Metallothionein (MT) was discovered 40 years ago (1). By all accounts it is a most unusual and unconventional protein (2–6). One-third of its 60+ amino acids are cysteines and eight are lysines; it contains neither aromatic amino acids nor histidine. MT usually binds seven zinc atoms, but it also can contain copper, cadmium, and traces of other metals. In an evolutionary sense it is a very old protein, and the composition of its two major isoproteins has changed only imperceptibly over time. The number of genes that code for human MTs could be as high as 17. MT-1 and MT-2 are the two prevalent forms: they are expressed but their physiological functions are still unknown. MT-3 was discovered only recently in brains from patients afflicted with Alzheimer’s disease (7), a discovery based on the fact that MT-3 inhibits the growth of neurons. Thus far, it is the only isoform that is known to exhibit such a specific biological function; it contains zinc and copper (I), but not cadmium. Multiple factors, including members of the nuclear hormone receptor family, interferons, inducers of the acute phase response and metalloregulatory proteins, affect tissue-and isoprotein-specific gene expression. In addition, MT is induced by numerous other agents whose signaling pathways remain obscure. Thionein, the apoform of MT has never been isolated as such from any biological source. Apparently, at its formation, it instantaneously combines with zinc, whose “free” concentration in the cell has been reported to be exceedingly low, i.e., in the nanomolar to picomolar range. All these facts suggest that MT must be biologically essential. This hypothesis, indeed, now has been proven to be correct. MT-1 and MT-2 double-knockout mice become obese, demonstrating the involvement of MT in energy metabolism (8).
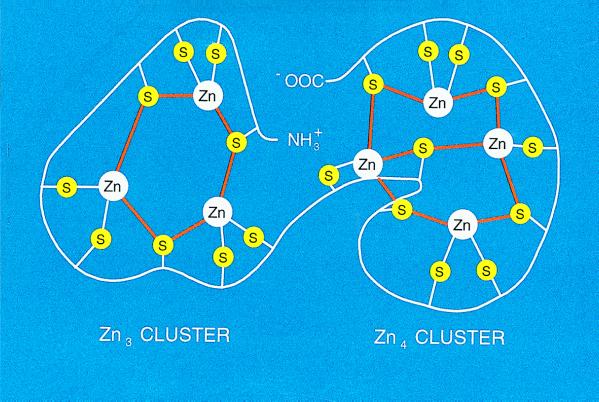
Although MT was discovered in the late 1950s, its three-dimensional structure by both x-ray crystallography (9) and NMR spectroscopy (10) was reported only seven years ago; the structure has proved critical to efforts designed to establish its function. The protein has the shape of a dumbbell and envelops the metals that it contains in two separate domains in a manner that effectively shields them from the environment. It is most remarkable that the metals are arranged in a cluster structure unique to biology. In one cluster, four metal atoms are bound to 11 cysteines, five of which bridge the metals; the other has three metal atoms and nine cysteines with three bridges (Fig. 1). Zinc is bound extremely tightly (KD about 10−13 M). Until today, there has been no indication as to how it is removed or added.
Figure 1.
Schematic drawing of the zinc thiolate clusters in MT. There are three bridging and six terminal cysteine ligands in the N-terminal β-domain (Zn3 cluster) and four bridging and six terminal ligands in the C-terminal α-domain (Zn4 cluster). Based on both x-ray diffraction and NMR spectroscopy data (for general reviews, see refs. 9 and 10).
Data that cast light on the circumstances under which zinc can be released from MT have appeared in the Proceedings (11, 12). In this issue of the Proceedings (13–15), additional findings by Vallee, Maret, and coworkers focus on the relevant theoretical and experimental facts and propose mechanisms that might underlie the function of MT. Zinc, in contrast to copper or iron, is redox-inert. Hence, the properties of zinc complexes are not altered by valence changes of the central atom in a manner akin to those in iron or copper complexes. However, the redox state of the sulfur ligands can be altered. The cluster structure provides the chemical basis by which the cysteine ligands can induce oxidoreductive properties (13). Hence, the cluster structure focuses on the significance of zinc/cysteine thiolate coordination as the critical arrangement for zinc in MT to render the complex oxidoreductive. This structure allows for thermodynamic stability of zinc in MT while permitting zinc to retain kinetic lability. This is demonstrated experimentally by the ensuing facile and fast zinc exchange between MT isoforms, between MT and the zinc cluster in the Gal4 transcription factor (11), and now between MT and the apoforms of various zinc proteins (14, 15). Thus kinetic lability can be introduced into the zinc-thiolate clusters by oxidoreduction of the sulfur donor atoms. Indeed, the data presented in this issue of the Proceedings demonstrate that a large number of agents oxidize the thiolate ligands even while they are coordinated to zinc. In point of fact, the reducing potential of MT remains sufficiently low to allow oxidation by a number of physiological oxidants including disulfides or selenium compounds. Thus, the existence of these clusters in MT seem to be unique to biology and have a role that could not have been predicted from either known zinc or sulfur chemistry. They embody a completely new utilization of Zn-S chemistry in conferring dynamic behavior on complexes with inherently high stability. This finding then establishes a hitherto unknown mechanism that has a role in cellular zinc distribution.
Two of the papers in this issue of the Proceedings (14, 15) provide further insight into how MT acts as an agent that controls zinc distribution. Zinc release and transfer to the apoform of a zinc enzyme (sorbitol dehydrogenase) is modulated by both reduced glutathione (GSH) and oxidized glutathione (GSSG). GSH alone inhibits zinc release in the absence of GSSG, indicating that MT is stabilized at relatively high cellular GSH concentrations. The presence of GSSG (or any other oxidizing agent) results in a release of zinc that is synergistically increased by GSH. Because zinc release from MT depends on both GSH and GSSG in a concentration-dependent manner, the authors propose that the supply of zinc from MT is redox-regulated in a concentration-dependent manner, i.e., zinc distribution is coupled both to energy metabolism and the cellular redox state. In vitro, MT does not act only as a zinc donor, but in the form of thionein, as a zinc acceptor. The data show that these reactions are not strictly reversible and depend on additional factors and conditions, thus adding a further level of sophistication to the kinetic partitioning of zinc.
In summary, zinc plays a central role in cellular metabolism, It is monitored and complexed within a unique structure, i.e., a cluster of atoms seen nowhere else than in biological matter. It is also not a very daring proposition to suggest that MT also is critically involved in energy metabolism. The biological importance of zinc does not require elaboration at this point, because its catalytic functions in enzymes and its structural functions in zinc finger proteins have been documented amply (16, 17). A third area is now emerging, i.e., the regulatory properties of zinc. Zinc is a modulator of synaptic transmission, which is an extracellular regulatory function. It is quite likely that zinc also serves as an intracellular regulator that operates at concentrations far below those of calcium and hence coordinates different sets of biochemical processes. Of course, many important problems remain to be resolved. For instance, how can the intracellular concentration of MT be accurately measured so that its level relative to other zinc-binding proteins be determined? Where does it localize and are there specific carrier proteins involved in its translocation and/or anchoring to subcellular elements? What is its turnover and the fate of the zinc released? The joining of inorganic and organic chemistry referred to above sets new horizons for further exploration.
References
- 1.Margoshes M, Vallee B L. J Am Chem Soc. 1957;79:4813–4814. [Google Scholar]
- 2.Vallee, B. L. (1979) Experientia 34, Suppl., 19–40. [DOI] [PubMed]
- 3.Vallee, B. L. (1987) Experientia 52, Suppl., 5–16. [DOI] [PubMed]
- 4.Vallee B L. Methods Enzymol. 1991;205:3–7. doi: 10.1016/0076-6879(91)05077-9. [DOI] [PubMed] [Google Scholar]
- 5.Vallee B L, Maret W. In: Metallothionein III. Suzuki K T, Imura N, Kimura M, editors. Basel: Birkhäuser; 1993. pp. 1–27. [Google Scholar]
- 6.Vallee B L. Neurochem Int. 1995;27:23–33. doi: 10.1016/0197-0186(94)00165-q. [DOI] [PubMed] [Google Scholar]
- 7.Uchida Y, Takio K, Titani K, Ihara Y, Tomonaga M. Neuron. 1991;7:337–347. doi: 10.1016/0896-6273(91)90272-2. [DOI] [PubMed] [Google Scholar]
- 8.Beattie J H, Wood A M, Newman A M, Bremner I, Choo K H A, Michalska A E, Duncan J S, Trayhurn P. Proc Natl Acad Sci USA. 1998;95:358–363. doi: 10.1073/pnas.95.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins A H, Stout C D. Methods Enzymol. 1991;205:485–502. doi: 10.1016/0076-6879(91)05134-h. [DOI] [PubMed] [Google Scholar]
- 10.Wüthrich K. Methods Enzymol. 1991;205:502–520. doi: 10.1016/0076-6879(91)05135-i. [DOI] [PubMed] [Google Scholar]
- 11.Maret W. Proc Natl Acad Sci USA. 1994;91:237–241. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maret W, Larsen K S, Vallee B L. Proc Natl Acad Sci USA. 1997;94:2233–2237. doi: 10.1073/pnas.94.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maret W, Vallee B L. Proc Natl Acad Sci USA. 1998;95:3478–3482. doi: 10.1073/pnas.95.7.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang L J, Maret W, Vallee B L. Proc Natl Acad Sci USA. 1998;95:3483–3488. doi: 10.1073/pnas.95.7.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob C, Maret W, Vallee B L. Proc Natl Acad Sci USA. 1998;95:3489–3494. doi: 10.1073/pnas.95.7.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallee B L, Falchuk K F. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 17.Vallee B L, Auld D S. Acc Chem Res. 1993;26:543–551. [Google Scholar]