Abstract
Actin cytoskeleton dynamics lie at the heart of cell mechanosensing signaling. In fibroblast cells, two perinuclear acto-myosin structures, the actin cap and the transmembrane actin-associated nuclear (TAN) line, are components of a physical pathway transducing extracellular physical signals to changes in nuclear shape and movements. We recently demonstrated the existence of a previously uncharacterized third apical perinuclear actin organization in epithelial cells that forms during epithelial–mesenchymal transition (EMT) mediated by TGFβ (TGFβ). A common regulatory mechanism for these different perinuclear actin architectures has emerged with the identification of a novel family of actin bundling proteins, the Refilins. Here we provide updates on some characteristics of Refilin proteins, and we discuss potential function of the Refilins in cell mechanosensing signaling.
Keywords: EMT, Epithelial Mesenchymal Transition, FAM101A, FAM101B, Filamin, LINC complex, RefilinA, RefilinB, TAN lines, actin cap
Introduction
Recent discoveries have established that mechanical properties of the cellular environment such as rigidity, geometry and external stresses play an important role in determining the cellular function and fate. Mechanical properties influence cell shape and orientation, regulate cell proliferation and differentiation, and even govern the development and organization of tissues.1 In eukaryotic cells, physical connections between the nuclear envelope and the cytoskeleton provide a mechanism to transmit extracellular and cytoskeletal forces to the nucleus to coordinate nuclear migration and anchorage, organize chromatin and gene transcription, and aid meiotic chromosome pairing.2-5 In fibroblasts, two related perinuclear actin structure anchored to the nuclear membrane have been characterized, the actin cap and TAN lines. These structures may play important role in cell mechanosensing and mechanotransduction, i.e., the ability of cells to sense and respond to changes in substrate rigidity and externally applied forces, respectively.6 Actin cap has been first described on fibroblast grown on adhesive micropatterned surfaces to control the overall cell shape.7,8 TAN lines were observed in NIH3T3 fibroblasts migrating into scratch wounds, where they are involved in nuclear movement and nuclear positioning.4,9 Actin cap and TAN lines are made of actomyosin fibers and actin bundles connected to the outer nuclear membrane through the LINC (linker of the nucleoskeleton and cytoskeleton) or SUN-KASH complex.4,7-9 To progress our understanding of the functions of perinuclear actin cytoskeleton in mechanosensing and mechanotransduction, an important step is to identify proteins that participate in their organization. In this context, we have identified a new family of short- lived actin regulatory proteins, Refilins (RefilinA and RefilinB), that organizes perinuclear actin cytoskeleton during cell differentiation switches. In cells, Refilins interact with the actin-binding proteins Filamins (FLNA, FLNB, FLNC) to exert their function.10,11 Filamins are a family of actin binding and scaffolding proteins that integrate cellular architectural and signaling parameters and are essential for normal fetal development.12-15 Here we aim to update the properties of Refilins to further highlight their implications in cell mechanosensing signaling.
The Refilins
Refilins (RefilinA and RefilinB) belong to the FAM101 gene family highly conserved in mammals, xenopus and zebrafish, but without homologs in the genomes of Drosophila or Caenorhabditis elegans. In situ hybridizing studies in early mouse embryo show that both RefilinA (cfm2) and RefilinB (cfm1) transcript levels are high at the time of brain development and in peripheral tissues when, concomitantly, proliferation and epithelial-mesenchymal transition (EMT) are observed.16
Refilins are small hydrophilic proteins enriched in proline with a secondary structure predicted to be composed of β sheet and coiled domains lacking α helices. Such remarkable primary and secondary structures cause recombinant protein to aggregate in bacteria, yeast, or following in vitro translation. Refilins are stabilized upon interaction with their target protein FLNA. Hence, Refilins can be purified for in vitro experiments only when complexed with FLNA.10 Refilins homodimerize through their N-termini. The N-termini of Refilins are also characterized by a conserved ubiquitin-independent PEST degradation signal (Pestfind score: 7.8 and 10.2 for RefilinA and B, respectively) which overlaps with a DSG(X)2–4S motif that targets protein for rapid proteasome-dependant degradation17 (Fig. 1).
Figure 1. Sequence analysis of the N-terminus of rat RefilinA and rat RefilinB. (A) Sequence alignment of the N-terminus of rat RefilinA (residues 1–99) and RefilinB (residues 1–112) proteins shows conserved regions with homologous (purple) or similar (blue) residues. A 15 amino-acid N-terminal sequence, harbouring a DSG(X)2–4S motif is fully conserved between the two proteins (blue rectangle), whereas a specific adjacent sequence is only found in RefilinB (red rectangle). (B) Sequence comparison of the DSG(X)2–4S motif within β-catenin, IκBα, EMI1, Snail, ATF4, NFκB1/p105, Refilin and Cdc25A proteins.
To our knowledge, only inhibitor of NFκB (IκB) proteins have both a PEST sequence and a DSG(X)2–4S motif that are responsible for the degradation of the free form and the NFκB-bound form of IκB, respectively.18 By treating transfected human astrocytoma cell line U373 MG with cycloheximide, the half-life of a recombinant RefilinA-Myc fusion protein was found less than 1 h (unpublished data). Remarkably, RefilinB-Myc showed a longer half-life than RefilinA (~7 h). We have now identified a sequence specifically present in RefilinB, contiguous to the PEST sequence (red rectangle in Fig. 1), that functions as an auto-inhibitory domain for degradation (unpublished data). Similar regulation has been observed for unbound IκBα.18 Studies are now in progress to determine the complex pathways associated with Refilins’ stability and degradation. The relative short half-life of Refilins suggests that in physiological situations Refilins may function in dynamic actin cytoskeleton regulations. Accordingly, endogenous Refilins localize primarily on perinuclear actin structures that transiently form in cells that respond to external stimulations by change in shape, migration and differentiation. These include rat neural progenitors stimulated to migrate out of neurospheres upon adhesion on poly-l-lysine substrate for glial differentiation (unpublished data), and murine epithelial NMuMG cells undergoing EMT in response to TGFβ stimulation.10
Refilin/FLNA-dependent actin bundling promotes cell-type-dependent actin cytoskeleton phenotypes
In vitro, Refilins bind to FLNA and convert FLNA from an actin branching protein into one that bundles.10 Among the impressive number of FLNA interacting proteins so far identified,15 Refilins are the only ones that promote such a switch in FLNA properties. The mechanistic of this process has been studied in detail.10 Taking into account that both Refilin and FLNA are capable of homodimerization, a model where Refilin dimer functions as a zipper to promote multimolecular FLNA complex to bundle F-actin has been proposed.10,11
In vivo, the short half-life of Refilin makes the protein hardly detectable by conventional immunological tools. Hence, princeps studies aimed at understanding the Refilin/FLNA complex functions used ectopic Refilin protein expression system. Actin bundling mediated by the Refilin/FLNA complex can convey very different actin cytoskeleton phenotypes depending on the cell type used for investigation. A straightforward illustration is provided by comparison of Refilin-dependent phenotypes in human astrocytoma U373MG cell line cells and in its derivative clone U373A selected for its tumorogenic properties by xenograft (Fig. 2). In the parental U373MG cells, ectopic expression of Refilin-GFP promotes a relocalization of FLNA primarily onto basal actin stress fibers that show increased F-actin staining by phalloidin (Fig. 2A). In U373A cells, ectopic expression of Refilins promotes the formation of robust apical perinuclear parallel actin bundles forming a cap and star-shaped actin superstructures that co-immunostain with FLNA (Fig. 2B). Formation of perinuclear actin bundles decreased nuclear height.10 These results clearly demonstrate that the Refilin/FLNA-mediated actin cytoskeleton re-organization, particularly the actin perinuclear and star shaped superstructures, is tightly dependent on the cell physiology and probably on other signaling molecules.
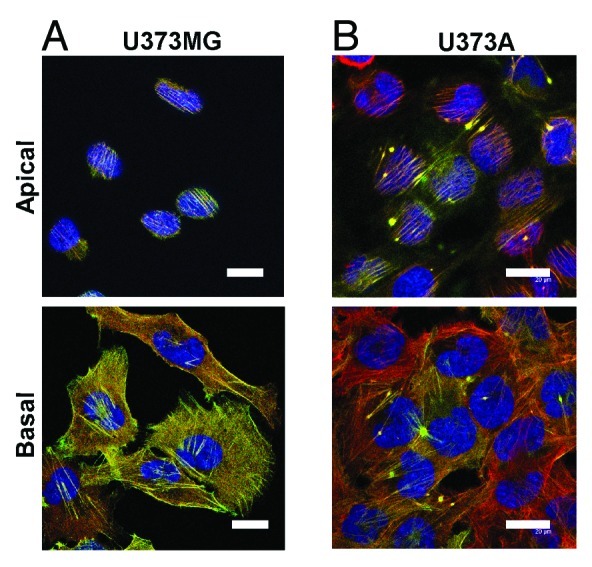
Figure 2. Localization of the Refilin/FLNA complex in U373 MG and U373A cells. U373MG cells and their tumorogenic U373A countepart were infected with recombinant adenovirus expressing RefilinB-GFP. Cells were fixed in paraformaldehyde and immunostained with antibodies against FLNA from USBiological (red). Bar = 20 µm.
Actin cap mediated by Refilin is regulated by cell geometry
The dependence of the Refilin/FLNA complex on cell signaling to promote formation of actin perinuclear structures has gained further support from studies in cells expressing endogenous RefilinB. In mouse NIH3T3 fibroblasts grown on plastic dishes, endogenously expressed RefilinB is stabilized when cell reach confluence. In RefilinB positive cells, RefilinB immunoreactivity co-localizes with FLNA on perinuclear actin cap.10 In NHI 3T3 cells stably transfected with shRNA against RefilinB, cells showed a disrupted actin cap implying RefilinB in actin cap dynamic regulations.10 We confirmed a correlation between RefilinB expression and perinuclear actin cap formation in the human retina-derived RPE1 cells grown to confluence (Fig. 3). As previously noticed with NHI 3T3 cells, not all cells express RefilinB and only those with perinuclear actin cap do show significant RefilinB immunoreactivity.
Figure 3. RefilinB localizes on apical actin cytoskeleton in confluent RPE1 cells. Confluente RPE1 cells were fixed with methanol and double immunostained with guinea pig anti RefilinB antibody (Red) and mouse anti-FLNA antibody (green). Bar = 20 µm.
In sparsely growing NIH3T3 or RPE1 cells, endogenous RefilinB concentration is below the detection limit and only few cell do show actin cap. Ectopic expression of RefilinB-GFP in sparsely growing cells was not sufficient to promote the formation of actin cap. In sparsely growing NIH3T3 the half-live of recombinant RefilinB-GFP, assessed trough cycloheximide chase and protein gel blot analyses, was much lower that in confluente cells and the protein showed diffuse cytoplasmic localization or localized with FLNA on basal actin stress fibers (unpublished data). Taken together these results indicate that RefilinB stabilization and actin cap formation are co-regulated. They also suggest that RefilinB stabilization as well as formation of the actin cap are linked to the cell responses to physical constraints imposed by confluence.
To confirm the dependence of actin perinuclear structure formation on cell physical status, we used U373A that spontaneously form actin cap in response to RefilinB-GFP expression, and analyzed the presence of the actin cap depending on the substrata and physical constraints imposed to the cells (Fig. 4). Perinuclear actin organization decorated with Refilin-GFP and FLNA are clearly visible with U373A cells grown on fibronectin (Fig. 4A, left column). As previously reported for fibroblast cells, the actin cap orients along the direction of the main axis of the cell.7 When U373A cells are plated onto collagen 1, cells adopt a more flattened morphology and the actin cap does not form, although the Refilin/FLNA complex still decorated the basal actin stress fibers (Fig. 4A, right column). Moreover, when individual U373A cells were grown on adhesive fibronectin-coated micropatterns that prevent cells from adopting an elongated morphology, the Refilin/FLNA-dependent actin perinuclear structure did not form (Fig. 4B). In these cells, the Refilin/FLNA complex localized onto basal stress fibers, tension fibers and actin superstructures. These observations suggest that actin perinuclear structure formation mediated by the Refilin/FLNA complex is dependent on adhesion signaling and cellular geometry, and support a function for the actin cap as a mediator of cellular mechanosensing and mechanotransduction.8
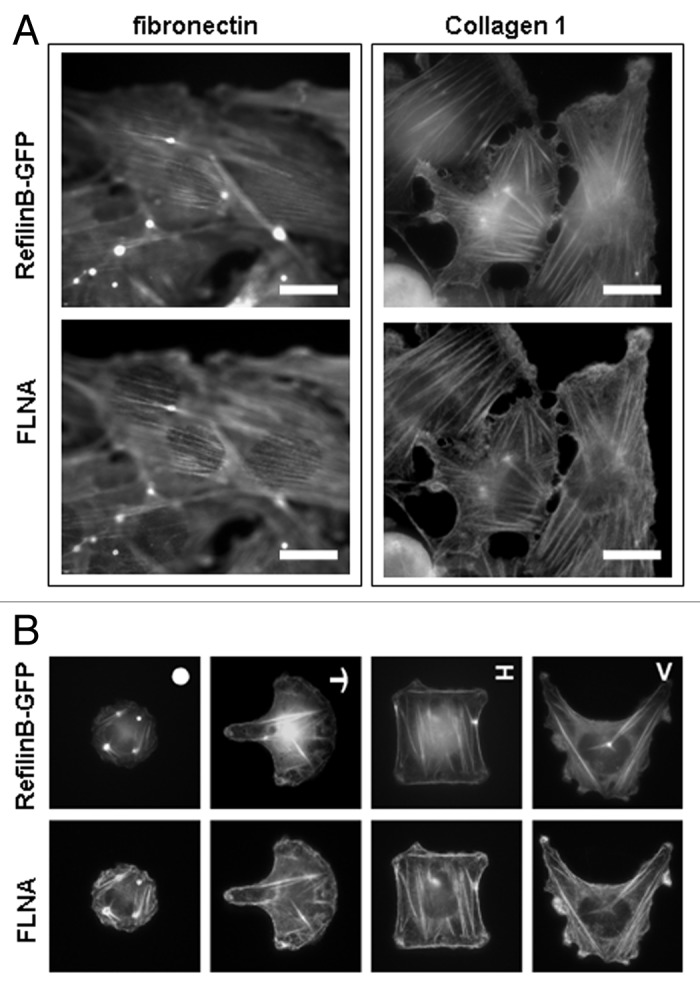
Figure 4. Parameters regulating formation of actin perinuclear strucuture by Refilins. (A) U373A cells plated on fibronectin (left column) or collagen 1 (right column) substrates were infected with RefilinB-GFP expressing adenoviral particles, fixed in 4% paraformaldehyde and immunostained with a mouse antibody against FLNA protein. Bar = 20 µm. (B) Single U373A cells plated on fibronectin micropatterns of different shapes (upper right of top image) were infected with RefilinB-GFP expressing adenoviral particles, fixed in 4% paraformaldehyde and immunostained with a mouse antibody against FLNA protein. RefilinB-GFP (top) and FLNA (bottom) co-localize on basal fibers but lack perinuclear staining. Similar results were obtained with microprinted fibronectin islands with the sizes of 700 µm2, 1100 µm2 and 1600 µm2.
Refilin-dependent actin bundling in EMT
EMT is a biological process that allows the transformation of a coherent layer of epithelial cells into a group of individual and migrating mesenchymal cells.19,20 A number of distinct molecular processes are engaged in order to initiate EMT. These include stabilization of master transcription factors, such as Snail and ATF4, which are normally degraded by the phosphorylation of serines of the DSG(X)2–4S motif, which leads to proteosomal degradation.21,22 Refilins share this consensus phosphorylation DSG(X)2–4S motif (Fig. 1B). Like Snail and ATF4, RefilinB protein level also specifically increases in epithelial cells stimulated with TGFβ suggesting that RefilinB is a downstream effector in the TGFβ pathway linked to EMT.10 It is now important to evaluate if TGFβ treatment regulates RefilinB stability through dephosphorylation of the serines of the DSG(X)2–4S motif and to identify the kinases responsible for its degradation in the absence of EMT.
It is admitted that EMT processes start with the disruption of cell-cell junctions associated with the functional loss of cell adhesion molecules and cortical actin cytoskeleton reorganization into basal stress fibers and focal adhesions.20,23 We add a level of complexity to this process by showing that RefilinB controls a novel apical actin-based network organization during early steps of EMT that is independent from basal stress fibers.10
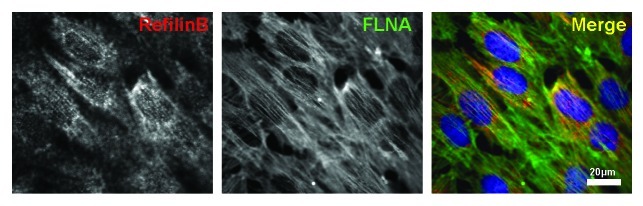
Confocal microscopy observations show that, in polarized epithelial NMuMG cells, TGFβ stimulation promotes rapid concentration of RefilinB protein at the cell-cell contact sites at the apical surface, where it co-localizes with FLNA (Fig. 5). The Refilin/FLNA complex then persists on actin bundles that organize above the nuclei. Interestingly, during TGFβ treatment, basal actin stress fibers will also form but show no Refilin/FLNA staining.10 This suggests that the Refilin/FLNA complex contributes to re-organization of the cortical actin cytoskeleton to promote the formation of the perinuclear actin bundles, and most likely to initiate its formation.
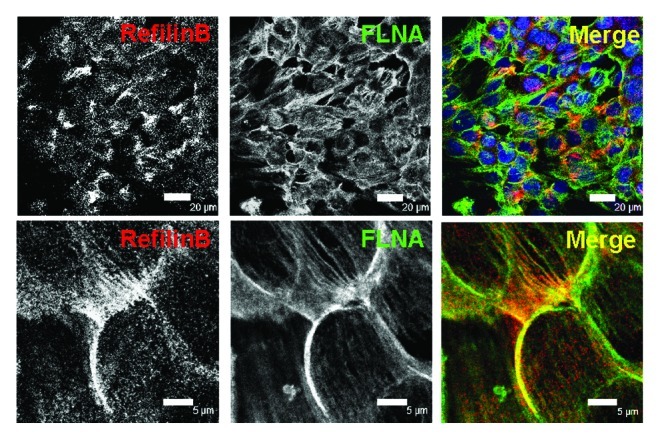
Figure 5. RefilinB and FLNA localize on apical actin cytoskeleton in NMuMG cells during epithelial-mesenchymal transition. NMuMG epithelial cells were cultured in the presence of TGFβ (2 ng/ml) for 12 h. Cells were then fixed with methanol and double immunostained with guinea pig anti RefilinB antibody (red) and mouse anti FilaminA antibody (green). Low (upper; Bar = 20 µm) and high (lower; Bar = 5 µm) magnification confocal microscopy images are shown.
In NMuMG cells stably expressing RefilinB shRNA, the perinuclear actin bundles did not form whereas basal actin stress fibers re-organization was left unchanged, confirming that the RefilinB/FLNA complex functions as a specific regulator of apical perinuclear actin dynamics during EMT.10 Additional studies are now required to understand the functional properties of this new perinuclear actin organization during EMT and to draw comparison with actin cap and TAN line. It is of prime interest to determine if the perinuclear actin network that forms in the initial steps of EMT is anchored to the nuclear membrane for force transduction to control nuclear movement, nuclear shape and/or transcription associated with EMT.
Conclusions
It is now well established that physical and mechanical cues regulate cell behavior, and that actin cytoskeletal tension behave as a bioregulator in mechanotransduction.1,5 It is thus likely that specific actin regulatory proteins are involved in mechanosensing and mechanoresponses. We have shown that Refilin form a novel family of actin bundling proteins whose activities depend on mechanical cues instructed by the substrata and by the cell geometry. An important characteristic of the proteins is their relatively short half-life in cells. This parameter is imperative to consider when analyzing Refilin-dependent cell phenotypes and functions. In confluent NIH3T3 fibroblasts and in RPE1 cells, endogenous RefilinB is stabilized when cells organize their actin cap suggesting that RefilinB is an actin cap-specific protein. The capacity of Refilin to promote actin cap has allowed for the first time to stimulate formation of actin caps in cells. In U373A cells transfected with Refilin plasmid, the ectopically expressed protein localizes with FLNA on both apical perinuclear actin cap and basal actin bundles, but only perinuclear actin cap was dependent on the substrata and the cell geometry (Fig. 4). These findings support a model where Refilin function in mechanosensing signaling and mechanotransduction through interaction with FLNA to organize/stabilize the actin cap. They open new perspectives toward understanding mechanisms of the actin cap formation, regulation and functions.
Identifying the role of Refilin/FLNA complex in organizing a perinuclear actin network during changes in cellular phenotype, such as those observed during EMT, should also have implications for understanding the process of intracellular mechanotransduction linked with cell differentiation especially during embryogenesis, when these regulations are prevalent.1,5 Dysregulation of perinuclear actin dynamics in EMT programs could lead to developmental defects or pathologies such as those observed in FLNA-null mouse embryos and syndromes associated with Filamin mutations. Loss of function of FLNA in mice or FLNA mutations in human are associated with severe organogenesis defects during embryonic development and adulthood.24-26 The mechanisms involved in EMT are also integrated with oncogenic pathways regulating tumor growth and metastasis.19,23,27 Unravelling the implication of the actin cap in mechanotransduction linked with the control of the EMT switch, could have a broad impact in understanding dysregulation associated with tumorogenic processes.
Acknowledgments
This work was supported by grants from Institut National du Cancer INCA-PL 114 and Association contre le Cancer ARC-SFI20101201517 (J.B.), and a fellowship from Ligue Nationale contre le Cancer (O.G.)
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/18246
References
- 1.Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137:1407–20. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starr DA. Cell biology. Nuclei get TAN lines. Science. 2010;329:909–10. doi: 10.1126/science.1194562. [DOI] [PubMed] [Google Scholar]
- 3.Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ Res. 2008;102:1307–18. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luxton GW, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science. 2010;329:956–9. doi: 10.1126/science.1189072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De R, Zemel A, Safran SA. Theoretical concepts and models of cellular mechanosensing. Methods Cell Biol. 2010;98:143–75. doi: 10.1016/S0091-679X(10)98007-2. [DOI] [PubMed] [Google Scholar]
- 6.Vogel V, Sheetz MP. Cell fate regulation by coupling mechanical cycles to biochemical signaling pathways. Curr Opin Cell Biol. 2009;21:38–46. doi: 10.1016/j.ceb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khatau SB, Hale CM, Stewart-Hutchinson PJ, Patel MS, Stewart CL, Searson PC, et al. A perinuclear actin cap regulates nuclear shape. Proc Natl Acad Sci USA. 2009;106:19017–22. doi: 10.1073/pnas.0908686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khatau SB, Kim DH, Hale CM, Bloom RJ, Wirtz D. The perinuclear actin cap in health and disease. Nucleus. 2010;1:337–42. doi: 10.4161/nucl.1.4.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luxton GG, Gomes ER, Folker ES, Worman HJ, Gundersen GG. TAN lines: A novel nuclear envelope structure involved in nuclear positioning. Nucleus. 2011;2:173–81. doi: 10.4161/nucl.2.3.16243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gay O, Gilquin B, Nakamura F, Jenkins ZA, McCartney R, Krakow D, et al. RefilinB (FAM101B) targets FilaminA to organize perinuclear actin networks and regulates nuclear shape. Proc Natl Acad Sci USA. 2011;108:11464–9. doi: 10.1073/pnas.1104211108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gay O, Nakamura F, Baudier J. Refilin holds the cap. Commun Integr Biol. 2011;4 doi: 10.4161/cib.17911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, et al. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–45. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- 13.Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol. 2004;6:1034–8. doi: 10.1038/ncb1104-1034. [DOI] [PubMed] [Google Scholar]
- 14.Popowicz GM, Schleicher M, Noegel AA, Holak TA. Filamins: promiscuous organizers of the cytoskeleton. Trends Biochem Sci. 2006;31:411–9. doi: 10.1016/j.tibs.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Zhou AX, Hartwig JH, Akyurek LM. Filamins in cell signaling, transcription and organ development. Trends Cell Biol 2010. [DOI] [PubMed]
- 16.Hirano M, Murata T, Furushima K, Kiyonari H, Nakamura M, Suda Y, et al. cfm is a novel gene uniquely expressed in developing forebrain and midbrain, but its null mutant exhibits no obvious phenotype. Gene Expr Patterns. 2005;5:439–44. doi: 10.1016/j.modgep.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Busino L, Donzelli M, Chiesa M, Guardavaccaro D, Ganoth D, Dorrello NV, et al. Degradation of Cdc25A by beta-TrCP during S phase and in response to DNA damage. Nature. 2003;426:87–91. doi: 10.1038/nature02082. [DOI] [PubMed] [Google Scholar]
- 18.Mathes E, Wang L, Komives E, Ghosh G. Flexible regions within I{kappa}B{alpha} create the ubiquitin-independent degradation signal. J Biol Chem. 2010;285:32927–36. doi: 10.1074/jbc.M110.107326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–72. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, et al. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–40. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T, Osumi N, Wakamatsu Y. Stabilization of ATF4 protein is required for the regulation of epithelial-mesenchymal transition of the avian neural crest. Dev Biol. 2010;344:658–68. doi: 10.1016/j.ydbio.2010.05.492. [DOI] [PubMed] [Google Scholar]
- 23.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Feng Y, Chen MH, Moskowitz IP, Mendonza AM, Vidali L, Nakamura F, et al. Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci USA. 2006;103:19836–41. doi: 10.1073/pnas.0609628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart AW, Morgan JE, Schneider J, West K, McKie L, Bhattacharya S, et al. Cardiac malformations and midline skeletal defects in mice lacking filamin A. Hum Mol Genet. 2006;15:2457–67. doi: 10.1093/hmg/ddl168. [DOI] [PubMed] [Google Scholar]
- 26.Robertson SP, Twigg SR, Sutherland-Smith AJ, Biancalana V, Gorlin RJ, Horn D, et al. Localized mutations in the gene encoding the cytoskeletal protein filamin A cause diverse malformations in humans. Nat Genet. 2003;33:487–91. doi: 10.1038/ng1119. [DOI] [PubMed] [Google Scholar]
- 27.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]