Abstract
AMP-activated protein kinase (AMPK), a phylogenetically conserved serine/threonine protein kinase, is a major regulator of cellular and whole-body energy homeostasis that coordinates metabolic pathways in order to balance nutrient supply with energy demand. It is now recognized that pharmacological activation of AMPK improves blood glucose homeostasis, lipid profile and blood pressure in insulin-resistant rodents. Indeed, AMPK activation mimics the beneficial effects of physical activity or those of calorie restriction by acting on multiple cellular targets. In addition it is now demonstrated that AMPK is one of the probable (albeit indirect) targets of major antidiabetic drugs including, the biguanides (metformin) and thiazolidinediones, as well as of insulin sensitizing adipokines (e.g., adiponectin). Taken together, such findings highlight the logic underlying the concept of targeting the AMPK pathway for the treatment of metabolic syndrome and type 2 diabetes.
Keywords: Anti-Obesity Agents; pharmacology; therapeutic use; Caloric Restriction; Cyclic AMP-Dependent Protein Kinases; antagonists & inhibitors; metabolism; Endothelium, Vascular; drug effects; physiology; Enzyme Inhibitors; pharmacology; therapeutic use; Exercise; physiology; Humans; Hypoglycemic Agents; pharmacology; therapeutic use; Hypolipidemic Agents; pharmacology; therapeutic use; Myocardial Ischemia; drug therapy
Keywords: AMP-activated protein kinase, energy balance, diabetes, obesity, therapeutic strategy
1. Introduction
Obesity (defined as a body-mass index (BMI) of >30 kg.m−2) and the metabolic syndrome are related conditions that can be considered as precursors of T2D and increases the risk of developing this disease by >20-fold (Willett et al. 1999). Although these conditions clearly have a genetic component, as indicated by the high prevalence in certain ethnic group, the rapid increase in the prevalence of these conditions in populations throughout the world suggests the contribution of environmental factors. A widely accepted explanation for the increasing prevalence of these conditions lays on the frequent consumption of processed foods with high energy and low fibers content and the reduction in physical exercise due to sedentary lifestyle in modern urban environment. Thus, obesity arises due to an imbalance between energy intake and energy expenditure where caloric excess accumulates preferentially as lipids in adipose tissue but also into muscle and liver. Disruption of energy balance has led to an increased prevalence of T2D and related comorbidities such as coronary heart disease, heart failure, hypertension and renal failure (Wing et al. 2001).
Type 2 diabetes (T2D) has a high prevalence worldwide and its treatment produces considerable costs for the health budgets. Prevention and management of T2D has become a major public health challenge around the world. Diabetes is defined by a fasting plasma glucose higher than 7 mM (Alberti and Zimmet 1998). T2D is characterized by altered lipid and glucose metabolism (fasting or postprandial hyperglycaemia, dyslipidemia) as a consequence of combined insulin resistance in skeletal muscle, liver and adipose tissue and relative defects of insulin secretion by β-cells that may arise due to an imbalance between energy intake and expenditure (Saltiel and Kahn 2001). Insulin is the primary anabolic hormone that stimulates uptake and storage of fuel substrates, while inhibiting substrate production in peripheral tissues (Kahn et al. 2006). It lowers blood glucose levels by facilitating glucose uptake, mainly into skeletal muscle and fat tissue, and by inhibiting endogenous glucose production in the liver. Insulin resistance occurs when a normal dose of insulin is unable to elicit its metabolic responses. Peripheral insulin resistance is associated with lipid partitioning in specific compartments, i.e. muscle and liver, more than with obesity per se (DeFronzo and Tripathy 2009; Unger 1995). In the natural history of type 2 diabetes, pancreatic β-cells initially compensate for insulin resistance by increasing insulin secretion, but with time, progressive β-cell failure leads to insulin deficiency, and hyperglycaemia ensues (Fonseca 2009).
Lifestyle intervention is now recognized as the first-line strategy for the management of T2D and remains important for optimization of metabolic control. This is supported by observational studies and clinical trials comparing the respective effects of diet, drugs or exercise, in persons at high risk for type 2 diabetes (Knowler et al. 2002; Pan et al. 1997; Tuomilehto et al. 2001). The Diabetes Prevention Program (DPP) Research Group conducted a large, randomized clinical trial involving adults in the United States who were at high risk for the development of this disease (Knowler et al. 2002). In this study, the lifestyle intervention was particularly effective (and more than an oral hypoglycaemic drug) to prevent the onset of diabetes. In clinical practice, when lifestyle modification fails to achieve or sustain adequate glycaemic control, insulin or oral anti-diabetic agents are typically used to manage the disease (Nathan et al. 2009). Treatment options with oral agents are quite diverse, including metformin, thiazolidinediones (TZDs), α-glucosidase inhibitors, sulphonylureas, DPP-4 inhibitors, and GLP-1 analogs. The currently available classes of oral agents differ in mechanism and duration of action, and the degree to which they lower blood glucose and their side-effect profile (including hypoglycaemia, weight gain, dema, fractures, lactic acidosis, and gastrointestinal intolerance). Because it is recognized that T2D is a progressive disease worsening with time, all available drugs can be used alone or in varied associations.
There is a pressing need to develop new therapeutic strategies to prevent and treat T2D. Exciting recent developments have shown that AMP-activated protein kinase (AMPK), a phylogenetically conserved serine/threonine protein kinase, acts as an integrator of regulatory signals monitoring systemic and cellular energy balance, thus providing the emerging concept, as first suggested by Winder and Hardie in 1999 (Winder and Hardie 1999), that AMPK is an attractive therapeutic target for intervention in many conditions of disordered energy balance including T2D and insulin resistance.
2. Rational for a pharmacological management of T2D by targeting AMPK
Physical activity is an important determinant to prevent and control T2D. Current guidelines recommend practical, regular and moderate regimens of physical activity. The multiple metabolic adaptations that occur in response to physical activity can improve glycaemic control for individuals with T2D or delay the onset of the disease. Indeed, it is now recognized that beneficial effects of physical activity are still maintained in insulin resistant populations. This suggests that some metabolic actions of exercise (as increase in muscular glucose uptake) are dependent of specific intracellular pathways that bypass signalling altered by insulin resistance. In consequence, any drug inducing favourable changes similar to those of physical exercise on whole body metabolism are attractive candidates for treatment and prevention of obesity, metabolic syndrome and T2D. Interestingly, it is now well established that muscle contraction is a prototypical AMPK activator (Hayashi et al. 1998). Thus, it is expected that part of the effect of physical activity in preventing the development of metabolic disorders related to a sedentary lifestyle is due to activation of AMPK. Indeed, it has been documented that pharmacological AMPK activation may recapitulate some of the exercise-induced short-term adaptations and is likely to mediate beneficial effects of exercise on insulin sensitivity and glucose transport in skeletal muscle (Bergeron et al. 1999; Fisher et al. 2002). In addition, pharmacological AMPK activation resulted in long-term adaptation similar to those induced by endurance exercise training with the induction of genes linked to oxidative metabolism and enhanced running endurance (Narkar et al. 2008).
In the Diabetes Prevention Program (DPP) the incidence of diabetes was reduced by 58% with a low-calorie, low-fat diet, as compared with placebo after 3 years of follow-up (Knowler et al. 2002). The beneficial effect of calorie restriction in reducing T2D incidence was confirmed by other clinical studies (Pan et al. 1997; Tuomilehto et al. 2001). In overweight and obese humans, calorie restriction improves glucose tolerance, lipid profile and insulin action and reduces mortality associated with T2D (Hammer et al. 2008; Jazet et al. 2008; Larson-Meyer et al. 2006; Weiss et al. 2006). In order to produce a metabolic profile similar to those of calorie restriction in diabetic patients, there is an increased interest in developing pharmacological agents acting as “calorie-restriction” mimetics. Such agents could provide the beneficial metabolic, hormonal and physiological effects of calorie restriction without altering dietary intake or experiencing any potential adverse consequences of excessive restriction. To this purpose, phytochemicals mimicking the effects of calorie restriction (polyphenols) were recently identified as potent activators for AMPK in vitro and in vivo (Baur et al. 2006; Collins et al. 2007; Zang et al. 2006).
Additionally, it is now recognized that a dysfunction in AMPK signalling pathway might have sustained, deleterious effects at the systemic levels and might contribute to the events that lead to the metabolic syndrome. It is interesting to note that there is a strong correlation between low activation state of AMPK with metabolic disorders associated with insulin resistance, obesity and sedentary activities (Lee et al. 2005a; Lee et al. 2005b; Luo et al. 2005; Martin et al. 2006). Recent studies showed that AMPK is likely to be under both endocrine and autocrine control in rodents. Thus, in addition to exercise and starvation, AMPK is activated by the fat-cell-derived hormones adiponectin and leptin (Minokoshi et al. 2002; Tomas et al. 2002; Yamauchi et al. 2002) and interleukin-6 (IL-6) (Kelly et al. 2004). Conversely, AMPK activity is suppressed in muscle and liver by sustained hyperglycaemia, in liver by re-feeding after starvation (Assifi et al. 2005) and by increases in the plasma concentration of others adipocyte-derived hormones, resistin (Banerjee et al. 2004) and tumor necrosis factor-α (TNF-α) (Steinberg et al. 2006). In addition to its role in the periphery, AMPK also regulates energy intake and body weight by mediating opposing effects of anorexigenic and orexigenic signals in the hypothalamus (Andersson et al. 2004; Kim et al. 2004; Kola et al. 2005; Minokoshi et al. 2004). In addition, many therapies that are useful in treating the metabolic syndrome and associated disorders in humans, including TZDs (Fryer et al. 2002; Saha et al. 2004), metformin (Zhou et al. 2001), calorie deprivation and exercise, have been shown to activate AMPK system. Lastly, the development of transgenic and knockout (KO) mouse models (see below) have made possible to better understand the physiological role of AMPK and confirm that disruption of AMPK pathway in various tissues induces various phenotypes mimicking the metabolic syndrome observed in humans.
Taken together the physiological functions of AMPK and the suspected role of AMPK in metabolic disorders, activation of AMPK pathway appears as a promising tool to prevent and/or to treat metabolic disorders.
3. Structure and regulation of AMPK
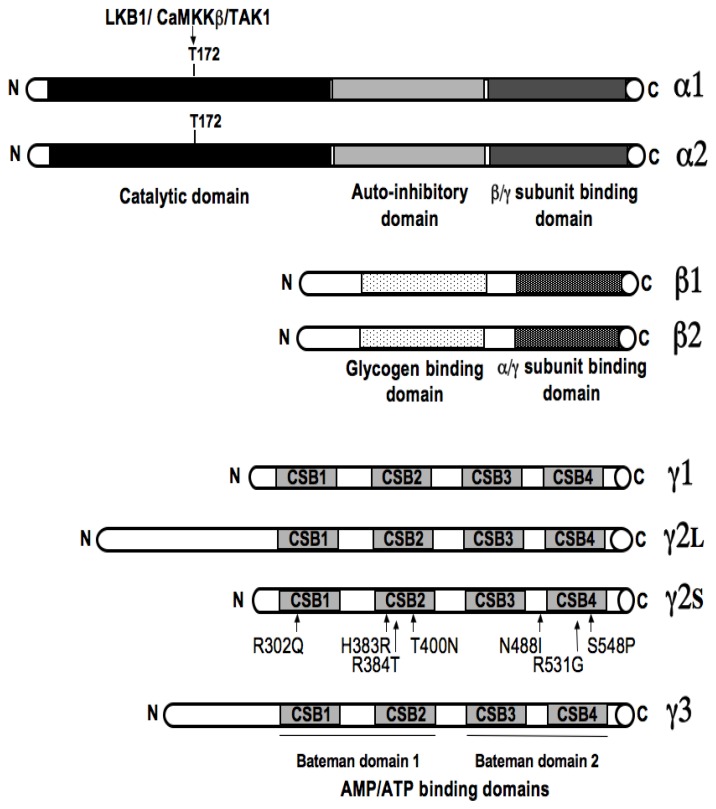
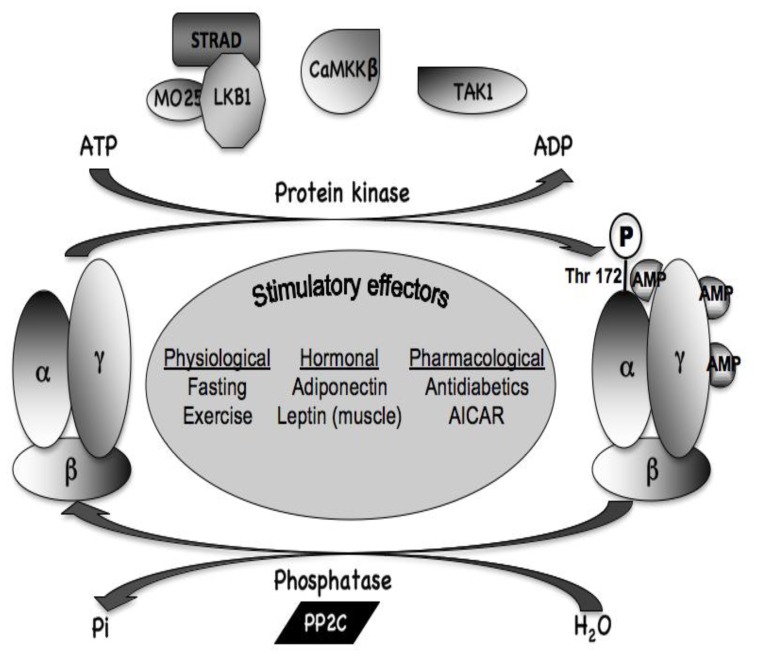
AMPK is a major regulator of cellular and whole-body energy homeostasis that coordinates metabolic pathways in order to balance nutrient supply with energy demand. Activation of AMPK switches off ATP-consuming anabolic pathways and switches on ATP-producing catabolic pathways (Viollet et al. 2003). This would typically occur when AMPK is activated as a result of energy deprivation linked to alterations of the intracellular AMP/ATP ratio (e.g., hypoxia, glucose deprivation, muscle contraction), changes in calcium concentration as well as the action of various adipocytokines. AMPK is composed of three different subunits α, β and γ appearing in several isoforms with different action properties (Figure 1). The α-subunit contains the catalytic site whereas regulatory β- and γ-subunits are important to maintain the stability of the heterotrimeric complex. The β subunit contains a central region that allows AMPK complex to bind glycogen. The γ subunit contains four tandem repeats known as cystathionine β-synthase (CBS) motifs that bind together two molecules of AMP or ATP in a mutually exclusive manner. Binding of AMP (on γ subunit) activates AMPK via a complex mechanism involving direct allosteric activation and phosphorylation of α subunit on Thr172 by upstream kinases as the protein kinase LKB1 (a tumour suppressor whose germline mutations in humans are the cause of Peutz-Jeghers syndrome), the CaMKKβ (calmodulin-dependent protein kinase kinase β) and TAK1 (mammalian transforming growth factor β-activated kinase) (Figure 2). Although it was originally proposed that AMP binding promoted AMPK phosphorylation by upstream kinases, recent work suggested entirely inhibition of dephosphorylation of Thr 172 to be critical (Sanders et al. 2007; Suter et al. 2006) (Figure 2).
Figure 1. Domain organization of the catalyticα and regulatory β and γ subunits of AMPK.
Each AMPK molecule is comprised of a α catalytic (α1 and α2) and regulatory β (β1 and β2) and γ (γ1, γ2 and γ3) subunits. The catalytic α subunit is phosphorylated at threonine 172 by upstream kinases (LKB1, CaMKKβ and TAK1), leading to enzyme activation. The β subunit contains a glycogen-binding domain. The γ subunit contains 4 nucleotide-binding modules (CBS domains) capable of cooperative binding to 2 molecules of either ATP or AMP. Mutations in the human γ2 subunit gene (PRKAG2) causing cardiac hypertrophy associated with abnormal glycogen accumulation and conduction system disease are shown.
Figure 2. Regulation of AMPK by upstream kinases and phosphatases.
The major upstream kinase is a complex between the tumour suppressor kinase LKB1 and two accessory subunits, STRAD and MO25 which appears to be constitutively active. The CaMKKβ could also phosphorylate Thr-172 and activate AMPK following a rise in cytosolic Ca2+. A third potential upstream kinase is TAK1 but its physiological significance is uncertain. Thr-172 phosphorylation is removed by PP2C phosphatase. Physiological, hormonal and pharmacological stimulatory effectors of AMPK complex are listed.
4. Beneficial metabolic effects of targeting AMPK pathway
4.1. Mimicking the beneficial effects of physical exercise
It has been confirmed in large scale epidemiological and interventional studies that regular physical activity is of great benefit for the metabolic control of subjects with metabolic syndrome or impaired glucose tolerance or T2D (Knowler et al. 2002; Pan et al. 1997; Tuomilehto et al. 2001). Although appropriate diet and exercise regimes should therefore be the first choice of treatment and prevention of type 2 diabetes, in some patients such management is not appropriate for other medical reasons, or when compliance is difficult because of social factors or poor motivation. In these cases, drugs that act on the signalling pathways involved in physical activity are attractive candidates for treatment and prevention. It is now clearly demonstrated that AMPK is activated by physical training in an intensity-dependent manner both in humans and in rodents (Steinberg and Kemp 2009). AMPK activation during muscle contraction is a physiological adaptation in front of increased energy demand and ATP turnover. It has been demonstrated that AMPK activation may recapitulate some of the exercise-induced adaptations and is likely to mediate beneficial effects of exercise not only on insulin sensitivity and glucose transport in skeletal muscle (Fisher et al. 2002) but also for additional metabolic benefits coming from AMPK activation by exercise in liver and in adipose tissue (Park et al. 2002). Conversely, it has also been demonstrated that disruption of muscular AMPK signalling can be a key factor in the pathophysiology of metabolic disorders. Indeed, reduction of muscular AMPK activity exacerbates the development of insulin resistance and glucose intolerance during high-fat feeding, disturbs muscle energy balance during exercise (as indicated by a reduced muscular ATP content during muscle contraction) and abolishes mitochondrial biogenesis (Fujii et al. 2008; Jorgensen et al. 2005; Zong et al. 2002).
As a proof of concept, studies with AMPK activators in animal models of T2D have provided promising results. The first evidence came from in vivo treatment with the pharmacological compound AICAR (5-amino-imidazole-4-carboxamide-1βD-ribofuranoside, metabolized to ZMP which is an analog of AMP) of various animal models of insulin resistance, causing improvement in most, if not all, of the metabolic disturbances of these animals (Bergeron et al. 2001a; Buhl et al. 2002; Iglesias et al. 2002; Pold et al. 2005; Song et al. 2002). In addition, long-term AICAR administration prevents the development of hyperglycaemia in Zucker diabetic fatty (ZDF) rats, improves peripheral insulin sensitivity in skeletal muscle and delays β-cell dysfunction associated with type 2 diabetes (Pold et al. 2005). AICAR increases muscle glucose uptake concomitantly with glucose transporter 4 (GLUT4) translocation to the plasma membrane in insulin-resistant animal models and in humans (Koistinen et al. 2003; Kurth-Kraczek et al. 1999; Merrill et al. 1997). Interestingly, AMPK-induced glucose transport occurs through a mechanism distinct from that utilized by the classical insulin signalling pathway because it is not blocked by inhibitors of phosphatidylinositol 3-kinase, and also because the effects of insulin and AMPK activators are additive (Hayashi et al. 1998). This metabolic improvement can be also explained partly by increased expression of specific muscle proteins mimicking some of the effects of exercise training following chronic pharmacological activation of AMPK in vivo. Thus, AICAR or chronic intake of the creatine analogue β-guanadinopropionic acid (β-GPA, which competitively inhibits creatine uptake and lowers ATP content) (Bergeron et al. 2001b) in rodent increases muscle expression of glucose transporter GLUT4 and hexokinase II, an effect partly mediated by the transcriptional coactivator peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) (Holmes et al. 1999; Michael et al. 2001). It has been proposed that the development of skeletal muscle insulin resistance may be partly linked to decreased mitochondrial density (Petersen et al. 2003). Interestingly, chronic activation of AMPK with AICAR or β-GPA increases mitochondrial content and expression of mitochondrial proteins, leading to a mitochondrial biogenesis (Bergeron et al. 2001b; Winder et al. 2000; Zong et al. 2002). All of these data argue for AMPK as a key factor for the metabolic adaptation of skeletal muscle to physical exercise. Supporting this, the effects of chronic activation of AMPK mimicking physical activity on gene expression and mitochondrial biogenesis are abolished in AMPKα2 knock-out (KO) and mAMPK-KD (transgenic mice overexpressing a kinase-dead AMPKα2 mutant [K45R mutation] in skeletal muscle) mice (Holmes et al. 2004; Jorgensen et al. 2005; Zong et al. 2002). Increased mitochondrial biogenesis after chronic activation of AMPK is partly explained by increased expression of nuclear respiratory factor-1 and -2 (which are critical regulators of genes encoding electron chain complexes) (Bergeron et al. 2001b). Another critical factor for mitochondrial biogenesis is the inducible coactivator of nuclear receptors, PGC-1α. Regulation of PGC-1α by AMPK is complex. First, it has been demonstrated that AMPK directly phosphorylates and activates PGC-1α (Jager et al. 2007). In addition, activated PGC-1α in turn increased the expression of PGC-1α and of mitochondrial oxidative genes (cytochrome c, uncoupling protein 1). Interestingly, PGC-1α activity and expression are reduced in type 2 diabetes in humans (Mootha et al. 2003). Thus, AMPK activators could be used in order to reverse this defect. Additionally, activation of AMPK in response to physical exercise has been also observed in extra-muscular tissues such as liver and adipose tissue (Park et al. 2002) and might accounted for additional metabolic benefits. Physical training increases circulating adiponectin and mRNA expression of its receptors in muscle, which may mediate the improvement of insulin resistance and the metabolic syndrome in response to exercise by activation of AMPK.
Lastly, increase in blood supply is critical for physiological adaptation during physical activity. Vasodilatation is a vital mechanism of systemic blood flow regulation that occurs during periods of increased energy demand. Thus, because AMPK plays a central role in the adaptation to metabolic stress, it is tempting to speculate that AMPK could be involved in the regulation of metabolic vasomotion. It is well known that moderate-intensity exercise increases nitric oxide synthase (NOS) activity (Roberts et al. 1999). Interestingly, it has been recently reported that mAMPK-KD mice are unable to increase total NOS activity during moderate-intensity exercise and may cause an impairment in muscle blood flow (Lee-Young et al. 2009). This finding is supported by the close association between AMPK and nNOSμ phosphorylation following moderate-intensity exercise (Chen et al. 2000; Stephens et al. 2002) and reduced expression of nNOSμ in mAMPK-KD mice (Lee-Young et al. 2009). This indicates how changes in tissue metabolism can direct blood flow according to demand. In addition, the lower skeletal muscle capillarization in mAMPK-KD mice might also contribute to the reduced blood flow during exercise (Zwetsloot et al. 2008). Nitric oxide (NO) plays a fundamental role in vascular homeostasis and it has been suggested that impaired NO efflux from contracting mAMPK-KD mice suppressed exercise-induced vascular relaxation (Lee-Young et al. 2009). Furthermore, it has suggested that AMPK activation is in part regulated by endogenous NO in a positive feedback mechanism, such that increase NO activates AMPK, which further augments NOS activity and NO production (Lira et al. 2007; Zhang et al. 2008). Accordingly, the exercise-induced increase in AMPK signalling was ablated in skeletal muscle of eNOS KO mice (Lee-Young et al.). Therefore, AMPK-eNOS interaction might play an important role in the adaptation processes during exercise in order to maintain cellular energy levels by amending vascular function.
4.2. Mimicking the beneficial effects of calorie/dietary restriction
Excessive calorie intake increases the risk of developing chronic disease such as obesity, metabolic syndrome, T2D, systemic low grade inflammation, cardiovascular event and premature mortality. Conversely, calorie restriction improves glucose tolerance and insulin action and reduces mortality linked to type 2 diabetes and cardiovascular diseases (Hammer et al. 2008; Jazet et al. 2008; Larson-Meyer et al. 2006; Weiss et al. 2006). Because it is difficult to maintain long-term calorie restriction in modern society, there has been an increased interest in developing pharmacological agents that act as “calorie-restriction” mimetics. Among them, plant-derived polyphenolic compounds, such as resveratrol (which is present in grapes, peanuts, and several other plants) were first recognized as mimicking the effects of calorie restriction in lower eukaryote (Howitz et al. 2003). Additionally, resveratrol administration prevents the deleterious effects of high calorie intake on insulin resistance and metabolic syndrome components in rodents (Baur et al. 2006; Lagouge et al. 2006; Milne et al. 2007; Sun et al. 2007; Zang et al. 2006). Resveratrol has been described as a potent activator of the NAD(+)-dependent deacetylases sirtuins including SIRT1, one of the seven mammalian sirtuin genes (Howitz et al. 2003). However, recent findings indicate that resveratrol is not direct SIRT1 activator (Pacholec et al. 2010). Resveratrol, like other polyphenols, also activates AMPK (Baur et al. 2006; Collins et al. 2007; Zang et al. 2006). Acute activation of AMPK by resveratrol appears to be independent of SIRT1 (Dasgupta and Milbrandt 2007), probably through changes in AMP/ATP ratio as resveratrol inhibits the mitochondrial F1 ATPase (Gledhill et al. 2007). Furthermore, resveratrol increased the NAD(+)/NADH ratio in an AMPK-dependent manner, which may explain how it may activate SIRT1 indirectly (Canto et al. 2009; Um et al. 2010). SIRT1 has been suggested to prime the organism in order to reduce the deleterious effects of insulin resistance on energy balance and metabolic homeostasis. Thus, SIRT1 activation increases hepatic insulin sensitivity, decreases whole-body energy requirements (Banks et al. 2008; Sun et al. 2007) promotes adaptation of insulin secretion during insulin resistance development (Bordone et al. 2006; Moynihan et al. 2005) and coordinates lipid mobilization and utilization (Picard et al. 2004). The knowledge of SIRT1 action at the molecular level has been more delineated by using chronic treatments with resveratrol and it has been suggested that SIRT1 promotes LKB1-dependent AMPK stimulation through the direct deacetylation and activation of LKB1 (Hou et al. 2008; Lan et al. 2008). Thus, polyphenols as resveratrol are now recognized as compounds with potential great interest to improve and/or delay or prevent metabolic disorders linked to western life style by activating the complementary metabolic stress sensors SIRT1 and AMPK (Canto et al. 2009). Accordingly, it has been recently established that AMPK acts as the prime initial sensor for fasting-induced adaptations in skeletal muscle and that SIRT1 downstream signalling was blunted in the absence of AMPK (Canto et al. 2010). In addition, recent studies demonstrated that resveratrol failed to increase the metabolic rate, insulin sensitivity, glucose tolerance, mitochondrial biogenesis and physical endurance in the absence of either AMPKα1 or AMPKα2 (Um et al. 2010).
4.3. Mimicking the beneficial effects of hypoglycaemic agents
4.3.1. AMPK action in liver
T2D is the result of an imbalance between glucose production and glucose uptake by peripheral tissues. Elevated hepatic glucose production is a major cause of fasting hyperglycaemia in diabetic subjects (Saltiel and Kahn 2001). From various effectors, AMPK signalling is a key factor that control hepatic glucose production. Indeed, systemic infusion of AICAR in normal and insulin-resistant obese rats leads to the inhibition of hepatic glucose production (Bergeron et al. 2001a). Additionally, short-term hepatic expression of a constitutively active form of the α2 catalytic subunit (AMPKα2-CA) leads to mild hypoglycaemia in normal mice (Foretz et al. 2005; Viana et al. 2006) and abolishes hyperglycaemia in diabetic ob/ob and streptozotocin-induced diabetic mice (Foretz et al. 2005; Viana et al. 2006) by inhibition of gluconeogenesis (Foretz et al. 2005; Lochhead et al. 2000; Viana et al. 2006). This effect is achieved at least to a large extent via the regulation of a transcriptional coactivator, transducer of regulated CREB activity 2 (TORC2) (Koo et al. 2005) which is known to mediate CREB-dependent transcription of PGC1α and its subsequent gluconeogenic targets PEPCK and G6Pase genes. AMPK activation causes TORC2 phosphorylation and sequesters the coactivator in the cytoplasm, thus blunting the expression of the gluconeogenic program (Koo et al. 2005). Control of hepatic glucose production by activated AMPK is also demonstrated in resistin KO mice and in adiponectin treated rodents (Banerjee et al. 2004; Yamauchi et al. 2002) suggesting that hepatic AMPK is specifically a target of both adipocytokines, the former acting as an AMPK inhibitor, the latter as an activator. This was also demonstrated by lack of systemic adiponectin infusion effect on hepatic glucose production in liver-specific AMPKα2 KO mice (Andreelli et al. 2006).
4.3.2. AMPK action in skeletal muscle
After a meal or during the euglycaemic hyperinsulinemic clamp, both situations with high circulating levels of insulin, skeletal muscle is the main site for glucose disposal in the body. This is sustained by the insulin-dependent translocation of glucose transporter GLUT4 from intracellular vesicles to the cell surface, which is impaired in T2D patients. As described above, it has been clearly demonstrated that muscular AMPK activation, either by exercise or by AICAR, stimulates muscle glucose uptake. Interestingly, even if AMPK and insulin acts through phosphorylation of downstream target of Akt (Akt substrate of 160kDa, AS160) (Dreyer et al. 2008), AMPK-dependent and insulin-dependent GLUT4 translocation are distinct pathways (Treebak et al. 2007). Additionally, exercise-induced muscular AMPK activation and AS160 phosphorylation are both reduced in obese non-diabetic and obese type 2 diabetes subjects (Musi et al. 2001) but maintained in lean type 2 diabetes patients (Bruce et al. 2005) suggesting that dysregulation of muscular AMPK is more dependent of obesity than of hyperglycaemia. Discovery of muscular AMPK activators in order to mimic regular physical activity metabolic effects is an important challenge. It was first demonstrated that some adipokines stimulate glucose transport in skeletal muscle in an AMPK-dependent manner. Indeed, leptin is known to stimulate glucose uptake in peripheral tissue (Kamohara et al. 1997; Minokoshi et al. 1999) by stimulating AMPKα2 phosphorylation and activation in skeletal muscle (Minokoshi et al. 2002). Adiponectin, another adipokine, has also been shown to increase glucose transport in both lean and obese skeletal muscle, although the effect was less significant in obese skeletal muscle (Bruce et al. 2005). It has also recently been recognized that IL-6 (also called “myokine” (Febbraio and Pedersen 2005)) is released acutely from the skeletal muscle during prolonged exercise, activates AMPK and improves peripheral glucose uptake and insulin sensitivity at the whole body level (Glund et al. 2007). In contrast, chronic exposure of IL-6 (as observed in obesity) promotes insulin resistance both in vitro and in vivo (Nieto-Vazquez et al. 2008). The dual effect of IL-6 on insulin sensitivity probably explains some conflicting results recently discussed in more details elsewhere (Nieto-Vazquez et al. 2008). Importantly, it has been also suggested that AICAR, in addition to activating AMPK, suppresses chronic IL-6 release by an AMPK independent mechanism in insulin-resistant models (Glund et al. 2009). This strongly suggested that AMPK activators can act at a multi-tissular level in order to restore metabolic inter-organs cooperation.
Interestingly, available hypoglycaemic drugs as metformin and TZDs have been reported to activate AMPK (Fryer et al. 2002; Zhou et al. 2001). Even if it was postulated that blood glucose lowering effects of metformin are mediated by AMPK activation from studies of mice that are deficient in the upstream AMPK kinase, LKB1, in the liver (Shaw et al. 2005) recent studies have shown that LKB1 phosphorylates and activates at least 12 AMPK-related kinases. These data raised the question whether the glucose-lowering function of LKB1 is mediated by AMPK-related kinases rather than AMPK itself.
Because circulating levels of adiponectin are decreased in individuals with obesity and insulin resistance, adiponectin replacement in humans may be a promising approach. It has been demonstrated that full-length adiponectin activates AMPK in the liver, while globular adiponectin did so both in muscle and the liver (Yamauchi et al. 2002). Blocking AMPK activation by the use of a dominant negative mutant inhibited the action of full length adiponectin on glucose hepatic production (Yamauchi et al. 2002). In addition, lack of action of adiponectin on hepatic glucose production when AMPKα2 catalytic subunit is missing, strongly supports the concept that adiponectin effect is strictly dependent on AMPK (Andreelli et al. 2006). Awaiting adiponectin analogs development, alternative ways to restore adiponectin effects have been suggested recently. Improved metabolic disorders following TZDs administration are in part mediated through adiponectin-dependent activation of AMPK since activation of AMPK by rosiglitazone treatment is diminished in adiponectin KO mice (Nawrocki et al. 2006). TZDs can markedly enhance the expression and secretion of adiponectin in vitro and in vivo through the activation of its promoter and also antagonize the suppressive effect of TNF-α on the production of adiponectin (Maeda et al. 2001). Interestingly, in human adipose tissue, AICAR has been shown to increase the expression of adiponectin (Lihn et al. 2004; Sell et al. 2006) while no change in serum adiponectin concentration or adipocyte adiponectin content was found in type 2 diabetic patients treated with metformin (Phillips et al. 2003).
4.3.3. AMPK action in β-cells
β-cell failure is a strong determinant in the pathogenesis of type 2 diabetes. This defect inexorably aggravates with time as demonstrated in prospective clinical studies (1995). According to the glucolipotoxicity hypothesis (Prentki et al. 2002) chronic high glucose dramatically influences β-cell metabolism. Indeed, it has been observed in high glucose condition an increase of cytosolic fatty acyl-CoA partitioning toward potentially toxic cellular products (e.g., diacylglycerol, ceramide and lipid peroxides) leading to impaired insulin secretory response to glucose and ultimately apoptosis (Donath et al. 2005). Indeed, decrease in β-cell mass is likely to play a role in the pathogenesis of human type 2 diabetes (Butler et al. 2003) as it does in rodent models of the disease (Kaiser et al. 2003; Rhodes 2005).
Pathways regulating β-cell turnover are also implicated in β-cell insulin secretory function. In consequence, decrease in β-cell mass is not dissociable from an intrinsic secretory defect. Because AMPK is important for the balance of intracellular energy homeostasis it was interesting to analyze to what extend AMPK regulates β-cell function/survival. AICAR dose dependently improves β-cell function probably by reducing apoptosis induced by prolonged hyperglycaemia (Nyblom et al. 2008). In addition to AICAR, β-cell AMPK activation (by metformin, TZDs or adenovirus-mediated over-expression of AMPKα1-CA) favours fatty acid β-oxidation and prevents glucolipotoxicity-induced insulin secretory dysfunction in β-cells (El-Assaad et al. 2003; Eto et al. 2002; Higa et al. 1999; Lupi et al. 2002). In contrast, the role of AMPK in the control of β-cell death survival remains controversial (Kefas et al. 2003a; Kefas et al. 2003b; Kim et al. 2007; Riboulet-Chavey et al. 2008; Richards et al. 2005).
Beyond a potential role of AMPK for long-term regulation of β-cell function and survival, AMPK may also regulate acutely insulin secretion. Thus, AMPK activity is rapidly decreased when glucose levels increased over the physiological range suggesting that AMPK could be one of the regulator of insulin secretion through its capacity to sense intracellular energy (da Silva Xavier et al. 2003; Leclerc et al. 2004). Interestingly, activation of AMPK by AICAR, berberine, metformin and TZDs or by overexpression of AMPKα1-CA markedly reduced glucose-stimulated insulin secretion in β-cell lines and in rodent and human islets (Eto et al. 2002; Leclerc et al. 2004; Wang et al. 2007; Zhou et al. 2008). Similarly, activation of AMPK selectively in β-cells in AMPKα1-CA transgenic mice decreased glucose-stimulated insulin secretion (Sun et al. 2010). This could be considered as a deleterious effect of AMPK activation. But it is hypothesized that pharmacological activation of AMPK and its subsequent decrease in insulin secretion, could be appropriate in insulin resistant conditions characterized by high insulin levels. Indeed, it has been suggested that reduction of the pathological hyperinsulinemia is potentially a mechanism to protect β-cell mass. Consistent with this assumption, systemic AICAR infusion in prediabetic Zucker fatty rats prevented the development of hyperglycaemia and preserved β-cell mass (Pold et al. 2005).
Taken together, these data suggest that AMPK is an emergent factor that could protect by different ways β-cell function and β-cell mass from the deleterious effects of glucolipotoxicity.
4.4. Mimicking the beneficial effects of hypolipidemic agents
Dyslipidemia of both insulin resistance and type 2 diabetes is a recognized risk factor for cardiovascular disease. Diabetic dyslipidemia is a cluster of potentially atherogenic lipid and lipoprotein abnormalities that are metabolically interrelated. Activated AMPK inhibits cholesterol and fatty acid synthesis. Thus, AMPK suppresses expression of lipogenesis-associated genes such as fatty acid synthase, pyruvate kinase and acetyl CoA carboxylase (ACC) (Foretz et al. 2005; Foretz et al. 1998; Leclerc et al. 1998; Leclerc et al. 2001; Woods et al. 2000), and 3-Hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase). HMG-CoA reductase activity is inhibited by phosphorylation of Ser-872 by AMPK (Clarke and Hardie 1990). Adiponectin activates AMPK and inhibits cholesterol synthesis in vivo suggesting that AMPK is a key regulator of cholesterol pathways (Ouchi et al. 2001). Inhibition of ACC by AMPK leads to a drop in malonyl-CoA content and a subsequent decrease in fatty acid synthesis and increase in fatty acid oxidation, thus reducing excessive storage of triglycerides. Consistently, overexpression of AMPKα2-CA in the liver or treatment with AICAR, metformin or A769662 (a small-molecule AMPK activator) in lean and obese rodents decreases plasma triglyceride levels, concomitantly with an increase in plasma β-hydroxybutyrate levels, suggesting elevated hepatic lipid oxidation (Bergeron et al. 2001a; Cool et al. 2006; Foretz et al. 2005; Zhou et al. 2001). Conversely, liver-specific AMPKα2 deletion leads to increased plasma triglyceride levels and enhanced hepatic lipogenesis (Andreelli et al. 2006). These data emphasize the critical role for AMPK in the control of hepatic lipid deposition via decreased lipogenesis and increased lipid oxidation, thus improving lipid profile in type 2 diabetes.
It is well documented that changes in adipose tissue mass are frequently associated with alterations in insulin sensitivity (Eckel et al. 2005; Katsuki et al. 2003). AMPK evidenced recently as a regulator of fat mass. Indeed, activation of AMPK in white adipocytes is concomitant with a decreased expression of genes coding lipogenic enzyme (Orci et al. 2004) and leads to a decreased lipogenic flux and a decreased triglyceride synthesis (Daval et al. 2005; Sullivan et al. 1994). In white adipocytes, AMPK activation using AICAR or overexpression of AMPK-CA has been shown to inhibit β-adrenergic-induced lipolysis (Corton et al. 1995; Sullivan et al. 1994). Hormone sensitive lipase (HSL), one of the key protein responsible for the lipolytic activity, is activated by PKA phosphorylation at serines 563, 659 and 660 (Anthonsen et al. 1998). AMPK reduces this activation through phosphorylation at serine 565 (Garton et al. 1989; Garton and Yeaman 1990). This effect has been demonstrated both in white adipocytes and in skeletal muscle in both resting and contracting conditions (Muoio et al. 1999; Smith et al. 2005; Watt et al. 2006). Thus, inhibition of HSL by AMPK represents a mechanism to limit this recycling and ensure that the rate at which fatty acids are released by lipolysis does not exceed the rate at which they could be disposed of by export or by internal oxidation.
Beyond its hypolipidemic properties, AMPK system can be also a regulator of ectopic lipids metabolism. Depot of lipids in tissue is a hallmark defect in metabolic syndrome in humans. According to this lipotoxicity hypothesis, insulin resistance develops when excess lipids are deposited in insulin-sensitive cell types. The balance between lipids oxidation and lipids storage in cells is mainly regulated by malonyl-CoA, generated by ACC. Malonyl-CoA is known to inhibit transport of fatty acids into mitochondria via allosteric regulation of carnitine palmitoyltransferase-1, thereby preventing them from being metabolized. Activated AMPK inhibits malonyl-CoA synthesis and shifts the balance towards mitochondrial fatty acid oxidation and away from fat storage. Several studies have shown that activation of AMPK with AICAR, α-lipoic acid, leptin, adiponectin and IL-6 enhances muscle fatty acid βoxidation (Carey et al. 2006; Lee et al. 2005b; Merrill et al. 1997; Minokoshi et al. 2002; Yamauchi et al. 2002). Chronic leptin treatment increases skeletal muscle fatty acid oxidation in an AMPK-dependent manner by increasing AMP/ATP ratio in oxidative muscle fibers and by increasing AMPKα2 nuclear translocation and PPARα transcription (Suzuki et al. 2007). Studies in transgenic animals support these observations since expression of the activating AMPKγ3 R225Q mutation in muscle increased fatty acid oxidation and protected against excessive triglyceride accumulation and insulin resistance in skeletal muscle (Barnes et al. 2004). Interestingly, recent data have shown that resistin lowers AMPK signalling in muscle cells and that this reduction is associated with suppressed fatty acid oxidation (Palanivel and Sweeney 2005).
Non-alcoholic fatty liver disease is a serious consequence of obesity increasing the risk of liver cancer or cirrhosis. The origin of this disease is unknown and probably multifactorial. Nevertheless, because insulin resistance is recognized as an associate and/or promoting mediator of the disease, management of insulin resistance becomes an important challenge. For this specific point and because AMPK is a key factor in lipids partitioning (balance between synthesis and oxidation), management of non-alcoholic fatty liver disease by activators of AMPK represents a new therapeutic strategy. Adiponectin treatment restores insulin sensitivity and decreases hepatic steatosis of obese mice (Xu et al. 2003). This effect is linked to an activation of AMPK in the liver that decreases fatty acid biosynthesis and increases mitochondrial fatty acid oxidation (Yamauchi et al. 2001). Reduction of liver steatosis when AMPK is activated has been also confirmed by a decrease in liver triglyceride content in lean and obese rodents during AICAR infusion (Bergeron et al. 2001a; Cool et al. 2006) and after treatment with small-molecule AMPK activators (Cool et al. 2006). The synthesis of triglycerides is regulated by both the supply of glycerol-3-phosphate (from carbohydrate metabolism) and of fatty acyl-coenzyme A. The first step of triglycerides synthesis is catalyzed by glycerol-3-phosphate acyl-transferase (GPAT). AICAR or exercise induced AMPK activation reduces hepatic GPAT activity and triglycerides esterification (Muoio et al. 1999; Park et al. 2002). Fasting, that increases hepatic AMPK inhibits GPAT activity (Witters et al. 1994). In the same way, AMPK activation by resveratrol protects against lipid accumulation in the liver of diabetic mice (Zang et al. 2006) in association with increased mitochondrial number (Baur et al. 2006) and SIRT1-dependent deacetylation of peroxisome proliferator-activated receptor coactivator (PGC)-1α, a master regulator of mitochondrial biogenesis (Baur et al. 2006; Rodgers and Puigserver 2007). The efficacy of metformin as a treatment for fatty liver disease has been confirmed in obese, ob/ob mice, which develop hyperinsulinemia, insulin resistance and fatty livers (Lin et al. 2000).
The discovery of new strategies of management of hepatic steatosis in humans is of considerable interest. AMPK activation could be one of them as suggested by recent clinical studies in type 2 diabetic patients. Indeed, it has been demonstrated that AICAR infusion results in significant decline in circulating plasma non-esterified fatty acids (NEFA) levels, suggesting stimulation of hepatic fatty acid oxidation and/or a reduction in whole body lipolytic rate (Boon et al. 2008). Management of hepatic steatosis by targeting AMPK is also suggested by recent successes in treating this disorder with diet, exercise, and TZDs all known as AMPK activators (Carey et al. 2002; Neuschwander-Tetri and Caldwell 2003). Other studies are needed to analyze the beneficial effect of AMPK activation for the management of fatty liver diseases in humans.
4.5. Mimicking the beneficial effects of an anti-obesity drug
Weight reduction is best achieved by behavioural change to reduce energy intake and by increasing physical activity to enhance energy expenditure. Therefore, the AMPK system may be an important pharmacological target to reduce fatty acid storage in adipocytes and to treat obesity. By inducing fatty acid oxidation within the adipocyte, activation of AMPK would reduce fat cell size and also prevent fatty acids from being exported to peripheral tissues and cause deleterious effects. Direct evidence linking AMPK activation to diminished adiposity was first obtained by chronic administration of AICAR to lean and obese rats, an effect attributable, at least in part, to an increase in energy expenditure (Buhl et al. 2002; Winder et al. 2000). Furthermore, the anti-obesity hormone leptin increases fatty-acid oxidation in skeletal muscle by activating AMPK (this process involves an increase in the AMP/ATP ratio) (Minokoshi et al. 2002), depletes body fat stores by activating AMPK activity and by increasing uncoupling mitochondrial protein (UCP)-1 and UCP-2 expression (Orci et al. 2004). β3-adrenoceptor (β3-AR) agonists were also found to have remarkable anti-obesity and anti-diabetic effects in rodents and these compounds were found to stimulate AMPK in fat cells (Moule and Denton 1998). In addition, overexpression of UCP-1 in adipocytes leads to an increase in the AMP/ATP ratio and activation of AMPK, inactivation of ACC and a decreased lipogenesis (Matejkova et al. 2004). Additionally, a strong mitochondrial biogenesis in response to increased UCP-1 expression in adipocytes has been demonstrated (Orci et al. 2004; Rossmeisl et al. 2002), features that could enhance the fatty acid oxidation capacity of adipocytes in response to AMPK activation. During chronic AICAR treatment, activated AMPK increases UCP-3 expression in muscle independently of changes in mitochondrial biogenesis (Stoppani et al. 2002; Zhou et al. 2000). This effect can also explain changes in energy expenditure during AMPK activation.
5. Benefits of targeting AMPK pathway for metabolic complications
5.1. AMPK and ischemic heart
Type 2 diabetes is recognized as an important risk factor for cardiovascular diseases and mortality. In ischemic heart, balance between glucose and lipids is altered. In this situation, activation of AMPK is considered as a metabolic adaptation to rescue energy supply. Indeed, AMPK stimulates glycolysis and sustains energy supply during ischemic stress. Convincing evidence suggests that the more AMPK is activated in ischemic myocardial tissue, the more the size of infarcted tissue is reduced. Because the size of myocardial infarcted tissue is one of the variables that determine the risk of sudden death and the risk of cardiac insufficiency in humans, reduction of the volume of ischemic tissue is an important therapeutic challenge. Thus, promotion of glucose oxidation or inhibition of fatty acid oxidation in ischemic/reperfused hearts could be a promising novel therapeutic approach during myocardial ischemia. Such a mechanism has been demonstrated during the phenomenon called ischemic preconditioning. This phenomenon (consisting in repeated brief episodes of myocardial ischemia) (Murry et al. 1986) induces endogenous protective mechanisms in the heart which becomes more resistant to subsequent ischemic episodes. The molecular mechanism of this protective effect is based on AMPK activation in a PKC-dependent manner and promotion of glucose utilization in myocardial cells (Nishino et al. 2004). Attractively, adiponectin protects the heart from ischemia by activating AMPK and increasing the energy supply to heart cells (Shibata et al. 2005). For example, high blood levels of adiponectin are associated with a lower risk of heart attack, and vice versa (Pischon et al. 2004). Additionally, adiponectin levels rapidly decline after the onset of acute myocardial infarction. Similarly, in mice, deletion of adiponectin induces increased heart damage after reperfusion that was associated with diminished AMPK signalling in the myocardium (Shibata et al. 2005). In addition, it has also been reported that adiponectin attenuated cardiac hypertrophy through activation of AMPK signalling pathway (Liao et al. 2005; Shibata et al. 2004a). These findings clearly show that adiponectin has a cardioprotective role in vivo during ischemia through AMPK-dependent mechanisms.
Since AMPK regulates the balance between glucose and fatty acid metabolism at the cellular level, the metabolic response of the heart to global ischemia was studied in AMPKα2−/− mice. These hearts displayed a more rapid onset of ischemic contracture, which was associated with a decrease in ATP content, in lactate production, in glycogen content and in the phosphorylation state of ACC (Zarrinpashneh et al. 2006). Importance of metabolic adaptation via AMPK activation during ischemia was also documented in another transgenic mouse model overexpressing a dominant negative form of AMPKα2 in the heart (Russell et al. 2004). These studies indicate that the α2 isoform of AMPK is required for the metabolic response of the heart to ischemia suggesting that AMPK is cardioprotective. Thus, AMPK activators could be of particular interest for the management of myocardial ischemia. Nevertheless, inappropriate activation of AMPK can have deleterious consequences in the heart. Indeed, in humans, a variety of mutations in the γ2 subunit (Figure 1) have been shown to produce a glycogen storage cardiomyopathy characterized by ventricular pre-excitation, conduction defects and cardiac hypertrophy (Dyck and Lopaschuk 2006). This argues for a restrictive use of AMPK activators during the acute phase of heart ischemia and not for a chronic activation of cardiac AMPK. Thus, the balance between benefits and deleterious cardiac effects of AMPK activation has to be studied in more details.
5.2. AMPK and endothelial dysfunction
Endothelial cell dysfunction, as manifested by impaired vascular relaxation or an increase in circulating vascular cell adhesion molecules, is present in patients with type 2 diabetes, and it is thought to be one component of the inflammatory process that initiates atherogenesis (Van Gaal et al. 2006). Based on studies using genetically modified mice, the production of NO via eNOS is crucial in the regulation of vascular tone (Lau et al. 2000; Maxwell et al. 1998). The activity of eNOS is largely determined by posttranslational modifications such as multisite phosphorylation and protein interactions. Interestingly, AMPK enhances eNOS activity by direct phosphorylation of Ser1177 (Chen et al. 2000; Chen et al. 1999), Ser633 (Chen et al. 2009) and by promoting its association with heat shock protein 90 (Davis et al. 2006) leading to endothelial NO production. In this respect, metformin has been proposed to improve endothelium function in diabetes by favouring phosphorylation of eNOS by AMPK activation (Davis et al. 2006). Metformin was also shown to relax endothelium-denuded rat aortic rings pre-contracted with phenylephrine, showing that AMPK can induce vasorelaxation in an endothelium- and NOS-independent manner (Majithiya and Balaraman 2006). Accordingly, AMPK activation in response to hypoxia or metabolic challenge can induce vasorelaxation of big vessels (Evans et al. 2005; Rubin et al. 2005), thereby favouring blood flow. Interestingly, AMPK-dependent adiponectin vascular effects have been demonstrated for angiogenic repair in an ischemic hind limbs model (Shibata et al. 2004b). Similarly, α-lipoic acid improves vascular dysfunction by normalizing triglyceride and lipid peroxide levels and NO synthesis in endothelial cells from obese rat by activating AMPK (Lee et al. 2005a). Attractively, adiponectin exhibits potent anti-atherosclerotic effects and suppresses endothelial cell proliferation via AMPK activation (Kubota et al. 2002; Yamauchi et al. 2003).
Beyond the vascular effects of AMPK activation, it has been recently demonstrated that AMPK can regulate blood pressure. Thus, long-term administration of AICAR reduces systolic blood pressure in an insulin-resistant animal model (Buhl et al. 2002). In this process, a potential role for AMPK could be the regulation of ion channels or sodium cotransporters including ENaC and the Na-K-2Cl cotransporter (Carattino et al. 2005; Fraser et al. 2003). These data provide additional support to the hypothesis that AMPK activation might be a potential future pharmacological strategy for treating the cardiovascular risk factors linked to the metabolic syndrome.
6. Conclusion
Lifestyle modifications are recognized as an important preventive and therapeutic intervention for impaired glucose tolerance, insulin resistance and type 2 diabetic patients. AMPK activators are potential new therapeutic agents for the treatment of type 2 diabetes by mimicking the beneficial effects of physical activity and of calorie restriction. Accordingly, AMPK-activating agents could also be used as regulators of hyperglycaemia, obesity, lipids disorders, lipotoxicity and cardiovascular risk by targeting specific cellular pathways (Figure 3). Resveratrol, metformin, TZDs, adiponectin and leptin are now considered as AMPK activators. However, many other effects of AMPK activation should be carefully evaluated and many questions are not resolved: are new AMPK activators tissue specific? What are the consequences of a long term pharmacological AMPK activation? Additional studies are required to address these critical points.
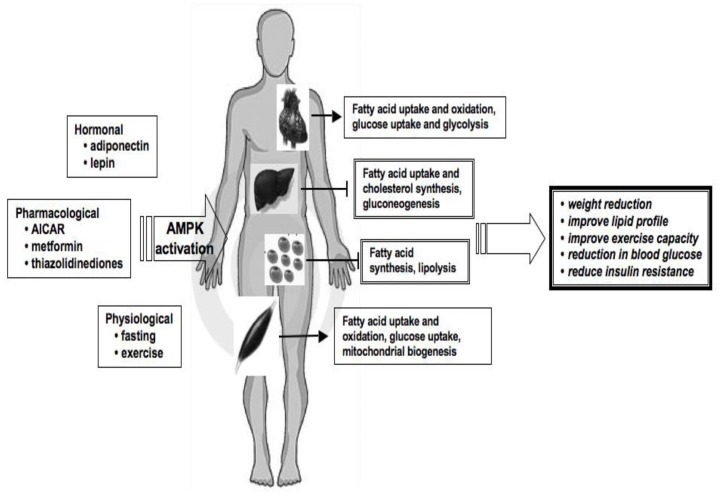
Figure 3. AMPK, a potential therapeutic target in metabolic disease.
AMPK pathway has become the focus of a great deal of attention as a novel therapeutic target in metabolic disease because it has been demonstrated that physiological and pharmacological activation of AMPK results in remodelling different metabolic pathways. AMPK has several important metabolic effects, mimicking the beneficial effects of exercise, including modulation of lipid metabolism, enhanced muscle glucose uptake, increased mitochondrial biogenesis, improvement in insulin sensitivity and reduction in blood glucose. Activation of AMPK by pharmacological agents presents a unique challenge to prevent and treat the metabolic abnormalities associated with the metabolic syndrome.
Acknowledgments
This work was supported by the European Commission integrated project (LSHM-CT-2004-005272/exgenesis), Agence Nationale de la Recherche (ANR-06-PHYSIO-026), Association Française contre les Myopathies (AFM), Association pour l’Etude des Diabètes et des Maladies Métaboliques (ALFEDIAM) and Institut Benjamin Delessert.
References
- Alberti KG, Zimmet PZ. New diagnostic criteria and classification of diabetes--again? Diabet Med. 1998;15:535–536. doi: 10.1002/(SICI)1096-9136(199807)15:7<535::AID-DIA670>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Andersson U, Filipsson K, et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Andreelli F, Foretz M, et al. Liver adenosine monophosphate-activated kinase-alpha2 catalytic subunit is a key target for the control of hepatic glucose production by adiponectin and leptin but not insulin. Endocrinology. 2006;147:2432–2441. doi: 10.1210/en.2005-0898. [DOI] [PubMed] [Google Scholar]
- Anthonsen MW, Ronnstrand L, et al. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- Assifi MM, Suchankova G, et al. AMP-activated protein kinase and coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am J Physiol Endocrinol Metab. 2005;289:E794–800. doi: 10.1152/ajpendo.00144.2005. [DOI] [PubMed] [Google Scholar]
- Banerjee RR, Rangwala SM, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- Banks AS, Kon N, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes BR, Marklund S, et al. The 5′-AMP-activated protein kinase gamma3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J Biol Chem. 2004;279:38441–38447. doi: 10.1074/jbc.M405533200. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, et al. Resveratrol improves health and survival of mice on a high-calorie diet. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron R, Russell RR, 3rd, et al. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am J Physiol. 1999;276:E938–944. doi: 10.1152/ajpendo.1999.276.5.E938. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Previs SF, et al. Effect of 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes. 2001a;50:1076–1082. doi: 10.2337/diabetes.50.5.1076. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Ren JM, et al. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab. 2001b;281:E1340–1346. doi: 10.1152/ajpendo.2001.281.6.E1340. [DOI] [PubMed] [Google Scholar]
- Boon H, Bosselaar M, et al. Intravenous AICAR administration reduces hepatic glucose output and inhibits whole body lipolysis in type 2 diabetic patients. Diabetologia. 2008;51:1893–1900. doi: 10.1007/s00125-008-1108-7. [DOI] [PubMed] [Google Scholar]
- Bordone L, Motta MC, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce CR, Mertz VA, et al. The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes. 2005;54:3154–3160. doi: 10.2337/diabetes.54.11.3154. [DOI] [PubMed] [Google Scholar]
- Buhl ES, Jessen N, et al. Long-term AICAR administration reduces metabolic disturbances and lowers blood pressure in rats displaying features of the insulin resistance syndrome. Diabetes. 2002;51:2199–2206. doi: 10.2337/diabetes.51.7.2199. [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Jiang LQ, et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattino MD, Edinger RS, et al. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J Biol Chem. 2005;280:17608–17616. doi: 10.1074/jbc.M501770200. [DOI] [PubMed] [Google Scholar]
- Carey AL, Steinberg GR, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- Carey DG, Cowin GJ, et al. Effect of rosiglitazone on insulin sensitivity and body composition in type 2 diabetic patients [corrected] Obes Res. 2002;10:1008–1015. doi: 10.1038/oby.2002.137. [DOI] [PubMed] [Google Scholar]
- Chen Z, Peng IC, et al. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZP, Mitchelhill KI, et al. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- Chen ZP, McConell GK, et al. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab. 2000;279:E1202–1206. doi: 10.1152/ajpendo.2000.279.5.E1202. [DOI] [PubMed] [Google Scholar]
- Clarke PR, Hardie DG. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. Embo J. 1990;9:2439–2446. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins QF, Liu HY, et al. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J Biol Chem. 2007;282:30143–30149. doi: 10.1074/jbc.M702390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool B, Zinker B, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, et al. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- da Silva Xavier G, Leclerc I, et al. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J. 2003;371:761–774. doi: 10.1042/BJ20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daval M, Diot-Dupuy F, et al. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Xie Z, et al. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55:496–505. doi: 10.2337/diabetes.55.02.06.db05-1064. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath MY, Ehses JA, et al. Mechanisms of beta-cell death in type 2 diabetes. Diabetes. 2005;54(Suppl 2):S108–113. doi: 10.2337/diabetes.54.suppl_2.s108. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Drummond MJ, et al. Resistance exercise increases human skeletal muscle AS160/TBC1D4 phosphorylation in association with enhanced leg glucose uptake during post-exercise recovery. J Appl Physiol. 2008 doi: 10.1152/japplphysiol.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol. 2006;574:95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, et al. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- El-Assaad W, Buteau J, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–4163. doi: 10.1210/en.2003-0410. [DOI] [PubMed] [Google Scholar]
- Eto K, Yamashita T, et al. Genetic manipulations of fatty acid metabolism in beta-cells are associated with dysregulated insulin secretion. Diabetes. 2002;51(Suppl 3):S414–420. doi: 10.2337/diabetes.51.2007.s414. [DOI] [PubMed] [Google Scholar]
- Evans AM, Mustard KJ, et al. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J Biol Chem. 2005;280:41504–41511. doi: 10.1074/jbc.M510040200. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev. 2005;33:114–119. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Gao J, et al. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab. 2002;282:E18–23. doi: 10.1152/ajpendo.2002.282.1.E18. [DOI] [PubMed] [Google Scholar]
- Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151–156. doi: 10.2337/dc09-S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz M, Carling D, et al. AMP-activated protein kinase inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J Biol Chem. 1998;273:14767–14771. doi: 10.1074/jbc.273.24.14767. [DOI] [PubMed] [Google Scholar]
- Foretz M, Ancellin N, et al. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 2005;54:1331–1339. doi: 10.2337/diabetes.54.5.1331. [DOI] [PubMed] [Google Scholar]
- Fraser SA, Mount PF, et al. Inhibition of the Na-K-2Cl cotransporter by novel interaction with the metabolic sensor AMP-activated protein kinase. J Am Soc Nephrol. 2003;14:545A. [Google Scholar]
- Fryer LG, Parbu-Patel A, et al. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- Fujii N, Ho RC, et al. Ablation of AMP-activated Protein Kinase {alpha}2 Activity Exacerbates Insulin Resistance Induced by High-fat Feeding of Mice. Diabetes. 2008;57:2958–2966. doi: 10.2337/db07-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garton AJ, Campbell DG, et al. Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. Eur J Biochem. 1989;179:249–254. doi: 10.1111/j.1432-1033.1989.tb14548.x. [DOI] [PubMed] [Google Scholar]
- Garton AJ, Yeaman SJ. Identification and role of the basal phosphorylation site on hormone-sensitive lipase. Eur J Biochem. 1990;191:245–250. doi: 10.1111/j.1432-1033.1990.tb19116.x. [DOI] [PubMed] [Google Scholar]
- Gledhill JR, Montgomery MG, et al. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc Natl Acad Sci U S A. 2007;104:13632–13637. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glund S, Deshmukh A, et al. Interleukin-6 directly increases glucose metabolism in resting human skeletal muscle. Diabetes. 2007;56:1630–1637. doi: 10.2337/db06-1733. [DOI] [PubMed] [Google Scholar]
- Glund S, Treebak JT, et al. Role of adenosine 5′-monophosphate-activated protein kinase in interleukin-6 release from isolated mouse skeletal muscle. Endocrinology. 2009;150:600–606. doi: 10.1210/en.2008-1204. [DOI] [PubMed] [Google Scholar]
- Hammer S, Snel M, et al. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol. 2008;52:1006–1012. doi: 10.1016/j.jacc.2008.04.068. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hirshman MF, et al. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- Higa M, Zhou YT, et al. Troglitazone prevents mitochondrial alterations, beta cell destruction, and diabetes in obese prediabetic rats. Proc Natl Acad Sci U S A. 1999;96:11513–11518. doi: 10.1073/pnas.96.20.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes BF, Kurth-Kraczek EJ, et al. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol. 1999;87:1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- Holmes BF, Lang DB, et al. AMP kinase is not required for the GLUT4 response to exercise and denervation in skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287:E739–743. doi: 10.1152/ajpendo.00080.2004. [DOI] [PubMed] [Google Scholar]
- Hou X, Xu S, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Iglesias MA, Ye JM, et al. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51:2886–2894. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, et al. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazet IM, Schaart G, et al. Loss of 50% of excess weight using a very low energy diet improves insulin-stimulated glucose disposal and skeletal muscle insulin signalling in obese insulin-treated type 2 diabetic patients. Diabetologia. 2008;51:309–319. doi: 10.1007/s00125-007-0862-2. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Wojtaszewski JF, et al. Effects of alpha-AMPK knockout on exercise-induced gene activation in mouse skeletal muscle. Faseb J. 2005;19:1146–1148. doi: 10.1096/fj.04-3144fje. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, et al. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- Kaiser N, Leibowitz G, et al. Glucotoxicity and beta-cell failure in type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2003;16:5–22. doi: 10.1515/jpem.2003.16.1.5. [DOI] [PubMed] [Google Scholar]
- Kamohara S, Burcelin R, et al. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- Katsuki A, Sumida Y, et al. Increased visceral fat and serum levels of triglyceride are associated with insulin resistance in Japanese metabolically obese, normal weight subjects with normal glucose tolerance. Diabetes Care. 2003;26:2341–2344. doi: 10.2337/diacare.26.8.2341. [DOI] [PubMed] [Google Scholar]
- Kefas BA, Cai Y, et al. AMP-activated protein kinase can induce apoptosis of insulin-producing MIN6 cells through stimulation of c-Jun-N-terminal kinase. J Mol Endocrinol. 2003a;30:151–161. doi: 10.1677/jme.0.0300151. [DOI] [PubMed] [Google Scholar]
- Kefas BA, Heimberg H, et al. AICA-riboside induces apoptosis of pancreatic beta cells through stimulation of AMP-activated protein kinase. Diabetologia. 2003b;46:250–254. doi: 10.1007/s00125-002-1030-3. [DOI] [PubMed] [Google Scholar]
- Kelly M, Keller C, et al. AMPK activity is diminished in tissues of IL-6 knockout mice: the effect of exercise. Biochem Biophys Res Commun. 2004;320:449–454. doi: 10.1016/j.bbrc.2004.05.188. [DOI] [PubMed] [Google Scholar]
- Kim MS, Park JY, et al. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- Kim WH, Lee JW, et al. AICAR potentiates ROS production induced by chronic high glucose: roles of AMPK in pancreatic beta-cell apoptosis. Cell Signal. 2007;19:791–805. doi: 10.1016/j.cellsig.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinen HA, Galuska D, et al. 5-amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes. Diabetes. 2003;52:1066–1072. doi: 10.2337/diabetes.52.5.1066. [DOI] [PubMed] [Google Scholar]
- Kola B, Hubina E, et al. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem. 2005;280:25196–25201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- Koo SH, Flechner L, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- Kurth-Kraczek EJ, Hirshman MF, et al. 5′ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48:1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lan F, Cacicedo JM, et al. SIRT1 modulation of the acetylation status, cytosolic localization and activity of LKB1; possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer DE, Heilbronn LK, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau KS, Grange RW, et al. nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol Genomics. 2000;2:21–27. doi: 10.1152/physiolgenomics.2000.2.1.21. [DOI] [PubMed] [Google Scholar]
- Leclerc I, Kahn A, et al. The 5′-AMP-activated protein kinase inhibits the transcriptional stimulation by glucose in liver cells, acting through the glucose response complex. FEBS Lett. 1998;431:180–184. doi: 10.1016/s0014-5793(98)00745-5. [DOI] [PubMed] [Google Scholar]
- Leclerc I, Lenzner C, et al. Hepatocyte nuclear factor-4alpha involved in type 1 maturity-onset diabetes of the young is a novel target of AMP-activated protein kinase. Diabetes. 2001;50:1515–1521. doi: 10.2337/diabetes.50.7.1515. [DOI] [PubMed] [Google Scholar]
- Leclerc I, Woltersdorf WW, et al. Metformin, but not leptin, regulates AMP-activated protein kinase in pancreatic islets: impact on glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2004;286:E1023–1031. doi: 10.1152/ajpendo.00532.2003. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Lee IK, et al. Alpha-lipoic acid prevents endothelial dysfunction in obese rats via activation of AMP-activated protein kinase. Arterioscler Thromb Vasc Biol. 2005a;25:2488–2494. doi: 10.1161/01.ATV.0000190667.33224.4c. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Song KH, et al. Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem Biophys Res Commun. 2005b;332:885–891. doi: 10.1016/j.bbrc.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Lee-Young RS, Griffee SR, et al. Skeletal muscle AMP-activated protein kinase is essential for the metabolic response to exercise in vivo. J Biol Chem. 2009;284:23925–23934. doi: 10.1074/jbc.M109.021048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Young RS, Ayala JE, et al. Endothelial Nitric Oxide Synthase is Central to Skeletal Muscle Metabolic Regulation and Enzymatic Signaling during Exercise In Vivo. Am J Physiol Regul Integr Comp Physiol. 2010 doi: 10.1152/ajpregu.00004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Takashima S, et al. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc Res. 2005;67:705–713. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Lihn AS, Jessen N, et al. AICAR stimulates adiponectin and inhibits cytokines in adipose tissue. Biochem Biophys Res Commun. 2004;316:853–858. doi: 10.1016/j.bbrc.2004.02.139. [DOI] [PubMed] [Google Scholar]
- Lin HZ, Yang SQ, et al. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- Lira VA, Soltow QA, et al. Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Am J Physiol Endocrinol Metab. 2007;293:E1062–1068. doi: 10.1152/ajpendo.00045.2007. [DOI] [PubMed] [Google Scholar]
- Lochhead PA, Salt IP, et al. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 2000;49:896–903. doi: 10.2337/diabetes.49.6.896. [DOI] [PubMed] [Google Scholar]
- Luo Z, Saha AK, et al. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Lupi R, Del Guerra S, et al. Lipotoxicity in human pancreatic islets and the protective effect of metformin. Diabetes. 2002;51(Suppl 1):S134–137. doi: 10.2337/diabetes.51.2007.s134. [DOI] [PubMed] [Google Scholar]
- Maeda N, Takahashi M, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- Majithiya JB, Balaraman R. Metformin reduces blood pressure and restores endothelial function in aorta of streptozotocin-induced diabetic rats. Life Sci. 2006;78:2615–2624. doi: 10.1016/j.lfs.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Martin TL, Alquier T, et al. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem. 2006;281:18933–18941. doi: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- Matejkova O, Mustard KJ, et al. Possible involvement of AMP-activated protein kinase in obesity resistance induced by respiratory uncoupling in white fat. FEBS Lett. 2004;569:245–248. doi: 10.1016/j.febslet.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Maxwell AJ, Schauble E, et al. Limb blood flow during exercise is dependent on nitric oxide. Circulation. 1998;98:369–374. doi: 10.1161/01.cir.98.4.369. [DOI] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, et al. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- Michael LF, Wu Z, et al. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci U S A. 2001;98:3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Haque MS, et al. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287–291. doi: 10.2337/diabetes.48.2.287. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Alquier T, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- Moule SK, Denton RM. The activation of p38 MAPK by the beta-adrenergic agonist isoproterenol in rat epididymal fat cells. FEBS Lett. 1998;439:287–290. doi: 10.1016/s0014-5793(98)01392-1. [DOI] [PubMed] [Google Scholar]
- Moynihan KA, Grimm AA, et al. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Seefeld K, et al. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J. 1999;338 ( Pt 3):783–791. [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, et al. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Musi N, Fujii N, et al. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes. 2001;50:921–927. doi: 10.2337/diabetes.50.5.921. [DOI] [PubMed] [Google Scholar]
- Narkar VA, Downes M, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DM, Buse JB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrocki AR, Rajala MW, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- Nieto-Vazquez I, Fernandez-Veledo S, et al. Dual Role of Interleukin-6 in Regulating Insulin Sensitivity in Murine Skeletal Muscle. Diabetes. 2008 doi: 10.2337/db07-1062. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nishino Y, Miura T, et al. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovascular research. 2004;61:610–619. doi: 10.1016/j.cardiores.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Nyblom HK, Sargsyan E, et al. AMP-activated protein kinase agonist dose dependently improves function and reduces apoptosis in glucotoxic beta-cells without changing triglyceride levels. J Mol Endocrinol. 2008;41:187–194. doi: 10.1677/JME-08-0006. [DOI] [PubMed] [Google Scholar]
- Orci L, Cook WS, et al. Rapid transformation of white adipocytes into fat-oxidizing machines. Proc Natl Acad Sci U S A. 2004;101:2058–2063. doi: 10.1073/pnas.0308258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanivel R, Sweeney G. Regulation of fatty acid uptake and metabolism in L6 skeletal muscle cells by resistin. FEBS Lett. 2005;579:5049–5054. doi: 10.1016/j.febslet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Pan XR, Li GW, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- Park H, Kaushik VK, et al. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002;277:32571–32577. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SA, Ciaraldi TP, et al. Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes. 2003;52:667–674. doi: 10.2337/diabetes.52.3.667. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischon T, Girman CJ, et al. Plasma adiponectin levels and risk of myocardial infarction in men. Jama. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- Pold R, Jensen LS, et al. Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes. 2005;54:928–934. doi: 10.2337/diabetes.54.4.928. [DOI] [PubMed] [Google Scholar]
- Prentki M, Joly E, et al. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: role in beta-cell adaptation and failure in the etiology of diabetes. Diabetes. 2002;3(51 Suppl):S405–413. doi: 10.2337/diabetes.51.2007.s405. [DOI] [PubMed] [Google Scholar]
- Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- Riboulet-Chavey A, Diraison F, et al. Inhibition of AMP-activated protein kinase protects pancreatic beta-cells from cytokine-mediated apoptosis and CD8+ T-cell-induced cytotoxicity. Diabetes. 2008;57:415–423. doi: 10.2337/db07-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards SK, Parton LE, et al. Over-expression of AMP-activated protein kinase impairs pancreatic {beta}-cell function in vivo. J Endocrinol. 2005;187:225–235. doi: 10.1677/joe.1.06413. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Barnard RJ, et al. Acute exercise increases nitric oxide synthase activity in skeletal muscle. Am J Physiol. 1999;277:E390–394. doi: 10.1152/ajpendo.1999.277.2.E390. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci U S A. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmeisl M, Barbatelli G, et al. Expression of the uncoupling protein 1 from the aP2 gene promoter stimulates mitochondrial biogenesis in unilocular adipocytes in vivo. Eur J Biochem. 2002;269:19–28. doi: 10.1046/j.0014-2956.2002.02627.x. [DOI] [PubMed] [Google Scholar]
- Rubin LJ, Magliola L, et al. Metabolic activation of AMP kinase in vascular smooth muscle. J Appl Physiol. 2005;98:296–306. doi: 10.1152/japplphysiol.00075.2004. [DOI] [PubMed] [Google Scholar]
- Russell RR, 3rd, Li J, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha AK, Avilucea PR, et al. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314:580–585. doi: 10.1016/j.bbrc.2003.12.120. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Grondin PO, et al. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell H, Dietze-Schroeder D, et al. Cytokine secretion by human adipocytes is differentially regulated by adiponectin, AICAR, and troglitazone. Biochem Biophys Res Commun. 2006;343:700–706. doi: 10.1016/j.bbrc.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Ouchi N, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004a;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Ouchi N, et al. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004b;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- Shibata R, Sato K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Bruce CR, et al. AMP kinase activation with AICAR further increases fatty acid oxidation and blunts triacylglycerol hydrolysis in contracting rat soleus muscle. J Physiol. 2005;565:547–553. doi: 10.1113/jphysiol.2004.081687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XM, Fiedler M, et al. 5-Aminoimidazole-4-carboxamide ribonucleoside treatment improves glucose homeostasis in insulin-resistant diabetic (ob/ob) mice. Diabetologia. 2002;45:56–65. doi: 10.1007/s125-002-8245-8. [DOI] [PubMed] [Google Scholar]
- Steinberg GR, Michell BJ, et al. Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006;4:465–474. doi: 10.1016/j.cmet.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- Stephens TJ, Chen ZP, et al. Progressive increase in human skeletal muscle AMPKalpha2 activity and ACC phosphorylation during exercise. Am J Physiol Endocrinol Metab. 2002;282:E688–694. doi: 10.1152/ajpendo.00101.2001. [DOI] [PubMed] [Google Scholar]
- Stoppani J, Hildebrandt AL, et al. AMP-activated protein kinase activates transcription of the UCP3 and HKII genes in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E1239–1248. doi: 10.1152/ajpendo.00278.2002. [DOI] [PubMed] [Google Scholar]
- Sullivan JE, Brocklehurst KJ, et al. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994;353:33–36. doi: 10.1016/0014-5793(94)01006-4. [DOI] [PubMed] [Google Scholar]
- Sun C, Zhang F, et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Sun G, Tarasov AI, et al. Ablation of AMP-activated protein kinase alpha1 and alpha2 from mouse pancreatic beta cells and RIP2.Cre neurons suppresses insulin release in vivo. Diabetologia. 2010;53:924–936. doi: 10.1007/s00125-010-1692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter M, Riek U, et al. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J Biol Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Okamoto S, et al. Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the alpha2 form of AMP-activated protein kinase. Mol Cell Biol. 2007;27:4317–4327. doi: 10.1128/MCB.02222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas E, Tsao TS, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treebak JT, Birk JB, et al. AS160 phosphorylation is associated with activation of alpha2beta2gamma1- but not alpha2beta2gamma3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab. 2007;292:E715–722. doi: 10.1152/ajpendo.00380.2006. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Lindstrom J, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- UK prospective diabetes study group. U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44:1249–1258. [PubMed] [Google Scholar]
- Um JH, Park SJ, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Mertens IL, et al. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- Viana AY, Sakoda H, et al. Role of hepatic AMPK activation in glucose metabolism and dexamethasone-induced regulation of AMPK expression. Diabetes Res Clin Pract. 2006;73:135–142. doi: 10.1016/j.diabres.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Viollet B, Andreelli F, et al. Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem Soc Trans. 2003;31:216–219. doi: 10.1042/bst0310216. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhou L, et al. Troglitazone acutely activates AMP-activated protein kinase and inhibits insulin secretion from beta cells. Life Sci. 2007;81:160–165. doi: 10.1016/j.lfs.2007.04.034. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Holmes AG, et al. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab. 2006;290:E500–508. doi: 10.1152/ajpendo.00361.2005. [DOI] [PubMed] [Google Scholar]
- Weiss EP, Racette SB, et al. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–1042. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett WC, Dietz WH, et al. Guidelines for healthy weight. N Engl J Med. 1999;341:427–434. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- Winder WW, Holmes BF, et al. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- Wing RR, Goldstein MG, et al. Behavioral science research in diabetes: lifestyle changes related to obesity, eating behavior, and physical activity. Diabetes Care. 2001;24:117–123. doi: 10.2337/diacare.24.1.117. [DOI] [PubMed] [Google Scholar]
- Witters LA, Gao G, et al. Hepatic 5′-AMP-activated protein kinase: zonal distribution and relationship to acetyl-CoA carboxylase activity in varying nutritional states. Arch Biochem Biophys. 1994;308:413–419. doi: 10.1006/abbi.1994.1058. [DOI] [PubMed] [Google Scholar]
- Woods A, Azzout-Marniche D, et al. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol. 2000;20:6704–6711. doi: 10.1128/mcb.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Wang Y, et al. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- Zang M, Xu S, et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- Zarrinpashneh E, Carjaval K, et al. Role of the alpha2 isoform of AMP-activated protein kinase in the metabolic response of the heart to no-flow ischemia. Am J Physiol Heart Circ Physiol. 2006 doi: 10.1152/ajpheart.01032.2005. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xie Z, et al. Identification of nitric oxide as an endogenous activator of the AMP-activated protein kinase in vascular endothelial cells. J Biol Chem. 2008;283:27452–27461. doi: 10.1074/jbc.M802578200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhou G, Myers R, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Wang X, et al. Berberine acutely inhibits insulin secretion from beta-cells through 3′,5′-cyclic adenosine 5′-monophosphate signaling pathway. Endocrinology. 2008;149:4510–4518. doi: 10.1210/en.2007-1752. [DOI] [PubMed] [Google Scholar]
- Zhou M, Lin BZ, et al. UCP-3 expression in skeletal muscle: effects of exercise, hypoxia, and AMP-activated protein kinase. Am J Physiol Endocrinol Metab. 2000;279:E622–629. doi: 10.1152/ajpendo.2000.279.3.E622. [DOI] [PubMed] [Google Scholar]
- Zong H, Ren JM, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwetsloot KA, Westerkamp LM, et al. AMPK regulates basal skeletal muscle capillarization and VEGF expression, but is not necessary for the angiogenic response to exercise. J Physiol. 2008;586:6021–6035. doi: 10.1113/jphysiol.2008.159871. [DOI] [PMC free article] [PubMed] [Google Scholar]