Use of transgenic mouse eggs expressing human zona pellucida proteins identifies an N-terminal domain of ZP2 that is essential for human sperm–egg binding.
Abstract
Fertilization requires taxon-specific gamete recognition, and human sperm do not bind to zonae pellucidae (ZP1–3) surrounding mouse eggs. Using transgenesis to replace endogenous mouse proteins with human homologues, gain-of-function sperm-binding assays were established to evaluate human gamete recognition. Human sperm bound only to zonae pellucidae containing human ZP2, either alone or coexpressed with other human zona proteins. Binding to the humanized matrix was a dominant effect that resulted in human sperm penetration of the zona pellucida and accumulation in the perivitelline space, where they were unable to fuse with mouse eggs. Using recombinant peptides, the site of gamete recognition was located to a defined domain in the N terminus of ZP2. These results provide experimental evidence for the role of ZP2 in mediating sperm binding to the zona pellucida and support a model in which human sperm–egg recognition is dependent on an N-terminal domain of ZP2, which is degraded after fertilization to provide a definitive block to polyspermy.
Introduction
Mammalian development begins when a single spermatozoon fertilizes an ovum in the ampulla of the female oviduct. Unlike free-spawning marine invertebrates, this seminal event occurs internally in eutherian mammals. Prefertilization and postzygotic incompatibilities (habitation, physiognomy, and aneuploidy) moot many potential taxonomic crosses. In appropriate mating within a species, gamete recognition must ensure fertilization with a single sperm while preventing polyspermy that results in embryonic lethality. Although a variety of mechanisms have evolved, the initial permissive–nonpermissive dichotomy of gamete recognition in mouse and human is mediated primarily by the zona pellucida that surrounds ovulated eggs.
Human and mouse genomes have syntenic genetic loci (ZP1, ZP2, ZP3, and ZP4) that encode components of the zona matrix (Hoodbhoy et al., 2005), but ZP4 protein is not present in mice because of multiple stop and missense codons (Lefièvre et al., 2004). Based on its ability to inhibit sperm binding to ovulated eggs in vitro, mouse ZP3 was initially identified as the zona ligand for sperm binding (Bleil and Wassarman, 1980). With time, this model became increasingly precise to implicate O-glycans attached to Ser332 and Ser334 on ZP3 as essential for gamete recognition with their postfertilization release accounting for the inability of sperm to bind to two-cell embryos (Florman and Wassarman, 1985; Chen et al., 1998). However, genetic ablation of glycosyltransferases required for candidate glycans did not prevent fertility (Lopez et al., 1985; Thall et al., 1995; Asano et al., 1997; Lowe and Marth, 2003; Shi et al., 2004; Williams et al., 2007), and glycosylation at the designated serine residues was not observed by mass spectrometry of native zonae pellucidae (Boja et al., 2003). Moreover, specific genetic mutation of the implicated serine residues to preclude attachment of O-glycans did not affect sperm recognition or fertility in transgenic mice (Liu et al., 1995), even in the absence of endogenous normal mouse ZP3 (Gahlay et al., 2010).
These observations prompt consideration of other zona pellucida proteins as mediators of sperm–egg recognition. Zp1-null mice are fertile, albeit with decreased fecundity (Rankin et al., 1999), which excludes an essential role for ZP1 in gamete recognition. Zp4 is a pseudogene in mice (Lefièvre et al., 2004) and is not sufficient to support human sperm binding in transgenic mice expressing recombinant human ZP4 (Yauger et al., 2011). Thus, ZP2 draws attention. After fertilization, cortical granules in the periphery of the egg exocytose their contents (Ducibella et al., 2002), and sperm do not bind to the zona pellucida of two-cell embryos (Inoue and Wolf, 1975). Although other changes have been inferred, only cleavage of ZP2 has been biochemically documented in mouse and human (Bleil et al., 1981; Bauskin et al., 1999). Recently, the site on mouse ZP2 has been molecularly characterized as 166LA↓DE169, and ovastacin has been defined as the egg cortical granule metalloendoprotease responsible for its cleavage. Mutation of the ZP2 site or genetic ablation of ovastacin in transgenic mice prevents ZP2 cleavage, and sperm bind to two-cell embryos, despite fertilization and cortical granule exocytosis (Gahlay et al., 2010; Burkart et al., 2012).
Although these results document the dependency of gamete recognition on intact ZP2, the sperm binding site on the zona pellucida remained undefined. We now report the systematic evaluation of human sperm binding to transgenic mouse zonae pellucidae containing human ZP1, ZP2, ZP3, or ZP4. In these gain-of-function assays, human ZP2, within the context of the zona matrix, is necessary and sufficient to support human sperm binding, and the site of gamete interaction is localized to the N terminus of ZP2, upstream of the postfertilization cleavage site.
Results and discussion
Taxon-specific sperm-binding assay with humanized transgenic mouse eggs
Human sperm bind to Homo sapiens, Gorilla gorilla, and Hylobates lar (gibbon) zonae pellucidae but not to those of nonhumanoid primates (baboon and rhesus) or other mammals, including mice (Bedford, 1977; Lanzendorf et al., 1992). Taking advantage of this fastidious nature, we have established a gain-of-function human sperm-binding assay in transgenic mice.
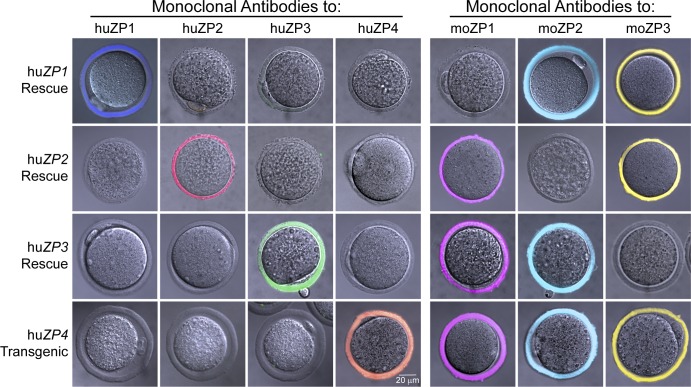
Human ZP1 mice (ZP1huTG) were produced and crossed into the mouse Zp1-null background (Rankin et al., 1999) to establish huZP1 rescue mice (ZP1huTG, Zp1tm/tm, Zp2+/+, and Zp3+/+), in which TG and tm represent the transgene and null allele, respectively. HuZP1 transgenic and rescue mice had normal fertility (Table S1) and, other than expressing the transgene, were indistinguishable from normal controls (Fig. S1). HuZP2 (ZP2huTG, Zp1+/+, Zp2tm/tm, and Zp3+/+), huZP3 (ZP3huTG, Zp1+/+, Zp2+/+, and Zp3tm/tm) rescue, and huZP4 transgenic (ZP4huTG, Zp1+/+, Zp2+/+, and Zp3+/+) mice have been described previously (Rankin et al., 1998, 2003; Yauger et al., 2011). To confirm the composition of zonae pellucidae from the four humanized mouse lines, ovulated eggs were stained with mAb specific to each mouse and human zona protein and imaged by confocal microscopy (Fig. 1). In each mouse line, both the human and mouse proteins were detected throughout the zona pellucida, and although the width of the zona matrix varied among the humanized mouse lines, there were no other apparent abnormalities.
Figure 1.
Human zona proteins are incorporated into the zona pellucida of transgenic mouse eggs. Ovulated eggs from human ZP1 (huZP1), huZP2, and huZP3 rescue as well as huZP4 transgenic mice were fixed and stained with mAb to human ZP1 (huZP1), huZP2, huZP3, huZP4, mouse ZP1 (moZP1), moZP2, and moZP3. Antibody binding to human and mouse zona proteins was detected by confocal microscopy and faux colored dark blue (huZP1), red (huZP2), green (huZP3), orange (huZP4), magenta (moZP1), light blue (moZP2), or yellow (moZP3). Fluorescent and DIC images were merged.
To analyze the ability of human sperm to bind to humanized eggs, a gain-of-function assay based on human in vitro fertilization (Yauger et al., 2011) was developed (Fig. 2 A). Eggs in cumulus were isolated from transgenic mice and incubated with human sperm (106 ml−1) for 4 h to ensure sperm capacitation. Gametes were then washed serially before obtaining videos and quantifying sperm binding by confocal microscopy. Each assay contained noninseminated human oocytes and Zp3EGFP transgenic mouse eggs (Zhao et al., 2002) as positive and negative controls, respectively. Capacitated human sperm bound to neither normal nor Zp3EGFP mouse eggs (Fig. 2 B).
Figure 2.
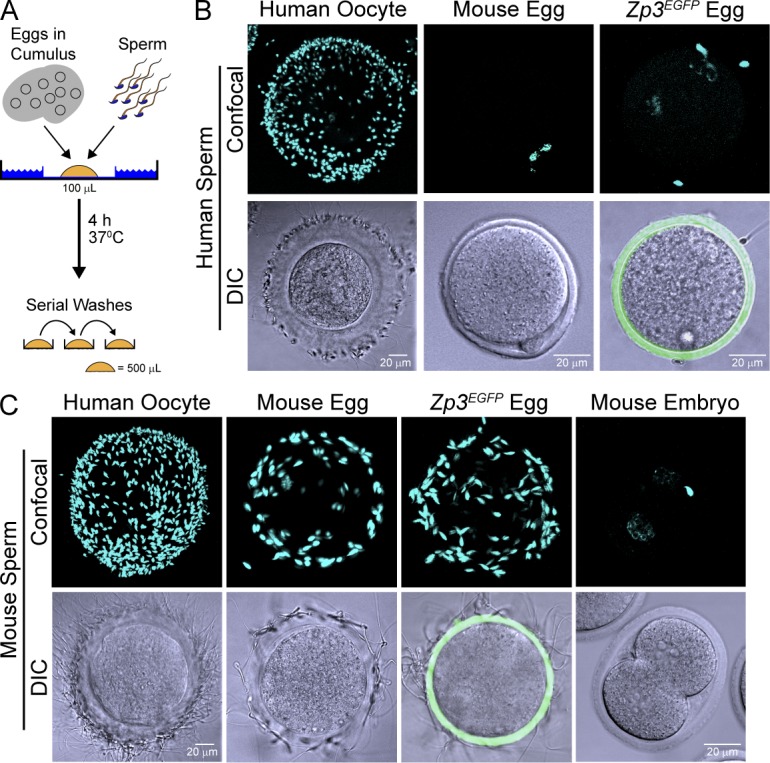
Taxon-specific sperm binding. (A) Sperm-binding assay in which 10–20 eggs in cumulus were added to 106 ml−1 sperm in HTF (supplemented with 0.5% BSA) and incubated for 4 h at 37°C. After serial washing with control eggs or two-cell embryos, eggs were fixed to determine the number of bound sperm. (B) Capacitated human sperm binding to noninseminated human oocytes, normal mouse, and Zp3EGFP transgenic eggs. (top) Confocal (blue channel) z projections were obtained after staining with Hoechst. (bottom) DIC and confocal (green channel) images were merged to detect zonae pellucidae containing Zp3EGFP. (C) Same as B but with capacitated mouse sperm incubated for 1 h.
Similar assay conditions were used to assess mouse sperm binding to humanized eggs, except that incubation was for 40 min and mouse two-cell embryos served as negative wash controls (Fig. 2 C). Mouse sperm bound as well to Zp3EGFP as normal eggs. Thus, ZP3EGFP within the zona pellucida did not affect mouse sperm binding (Fig. 2 C) or support human sperm binding (Fig. 2 B), and Zp3EGFP eggs were used in subsequent assays to distinguish normal from humanized mouse eggs.
Human sperm bind to transgenic zonae pellucidae containing human ZP2
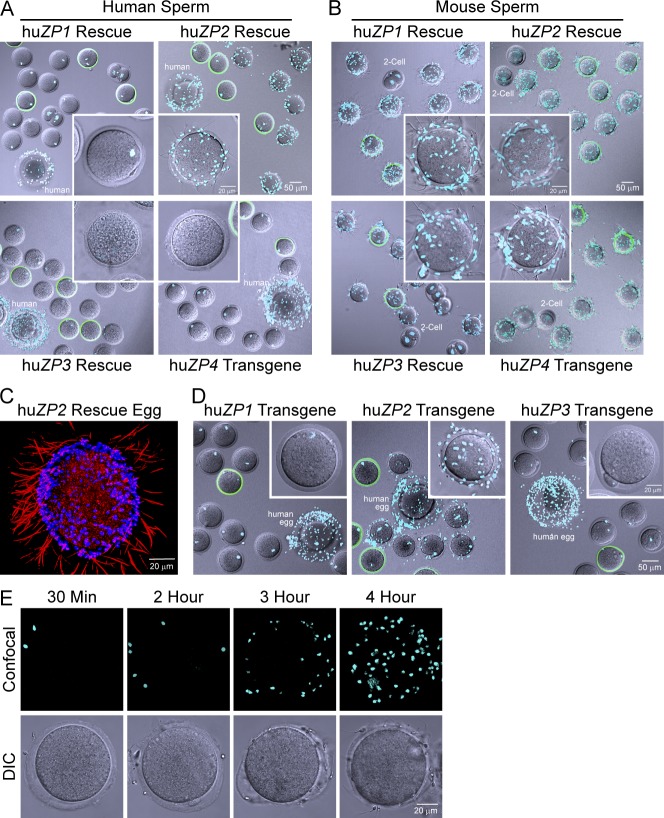
Using the aforementioned gamete recognition assay, human sperm bound avidly to huZP2 rescue (57.1 ± 2.6 sperm/egg) but not to huZP1 rescue, huZP3 rescue, or huZP4 transgenic eggs (Fig. 3 A). In controls, mouse sperm bound comparably well to huZP1 rescue (70.4 ± 6.2 sperm/egg), huZP2 rescue (63.0 ± 2.3 sperm/egg), huZP3 rescue (49.1 ± 3.9 sperm/egg), and huZP4 transgenic (61.1 ± 4.3 sperm/egg) eggs (Fig. 3 B). Reconstruction of a z stack from confocal microscopy documented that capacitated human sperm on the surface of huZP2 rescue eggs bound by their heads with tails protruding into the media (Fig. 3 C).
Figure 3.
Human sperm bind to huZP2 rescue eggs. (A) Human sperm binding to human ZP1 (huZP1), huZP2, and huZP3 rescue and huZP4 transgenic eggs. Noninseminated human oocytes (human) and Zp3EGFP transgenic eggs (green zona) were positive and negative controls, respectively. Images obtained by confocal and DIC microscopy after staining with Hoechst were merged. Insets show magnification of a single humanized egg. (B) Same as A but with mouse sperm. Positive and negative controls were Zp3EGFP transgenic eggs and two-cell embryos, respectively. (C) 3D reconstruction of a z stack of confocal images of human sperm binding to the huZP2 rescue egg. Sperm heads binding to the zona pellucid were visualized by Hoechst staining, and the projection of sperm tails into the media was detected by single wavelength reflection. (D) Same as A but with human ZP1 (huZP1), huZP2, and huZP3 transgenic eggs before crossing into the corresponding null background. (E) Human sperm binding to huZP2 rescue eggs after sperm capacitation for 30 min, 2 h, 3 h, and 4 h. Confocal z projections (top) and DIC (bottom) images after staining with Hoechst.
Within a lawn of highly motile human sperm, huZP2 rescue eggs with bound human sperm rotated and moved, but in the absence of bound sperm, huZP1 rescue, huZP3 rescue, and huZP4 transgenic eggs moved little, if at all (Videos 1, 2, 3, and 4). Thus, human ZP2 was sufficient to support human sperm binding to the zona pellucida of transgenic mice lacking endogenous mouse ZP2. To determine whether the gain of function was a dominant effect, eggs from huZP1, huZP2, and huZP3 transgenic mice (before crossing into the corresponding null background) were assayed. Although fewer in number, human sperm bound to huZP2 transgenic (25.7 ± 2.6 sperm/egg) as well as they did to huZP2 rescue eggs and did not bind to huZP1 or huZP3 transgenic eggs (Fig. 3 D). Thus, the presence of human ZP2, rather than the absence of mouse ZP2, is required for human sperm attachment.
Human and mouse sperm must be capacitated by passage through the female reproductive tract or by in vitro incubation, usually in the presence of serum proteins or cyclodextrin (Choi and Toyoda, 1998), to bind to the zona pellucida, undergo acrosome exocytosis, and fertilize ovulated eggs (Bailey, 2010). Initially defined operationally (Austin, 1951; Chang, 1951), the molecular basis of capacitation remains incompletely understood. Capacitation of mouse sperm occurs within 1 h but requires 4 h of coincubation of gametes for human sperm (Plachot et al., 1986; Bavister, 2002). Using conditions for mouse in vitro fertilization, we previously reported that human sperm capacitated for <2 h did not bind to ovulated huZP2 rescue eggs (Rankin et al., 2003). These observations were confirmed in our current assay using human in vitro fertilization conditions and noninseminated human oocytes as positive controls. However, after 3 h of incubation, human sperm began to bind, and by 4 h, robust binding was observed (Fig. 3 E). Thus, human sperm need to be capacitated for ∼4 h to bind to the humanized zona pellucida, indicating physiological relevance of the observed gamete interactions.
Sperm bind an N-terminal domain on human ZP2
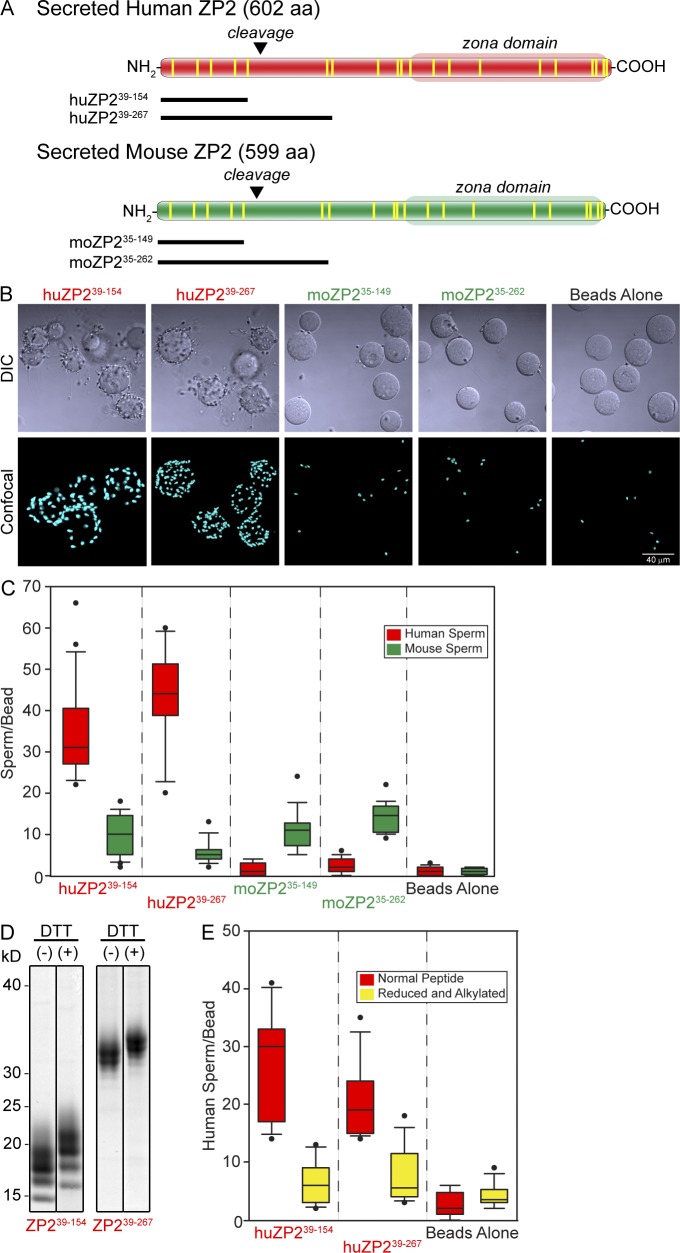
Postfertilization proteolytic cleavage at the N terminus of ZP2 prevents sperm binding to the zona pellucida (Bleil et al., 1981; Bauskin et al., 1999; Gahlay et al., 2010; Burkart et al., 2012). However, whether cleavage was indirectly affecting the 3D structure of the zona matrix or a specific binding site remained unresolved. To investigate a direct role in gamete recognition, recombinant baculovirus encoding N-terminal human and mouse ZP2 peptides were constructed (Fig. 4 A). Each peptide had a honeybee mellitin signal peptide (23 aa) at its N terminus to ensure secretion and a 6-His tag at its C terminus to facilitate purification. Human ZP239–154 and ZP239–267 start at the N terminus of the secreted ectodomain and terminate before and after the previously described 171LA↓DD174 cleavage site (Gahlay et al., 2010), respectively. Each contained an even number of cysteine residues capable of forming two (ZP239–154) or three (ZP239–267) disulfide bonds (Fig. 4 E), and each contained N-linked glycans that could be removed with PNGase F (Fig. S2 A). The corresponding mouse ZP235–149 and ZP235–262 recombinant peptides, the C termini of which flank the mouse 166LA↓DE169 cleavage site, were also expressed and purified from insect cells (Fig. S2 B).
Figure 4.
Sperm binding to recombinant ZP2 peptides. (A) Schematic representation of secreted human and mouse ZP2 with zona domains at their C termini and postfertilization cleavage sites near their N termini. Vertical lines (yellow) represent conserved cysteine residues. Recombinant human and mouse N-terminal peptides that end either before or after the initial postfertilization cleavage site (inverted triangles) are indicated below each protein. (B) Capacitated human sperm binding to 6-His–tagged recombinant ZP2 peptides (huZP239–154, huZP239–267, moZP235–149, and moZP235–262) attached to IMAC Sepharose beads. Beads alone provide a negative control. DIC (top) and confocal z projection (bottom) images after staining with Hoechst. (C) Quantification of capacitated human (red) and mouse (green) sperm binding to recombinant human (huZP239–154 and huZP239–267) and mouse (moZP235–149 and moZP235–262) peptide beads. Box plots reflect the median (lines) and data points within the 10th and 90th percentiles (error bars). Boxes include the middle two quartiles, and outliers are indicated by dots. (D) Coomassie blue–stained SDS-PAGE of huZP239–154 and huZP239–267 before (−) and after (+) reduction and alkylation of the disulfide bonds. Molecular masses are indicated on the left. (E) Same as C for capacitated human sperm binding to recombinant human (huZP239–154 and huZP239–267) peptide beads before (red) and after (yellow) reduction and alkylation of peptides to disrupt disulfide bonds. Molecular masses are indicated on the left.
Human sperm bound well to beads coated with recombinant human ZP239–154 or ZP239–267 peptides but poorly to beads coated with the homologous mouse peptides (Fig. 4 B), an observation that was quantified with box plots to capture all of the experimental data (Fig. 4 C). Reduction of disulfide bonds and alkylation of free sulfhydryl groups (Fig. 4 D) significantly decreased human sperm binding to ZP239–154 or ZP239–267 peptides (Fig. 4 E), indicating a dependence on secondary structures defined, at least in part, by disulfide bonds. The binding of human sperm to huZP2 rescue eggs (as well as to huZP239–154 peptide beads) was inhibited by excess huZP239–154, but not moZP235–149 peptides, which corroborated the specificity of the binding site on ZP2 (Fig. S2, C and D). Although fewer in number, mouse sperm bound comparably to mouse and human ZP2 peptide beads consistent with its observed lack of taxon specificity in binding to mouse and human eggs (Bedford, 1977). From these results, we conclude that the N terminus of human ZP2 plays an important role in gamete recognition on the surface of the zona pellucida.
Sperm binding to humanized zonae pellucidae
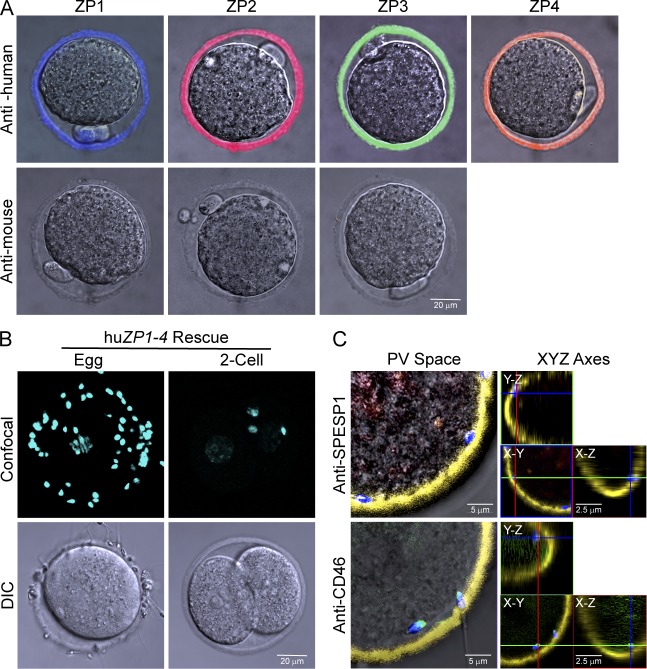
To analyze mice with a more physiologically relevant human zona pellucida, the four mouse lines expressing human zona proteins were crossed to establish huZP1–4 rescue mice (ZP1huTG, ZP2huTG, ZP3huTG, ZP4huTG, Zp1tm/tm, Zp2tm/tm, and Zp3tm/tm) that expressed the four human and none of the three mouse zona proteins (Fig. 5 A). The resultant line was difficult to maintain, as transgenic females were variably fertile with roughly 50% producing litters of decreased size (two to four pups), only half of which were female. Nevertheless, in the presence of all four human zona proteins, capacitated human sperm bound in vitro to huZP1–4 rescue eggs but not to two-cell embryos obtained after fertilization with mouse sperm (Fig. 5 B). The sperm that bound to the surface of the zona pellucida penetrated the zona matrix and accumulated in the perivitelline space between the inner aspect of the zona matrix and the egg plasma membrane.
Figure 5.
Human sperm bind and penetrate humanized zonae pellucidae. (A) Ovulated eggs from huZP1–4 rescue mice expressing human ZP1, ZP2, ZP3, and ZP4 but not mouse ZP1, ZP2, or ZP3 after staining with monoclonal antibodies to individual zona proteins. Antibody binding was detected by confocal microscopy as described in Fig. 1. (B) Human sperm binding to huZP1–4 rescue eggs and two-cell embryos. Confocal z projections (top) and DIC (bottom) images after staining with Hoechst. (C) Acrosome status of human sperm within the perivitelline (PV) space of huZP1–4 rescue eggs was determined by confocal microscopy after staining with Hoechst and using SPESP1 (red) and CD46 (green) antibodies that detect acrosome-intact and -reacted sperm, respectively (Fig. S3). The localization of sperm within the perivitelline space was confirmed by x-y, x-z, and y-z orthogonal views, where the x, y, and z axes are colored green, red, and blue, respectively.
Human sperm are relatively short (∼60 µm) with spatulate-shaped heads (3 × 4.5 µm) that contain an acrosome, a Golgi-derived subcellular organelle underlying the anterior plasma membrane. In association with fertilization, the plasma membrane overlying the sperm head and the outer acrosomal membranes fuse to release acrosomal contents, and only acrosome-reacted sperm are present in the perivitelline space (Austin, 1975; Saling et al., 1979). The acrosome status of human sperm (Fig. S3) can be assessed with antibodies to SPESP1 (sperm equatorial segment protein 1) that bind to acrosome-intact sperm (Wolkowicz et al., 2008; Fujihara et al., 2010) and with antibodies that recognize CD46, an antigen present on the inner acrosomal membrane and only accessible after acrosome exocytosis (Fénichel et al., 1990).
Despite the paucity of biological material (14 eggs), only acrosome-reacted human sperm (n = 76, mean of 5.4 ± 1.2 SEM) were detected in the perivitelline space (Fig. 5 C). As previously reported for zona-free mouse eggs (Quinn, 1979; Yanagimachi, 1984), the acrosome-reacted human sperm were unable to fuse with mouse eggs. We conclude that the reconstituted humanized zona pellucida containing human ZP1–4 is a physiologically relevant platform to which human sperm bind, penetrate, and accumulate in the perivitelline space.
ZP2 model of gamete recognition
Collectively, these observations provide a simple, unifying formulation for the molecular basis of gamete recognition in which sperm bind to ZP2 in the extracellular zona pellucida surrounding ovulated eggs. In this model, capacitated sperm attach to an N-terminal domain (∼115 aa) of ZP2 before penetration of the zona matrix and entrance into the perivitelline space. After fertilization, ovastacin, an oocyte-specific member of the astacin family of metalloendoproteases (Quesada et al., 2004), is exocytosed from egg cortical granules and cleaves extracellular ZP2 (Burkart et al., 2012). After the initial cleavage (166LA↓DE169 in mouse; 171LA↓DD174Z in human), further digestion of ZP2 at additional diacidic motifs (54DE55 and 127DD128 in mice; 56DE57 and 144EE145 in humans) effectively destroys the sperm-binding domain (Greenhouse et al., 1999; Gahlay et al., 2010; Burkart et al., 2012) and accounts for the inability of sperm to bind to two-cell embryos.
ZP2 is present in a wide range of vertebrates, including birds, fish, and reptiles (Spargo and Hope, 2003). Although earlier studies implicated ZP2 as a secondary zona ligand that binds acrosome-reacted sperm (Bleil et al., 1988; Tsubamoto et al., 1999; Kerr et al., 2002; Chakravarty et al., 2008; Chiu et al., 2008), there is precedence for ZP2 acting in primary gamete recognition. Native gp69/64, the Xenopus laevis ZP2 homologue, inhibits sperm binding to eggs in a competition assay (Tian et al., 1997a). After fertilization and cleavage at the conserved 155FD↓DD158 site, neither a recombinant N-terminal 27-mer peptide (gp69/64130–156) nor the C-terminal native gp69/64 glycopeptide was inhibitory in a sperm-binding assay. These results were attributed to either the lack of normal posttranslational glycosylation of the small recombinant peptide (gp69/64130–156) or truncation of the C-terminal glycopeptide (Tian et al., 1997b, 1999). Alternatively, we note that the small Xenopus gp69/64130–156 peptide is bounded by diacidic residues (130DE131 and 157DE158) and could represent a proteolytic fragment of a larger (i.e., gp69/6434–156) sperm-binding domain and, thus, incapable of inhibiting gamete binding on its own. Xenopus ZP2 is cleaved by a zinc metalloprotease (Lindsay and Hedrick, 2004), and postfertilization proteolysis of the N-terminal domain of gp69/64 could account for the inability of sperm to bind to early Xenopus embryos as it does in mice. Thus, it seems likely that the ZP2 model of gamete recognition pertains more broadly among vertebrates.
If prior focus on ZP3 was misplaced, the current molecular definition of ZP2 as a zona ligand may provide a path forward in identification of the cognate sperm surface receptor and investigating its role in gamete recognition and induction of acrosome exocytosis, both necessary for sperm–egg fusion and fertilization. In addition, humanized transgenic eggs to which human sperm bind should provide a useful proxy for investigating human gamete interactions with potential translational applications for reproductive medicine.
Materials and methods
Establishment of transgenic mouse lines
Human ZP1 (chromosome 11: 60,635,015–60,643,164) was retrieved from bacterial artificial chromosome RP11-1080G11 (Children’s Hospital Oakland Research Institute) into a bacterial plasmid by DNA recombineering (Liu et al., 2003). Oligonucleotide primers were designed to PCR amplify left (huZP1LFW1-29-08 forward, 5′-GCGGCCGCAAGGAAAGCATTATC-3′; and HuZP1LRV1-29-08 reverse, 5′-AAGCTTAGAGGGGAATGGCACAC-3′) and right (HuZP1RFW1-29-08 forward, 5′-AAGCTTCCCAAGCCTATTGCTTAC-3′; and HuZP1RRV1-29-08 reverse, 5′-ACTAGTCCAACCAGCCAAGCTAAG-3′) arms bounded by NotI and SalI and separated by a HindIII restriction enzyme site (Yauger et al., 2011).
The NotI and SalI human ZP1 DNA fragment was injected into pronuclei of one-cell embryos to establish transgenic mouse lines. Mice were genotyped by PCR of tail DNA using exon-specific oligonucleotides (HuZP1EX2-3F2/08 forward primer, 5′-CTTCTCGGCCGATTACAGAG-3′; and HuZP1EX2-3RV2/08 reverse primer, 5′-CGTTTGTTCACATCCCAGTG-3′). The presence of the transgene in founder lines was confirmed by Southern hybridization using full-length human ZP1 cDNA (GeneArt AG) in 7 out of 31 pups (22.6%). Three mice (two female and one male) were used to establish stable transgenic lines, one of which (ZP1huTG) was used in the current experiments. Human ZP1 expression was detected by PCR using the aforementioned exon primer pair, and total RNA was isolated from 4–8-wk-old mouse tissues. Glyceraldehyde 3-phosphate dehydrogenase served as a control for RNA integrity. The ZP1huTG line was crossed into the mouse Zp1-null background (Rankin et al., 1999) to establish huZP1 rescue mice (ZP1huTG, Zp1tm/tm, Zp2+/+, and Zp3+/+).
Sense and antisense 35S-labeled human ZP1 cRNA (1–693 nt) was used for in situ hybridization of 7-µm sections from 16-d-old ovaries that were fixed in 4% formaldehyde and embedded in Tissue-Tek OCT (Sakura; Yauger et al., 2011). Slides were counterstained with cresyl violet and imaged using bright- and dark-field microscopy. Ovaries from 3–4-wk-old mice were fixed in glutaraldehyde, embedded in plastic, and stained with periodic acid Schiff’s reagent to visualize the zonae pellucidae. Ovulated eggs were obtained after gonadotropin stimulation and imaged by differential interference contrast (DIC) microscopy (Yauger et al., 2011). Zona proteins from noninseminated, immature human oocytes (Shady Grove Fertility Center) and huZP1 and huZP4 transgenic eggs were resolved on Novex Bis-Tris Gels (NuPAGE; Invitrogen), transferred onto polyvinylidene difluoride membranes, and probed with mAb to human ZP1 (Ganguly et al., 2010). The primary antibody (1:2,000) was detected using a goat HRP–conjugated secondary antibody (1:10,000) and a luminescent image analyzer (LAS-3000; Fujifilm).
Confocal microscopy
To detect human zona proteins in transgenic zonae pellucidae, ovulated eggs were fixed in 2% paraformaldehyde and stained with mAb to mouse ZP1 (Rankin et al., 1999), ZP2 (East and Dean, 1984), and ZP3 (East et al., 1985) and human ZP1 (Ganguly et al., 2010), ZP2 (Rankin et al., 2003), ZP3 (Rankin et al., 1998), and ZP4 (Bukovsky et al., 2008). Monoclonal antibodies to human ZP1 and ZP4 were kind gifts of S. Gupta (National Institute of Immunology, New Delhi, India). Antibody binding to human and mouse zona proteins was detected by confocal microscopy using goat anti–mouse Alexa Fluor 568 (1:100) and goat anti–rat Cy3 (1:100) antibodies, respectively. Capacitated human sperm, before and after treatment with 3 µmol A23187 to induce acrosome exocytosis, were fixed with 2% paraformaldehyde and stained with mouse antibodies to human SPESP1 (1:50; Abnova) or FITC-conjugated mouse anti–human CD46 (1:50; BD) to detect acrosome-intact and -reacted sperm, respectively, by confocal microscopy. Goat anti–mouse Alexa Fluor 564 (1:100) was used to detect the bound SPESP1 antibody.
Samples were mounted in PBS, and images of eggs, embryos, and beads were obtained with a confocal microscope (LSM 510; Carl Zeiss) using a 63×/1.2 NA water immersion objective (Baibakov et al., 2007; Yauger et al., 2011). LSM 510 images were exported as a full resolution TIF files and processed in Photoshop CS5.5 (Adobe) to adjust brightness and contrast. 3–5-µm optical sections were used for 3D reconstruction and orthogonal and z projections. Sperm tails were imaged by confocal single wavelength reflection using a 750-nm laser light passed through a pinhole and collected by a photomultiplier tube (Chen et al., 2009).
Fertility and sperm binding to ovulated eggs
To assay human sperm binding, individual 0.5-ml aliquots of liquefied human semen (Genetics & IVF Institute Cryobanks) were added to a 2.0-ml Eppendorf tube containing 0.5 ml of 40% PureSperm (Nidacon) layered over 0.5 ml of 80% PureSperm. After centrifugation (swinging bucket for 15 min × 300 g at 20°C) and removal of the supernatant, sperm were resuspended in the residual buffer and transferred to 1.0 ml human tubal fluid (HTF; EMD; Millipore) supplemented with 0.5% BSA (Sigma-Aldrich). After a second centrifugation (5 min × 300 g), sperm were resuspended in 0.2 ml HTF/BSA, and two to four aliquots were mixed before evaluation by a motility analyzer (HTM IVOS version 12.3; Hamilton Thorne Biosciences; Gahlay et al., 2010). Sperm were then diluted in HTF/BSA to 106 ml−1. Transgenic eggs in cumulus, normal eggs, Zp3EGFP eggs (Zhao et al., 2002), two-cell embryos from mice, and/or noninseminated, immature human oocytes were incubated in 100-µl droplets of sperm (105) in HTF/BSA under mineral oil for 4 h (37°C at 90% N2, 5% O2, and 5% CO2) in an incubator (BT37GP; Planer). Eggs/embryos/oocytes were washed by serial transfer through 500 µl HTF/BSA and either fixed for imaging or observed by video camera (KP-D20A/B; Hitachi). Two to three noninseminated human oocytes were used as controls.
To assay mouse sperm binding (Rankin et al., 2003), cauda epididymides were macerated in a dish of HTF/BSA to release sperm that were capacitated for 40 min (37°C with 90% N2, 5% O2, and 5% CO2) and added to eggs and embryos in 100 µl HTF/BSA. Normal mouse eggs and two-cell embryos served as positive and negative controls, respectively. Mouse and human samples were fixed in 2% paraformaldehyde and stained with Hoechst to identify nuclei. Bound sperm were quantified from z projections obtained by confocal microscopy (Baibakov et al., 2007), and results reflect the mean ± SEM from at least three independently obtained samples, each containing 6–12 mouse eggs/embryos.
Experiments with normal and transgenic mice were conducted in compliance with the guidelines of the Animal Care and Use Committee of the National Institutes of Health under a Division of Intramural Research, National Institutes of Diabetes and Digestive and Kidney Diseases–approved animal study protocol. Human sperm binding assays were conducted in compliance with an Institutional Review Board approved protocol and the Office of Human Subject Research determined that federal regulations did not apply to the anonymous human sperm and noninseminated, immature oocytes.
Sperm binding to ZP2 peptide beads
cDNA encoding N-terminal fragments of human (39–154 and 39–267 aa) and mouse (35–149 and 35–262 aa) ZP2 were cloned into the pFastBac HBM TOPO vector (Invitrogen) downstream of a polyhedron promoter and a 23-aa honeybee mellitin signal peptide. For purification, a 6-His tag was encoded in frame at the 3′ end of the open reading frame of each clone. Bacmid DNA, isolated after transformation into DH10Bac Escherichia coli, was used to transfect insect cells to produce recombinant baculovirus particles for infection of High Five cells using protocols provided by the manufacturer (Invitrogen). After incubation for 72 h at 21°C, the secretion of ZP2 peptides was confirmed by immunoblotting using mAb to ZP2 (East and Dean, 1984; Rankin et al., 2003), and peptides were isolated on immobilized metal affinity chromatography (IMAC) beads (GE Healthcare) per the manufacturer’s instructions. To examine the diversity of the recombinant human ZP2 glycoforms, huZP239–154 and huZP239–267 peptides were digested (1 h at 37°C and 300 rpm in a thermomixer; Eppendorf) with PNGase F (New England Biolabs, Inc.) according to the manufacturer’s instructions.
Beads alone or after conjugation with recombinant peptides were incubated with human or mouse sperm in 100 µl (see first paragraph in Fertility and sperm binding to ovulated eggs section) for 1–4 h (37°C with 90% N2, 5% O2, and 5% CO2), washed twice in 500 µl HTF/BSA, fixed in 2% paraformaldehyde, stained with Hoechst, and imaged by confocal microscopy. Human sperm binding to huZP2 rescue eggs and to huZP239–154 peptide beads (see previous section) was inhibited by the addition of recombinant huZP239–267, but not moZP235–149, peptides (20–50 µl of ∼5 µg/ml High Five cell supernatant) at the time of insemination.
To disrupt secondary structure determined by disulfide bonds, the human peptides were eluted from beads with 300 mM imidazole, diluted 10-fold, reduced with 5 mM Tris[2-carboxyethyl]phosphine, and alkylated with 30 mM iodoacetamide (Sigma-Aldrich) before reisolation on IMAC beads. Results reflect the mean ± SEM from at least three independently obtained samples, each containing 20–25 beads. Cloning, expression, and attachment to IMAC beads and protein modifications were performed in the Protein Expression Laboratory of the Advanced Technology Program, Science Applications International Corporation-Frederick, Inc.
Online supplemental material
Fig. S1 describes the phenotype of the human ZP1 transgenic mouse line. Fig. S2 characterizes the human and mouse ZP2 N-terminal peptides and their ability to inhibit human sperm binding. Fig. S3 defines the immunohistological assays used to determine the acrosome status of human sperm. Table S1 documents the fertility of human ZP1 transgenic mice. Videos 1–4 document human sperm binding to human ZP1 (Video 1), ZP2 (Video 2), and ZP3 (Video 3) rescue eggs as well as to human ZP4 (Video 4) transgenic eggs. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201203062/DC1.
Supplementary Material
Acknowledgments
We thank Ms. Lyn Gauthier for pronuclear injections to establish the human ZP1 transgenic mouse line and Drs. Dominic Esposito and William Gillete for expression of recombinant peptides (Protein Expression Laboratory, National Cancer Institute, Frederick, MD). Noninseminated, immature human oocytes were obtained from Shady Grove Fertility Clinic.
This research was support by the Intramural Research Program of the National Institutes of Health, National Institutes of Diabetes and Digestive and Kidney Diseases.
Footnotes
Abbreviations used in this paper:
- CCD
- charge-coupled device
- DIC
- differential interference contrast
- HTF
- human tubal fluid
- IMAC
- immobilized metal affinity chromatography
References
- Asano M., Furukawa K., Kido M., Matsumoto S., Umesaki Y., Kochibe N., Iwakura Y. 1997. Growth retardation and early death of beta-1,4-galactosyltransferase knockout mice with augmented proliferation and abnormal differentiation of epithelial cells. EMBO J. 16:1850–1857 10.1093/emboj/16.8.1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin C.R. 1951. Observations on the penetration of the sperm in the mammalian egg. Aust. J. Sci. Res., B. 4:581–596 [DOI] [PubMed] [Google Scholar]
- Austin C.R. 1975. Membrane fusion events in fertilization. J. Reprod. Fertil. 44:155–166 10.1530/jrf.0.0440155 [DOI] [PubMed] [Google Scholar]
- Baibakov B., Gauthier L., Talbot P., Rankin T.L., Dean J. 2007. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development. 134:933–943 10.1242/dev.02752 [DOI] [PubMed] [Google Scholar]
- Bailey J.L. 2010. Factors regulating sperm capacitation. Syst. Biol. Reprod. Med. 56:334–348 10.3109/19396368.2010.512377 [DOI] [PubMed] [Google Scholar]
- Bauskin A.R., Franken D.R., Eberspaecher U., Donner P. 1999. Characterization of human zona pellucida glycoproteins. Mol. Hum. Reprod. 5:534–540 10.1093/molehr/5.6.534 [DOI] [PubMed] [Google Scholar]
- Bavister B.D. 2002. Early history of in vitro fertilization. Reproduction. 124:181–196 10.1530/rep.0.1240181 [DOI] [PubMed] [Google Scholar]
- Bedford J.M. 1977. Sperm/egg interaction: the specificity of human spermatozoa. Anat. Rec. 188:477–487 10.1002/ar.1091880407 [DOI] [PubMed] [Google Scholar]
- Bleil J.D., Wassarman P.M. 1980. Mammalian sperm-egg interaction: identification of a glycoprotein in mouse egg zonae pellucidae possessing receptor activity for sperm. Cell. 20:873–882 10.1016/0092-8674(80)90334-7 [DOI] [PubMed] [Google Scholar]
- Bleil J.D., Beall C.F., Wassarman P.M. 1981. Mammalian sperm-egg interaction: fertilization of mouse eggs triggers modification of the major zona pellucida glycoprotein, ZP2. Dev. Biol. 86:189–197 10.1016/0012-1606(81)90329-8 [DOI] [PubMed] [Google Scholar]
- Bleil J.D., Greve J.M., Wassarman P.M. 1988. Identification of a secondary sperm receptor in the mouse egg zona pellucida: role in maintenance of binding of acrosome-reacted sperm to eggs. Dev. Biol. 128:376–385 10.1016/0012-1606(88)90299-0 [DOI] [PubMed] [Google Scholar]
- Boja E.S., Hoodbhoy T., Fales H.M., Dean J. 2003. Structural characterization of native mouse zona pellucida proteins using mass spectrometry. J. Biol. Chem. 278:34189–34202 10.1074/jbc.M304026200 [DOI] [PubMed] [Google Scholar]
- Bukovsky A., Gupta S.K., Bansal P., Chakravarty S., Chaudhary M., Svetlikova M., White R.S., Copas P., Upadhyaya N.B., Van Meter S.E., Caudle M.R. 2008. Production of monoclonal antibodies against recombinant human zona pellucida glycoproteins: utility in immunolocalization of respective zona proteins in ovarian follicles. J. Reprod. Immunol. 78:102–114 10.1016/j.jri.2007.10.004 [DOI] [PubMed] [Google Scholar]
- Burkart A.D., Xiong B., Baibakov B., Jiménez-Movilla M., Dean J. 2012. Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. J. Cell Biol. 197:37–44 10.1083/jcb.201112094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty S., Kadunganattil S., Bansal P., Sharma R.K., Gupta S.K. 2008. Relevance of glycosylation of human zona pellucida glycoproteins for their binding to capacitated human spermatozoa and subsequent induction of acrosomal exocytosis. Mol. Reprod. Dev. 75:75–88 10.1002/mrd.20726 [DOI] [PubMed] [Google Scholar]
- Chang M.C. 1951. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 168:697–698 10.1038/168697b0 [DOI] [PubMed] [Google Scholar]
- Chen J., Litscher E.S., Wassarman P.M. 1998. Inactivation of the mouse sperm receptor, mZP3, by site-directed mutagenesis of individual serine residues located at the combining site for sperm. Proc. Natl. Acad. Sci. USA. 95:6193–6197 10.1073/pnas.95.11.6193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.L., Chou C.K., Lin M.G., Chen Y.F., Jee S.H., Tan H.Y., Tsai T.H., Kim K.H., Kim D., So P.T., et al. 2009. Single-wavelength reflected confocal and multiphoton microscopy for tissue imaging. J. Biomed. Opt. 14:054026 10.1117/1.3247157 [DOI] [PubMed] [Google Scholar]
- Chiu P.C., Wong B.S., Lee C.L., Pang R.T., Lee K.F., Sumitro S.B., Gupta S.K., Yeung W.S. 2008. Native human zona pellucida glycoproteins: purification and binding properties. Hum. Reprod. 23:1385–1393 10.1093/humrep/den047 [DOI] [PubMed] [Google Scholar]
- Choi Y.H., Toyoda Y. 1998. Cyclodextrin removes cholesterol from mouse sperm and induces capacitation in a protein-free medium. Biol. Reprod. 59:1328–1333 10.1095/biolreprod59.6.1328 [DOI] [PubMed] [Google Scholar]
- Ducibella T., Huneau D., Angelichio E., Xu Z., Schultz R.M., Kopf G.S., Fissore R., Madoux S., Ozil J.P. 2002. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Dev. Biol. 250:280–291 10.1006/dbio.2002.0788 [DOI] [PubMed] [Google Scholar]
- East I.J., Dean J. 1984. Monoclonal antibodies as probes of the distribution of ZP-2, the major sulfated glycoprotein of the murine zona pellucida. J. Cell Biol. 98:795–800 10.1083/jcb.98.3.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- East I.J., Gulyas B.J., Dean J. 1985. Monoclonal antibodies to the murine zona pellucida protein with sperm receptor activity: effects on fertilization and early development. Dev. Biol. 109:268–273 10.1016/0012-1606(85)90454-3 [DOI] [PubMed] [Google Scholar]
- Fénichel P., Dohr G., Grivaux C., Cervoni F., Donzeau M., Hsi B.L. 1990. Localization and characterization of the acrosomal antigen recognized by GB24 on human spermatozoa. Mol. Reprod. Dev. 27:173–178 10.1002/mrd.1080270214 [DOI] [PubMed] [Google Scholar]
- Florman H.M., Wassarman P.M. 1985. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell. 41:313–324 10.1016/0092-8674(85)90084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara Y., Murakami M., Inoue N., Satouh Y., Kaseda K., Ikawa M., Okabe M. 2010. Sperm equatorial segment protein 1, SPESP1, is required for fully fertile sperm in mouse. J. Cell Sci. 123:1531–1536 10.1242/jcs.067363 [DOI] [PubMed] [Google Scholar]
- Gahlay G., Gauthier L., Baibakov B., Epifano O., Dean J. 2010. Gamete recognition in mice depends on the cleavage status of an egg’s zona pellucida protein. Science. 329:216–219 10.1126/science.1188178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A., Bukovsky A., Sharma R.K., Bansal P., Bhandari B., Gupta S.K. 2010. In humans, zona pellucida glycoprotein-1 binds to spermatozoa and induces acrosomal exocytosis. Hum. Reprod. 25:1643–1656 10.1093/humrep/deq105 [DOI] [PubMed] [Google Scholar]
- Greenhouse S., Castle P.E., Dean J. 1999. Antibodies to human ZP3 induce reversible contraception in transgenic mice with ‘humanized’ zonae pellucidae. Hum. Reprod. 14:593–600 10.1093/humrep/14.3.593 [DOI] [PubMed] [Google Scholar]
- Hoodbhoy T., Joshi S., Boja E.S., Williams S.A., Stanley P., Dean J. 2005. Human sperm do not bind to rat zonae pellucidae despite the presence of four homologous glycoproteins. J. Biol. Chem. 280:12721–12731 10.1074/jbc.M413569200 [DOI] [PubMed] [Google Scholar]
- Inoue M., Wolf D.P. 1975. Sperm binding characteristics of the murine zona pellucida. Biol. Reprod. 13:340–346 10.1095/biolreprod13.3.340 [DOI] [PubMed] [Google Scholar]
- Kerr C.L., Hanna W.F., Shaper J.H., Wright W.W. 2002. Characterization of zona pellucida glycoprotein 3 (ZP3) and ZP2 binding sites on acrosome-intact mouse sperm. Biol. Reprod. 66:1585–1595 10.1095/biolreprod66.6.1585 [DOI] [PubMed] [Google Scholar]
- Lanzendorf S.E., Holmgren W.J., Johnson D.E., Scobey M.J., Jeyendran R.S. 1992. Hemizona assay for measuring zona binding in the lowland gorilla. Mol. Reprod. Dev. 31:264–267 10.1002/mrd.1080310407 [DOI] [PubMed] [Google Scholar]
- Lefièvre L., Conner S.J., Salpekar A., Olufowobi O., Ashton P., Pavlovic B., Lenton W., Afnan M., Brewis I.A., Monk M., et al. 2004. Four zona pellucida glycoproteins are expressed in the human. Hum. Reprod. 19:1580–1586 10.1093/humrep/deh301 [DOI] [PubMed] [Google Scholar]
- Lindsay L.L., Hedrick J.L. 2004. Proteolysis of Xenopus laevis egg envelope ZPA triggers envelope hardening. Biochem. Biophys. Res. Commun. 324:648–654 10.1016/j.bbrc.2004.09.099 [DOI] [PubMed] [Google Scholar]
- Liu C., Litscher E.S., Wassarman P.M. 1995. Transgenic mice with reduced numbers of functional sperm receptors on their eggs reproduce normally. Mol. Biol. Cell. 6:577–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Jenkins N.A., Copeland N.G. 2003. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 13:476–484 10.1101/gr.749203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez L.C., Bayna E.M., Litoff D., Shaper N.L., Shaper J.H., Shur B.D. 1985. Receptor function of mouse sperm surface galactosyltransferase during fertilization. J. Cell Biol. 101:1501–1510 10.1083/jcb.101.4.1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J.B., Marth J.D. 2003. A genetic approach to mammalian glycan function. Annu. Rev. Biochem. 72:643–691 10.1146/annurev.biochem.72.121801.161809 [DOI] [PubMed] [Google Scholar]
- Plachot M., Junca A.M., Mandelbaum J., Cohen J., Salat-Baroux J., Da Lage C. 1986. Timing of in-vitro fertilization of cumulus-free and cumulus-enclosed human oocytes. Hum. Reprod. 1:237–242 [DOI] [PubMed] [Google Scholar]
- Quesada V., Sánchez L.M., Alvarez J., López-Otín C. 2004. Identification and characterization of human and mouse ovastacin: a novel metalloproteinase similar to hatching enzymes from arthropods, birds, amphibians, and fish. J. Biol. Chem. 279:26627–26634 10.1074/jbc.M401588200 [DOI] [PubMed] [Google Scholar]
- Quinn P. 1979. Failure of human spermatozoa to penetrate zona free mouse and rat ova in vitro. J. Exp. Zool. 210:497–505 10.1002/jez.1402100312 [DOI] [PubMed] [Google Scholar]
- Rankin T.L., Tong Z.-B., Castle P.E., Lee E., Gore-Langton R., Nelson L.M., Dean J. 1998. Human ZP3 restores fertility in Zp3 null mice without affecting order-specific sperm binding. Development. 125:2415–2424 [DOI] [PubMed] [Google Scholar]
- Rankin T., Talbot P., Lee E., Dean J. 1999. Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development. 126:3847–3855 [DOI] [PubMed] [Google Scholar]
- Rankin T.L., Coleman J.S., Epifano O., Hoodbhoy T., Turner S.G., Castle P.E., Lee E., Gore-Langton R., Dean J. 2003. Fertility and taxon-specific sperm binding persist after replacement of mouse sperm receptors with human homologs. Dev. Cell. 5:33–43 10.1016/S1534-5807(03)00195-3 [DOI] [PubMed] [Google Scholar]
- Saling P.M., Sowinski J., Storey B.T. 1979. An ultrastructural study of epididymal mouse spermatozoa binding to zonae pellucidae in vitro: sequential relationship to the acrosome reaction. J. Exp. Zool. 209:229–238 10.1002/jez.1402090205 [DOI] [PubMed] [Google Scholar]
- Shi S., Williams S.A., Seppo A., Kurniawan H., Chen W., Ye Z., Marth J.D., Stanley P. 2004. Inactivation of the Mgat1 gene in oocytes impairs oogenesis, but embryos lacking complex and hybrid N-glycans develop and implant. Mol. Cell. Biol. 24:9920–9929 10.1128/MCB.24.22.9920-9929.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spargo S.C., Hope R.M. 2003. Evolution and nomenclature of the zona pellucida gene family. Biol. Reprod. 68:358–362 10.1095/biolreprod.102.008086 [DOI] [PubMed] [Google Scholar]
- Thall A.D., Malý P., Lowe J.B. 1995. Oocyte Gal alpha 1,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J. Biol. Chem. 270:21437–21440 10.1074/jbc.270.37.21437 [DOI] [PubMed] [Google Scholar]
- Tian J., Gong H., Thomsen G.H., Lennarz W.J. 1997a. Gamete interactions in Xenopus laevis: identification of sperm binding glycoproteins in the egg vitelline envelope. J. Cell Biol. 136:1099–1108 10.1083/jcb.136.5.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Gong H., Thomsen G.H., Lennarz W.J. 1997b. Xenopus laevis sperm-egg adhesion is regulated by modifications in the sperm receptor and the egg vitelline envelope. Dev. Biol. 187:143–153 10.1006/dbio.1997.8607 [DOI] [PubMed] [Google Scholar]
- Tian J., Gong H., Lennarz W.J. 1999. Xenopus laevis sperm receptor gp69/64 glycoprotein is a homolog of the mammalian sperm receptor ZP2. Proc. Natl. Acad. Sci. USA. 96:829–834 10.1073/pnas.96.3.829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubamoto H., Hasegawa A., Nakata Y., Naito S., Yamasaki N., Koyama K. 1999. Expression of recombinant human zona pellucida protein 2 and its binding capacity to spermatozoa. Biol. Reprod. 61:1649–1654 10.1095/biolreprod61.6.1649 [DOI] [PubMed] [Google Scholar]
- Williams S.A., Xia L., Cummings R.D., McEver R.P., Stanley P. 2007. Fertilization in mouse does not require terminal galactose or N-acetylglucosamine on the zona pellucida glycans. J. Cell Sci. 120:1341–1349 10.1242/jcs.004291 [DOI] [PubMed] [Google Scholar]
- Wolkowicz M.J., Digilio L., Klotz K., Shetty J., Flickinger C.J., Herr J.C. 2008. Equatorial segment protein (ESP) is a human alloantigen involved in sperm-egg binding and fusion. J. Androl. 29:272–282 10.2164/jandrol.106.000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R. 1984. Zona-free hamster eggs: Their use in assessing fertilizing capacity and examining chromosomes of human spermatozoa. Gamete Res. 10:187–232 10.1002/mrd.1120100210 [DOI] [Google Scholar]
- Yauger B., Boggs N.A., Dean J. 2011. Human ZP4 is not sufficient for taxon-specific sperm recognition of the zona pellucida in transgenic mice. Reproduction. 141:313–319 10.1530/REP-10-0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Gold L., Ginsberg A.M., Liang L.-F., Dean J. 2002. Conserved furin cleavage site not essential for secretion and integration of ZP3 into the extracellular egg coat of transgenic mice. Mol. Cell. Biol. 22:3111–3120 10.1128/MCB.22.9.3111-3120.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.