Abstract
Cholesterol reduction from statin therapy has been one of the greatest public health successes in modern medicine. Simvastatin is among the most commonly used prescription medications. A non-synonymous coding single-nucleotide polymorphism (SNP), rs4149056, in SLCO1B1 markedly increases systemic exposure to simvastatin and the risk of muscle toxicity. This guideline explores the relationship between rs4149056 (c.521T>C, p.V174A) and clinical outcome for all statins. The strength of the evidence is high for myopathy with simvastatin. We limit our recommendations accordingly.
BACKGROUND
The purpose of this study was to provide interpretive guidance when SLCO1B1 genotype is already available in the clinical environment. We do not argue directly in this guideline that SLCO1B1 genotyping is absolutely necessary. However, it seems that the implementation of a gene-based dosing approach is inevitable. We therefore provide guidance for clinicians treating patients with simvastatin in the event that SLCO1B1 genotype is available during routine care.
This guideline is written for providers attempting to manage myopathy risk. Because the strength of the evidence is highest for simvastatin, we limit our recommendations accordingly. We do not present guidelines for all statins. Furthermore, our primary goal is to reduce muscle toxicity and optimize patient adherence. Although efficacy studies are under way, we do not yet see a clear basis for making recommendations on the role of SLCO1B1 genotype in lipid-lowering efficacy.
FOCUSED LITERATURE REVIEW
A systematic literature review was conducted that focused on SLCO1B1 gene polymorphisms and statin-related end points in humans. Emphasis was placed on the SLCO1B1 variant rs4149056 and two primary end points: (i) statin pharmacokinetics (including parent drugs, lactones, acids, and derivatives related to phase I oxidation and/or phase II conjugation) and (ii) relevant studies of clinical outcome. Because rs4149056 acts via its damaging effect on OATP1B1 protein function, it is assumed that other damaging SLCO1B1 gene variants may have similar clinical impact. However, we limit our recommendations to rs4149056 because the vast majority of pharmacokinetic and clinical outcome data have been published for this variant only.
At present, our recommended approach does not include other pharmacokinetic genes. The quality of evidence linking other pharmacokinetic candidates to myopathy is low to moderate, and additional study is needed. The evidence linking pharmacodynamic candidates to statin-induced myopathy is sparse. The mechanism underlying this adverse drug reaction (ADR) is only partly understood. Although some data show promise in terms of identifying pharmacodynamic variants, the clinical utility of typing these variants remains unclear.
SCOPE OF THE PROBLEM
The first statin to be approved by the US Food and Drug Administration (FDA) was lovastatin in 1987, followed by simvastatin in 1988, pravastatin in 1991, fluvastatin in 1994, atorvastatin in 1997, cerivastatin in 1998, rosuvastatin in 2003, and pitavastatin in 2009.1,2 Cerivastatin was withdrawn in 2001, because of its higher frequency of rhabdomyolysis. Seven of these agents remain in clinical use; and five are currently available as generic formulations (atorvastatin, fluvastatin, lovastatin, pravastatin, and simvastatin). In 2010, simvastatin was the most commonly prescribed generic statin formulation and the third most commonly prescribed drug in the United States (http://www.pharmacytimes.com/publications/issue/2011/May2011/Top-200-Drugs-of-2010).
In general, statins are safe. The most common statin-related ADR is skeletal muscle toxicity.3 Statin-related muscle problems include myalgias (pain), myopathy (pain with evidence of muscle degradation), and rhabdomyolysis (severe muscle damage with acute kidney injury). Frequency varies by definition, but, overall, statin-related myalgias are common, occurring in 1–5% of subjects exposed. In patients without arthritis, National Health and Nutrition Examination Survey data suggest a “number needed to harm” of 17 (ref. 4).
Clinicians often measure serum creatine kinase (CK) levels, as a rough proxy for severity, but the correlation between symptoms and CK level is incomplete. Therefore, the clinical interpretation of CK level is complex. Elevations less than threefold the upper limit of normal (ULN) are typically of little consequence. Conversely, clinicians often intervene (change dose or change drug) when CK levels exceed threefold the ULN. At present, the literature support three diagnostic strata: (i) incipient myopathy (CK above 3-fold the ULN, less than 10-fold the ULN), (ii) myopathy (CK above 10-fold the ULN, less than 50-fold the ULN), and (iii) rhabdomyolysis (CK above 50-fold the ULN).5,6 In the context of statin monotherapy, the frequency of myopathy is ∼1/1,000, and the frequency of rhabdomyolysis is ∼1/100,000. As discussed further below, frequency increases with dose.
CLINICAL DETERMINANTS OF RISK
Clinical factors known to influence a patient’s risk for developing statin-induced muscle toxicity include increased statin dose, advanced age, low body mass index, female gender, metabolic comorbidities (e.g., hypothyroidism), intense physical exercise, and Asian or African ancestry.3,7–10 Because polypharmacy is common in the elderly, the association with age is partly attributable to drug–drug interactions as well as to increases in chronic renal or hepatic disease.11
After studying the medical records of more than 250,000 patients, Schech et al. reported that statin users over age 65 years have four times the risk of hospitalization due to muscle toxicity than younger statin users (composite end point including myositis, myopathy, and/or rhabdomyolysis) (odds ratio (OR) 4.36; 95% confidence interval (CI) = 1.5–14.1).12 Although controversial, female gender may also increase risk because of smaller volumes of distribution.12,13 Although vigorous exercise often contributes to CK elevation in the context of statin exposure,14,15 the impact of physical activity can be difficult to interpret. Even in the absence of drug exposure or underlying muscle pathology, strenuous exercise can be associated with elevated serum CK levels exceeding 10-fold ULN.
Statin dose is the strongest independent predictor of risk. McClure and colleagues have reported an incidence rate for myotoxicity roughly sixfold higher in patients on high-dose statin therapy.16 Although the dose dependence of this relationship appears to be a class effect, a growing body of evidence suggests that the influence of dose may be greatest for simvas-tatin.17 Therefore, in 2011 the FDA announced an update to the simvastatin product label recommending against initiation of the 80-mg dose and cautioning against continuation of an 80-mg dose unless the patient has already tolerated it without muscle problems for more than 1 year.
DRUG-DRUG INTERACTIONS
In the context of statin monotherapy, myopathy rates are low.13 The frequency of this ADR increases with coadministration of medications altering the pharmacokinetic handling of statins. Between 1998 and 2001, more than 40 cases of muscle toxicity associated with the use of cerivastatin were found to be fatal. Many of these occurred within the context of use of gemfibrozil, a drug that strongly inhibits the cytochrome P450 (CYP) 2C8-catalyzed biotransformation of cerivastatin and also inhibits membrane transport and phase II conjugation of statins.18,19
The biological disposition of this class of drugs differs on a drug-by-drug basis. Some statins (atorvastatin, fluvastatin, lovastatin, and simvastatin) undergo extensive phase I oxidation; others (pitavastatin, pravastatin, and rosuvastatin) do not. CYP3A4 inhibitors (e.g., azole antifungals, protease inhibitors, amiodarone, and many calcium channel blockers) increase risk of myopathy for statins metabolized by CYP3A4/5 (e.g., simvastatin, lovastatin, and atorvastatin).20
Many statins also undergo additional modification through phase II conjugation by enzymes in the UDP-glucuronosyltransferase-1 (UGT1) family. This process can be altered by concomitant administration of fibric acids.21 Gemfibrozil, a fibric acid derivative, alters pharmacokinetic handling of a variety of statins. By inhibiting the glucuronidation and membrane transport of simvastatin hydroxy acids, gemfibrozil increases systemic exposure to active simvastatin acid,22 placing patients at increased risk for developing myopathy. Because of interactions such as these, the FDA label update also recommends reducing the dose of simvastatin in patients using concomitant medications known to alter its pharmacokinetics (details in Supplementary Table S1 online).
SLCO1B1
OATP1B1 (encoded by SLCO1B1) facilitates the hepatic uptake of statins as well as numerous endogenous compounds (e.g., bilirubin and 17-beta-glucuronosyl estradiol).23,24 Changes in the activity of this transporter (as occur during drug–drug interaction) can markedly increase the severity of statin-related muscle damage. For example, cyclosporine, a strong inhibitor of CYP3A4 and OATP1B1, increases the area under the curve for simvastatin acid three- to eightfold.24
Beyond acquired inhibition, genetic variability in SLCO1B1 also alters plasma concentration of statins. The overall pharmacokinetic profiles appear to be altered more for simvastatin than for any other drug in the class.24,25 Other transporters potentially influencing the distribution and tissue uptake of statins include OATP1B3 (encoded by SLCO1B3), OATP2B1 (encoded by SLCO2B1), OATP1A2 (encoded by SLCO1A2), and sodium-dependent taurocholate cotransporting polypeptide, NTCP.23,24
NOMENCLATURE
The SLCO1B1 gene locus occupies 109 kb on chromosome 12 (Chr 12p12.2). Although many single-nucleotide polymorphisms (SNPs) have been identified in SLCO1B1, only a few are known to have functional effects.24,26 The common c.521T>C variant rs4149056 produces a p.V174A substitution. The minor C allele at this locus has been associated with decreased transport function in vitro27,28 and decreased clearance for a number of drugs in vivo.29–31 Assignment of OAT1B1 phenotype has been summarized in Table 1, based on rs4149056. In general, this variant is present at a minor allele frequency between 5 and 20% in most populations (see Supplementary Tables S2–S4 online).
Table 1.
Assignment of likely SLCO1B1 phenotype based on genotype
| Genotype definitions | Diplotypes observed | Genotype at rs4149056 | Observed phenotype |
|---|---|---|---|
| An individual carrying two functional alleles | *1/*1 | TT | Homozygous wild-type or normal (55–88% of patients) high activity |
| An individual carrying one functional allele plus one reduced-function allele | *1/*5 (or *1/*15, *1/*16, or *1/*17) | TC | Heterozygous (11–36% of patients) intermediate activity |
| An individual carrying two reduced-function alleles | *5/*5 (or *5/*15, *5/*16, *5/*17, *15/*15, *15/*16, *15/*17, *16/*16, *16/*17, or *17/*17) | CC | Homozygous variant or mutant (0–6% of patients) low activity |
SLCO1B1 alleles are often named using *nomenclature, representing various SNPs alone or in combination (http://www.pharmgkb.org/gene/PA134865839#tabview=tab4&subtab=33). SLCO1B1 haplotypes are listed in Supplementary Table S2 online, including additional variants that are associated with low OAT1B1 protein expression or function. The minor C allele at rs4149056 is contained within SLCO1B1*5 (rs4149056 alone) as well as the *15, *16, and *17 haplotypes. As explained in detail below, although rs4149056 clearly influences the pharmacokinetic handling of simvastatin acid, the magnitude of this effect is similar for the *5, *15,*16, and *17 haplotypes.31
rs4149056 AND STATIN KINETICS
Simvastatin is administered as an inactive lactone, and hydrolyzed in vivo to the corresponding hydroxy acid by nonspecific carboxyesterases. Simvastatin acid is a potent inhibitor of HMG-CoA reductase. Simvastatin and lovastatin are the only statins requiring bioactivation. All other statins are administered directly as bio-active acid salts. For example, atorvastatin is administered in an active form (atorvastatin acid), and extensive first-pass metabolism by CYP3A4/5 yields ortho- and para-hydroxylated derivatives with similar activity to the parent compound.
SLCO1B1 polymorphisms clearly impact the pharmacokinetics of simvastatin and, to a lesser degree, the pharmacokinetics of other statins (Supplementary Table S5, online).32 Pasanen et al. determined that homozygous carriers of the C allele at rs4149056 (CC genotype) had much greater exposure to the active simvastatin acid (AUC0–12) than subjects homozygous for the ancestral T allele.31 In single-dose studies (Supplementary Figure S1 online), the observed plasma areas under the curve of active simvastatin acid, pitavastatin, atorvastatin, pravastatin, and rosuvastatin have been, respectively, 221, 162–191, 144, 57–130, and 62–117% higher in rs4149056 CC homozygotes than in rs4149056 TT homozygotes.
rs4149056 AND MYOPATHY RISK
In 2008, the SEARCH Collaborative Group (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) conducted a case–control study of simvastatin-induced myopathy using archived DNA from a randomized trial including more than 12,000 subjects who had received either low-dose simvastatin (20 mg daily) or high-dose simvastatin (80 mg daily) after myocardial infarction.17 During the course of the trial, 49 subjects in the high-dose arm developed myopathy (CK > 10-fold the ULN with pain). An additional 49 subjects in the same treatment arm developed “incipient” myopathy (CK > threefold the ULN and five times the patient’s baseline level). Of these combined incipient and definite myopathy cases, 85 underwent whole-genome scanning (with a platform containing 317,000 SNPs), and the results were compared with genome-wide data from 90 nonmyopathic controls (frequency-matched from within the same treatment arm). A single-nucleotide variant survived statistical correction for multiple testing: a base substitution in the SLCO1B1 gene. After genomic resequencing, the putative causative allele rs4149056 was retested for association in a subset of definite myopathy cases from the original cohort, revealing an OR for myopathy of 4.5 per copy of the minor C allele.17 Because ∼25% of the general population are carriers of this allele, and 1/100 patients taking high-dose simvastatin have an ADR, approximately 30 subjects would need to be genotyped to avoid one ADR.
The finding of an association between rs4149056 and statin-induced muscle toxicity has since been replicated in both a second independent trial and a practice-based longitudinal cohort. The Heart Protection Study enrolled more than 20,000 subjects with known vascular disease, or vascular risk factors and randomized each study participant to either 40 mg simvastatin daily or placebo.33,34 Twenty-four cases of myopathy (10 definite and 14 incipient) were identified from among the 10,269 participants receiving 40 mg of simvastatin, and 21 of these cases later underwent retrospective genotyping for rs4149056.17 In this validation cohort, the relative risk was 2.6 per copy of the minor C allele.17 Although both the SEARCH trial and the Heart Protection Study represent randomized controlled treatment trials, the effect size for rs4149056 was lower in the latter (i.e., at the 40-mg dose) than in SEARCH (i.e., at the 80-mg dose), underscoring the importance of dose.
Practice-based data suggest that the association between rs4149056 and muscle toxicity is stronger for simvastatin than for other drugs within the class. The C allele at rs4149056 has recently been shown to influence the rate of medication adherence for simvastatin.35 In the STRENGTH study, 509 hypercholesterolemic patients were randomized to simvastatin, atorvastatin, or pravastatin and followed for 16 weeks. The primary end point of the study was a composite of study drug discontinuation for any adverse effect, myalgia or muscle cramping, and/or elevated serum CK levels more than threefold the ULN. The overall effect size was striking for simvastatin (OR: 2.8, 95% CI: 1.3–6.0) but rather modest for atorvastatin (OR 1.6, 95% CI: 0.7–3.7). No significant association was observed for pravastatin (OR 1.0, 95% CI: 0.4–2.6). As in the STRENGTH study, other groups have reported a modest association between rs4149056 and atorvastatin intolerance based on adverse muscle symptoms,36 but the association between rs4149056 and laboratory-confirmed myopathy has been less compelling for atorvastatin.37
From the medical records of nearly 9,000 patients followed in an academic lipid clinic, Brunham and colleagues identified 25 laboratory-confirmed myopathy cases (frequency 0.26%).37 All 25 cases were genotyped for rs4149056, along with drug-exposed controls (frequency-matched 2:1), revealing an OR for myopathy of 2.3 per copy of the minor C allele at rs4149056. The OR was highest, 3.2 (95% CI 0.83–11.96), for subjects with the CC genotype exposed to simvastatin. No such relationship was observed using a similar number of atorvastatin cases (OR 1.06, 95% CI 0.22–4.80).37 These observations are consistent with reported differences in clearance (Supplementary Figure S1 online). To date, there is little evidence that rs4149056 genotype influences symptomatic intolerance or myopathy for pravastatin35 or rosuvastatin.36 Hence, the observed clinical association between rs4149056 and myopathy may not be a class effect.
AVAILABLE GENETIC-TEST OPTIONS
The commercially available clinical testing options listed in the Supplementary Material online and are freely available at http://www.PharmGKB.org. The rs4149056 SNP can be genotyped alone (e.g., PCR-based single SNP assay) or multiplexed on a variety of array-based platforms (e.g., with other pharmacokinetic variants). Array-based technologies are readily available within a Clinical Laboratory Improvement Amendments–approved environment for the Illumina VeraCode ADME array or the DMET Plus Affymetrix array. See Supplementary Material for details. This variant is also among the pharmacogenetic content available for participants in direct-to-consumer genomic offerings (e.g., 23andMe.com).
INCIDENTAL FINDINGS
Genetic variability in SLCO1B1 influences the hepatic uptake of other drugs (e.g., methotrexate)26 as well as important endogenous compounds (e.g., bilirubin)38 (Supplementary Material online).
GENE-BASED DOSING RECOMMENDATIONS
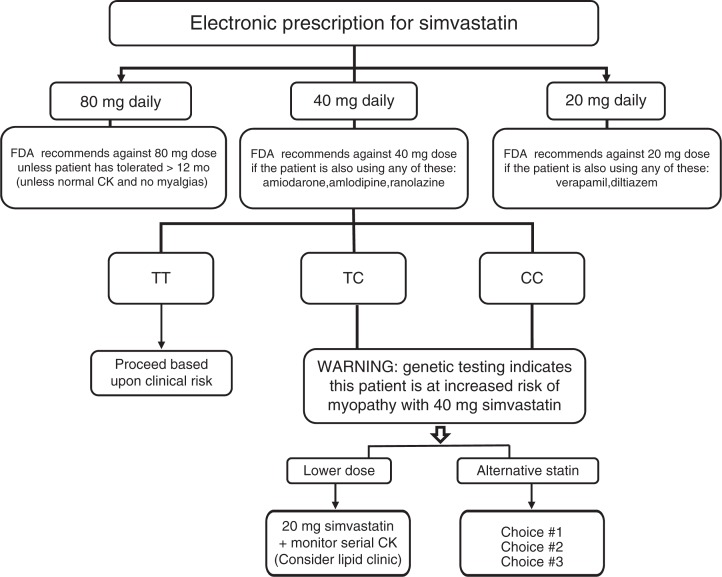
For simvastatin, the evidence linking myopathy to rs4149056 in SLCO1B1 is of high quality, and this association has been reproduced in randomized trials and clinical practice–based cohorts. Conversely, the association of rs4149056 with myopathy has been less compelling for other statins. We therefore focus this guideline on simvastatin. Our recommended approach (Table 2 and Figure 1) begins with product-label changes recently implemented by the FDA. (The agency’s recommendations are further summarized in Supplementary Table S1 online.) Decision-support tools are already being used to direct providers away from initiating the 80-mg simvastatin dose. At many of our institutions, SLCO1B1 genotype is further used to underscore this warning (i.e., myopathy risk for 80 mg of simvastatin) for subjects with a C allele at rs4149056 (see details in Supplementary Material).
Table 2.
Dosing recommendations for simvastatin, when rs4149056 genotype (or phenotype) is available
| Genotype at rs4149056 | Anticipated phenotype | Implications for simvastatin | Dosing recommendations for simvastatina | Classification of the recommendationb |
|---|---|---|---|---|
| TT | Normal activity | Normal myopathy risk | FDA recommends against 80 mg (unless already tolerated 12 months). Prescribe desired starting dose and adjust doses of simvastatin based on disease-specific guidelines. | Strong |
| TC | Intermediate activity | Intermediate myopathy risk | FDA recommends against 80 mg. Consider a lower dose; if suboptimal efficacy, consider an alternative statin. | Strong |
| CC | Low activity | High myopathy risk | FDA recommends against 80 mg. Prescribe a lower dose or consider an alternative statin; consider routine CK surveillance. | Strong |
CK, creatine kinase.
In all cases, the potential for drug–drug interaction should be evaluated prior to initiating a prescription.
See Supplementary Materials (text section entitled “Levels of Evidence”) online for additional details regarding the three-tiered system used to grade the quality of evidence. Further information is available within ref. 41.
Figure 1.
PREDICT (Pharmacogenomics Resource for Enhanced Decisions in Clinical Care and Treatment) decision support algorithm for simvastatin. Algorithm activated by provider selection of any simvastatin dose during the process of electronic prescribing at Vanderbilt University Medical Center. CK, creatine kinase.
At lower simvastatin doses (e.g., 40 mg daily), it is our position that this information should be used to warn providers about modest increases in myopathy risk for subjects with a C allele at rs4149056 (Figure 1). Under these circumstances, we also highlight the potential utility of routine CK surveillance (Table 2). If subjects with a C allele at rs4149056 do not achieve optimal low-density lipoprotein cholesterol–lowering efficacy with a lower dose of simvastatin, we recommend that the prescribing physician consider an alternative statin based on (i) potency differences, (ii) kinetic differences, (iii) comedications, (iv) hepatic function, (v) renal function, and (vi) relevant comorbidities.
POTENTIAL BENEFITS AND RISKS FOR THE PATIENT
Statins have a wide therapeutic index,39 and severe ADRs are therefore relatively uncommon. Nonetheless, even though the myopathy rate is low, the high prevalence of the clinical indication (hypercholesterolemia and cardiovascular disease) creates a situation in which the absolute number of ADRs is substantial. Furthermore, in the absence of severe myopathy, many patients still opt to discontinue statin therapy because of intermediate toxicity, and statin nonadherence thus has the potential to increase the burden of cardiovascular disease on our health-care infrastructure. It is with this perspective, a goal toward optimizing adherence, that we present the current guideline.
CAVEATS: APPROPRIATE USE AND/OR POTENTIAL MISUSE OF GENETIC TESTS
For the 40-mg simvastatin dose, the relative risk of myopathy is 2.6 per copy of the C allele at rs4149056. The risk is even higher for the 80-mg simvastatin dose (myopathy OR 4.5 for TC genotype, ∼20.0 for CC genotype). Nonetheless, simvastatin-related muscle toxicity can still occur in the absence of rs4149056. Thus, a TT genotype does not imply the absence of another potentially deleterious variant in SLCO1B1 or elsewhere. Furthermore, because rs4149056 can also be inherited in combination with other SLCO1B1 gene variants known to have protective effects, it should not be presumed that the C allele at rs4149056 confers risk with 100% certainty.
CURRENT STATE OF IMP LEMENTATION
Many academic medical centers have on-site array-based SNP genotyping available within a Clinical Laboratory Improvement Amendments–approved environment, and some have the capability to link this information directly to an electronic medical record.40 Few, however, integrate this information into the medical record preemptively; that is, before onset of the clinical indication requiring the drug. At present, there is no uniform approach to implementation of this information, but several institutions have begun providing automated decision support (examples provided in the Supplementary Material online).
Acknowledgments
The authors acknowledge assistance from Rita Burchett in the preparation of the manuscript.
Footnotes
CONFLICT OF INTEREST
The following National Institutes of Health grant support is acknowledged: R01DK080007 (R.A.W.), U01HL069757 (R.M.K.), U19HL065962 (D.M.R.), U01HG006378 (D.M.R.), K23HL086556 (R.M.C.-D.), and R24 GM61374 (L.G. and T.E.K.). M.W. is supported by the Swedish Research Council (Medicine 523-2008-5568), the Swedish Heart and Lung Foundation, EU FP7 (HEALTH-F2-2009-223062), and the Clinical Research Support (ALF) at Uppsala University. M.N. has grants from the Sigrid Jusélius Foundation (Helsinki, Finland), the Helsinki University Central Hospital (Helsinki, Finland), and the European Research Council (Brussels, Belgium). The funding organizations played no role in the writing of these guidelines. The other authors declared no conflict of interest.
Supplementary Material
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
References
- 1.Tobert JA. Lovastatin beyond the history of the HMG-CoA reductase inhibitors. Nat Rev Drug Discov. 2003;2:517–526. doi: 10.1038/nrd1112. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki J. Pitavastatin approved for treatment of primary hypercholesterolemia and combined dyslipidemia. Vasc Health Risk Manag. 2010;6:997–1005. doi: 10.2147/VHRM.S7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilke RA, et al. Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges. Nat Rev Drug Discov. 2007;6:904–916. doi: 10.1038/nrd2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buettner C, Rippberger MJ, Smith JK, Leveille SG, Davis RB, Mittleman MA. Statin use and musculoskeletal pain among adults with and without arthritis. Am J Med. 2012;125:176–182. doi: 10.1016/j.amjmed.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKenney JM, Davidson MH, Jacobson TA, Guyton JR. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C–94C. doi: 10.1016/j.amjcard.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Abd TT, Jacobson TA. Statin-induced myopathy a review and update. Expert Opin Drug Saf. 2011;10:373–387. doi: 10.1517/14740338.2011.540568. [DOI] [PubMed] [Google Scholar]
- 7.de Lemos JA, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 8.Chung JY, et al. Effect of OATP1B1 (SLCO1B1) variant alleles on the pharmacokinetics of pitavastatin in healthy volunteers. Clin Pharmacol Ther. 2005;78:342–350. doi: 10.1016/j.clpt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Lee E, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther. 2005;78:330–341. doi: 10.1016/j.clpt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Hippisley-Cox J, Coupland C. Individualising the risks of statins in men and women in England and Wales: population-based cohort study. Heart. 2010;96:939–947. doi: 10.1136/hrt.2010.199034. [DOI] [PubMed] [Google Scholar]
- 11.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 12.Schech S, et al. Risk factors for statin-associated rhabdomyolysis. Pharmacoepidemiol Drug Saf. 2007;16:352–358. doi: 10.1002/pds.1287. [DOI] [PubMed] [Google Scholar]
- 13.Graham DJ, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–2590. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- 14.Mareedu RK, et al. Use of an electronic medical record to characterize cases of intermediate statin-induced muscle toxicity. Prev Cardiol. 2009;12:88–94. doi: 10.1111/j.1751-7141.2009.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meador BM, Huey KA. Statin-associated myopathy and its exacerbation with exercise. Muscle Nerve. 2010;42:469–479. doi: 10.1002/mus.21817. [DOI] [PubMed] [Google Scholar]
- 16.McClure DL, Valuck RJ, Glanz M, Murphy JR, Hokanson JE. Statin and statin-fibrate use was significantly associated with increased myositis risk in a managed care population. J Clin Epidemiol. 2007;60:812–818. doi: 10.1016/j.jclinepi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Link E, et al. SEARCH Collaborative Group SLCO1B1 variants and statin-induced myopathy–a genomewide study. N Engl J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 18.Backman JT, Kyrklund C, Neuvonen M, Neuvonen PJ. Gemfibrozil greatly increases plasma concentrations of cerivastatin. Clin Pharmacol Ther. 2002;72:685–691. doi: 10.1067/mcp.2002.128469. [DOI] [PubMed] [Google Scholar]
- 19.Ronaldson KJ, O’Shea JM, Boyd IW. Risk factors for rhabdomyolysis with simvastatin and atorvastatin. Drug Saf. 2006;29:1061–1067. doi: 10.2165/00002018-200629110-00005. [DOI] [PubMed] [Google Scholar]
- 20.Rowan C, et al. Rhabdomyolysis reports show interaction between simvastatin and CYP3A4 inhibitors. Pharmacoepidemiol Drug Saf. 2009;18:301–309. doi: 10.1002/pds.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneck DW, et al. The effect of gemfibrozil on the pharmacokinetics of rosuvastatin. Clin Pharmacol Ther. 2004;75:455–463. doi: 10.1016/j.clpt.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Backman JT, Kyrklund C, Kivistö KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68:122–129. doi: 10.1067/mcp.2000.108507. [DOI] [PubMed] [Google Scholar]
- 23.Ho RH, et al. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130:1793–1806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 24.Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63:157–181. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- 25.Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158:693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsey LB, et al. Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition. Genome Res. 2012;22:1–8. doi: 10.1101/gr.129668.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem. 2001;276:35669–35675. doi: 10.1074/jbc.M103792200. [DOI] [PubMed] [Google Scholar]
- 28.Kameyama Y, Yamashita K, Kobayashi K, Hosokawa M, Chiba K. Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics. 2005;15:513–522. doi: 10.1097/01.fpc.0000170913.73780.5f. [DOI] [PubMed] [Google Scholar]
- 29.Niemi M, et al. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1) Pharmacogenetics. 2004;14:429–440. doi: 10.1097/01.fpc.0000114750.08559.32. [DOI] [PubMed] [Google Scholar]
- 30.Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2007;82:726–733. doi: 10.1038/sj.clpt.6100220. [DOI] [PubMed] [Google Scholar]
- 31.Pasanen MK, Neuvonen M, Neuvonen PJ, Niemi M. SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet Genomics. 2006;16:873–879. doi: 10.1097/01.fpc.0000230416.82349.90. [DOI] [PubMed] [Google Scholar]
- 32.Niemi M. Transporter pharmacogenetics and statin toxicity. Clin Pharmacol Ther. 2010;87:130–133. doi: 10.1038/clpt.2009.197. [DOI] [PubMed] [Google Scholar]
- 33.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 34.Heart Protection Study Collaborative Group. Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20 536 high-risk individuals: a randomised controlled trial. Lancet. 2011;378:2013–2020. doi: 10.1016/S0140-6736(11)61125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voora D, et al. The SLCO1B1*5 genetic variant is associated with statin-induced side effects. J Am Coll Cardiol. 2009;54:1609–1616. doi: 10.1016/j.jacc.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puccetti L, Ciani F, Auteri A. Genetic involvement in statins induced myopathy. Preliminary data from an observational case-control study. Atherosclerosis. 2010;211:28–29. doi: 10.1016/j.atherosclerosis.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 37.Brunham LR, et al. Differential effect of the rs4149056 variant in SLCO1B1 on myopathy associated with simvastatin and atorvastatin. Pharmacogenomics J. 2011 doi: 10.1038/tpj.2010.92. e-pub ahead of print 18 January 2011. [DOI] [PubMed] [Google Scholar]
- 38.van de Steeg E, et al. Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. J Clin Invest. 2012 doi: 10.1172/JCI59526. e-pub ahead of print 9 January 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilke RA, Reif DM, Moore JH. Combinatorial pharmacogenetics. Nat Rev Drug Discov. 2005;4:911–918. doi: 10.1038/nrd1874. [DOI] [PubMed] [Google Scholar]
- 40.Wilke RA, Dolan ME. Genetics and variable drug response. JAMA. 2011;306:306–307. doi: 10.1001/jama.2011.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Relling MV, Klein TE. Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther. 2011;89:464–467. doi: 10.1038/clpt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.