloramphenicol).
Other Procedures.
Detailed procedures are available in SI Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Drs. O. Schneewind and D. Missiakas at the University of Chicago for providing transposon mutants, and S. F. Reichard for editing the manuscript. This work was financially supported by National Institutes of Health National Institute of Allergy and Infectious Diseases Grants AI074658 and P50GM081892 (to C.H.), a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease Award (to C.H.), Shanghai Committee of Science and Technology Grant 10410703900 (to C.L.), and 863 Program Grant SS2012AA021103 (to H.J.). F.S. is a Scholar of the Chicago Biomedical Consortium with support from The Searle Funds at The Chicago Community Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200603109/-/DCSupplemental.
References
- 1.Camilli A, Bassler BL. Bacterial small-molecule signaling pathways. Science. 2006;311:1113–1116. doi: 10.1126/science.1121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuqua C, Greenberg EP. Listening in on bacteria: Acyl-homoserine lactone signalling. Nat Rev Mol Cell Biol. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 3.Novick RP, Geisinger E. Quorum sensing in staphylococci. Annu Rev Genet. 2008;42:541–564. doi: 10.1146/annurev.genet.42.110807.091640. [DOI] [PubMed] [Google Scholar]
- 4.Chen PR, Brugarolas P, He C. Redox signaling in human pathogens. Antioxid Redox Signal. 2011;14:1107–1118. doi: 10.1089/ars.2010.3374. [DOI] [PubMed] [Google Scholar]
- 5.Marshall HE, Merchant K, Stamler JS. Nitrosation and oxidation in the regulation of gene expression. FASEB J. 2000;14:1889–1900. doi: 10.1096/fj.00.011rev. [DOI] [PubMed] [Google Scholar]
- 6.Mongkolsuk S, Helmann JD. Regulation of inducible peroxide stress responses. Mol Microbiol. 2002;45:9–15. doi: 10.1046/j.1365-2958.2002.03015.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, et al. Vibrio cholerae anaerobic induction of virulence gene expression is controlled by thiol-based switches of virulence regulator AphB. Proc Natl Acad Sci USA. 2011;108:810–815. doi: 10.1073/pnas.1014640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 9.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 10.Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 11.Stauff DL, Skaar EP. The heme sensor system of Staphylococcus aureus. Contrib Microbiol. 2009;16:120–135. doi: 10.1159/000219376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung AL, Nishina KA, Trotonda MP, Tamber S. The SarA protein family of Staphylococcus aureus. Int J Biochem Cell Biol. 2008;40:355–361. doi: 10.1016/j.biocel.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geisinger E, Muir TW, Novick RP. agr receptor mutants reveal distinct modes of inhibition by staphylococcal autoinducing peptides. Proc Natl Acad Sci USA. 2009;106:1216–1221. doi: 10.1073/pnas.0807760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayville P, et al. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci USA. 1999;96:1218–1223. doi: 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Queck SY, et al. RNAIII-independent target gene control by the agr quorum-sensing system: Insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell. 2008;32:150–158. doi: 10.1016/j.molcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen PR, et al. An oxidation-sensing mechanism is used by the global regulator MgrA in Staphylococcus aureus. Nat Chem Biol. 2006;2:591–595. doi: 10.1038/nchembio820. [DOI] [PubMed] [Google Scholar]
- 17.Chen PR, et al. A new oxidative sensing and regulation pathway mediated by the MgrA homologue SarZ in Staphylococcus aureus. Mol Microbiol. 2009;71:198–211. doi: 10.1111/j.1365-2958.2008.06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballal A, Manna AC. Control of thioredoxin reductase gene (trxB) transcription by SarA in Staphylococcus aureus. J Bacteriol. 2010;192:336–345. doi: 10.1128/JB.01202-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luong TT, Dunman PM, Murphy E, Projan SJ, Lee CY. Transcription profiling of the mgrA regulon in Staphylococcus aureus. J Bacteriol. 2006;188:1899–1910. doi: 10.1128/JB.188.5.1899-1910.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlag S, et al. Characterization of the oxygen-responsive NreABC regulon of Staphylococcus aureus. J Bacteriol. 2008;190:7847–7858. doi: 10.1128/JB.00905-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun F, et al. AirSR, a [2Fe-2S] cluster-containing two-component system, mediates global oxygen sensing and redox signaling in Staphylococcus aureus. J Am Chem Soc. 2011 doi: 10.1021/ja2071835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker J, et al. Copper stress induces a global stress response in Staphylococcus aureus and represses sae and agr expression and biofilm formation. Appl Environ Microbiol. 2010;76:150–160. doi: 10.1128/AEM.02268-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothfork JM, et al. Inactivation of a bacterial virulence pheromone by phagocyte-derived oxidants: new role for the NADPH oxidase in host defense. Proc Natl Acad Sci USA. 2004;101:13867–13872. doi: 10.1073/pnas.0402996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimoto DF, et al. Staphylococcus aureus SarA is a regulatory protein responsive to redox and pH that can support bacteriophage lambda integrase-mediated excision/recombination. Mol Microbiol. 2009;74:1445–1458. doi: 10.1111/j.1365-2958.2009.06942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballal A, Manna AC. Regulation of superoxide dismutase (sod) genes by SarA in Staphylococcus aureus. J Bacteriol. 2009;191:3301–3310. doi: 10.1128/JB.01496-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung AL, Projan SJ. Cloning and sequencing of sarA of Staphylococcus aureus, a gene required for the expression of agr. J Bacteriol. 1994;176:4168–4172. doi: 10.1128/jb.176.13.4168-4172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sidote DJ, Barbieri CM, Wu T, Stock AM. Structure of the Staphylococcus aureus AgrA LytTR domain bound to DNA reveals a beta fold with an unusual mode of binding. Structure. 2008;16:727–735. doi: 10.1016/j.str.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng M, Aslund F, Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]
- 29.Helmann JD. OxyR: A molecular code for redox sensing? Sci STKE. 2002;2002:pe46. doi: 10.1126/stke.2002.157.pe46. [DOI] [PubMed] [Google Scholar]
- 30.Choi H, et al. Structural basis of the redox switch in the OxyR transcription factor. Cell. 2001;105:103–113. doi: 10.1016/s0092-8674(01)00300-2. [DOI] [PubMed] [Google Scholar]
- 31.Chen H, et al. The Pseudomonas aeruginosa multidrug efflux regulator MexR uses an oxidation-sensing mechanism. Proc Natl Acad Sci USA. 2008;105:13586–13591. doi: 10.1073/pnas.0803391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutchison KA, Matić G, Meshinchi S, Bresnick EH, Pratt WB. Redox manipulation of DNA binding activity and BuGR epitope reactivity of the glucocorticoid receptor. J Biol Chem. 1991;266:10505–10509. [PubMed] [Google Scholar]
- 33.Antelmann H, Helmann JD. Thiol-based redox switches and gene regulation. Antioxid Redox Signal. 2011;14:1049–1063. doi: 10.1089/ars.2010.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klomsiri C, Karplus PA, Poole LB. Cysteine-based redox switches in enzymes. Antioxid Redox Signal. 2011;14:1065–1077. doi: 10.1089/ars.2010.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNamara PJ, Iandolo JJ. Genetic instability of the global regulator agr explains the phenotype of the xpr mutation in Staphylococcus aureus KSI9051. J Bacteriol. 1998;180:2609–2615. doi: 10.1128/jb.180.10.2609-2615.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villaruz AE, et al. A point mutation in the agr locus rather than expression of the Panton-Valentine leukocidin caused previously reported phenotypes in Staphylococcus aureus pneumonia and gene regulation. J Infect Dis. 2009;200:724–734. doi: 10.1086/604728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Recsei P, et al. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol Gen Genet. 1986;202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 38.Dunman PM, et al. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J Bacteriol. 2001;183:7341–7353. doi: 10.1128/JB.183.24.7341-7353.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Somerville GA, et al. In vitro serial passage of Staphylococcus aureus: Changes in physiology, virulence factor production, and agr nucleotide sequence. J Bacteriol. 2002;184:1430–1437. doi: 10.1128/JB.184.5.1430-1437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Somerville GA, Proctor RA. At the crossroads of bacterial metabolism and virulence factor synthesis in Staphylococci. Microbiol Mol Biol Rev. 2009;73:233–248. doi: 10.1128/MMBR.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.