Abstract
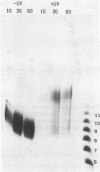
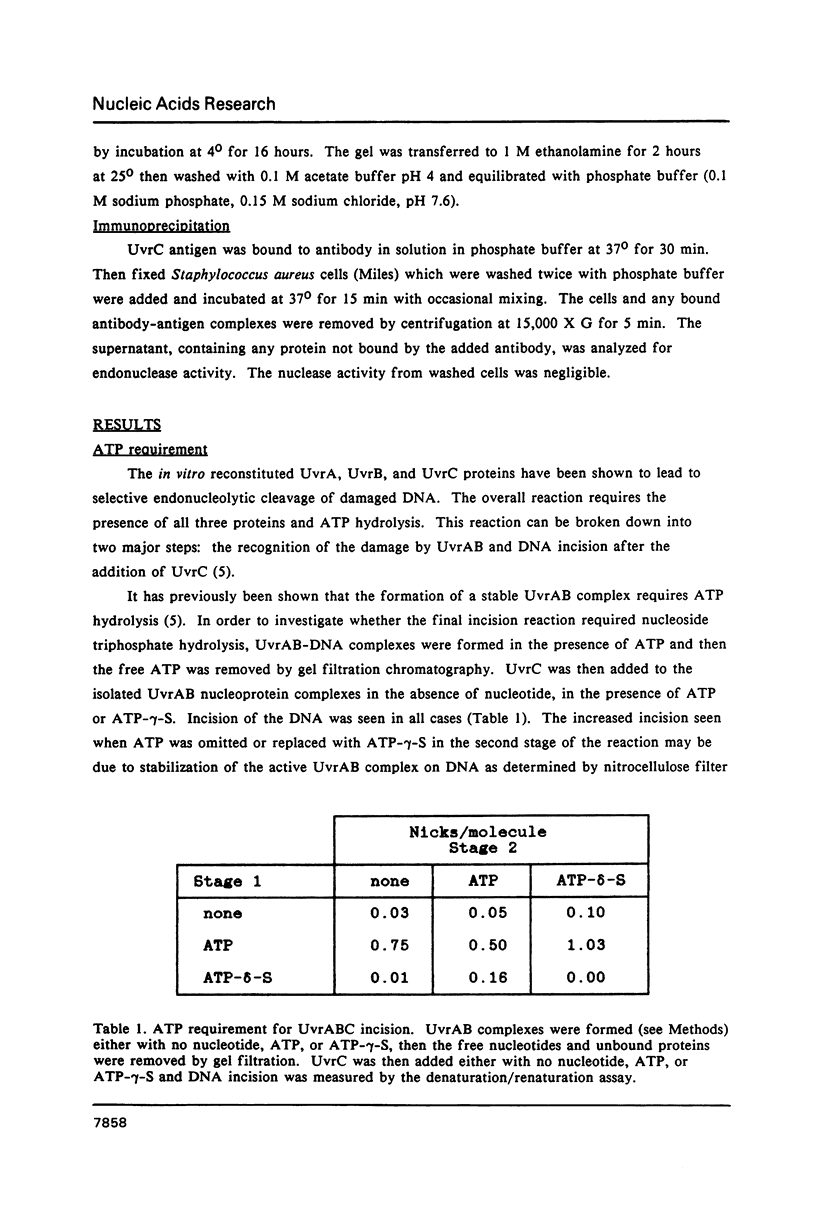
Incision of damaged DNA by the Escherichia coli UvrABC endonuclease requires the UvrA, UvrB, and UvrC proteins as well as ATP hydrolysis. This incision reaction can be divided into three steps: site recognition, preincision complex formation, and incision. UvrAB is able to execute the first two steps in the reaction while the addition of UvrC is required for the incision of DNA. This incision reaction does not require ATP hydrolysis and results in the formation of a tight UvrABC post-incision complex and the generation of an oligomer of approximately 12 nucleotides. At high UvrABC concentrations the specificity of the incision for damaged DNA is decreased and significant incision of undamaged DNA occurs. Analogous to damage specific incision, this type of incision leads to generation of an oligonucleotide, but in this case the size is approximately 9 nucleotides in length. Further evidence shows that the combination of UvrB and UvrC proteins can generate a significant amount of a similar size product on undamaged DNA. In addition, the UvrC protein alone can generate a small amount of the same product. Immunological characterization of the weak nuclease activity seen with UvrC indicates that the activity is very tightly associated with the purified UvrC protein.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck D. J., Popoff S., Sancar A., Rupp W. D. Reactions of the UVRABC excision nuclease with DNA damaged by diamminedichloroplatinum(II). Nucleic Acids Res. 1985 Oct 25;13(20):7395–7412. doi: 10.1093/nar/13.20.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichara M., Fuchs R. P. uvrC gene function has no specific role in repair of N-2-aminofluorene adducts. J Bacteriol. 1987 Jan;169(1):423–426. doi: 10.1128/jb.169.1.423-426.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. M., Gilham P. T. A new method for sequence analysis of oligodeoxyribonucleotides. Nucleic Acids Res. 1985 Apr 11;13(7):2433–2442. doi: 10.1093/nar/13.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A., Grossman L. An endonuclease from Escherichia coli that acts preferentially on UV-irradiated DNA and is absent from the uvrA and uvrB mutants. Proc Natl Acad Sci U S A. 1974 May;71(5):1838–1842. doi: 10.1073/pnas.71.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron P. R., Kushner S. R., Grossman L. Involvement of helicase II (uvrD gene product) and DNA polymerase I in excision mediated by the uvrABC protein complex. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4925–4929. doi: 10.1073/pnas.82.15.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain I., Van Houten B., Thomas D. C., Abdel-Monem M., Sancar A. Effect of DNA polymerase I and DNA helicase II on the turnover rate of UvrABC excision nuclease. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6774–6778. doi: 10.1073/pnas.82.20.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett R. H., Denis K. A., Tung A. S., Klinman N. R. Hybrid plasmacytoma production: fusions with adult spleen cells, monoclonal spleen fragments, neonatal spleen cells and human spleen cells. Curr Top Microbiol Immunol. 1978;81:77–91. doi: 10.1007/978-3-642-67448-8_13. [DOI] [PubMed] [Google Scholar]
- Kumura K., Sekiguchi M., Steinum A. L., Seeberg E. Stimulation of the UvrABC enzyme-catalyzed repair reactions by the UvrD protein (DNA helicase II). Nucleic Acids Res. 1985 Mar 11;13(5):1483–1492. doi: 10.1093/nar/13.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H., Kutter E., Nakanishi M. A restriction map of the bacteriophage T4 genome. Mol Gen Genet. 1980;179(2):421–435. doi: 10.1007/BF00425473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E. Y., Grossman L. Helicase properties of the Escherichia coli UvrAB protein complex. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3638–3642. doi: 10.1073/pnas.84.11.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E. Y., Grossman L. The effect of Escherichia coli Uvr protein binding on the topology of supercoiled DNA. Nucleic Acids Res. 1986 Nov 11;14(21):8557–8571. doi: 10.1093/nar/14.21.8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Franklin K. A., Sancar G., Tang M. S. Repair of psoralen and acetylaminofluorene DNA adducts by ABC excinuclease. J Mol Biol. 1985 Aug 20;184(4):725–734. doi: 10.1016/0022-2836(85)90316-x. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Seeberg E. Reconstitution of an Escherichia coli repair endonuclease activity from the separated uvrA+ and uvrB+/uvrC+ gene products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2569–2573. doi: 10.1073/pnas.75.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L., Blingsmo O. R. Two separable protein species which both restore uvrABC endonuclease activity in extracts from uvrC mutated cells. Biochimie. 1982 Aug-Sep;64(8-9):825–828. doi: 10.1016/s0300-9084(82)80137-5. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L. Purification and properties of the uvrA protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):988–992. doi: 10.1073/pnas.79.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Hearst J. E., Sancar A. Construction of DNA substrates modified with psoralen at a unique site and study of the action mechanism of ABC excinuclease on these uniformly modified substrates. J Biol Chem. 1986 Oct 25;261(30):14135–14141. [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Holbrook S. R., Hearst J. E., Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Sancar A., Hearst J. E. DNase I footprint of ABC excinuclease. J Biol Chem. 1987 Sep 25;262(27):13180–13187. [PubMed] [Google Scholar]
- Yeung A. T., Jones B. K., Capraro M., Chu T. The repair of psoralen monoadducts by the Escherichia coli UvrABC endonuclease. Nucleic Acids Res. 1987 Jun 25;15(12):4957–4971. doi: 10.1093/nar/15.12.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Grossman L. Protein complexes formed during the incision reaction catalyzed by the Escherichia coli UvrABC endonuclease. Nucleic Acids Res. 1986 Mar 25;14(6):2567–2582. doi: 10.1093/nar/14.6.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Oh E. Y., Grossman L. Enzymatic properties of purified Escherichia coli uvrABC proteins. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6157–6161. doi: 10.1073/pnas.80.20.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Mattes W. B., Oh E. Y., Yoakum G. H., Grossman L. The purification of the Escherichia coli UvrABC incision system. Nucleic Acids Res. 1986 Nov 11;14(21):8535–8556. doi: 10.1093/nar/14.21.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]