Abstract
γδ T cells are increasingly recognized as having important functional roles in a range of disease scenarios such as infection, allergy, autoimmunity and cancer. With this has come realization that γδ cells are not a homogeneous population of cells with a single physiological role. Instead, ever increasing complexity in both phenotype and function is being ascribed to γδ cell subsets from various tissues and locations, and in both mouse and human. Here, we review this complexity by describing how diverse γδ cell subsets are generated in the murine thymus, and how these events relate to subsequent γδ subset function in the periphery. We then review the two major γδ cell populations in human, highlighting the several similarities of Vδ1+ cells to certain murine γδ subsets, and describing the remarkable functional plasticity of human Vδ2+ cells. A better understanding of this spectrum of γδ cell phenotypes should facilitate more targeted approaches to utilise their tremendous functional potential in the clinic.
Keywords: human γδ T cell subsets, thymus
Introduction
The initial perception of γδ cells as innate cells with limited functional potential is now distinctly outdated. Instead, γδ cells display considerable subset heterogeneity, with complex patterns of effector function that range from T-cell help to antigen presentation. Understanding this heterogeneity, and how it develops, is central to understanding the role of γδ cells in complex diseases, and will provide an important foundation for harnessing these enigmatic cells for multiple future therapeutic opportunities in the clinic.
Commitment to the γδ cell lineage; pre-commitment and signal strength
In the murine thymus, commitment to the γδ lineage occurs at the immature CD4− CD8− double-negative (DN) stage of thymocyte development.1 Early DN cells with potential to develop as either γδ or αβ T cells rearrange their T-cell receptor-γ (TCR-γ), TCR-δ and TCR-β loci in an attempt to generate a productive TCR-γδ or pre-TCR (TCR-β paired with the invariant pre-TCR-α chain) that compete to drive γδ or αβ T-cell development, respectively.1–3 Despite initial suggestions that TCR-γδ and pre-TCR ‘instruct’ DN cells into their respective lineages, it now appears that signal strength, rather than identity of the expressed TCR complex, is the critical factor in fate determination; strong signalling committing DN cells to the γδ lineage; weak signalling committing cells to the αβ lineage.4,5 Nonetheless, this effectively equates to an instructional model, as under normal circumstances the pre-TCR signals weakly while TCR-γδ signalling is stronger.
Although this strength-of-signal model is now widely accepted, there is significant evidence to suggest that factors operating before, or contemporaneous with, TCR rearrangement and signalling may also influence γδ/αβ fate determination. Thus, CD44+ CD25+ DN2 cells that express CD127 [interleukin-7 receptor-α (IL-7Rα) chain],6 or Sox13,7 appear more likely to enter the γδ lineage, whereas adult DN cells, or DN cells from the later CD44− CD25+ DN3, or CD44− CD25− DN4 subsets appear biased toward αβ T-cell development.3,8 Thus, commitment to a γδ fate requires strong signalling from successfully rearranged TCR-γδ complexes in DN cells that are permissive for entry into the γδ cell lineage.3
Thymic γδ subsets in mouse; the consequence of strong TCR-γδ signals
The earliest murine TCR-γδ+ thymic progenitors are CD24+ CD25+ CD27+ cells that express relatively low levels of surface TCR-γδ, but are highly proliferative.9,10 At this stage, TCR-γδ signalling initiates, leading to CD25 down-regulation, up-regulation of TCR-γδ, and generation of cells with a CD24+ CD25− CD27+ phenotype.9 These uncommitted cells probably represent precursors of several distinct mature CD24− TCR-γδ+ thymocyte populations (Fig. 1a, and see below), that must arise from TCR-γδ signalling that exceeds a critical commitment threshold. Nonetheless, evidence now suggests that mechanisms of TCR-γδ signal initiation that underpin these commitment events may significantly differ.3,11,12
Figure 1.
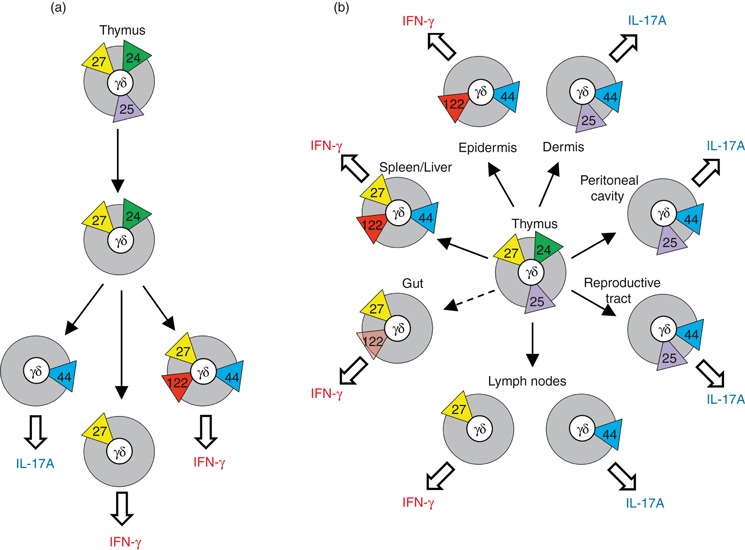
Mouse γδ cell subsets in the thymus and periphery. (a) Thymic γδ subsets described by surface expression of; yellow triangles – CD27; green triangles – CD24; blue triangles CD44; purple triangles – CD25; and red triangles – CD122. Proposed developmental relationships between subsets are indicated by arrows, and potential for cytokine secretion is shown. (b) Peripheral γδ subsets described by tissue location, potential for cytokine secretion and surface markers, as described for (a). Gut γδ cells (i.e. γδ intraepithelial lymphocytes) express only low levels of CD122. IL-17A, interleukin-17A; IFN-γ, interferon-γ.
Strong TCR signals of the type that commit DN cells to the γδ lineage are generally considered a consequence of agonist–ligand binding. However, a general TCR-γδ restricting element, analogous to MHC-I or MHC-II for TCR-αβ, has not been identified, and in mouse only one confirmed TCR-γδ-ligand, the MHC class Ib thymus leukaemia (TL) molecule, has been found.13 Nonetheless, there is reasonable evidence, although mostly indirect, for TCR-γδ-ligand binding during generation of thymic progenitors of at least three peripheral γδ cell subsets; dendritic epidermal T cells (DETC) that use TCR-γ chain variable region-5 (Vγ5) (nomenclature from ref. 14), and TCR-δ chain variable region-1 (Vδ1),11 natural killer T (NKT) -like γδ cells that use a Vγ1Vδ6.3/6.4+ TCR,15,16 and γδ cells whose TCRs recognize the TL molecules T10b and T22b (∼ 0·5–1·0% of all γδ cells).17–20 In each case, the TCRs used by these subsets already show restricted CDR3 length and amino acid composition in the thymus, indicative of thymic ligand-driven TCR selection.11,15,21 Moreover, these CD27+ thymic progenitors display a CD44+ CD62L− CD122+ phenotype that is generally associated with ligand engagement (Fig. 1a).
A further subset of mature TCR-γδ+ thymocytes that express a Vγ6Vδ1+ TCR, have also been reported to show evidence of CDR3-mediated TCR selection.21 These cells are progenitors of those that populate the female reproductive tract and peritoneal cavity, and appear shortly after Vγ5Vδ1+ DETC progenitors at approximately embryonic day 16.1 Notably, Vγ6Vδ1+γδ cells differ from DETC, NKT-like γδ cells and (ligand-selected) TL-specific γδ cells in their capacity to produce IL-17A.22 This IL-17A-secreting effector potential is shared by CD27− CCR6+γδ thymocytes9,23 (Fig. 1a), that are also CD44+ CD62L−, consistent with a previous TCR-γδ-ligand interaction. However, CD27−γδ cells do not express CD122, and conspicuously fail to develop in fetal thymic organ cultures supplemented with TCR-γδ antibodies that induce strong TCR signals.9,23 Hence, any putative ligand interaction for the development of CD27− IL-17A-secreting γδ cells must necessarily induce an attenuated (or at least qualitatively different) TCR signal that cannot readily be reproduced by classical antibody cross-linking of the TCR.9,11,19
The development of IL-17A-secreting γδ cells has also been suggested to result from ligand-independent TCR-γδ signalling in the thymus, possibly as a result of oligomerization of TCR-γδ induced by the variable domain of TCR-δ.19 However, a TCR-γδ that lacks both Vγ and Vδ can still signal effectively in DN thymocytes, resulting in generation of γδ thymocytes with interferon-γ (IFN-γ) -secreting potential.12 These cells evoke a subset of mature (i.e. CD24−) CD27+γδ thymocytes that show IFN-γ-secreting potential and display a ‘naive’ CD44− CD62L+ phenotype consistent with an absence of previous TCR-γδ-ligand interactions (Fig. 1a).9 Thus, several distinct subsets of mature γδ thymocytes can be identified that are probably the result of distinct mechanisms of thymic TCR-γδ signal initiation.3
Mouse peripheral γδ subsets
Several discrete populations of peripheral murine γδ cells have now been identified (Fig. 1b). Perhaps the best studied are Vγ5Vδ1+ DETC from the murine (but not human) epidermis that display ‘innate-like’ properties, in that they are thought to respond en masse to relatively few stress-associated TCR-ligands.24 These cells are CD44+ CD62L− CD103+, and express CD122 consistent with their dependence on IL-15.25,26 DETC secrete IFN-γ when activated, but can also drive IL-13-mediated T helper type 2 (Th2) -associated responses on recognition of NKG2D-ligands expressed on stressed epithelial cells.27 A CD44+ CD62L− CD122+ phenotype is also shared by a CD90dull CD27+ NKT-like γδ subset that uses a restricted Vγ1Vδ6·3/6·4+ TCR and secretes both IFN-γ and IL-4 when activated,15,28 and by IFN-γ-secreting TL-specific lymphoid γδ cells that develop in a T10b/T22b-expressing background.19
In contrast to DETC, which primarily make IFN-γ, γδ cells from the murine dermis predominantly secrete IL-17A. These cells are biased towards use of a Vγ4-containing TCR-γδ, are CD44+ CD122−, and express both CCR6 and SCART2.25,29–31 This phenotype closely resembles that of IL-17A-secreting γδ cells from the female reproductive tract, tongue and peritoneal cavity, that are CD27− CD25+ and predominantly use a Vγ6Vδ1+ TCR,22,32 and also of CD27−γδ cells from secondary lymphoid organs9 (Fig. 1b). A common feature of these γδ subsets is their potent IL-17A secretion en masse in response to cytokines such as IL-1β and IL-23.33,34 Indeed, this characteristic strongly predicts an innate-like role for these γδ subsets in diverse immune responses.
The capacity to secrete IFN-γ is also a feature of murine γδ cell populations that are found in secondary lymphoid tissues, and organs such as the lung.9 Unlike the IFN-γ-secreting DETC and NKT-like γδ subset, these CD27+γδ cells display a naive CD44− CD62L+ CD122− phenotype consistent with an absence of TCR ligation during development.3,9 They also possess a polyclonal TCR repertoire (using mainly Vγ1 or Vγ4), and expand extensively when activated through TCR-γδ.9,35 However, it remains to be determined whether these cells respond to environmental stimuli en masse in an innate-like manner, or whether their diverse TCR-γδ specificities and considerable proliferative potential allow adaptive-like TCR-driven clonal expansions in response to foreign antigen challenge.
A sizable yet enigmatic subset of γδ cells permanently resides in the epithelial layers of the gastrointestinal tract.36 These γδ intraepithelial lymphocytes (IELs) can be generated in gut-associated lymphoid tissue (e.g. cryptopatches)37 and are present (to ∼ 25% of normal levels) in athymic nude animals.38γδ IELs use predominantly Vγ1+ or Vγ7+ TCRs with limited junctional diversity, although their TCR specificities remain unknown.39γδ IELs lack expression of the conventional T-cell co-receptors CD4 and CD8αβ, but often express CD8αα.36 The vast majority of γδ IELs are CD27+ CD122lo CCR9+, but do not express either CD90 or CD2.9,18,40,41 Functionally, γδ IELs are cytolytic effector cells with an immunoprotective role in the gut,36,40 especially in young animals,42 and largely through the production of cytokines such as IFN-γ.43 Nonetheless, γδ IELs are also immunomodulatory. For example, adult mice lacking γδ IELs display exaggerated intestinal damage in response to Eimeria vermiformis infection, as a result of failure to control αβ T-cell responses.44
Human γδ cells
Human γδ cells, like their murine counterparts, are a minor population (1–10% of nucleated cells) in peripheral blood but are abundant in tissues, especially in epithelial layers.24 For identification purposes, they are usually sub-divided based on use of one of two variable regions of TCR-δ; Vδ1 or Vδ2.1 Vδ1+γδ cells are the predominant subset found at mucosal surfaces, and so share certain characteristics with murine γδ IELs (see below). By contrast, Vδ2+γδ cells (that are almost exclusively Vγ9+) largely dominate the peripheral blood (Vγ9 is often referred to as Vγ2 in an alternative nomenclature).45–48 Indeed, γδ cells expressing a Vγ9Vδ2+ TCR-γδ can sometimes identify > 50% of blood leucocytes after certain bacterial or parasitic infections.49
Vγ9Vδ2+γδ cells
A γδ population with the specific features of Vγ9Vδ2+ cells are found only in humans and higher primates (the absence of an equivalent subset in rodents making study of Vγ9Vδ2+ cells problematic). Vγ9Vδ2+ cells are unique in their recognition of low-molecular-weight non-peptide phosphoantigens; e.g. (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP), an intermediate metabolite from the 2-C-methyl-d-erythritol 4-phosphate pathway of microbial isoprenoid biosynthesis.50–52 Nanomolar concentrations of HMB-PP lead to rapid TCR-dependent activation of Vγ9Vδ2+ cells, enabling them to respond to a diverse range of pathogens, including Mycobacterium tuberculosis53 and Plasmodium falciparum.54 Vγ9Vδ2+ cells are also indirectly activated by aminobisphosphonates and alkylamines. These compounds inhibit farnesyl diphosphate synthase, an enzyme in the mevalonate pathway of isoprenoid synthesis (used by eukaryotic cells), leading to accumulation of the stimulatory phosphoantigen isopentenyl pyrophosphate (IPP).55 Interestingly, elevated IPP levels are also characteristic of many human tumours, rendering them potential targets for Vγ9Vδ2+ cells.56–58 Indeed, although the mechanism by which phosphoantigens activate the Vγ9Vδ2+ TCR remains unclear, administration of bisphosphonates such as zoledronate (plus IL-2) are generating encouraging Vγ9Vδ2+ cell responses against a range of tumours in the clinic.57,58
Heterogeneity within the Vγ9Vδ2+ subset
Vγ9Vδ2+ cells are often sub-divided on surface expression of CD45RA and CD27 (Fig. 2a); markers more commonly used to identify the naive, effector or memory status of conventional αβ T cells.59 Nonetheless, CD27 does not obviously identify a γδ subset in human comparable to the CD27+γδ subset in mouse (i.e. pre-committed to robust IFN-γ secretion). Instead, CD27 and CD45RA identify four Vγ9Vδ2+ subsets. ‘Naive’ (Tnaive) CD45RA+ CD27+ Vγ9Vδ2+ cells generally comprise 10–20% of those in peripheral blood (addition of a further marker; CD11a, suggests a slightly lower percentage),60 but are the major Vγ9Vδ2+ subset in lymph nodes, in keeping with their expression of CCR7 and CD62L but absence of CCR2, CCR5, CCR6 or CXCR3. Tnaive cells proliferate at relatively high concentrations of IPP (10−4–10−3 m), but do not secrete IFN-γ.59 After activation for 12 days with IPP+IL-2, Tnaive cells become largely CD45RA− CD27+. These ‘Central Memory’ (TCM) cells are CD45RO+, but remain CCR7+ CD62L+ (Fig. 2a). In healthy individuals TCM cells represent ∼ 25% and ∼ 50% of Vγ9Vδ2+ cells in lymph nodes and peripheral blood, respectively. TCM cells appear to proliferate at much lower concentrations of IPP (10−6–10−7 m), but can secrete only low levels of IFN-γ.59
Figure 2.

Human Vγ9Vδ2+γδ cells can be sub-divided using surface expression of CD45RA and CD27, and show remarkable functional plasticity after activation. (a) CD45RA+ CD27+ Tnaive cells give rise to CD45RA− CD27+ TCM cells on activation with isopentenyl pyrophosphate + interleukin-2 (IPP+IL-2), but do not secrete interferon-γ (IFN-γ). Central memory T (TCM) cells generate CD45RA− CD27− effector memory T (TEM) cells after activation with IPP+IL-2, or CD45RA+ CD27− TEMRA cells in the presence of IL-15. TEM cells can secrete abundant IFN-γ, whereas TEMRA cells are mainly cytotoxic. Prol; proliferative capacity, Kill; cytotoxic capacity; red triangles indicate CD27 expression; blue triangles indicate CD45RA expression; green triangles indicate CD62L expression; (b) Vγ9Vδ2+ cells display extensive plasticity after activation with phosphoantigen [(E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) or IPP] in the presence or absence of various cytokines as indicated. ‘?’ indicates uncertainty as to the potential of Tnaive, TCM, TEM and TEMRA to generate the indicated effector subsets. Expression of functionally relevant genes and characteristics are indicated. TGF-β, transforming growth factor-β; TNF-α, tumour necrosis factor-α.
After 12 days of activation with IPP+IL-2, TCM cells generate CD45RA− CD27−‘Effector Memory’ (TEM) cells that are CD45RO+, CCR7− CD62L−, but positive for the tissue-associated chemokine receptors CCR2, CCR5, CCR6 and CXCR359 (Fig. 2a). Unsurprisingly, TEM cells are scarce in lymph nodes, but are readily detected in blood and inflammatory sites. TEM cells secrete abundant IFN-γ and tumour necrosis factor-α (TNF-α) when activated with IPP+IL-2, but their capacity for proliferation is reduced compared with Tnaive and TCM cells. TCM cells also appear to generate a CD45RA+ effector memory (TEMRA) population when activated with IL-15.61 These cells are virtually absent from blood, are CD27−, CCR7− and CD62L−, but express CCR5 and CXCR3, a phenotype shared by a subset of CD8+αβ T cells that display robust cytotoxic potential. Consistent with this, TEMRA cells express abundant perforin, granulysin and N-alpha-benzyloxycarbonyl-L-lysine thiobenzyl ester (BLT)-esterase, and readily display cytolytic activity (but little production of IFN-γ). They also express CD16, KIR2DL1-3 and NKG2A/CD94. However, TEMRA cells are unresponsive to further TCR stimulation and have little proliferative capacity, a phenotype consistent with a terminally differentiated state.
Although Tnaive, TCM, TEM and TEMRA Vγ9Vδ2+ subsets can be identified, whether these represent true naive, effector and memory subsets, comparable to those observed for αβ T cells, is still unclear.62 Nonetheless, the assessment of Vγ9Vδ2+ cells on these criteria appears to correlate with objective clinical outcomes.63 For example, in a phase I trial of patients with advanced solid tumours, increased proportions of TCM and TEM from patients’ peripheral blood was predictive for good cell expansion in vitro with zoledronate+IL-2, which in turn correlated with better clinical responses after subsequent adoptive transfer.64
Notwithstanding the utility of CD45RA and CD27 to describe functional subsets of Vγ9Vδ2+ cells, further useful surface markers have also been identified. For example, cytotoxic potential appears to correlate with increased CD56 and CD16 expression following activation of Vγ9Vδ2+ cells with phosphoantigen and IL-2 for 10–14 days.65 The IPP-expanded CD56+, but not CD56−, Vγ9Vδ2+ cells efficiently killed several tumour lines in a perforin/granzyme-dependent manner that also required NKG2D. Interestingly, tracking individual clones using Vγ9 CDR3 regions appeared to reveal that the capacity for CD56 expression was a stable pre-existing characteristic of individual Vγ9Vδ2+ cells that was unrelated to TCR specificity.66 However, whether this reveals a cytotoxic lineage for Vγ9Vδ2+ cells remains uncertain.
Finally, approximately half the Vγ9Vδ2+ subset also expresses the skin homing receptor cutaneous lymphocyte-associated antigen (CLA).67 Dermal CLA+γδ cells were recently implicated in psoriasis, in which they secrete abundant IFN-γ and TNF-α, and high levels of CCL3, CCL4, CCL5 and CXCL8. However, as with CD56 and CD16, it is still unclear how the use of CLA to identify Vγ9Vδ2+ subsets overlaps with the method defined by CD45RA and CD27.
Remarkable plasticity of activated Vγ9Vδ2+ cells
For a cell type generally considered innate, the Vγ9Vδ2+ subset displays remarkable functional plasticity upon TCR activation that is easily comparable with their more illustrious cousin – the CD4+αβ T helper cell. Such plasticity was initially demonstrated in vitro through polarization of IPP-activated Vγ9Vδ2+ cells; IL-12 and anti-IL-4 antibody generating IFN-γ-secreting Th1-like cells; IL-4 plus anti-IL-12 antibody generating IL-4-producing Th2-like cells.68 An IFN-γ/TNF-α-secreting Th1-like phenotype is also generated following activation with HMB-PP plus IL-2, although the Th2-associated cytokines IL-5 and IL-13 are also produced69 (Fig. 2b). By contrast, HMB-PP activation of Vγ9Vδ2+ cells in the presence of IL-21 promotes a follicular helper (TFH) -like phenotype that is characterized by increased expression of IL-21R, CD244, CXCL10 and CXCL13, and trafficking to lymph node germinal centres.70 Somewhat surprisingly, Vγ9Vδ2+ cells have also been reported to express Foxp3 and to display regulatory activity after IPP activation with IL-15 and transforming growth factor-β,71 while 18–24 hr IPP stimulation (alone) of tonsillar Vγ9Vδ2+ cells appears to induce considerable antigen-presenting cell-like activity, with accompanying surface expression of MHC-II, CD80, CD86, CD40 and CD54.72
The production of IL-17A by human γδ cells, unlike for murine γδ cells, has been difficult to demonstrate.73 Nonetheless, Tnaive Vγ9Vδ2+ cells (especially those from neonates)74 can adopt an IL-17A-secreting Th17-like phenotype if cultured in the presence of various combinations of IL-1β, IL-6, transforming growth factor-β and IL-23 in media containing aromatic hydrocarbons.75 These IL-17A Vγ9Vδ2+ cells are CD161+ CCR6+ TRAIL+ FasL+ with a largely CD45RA+ CD27− TEMRA phenotype, and have been identified in psoriasis,67 and in the cerebrospinal fluid of patients with bacterial meningitis.75 However, these IL-17A-expressing TEMRA cells appear distinct from the previously described cytotoxic TEMRA cells because they do not express perforin or NKG2D.75
The extensive plasticity of activated Vγ9Vδ2+ cells contrasts sharply with murine γδ cells that demonstrate considerable pre-commitment to cytokine production in the thymus.9 However, it is still unclear whether this plasticity relates equally well to all CD45RA/CD27-defined Vγ9Vδ2+ subsets,75 or to what extent possible pre-commitment, for example to a CD56+ cytotoxic fate,66 regulates subsequent effector function.
Human Vδ1+ cells; the cousin of murine γδ IELs?
Human Vδ1+ cells are the major γδ population at epithelial sites such as the intestine and skin.76,77 Similar to murine γδ IELs, Vδ1+ cells frequently express CD8, and display a cytotoxic, Th1-like phenotype characterized by IFN-γ secretion.76 This notwithstanding, Vδ1+ cells also appear to play a significant role in tissue homeostasis and repair, as demonstrated by insulin-like growth factor-1 production in wound healing.78 Indeed, consistent with epithelial immunosurveillance,24 Vδ1+ cells kill a range of epithelial tumours,79,80 possibly through recognition of stress-induced MHC class I-related molecules MICA and MICB.80,81 Vδ1+ cells are also known to respond to autologous and/or endogenous phospholipids presented by CD1,82 and display TCR-driven clonal expansions in response to cytomegalovirus (along with the minor Vδ3+ and Vδ5+ subsets),83,84 and possibly HIV85 and malaria.86
Like Vδ2+ cells, Vδ1+ cells can be sub-divided based on CD45RA and CD27 expression.60 In contrast to Vδ2+ cells, the majority of adult blood Vδ1+ cells are CD45RA+, being evenly split into an IL-2-secreting CD27+ CD11alo‘naive’ subset, and an IFN-γ-secreting CD27− CD11ahi‘non-naive’ population.60 Unsurprisingly, ∼ 80% of cord blood Vδ1+ cells are naive (compared with ∼ 50–60% for Vδ2+ cells), dropping to ∼ 30–40% by 2 years of age.60 By contrast, < 5% of Vδ2+ cells are naive by 1 year of age, reflecting significant expansion of a restricted number of phosphoantigen-reactive Vγ9Vδ2+ clones. The fact that the percentage of naive Vδ1+ cells remains relatively constant in peripheral blood until late middle age may suggest a constant thymic production.60
In addition to expressing common surface markers such as CD2, ICAM-1 and NKG2D,87 Vδ1+ cells also display several notable differences when compared with Vδ2+ cells. For example, they are CD5dull CD28lo, but express abundant CD57 that correlates with high perforin expression.88 On activation of Vδ1+ cells through their TCR (e.g. with phytohaemagglutinin), in the presence of either IL-2 or IL-15, the natural cytotoxicity receptors NKp30, NKp44 and NKp46 are up-regulated, which correlates with potent tumour-directed cytotoxicity and CD56 expression.89,90 By contrast, HMB-PP+IL-2-activated Vδ2+ cells do not express natural cytotoxicity receptors; instead they use the NKG2D pathway as their main mechanism of targeting tumours.89,91
Concluding remarks
Recent studies have begun to characterize the subset complexity of mouse and human γδ cells. Interestingly, certain subsets, such as murine DETC or human Vγ9Vδ2+ cells, are restricted to certain species, whereas certain useful surface markers, such as CD27, do not appear to identify comparable subsets across species. Nonetheless, this methodical dissection of γδ cell repertoires has exposed the critically important innate-like, and possibly adaptive-like, functional roles for γδ cells in diverse disease settings. A further understanding of this largely unanticipated γδ cell biology should reveal much about the relationship between early tissue-associated immune surveillance and the powerful adaptive responses that follow and should perhaps provide unexpected therapeutic opportunities for the clinic.
Acknowledgments
We thank Neil McCarthy for critical review of the manuscript. D.J.Pa, N.S. and D.J.Pe are supported by the Wellcome Trust. J.F.N. was supported by the Fundação para a Ciência e Tecnologia of Portugal.
Disclosures
The authors have no financial disclosures.
References
- 1.Hayday AC. γδ cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 2.Pennington DJ, Silva-Santos B, Hayday AC. γδ T cell development – having the strength to get there. Curr Opin Immunol. 2005;17:108–15. doi: 10.1016/j.coi.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Turchinovich G, Pennington DJ. T cell receptor signalling in γδ cell development: strength isn’t everything. Trends Immunol. 2011;32:567–73. doi: 10.1016/j.it.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Haks MC, Lefebvre JM, Lauritsen JP, Carleton M, Rhodes M, Miyazaki T, Kappes DJ, Wiest DL. Attenuation of γδ TCR signaling efficiently diverts thymocytes to the αβ lineage. Immunity. 2005;22:595–606. doi: 10.1016/j.immuni.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Hayes SM, Li L, Love PE. TCR signal strength influences αβ/γδ lineage fate. Immunity. 2005;22:583–93. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Kang J, Volkmann A, Raulet DH. Evidence that γδ versus αβ T cell fate determination is initiated independently of T cell receptor signaling. J Exp Med. 2001;193:689–98. doi: 10.1084/jem.193.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melichar HJ, Narayan K, Der SD, et al. Regulation of γδ versus αβ T lymphocyte differentiation by the transcription factor SOX13. Science. 2007;315:230–3. doi: 10.1126/science.1135344. [DOI] [PubMed] [Google Scholar]
- 8.Bruno L, Scheffold A, Radbruch A, Owen MJ. Threshold of pre-T-cell-receptor surface expression is associated with αβ T-cell lineage commitment. Curr Biol. 1999;9:559–68. doi: 10.1016/s0960-9822(99)80259-0. [DOI] [PubMed] [Google Scholar]
- 9.Ribot JC, deBarros A, Pang DJ, et al. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17-producing γδ T cell subsets. Nat Immunol. 2009;10:427–36. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prinz I, Sansoni A, Kissenpfennig A, Ardouin L, Malissen M, Malissen B. Visualization of the earliest steps of γδ T cell development in the adult thymus. Nat Immunol. 2006;7:995–1003. doi: 10.1038/ni1371. [DOI] [PubMed] [Google Scholar]
- 11.Turchinovich G, Hayday AC. Skint-1 identifies a common molecular mechanism for the development of interferon-γ-secreting versus interleukin-17-secreting γδ T cells. Immunity. 2011;35:59–68. doi: 10.1016/j.immuni.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Mahtani-Patching J, Neves JF, Pang DJ, Stoenchev KV, Aguirre-Blanco AM, Silva-Santos B, Pennington DJ. PreTCR and TCRγδ signal initiation in thymocyte progenitors does not require domains implicated in receptor oligomerization. Sci Signal. 2011;4:ra47. doi: 10.1126/scisignal.2001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonneville M, Ito K, Krecko EG, et al. Recognition of a self major histocompatibility complex TL region product by γδ T-cell receptors. Proc Natl Acad Sci U S A. 1989;86:5928–32. doi: 10.1073/pnas.86.15.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heilig JS, Tonegawa S. Diversity of murine γ genes and expression in fetal and adult T lymphocytes. Nature. 1986;322:836–40. doi: 10.1038/322836a0. [DOI] [PubMed] [Google Scholar]
- 15.Azuara V, Levraud JP, Lembezat MP, Pereira P. A novel subset of adult γδ thymocytes that secretes a distinct pattern of cytokines and expresses a very restricted T cell receptor repertoire. Eur J Immunol. 1997;27:544–53. doi: 10.1002/eji.1830270228. [DOI] [PubMed] [Google Scholar]
- 16.Azuara V, Lembezat MP, Pereira P. The homogeneity of the TCRdelta repertoire expressed by the Thy-1dull γδ T cell population is due to cellular selection. Eur J Immunol. 1998;28:3456–67. doi: 10.1002/(SICI)1521-4141(199811)28:11<3456::AID-IMMU3456>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 17.Crowley MP, Fahrer AM, Baumgarth N, Hampl J, Gutgemann I, Teyton L, Chien Y. A population of murine γδ T cells that recognize an inducible MHC class Ib molecule. Science. 2000;287:314–16. doi: 10.1126/science.287.5451.314. [DOI] [PubMed] [Google Scholar]
- 18.Lin T, Yoshida H, Matsuzaki G, Guehler SR, Nomoto K, Barrett TA, Green DR. Autospecific γδ thymocytes that escape negative selection find sanctuary in the intestine. J Clin Invest. 1999;104:1297–305. doi: 10.1172/JCI7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen KD, Su X, Shin S, et al. Thymic selection determines γδ T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon γ. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams EJ, Chien YH, Garcia KC. Structure of a γδ T cell receptor in complex with the nonclassical MHC T22. Science. 2005;308:227–31. doi: 10.1126/science.1106885. [DOI] [PubMed] [Google Scholar]
- 21.Itohara S, Tonegawa S. Selection of γδ T cells with canonical T-cell antigen receptors in fetal thymus. Proc Natl Acad Sci U S A. 1990;87:7935–8. doi: 10.1073/pnas.87.20.7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata K, Yamada H, Nakamura R, Sun X, Itsumi M, Yoshikai Y. Identification of CD25+γδ T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol. 2008;181:5940–7. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 23.Haas JD, Gonzalez FH, Schmitz S, Chennupati V, Fohse L, Kremmer E, Forster R, Prinz I. CCR6 and NK1.1 distinguish between IL-17A and IFN-γ-producing γδ effector T cells. Eur J Immunol. 2009;39:3488–97. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 24.Hayday AC. γδ T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–96. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Sumaria N, Roediger B, Ng LG, et al. Cutaneous immunosurveillance by self-renewing dermal γδ T cells. J Exp Med. 2011;208:505–18. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Beneden K, De Creus A, Stevenaert F, Debacker V, Plum J, Leclercq G. Expression of inhibitory receptors Ly49E and CD94/NKG2 on fetal thymic and adult epidermal TCR Vγ3 lymphocytes. J Immunol. 2002;168:3295–302. doi: 10.4049/jimmunol.168.7.3295. [DOI] [PubMed] [Google Scholar]
- 27.Strid J, Sobolev O, Zafirova B, Polic B, Hayday A. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science. 2011;334:1293–7. doi: 10.1126/science.1211250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azuara V, Grigoriadou K, Lembezat MP, Nagler-Anderson C, Pereira P. Strain-specific TCR repertoire selection of IL-4-producing Thy-1 dull γδ thymocytes. Eur J Immunol. 2001;31:205–14. doi: 10.1002/1521-4141(200101)31:1<205::AID-IMMU205>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Gray EE, Suzuki K, Cyster JG. Cutting edge: identification of a motile IL-17-producing γδ T cell population in the dermis. J Immunol. 2011;186:6091–5. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kisielow J, Kopf M, Karjalainen K. SCART scavenger receptors identify a novel subset of adult γδ T cells. J Immunol. 2008;181:1710–16. doi: 10.4049/jimmunol.181.3.1710. [DOI] [PubMed] [Google Scholar]
- 31.Cai Y, Shen X, Ding C, et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shibata K, Yamada H, Sato T, et al. Notch-Hes1 pathway is required for the development of IL-17-producing γδ T cells. Blood. 2011;118:586–93. doi: 10.1182/blood-2011-02-334995. [DOI] [PubMed] [Google Scholar]
- 33.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–41. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–30. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 35.Ribot JC, Chaves-Ferreira M, d’Orey F, et al. Cutting edge: adaptive versus innate receptor signals selectively control the pool sizes of murine IFN-γ- or IL-17-producing γδ T cells upon infection. J Immunol. 2010;185:6421–5. doi: 10.4049/jimmunol.1002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 37.Saito H, Kanamori Y, Takemori T, Nariuchi H, Kubota E, Takahashi-Iwanaga H, Iwanaga T, Ishikawa H. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–8. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- 38.Bandeira A, Itohara S, Bonneville M, Burlen-Defranoux O, Mota-Santos T, Coutinho A, Tonegawa S. Extrathymic origin of intestinal intraepithelial lymphocytes bearing T-cell antigen receptor γδ. Proc Natl Acad Sci U S A. 1991;88:43–7. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pereira P, Gerber D, Huang SY, Tonegawa S. Ontogenic development and tissue distribution of Vγ1-expressing γδ T lymphocytes in normal mice. J Exp Med. 1995;182:1921–30. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shires J, Theodoridis E, Hayday AC. Biological insights into TCRγδ+ and TCR αβ+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE) Immunity. 2001;15:419–34. doi: 10.1016/s1074-7613(01)00192-3. [DOI] [PubMed] [Google Scholar]
- 41.Jensen KD, Shin S, Chien YH. Cutting edge: γδ intraepithelial lymphocytes of the small intestine are not biased toward thymic antigens. J Immunol. 2009;182:7348–51. doi: 10.4049/jimmunol.0900465. [DOI] [PubMed] [Google Scholar]
- 42.Ramsburg E, Tigelaar R, Craft J, Hayday A. Age-dependent requirement for γδ T cells in the primary but not secondary protective immune response against an intestinal parasite. J Exp Med. 2003;198:1403–14. doi: 10.1084/jem.20030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taguchi T, Aicher WK, Fujihashi K, Yamamoto M, McGhee JR, Bluestone JA, Kiyono H. Novel function for intestinal intraepithelial lymphocytes. Murine CD3+, γδ TCR+ T cells produce IFN-γ and IL-5. J Immunol. 1991;147:3736–44. [PubMed] [Google Scholar]
- 44.Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ, Hayday AC. T-cell αβ+ and γδ+ deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci U S A. 1996;93:11774–9. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forster A, Huck S, Ghanem N, Lefranc MP, Rabbitts TH. New subgroups in the human T cell rearranging V γ gene locus. EMBO J. 1987;6:1945–50. doi: 10.1002/j.1460-2075.1987.tb02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LeFranc MP, Forster A, Baer R, Stinson MA, Rabbitts TH. Diversity and rearrangement of the human T cell rearranging gamma genes: nine germ-line variable genes belonging to two subgroups. Cell. 1986;45:237–46. doi: 10.1016/0092-8674(86)90388-0. [DOI] [PubMed] [Google Scholar]
- 47.Strauss WM, Quertermous T, Seidman JG. Measuring the human T cell receptor γ-chain locus. Science. 1987;237:1217–19. doi: 10.1126/science.3498213. [DOI] [PubMed] [Google Scholar]
- 48.Quertermous T, Strauss WM, Van Dongen JJ, Seidman JG. Human T cell γ chain joining regions and T cell development. J Immunol. 1987;138:2687–90. [PubMed] [Google Scholar]
- 49.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 51.Altincicek B, Moll J, Campos N, et al. Cutting edge: human γδ T cells are activated by intermediates of the 2-C-methyl-d-erythritol 4-phosphate pathway of isoprenoid biosynthesis. J Immunol. 2001;166:3655–8. doi: 10.4049/jimmunol.166.6.3655. [DOI] [PubMed] [Google Scholar]
- 52.Hintz M, Reichenberg A, Altincicek B, et al. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 2001;509:317–22. doi: 10.1016/s0014-5793(01)03191-x. [DOI] [PubMed] [Google Scholar]
- 53.Kabelitz D, Bender A, Prospero T, Wesselborg S, Janssen O, Pechhold K. The primary response of human γδ+ T cells to Mycobacterium tuberculosis is restricted to Vγ9-bearing cells. J Exp Med. 1991;173:1331–8. doi: 10.1084/jem.173.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Behr C, Poupot R, Peyrat MA, Poquet Y, Constant P, Dubois P, Bonneville M, Fournie JJ. Plasmodium falciparum stimuli for human γδ T cells are related to phosphorylated antigens of mycobacteria. Infect Immun. 1996;64:2892–6. doi: 10.1128/iai.64.8.2892-2896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, Sarikonda G, Puan KJ, et al. Indirect stimulation of human Vγ2Vδ2 T cells through alterations in isoprenoid metabolism. J Immunol. 2011;187:5099–113. doi: 10.4049/jimmunol.1002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonneville M, Scotet E. Human Vγ9Vδ2 T cells: promising new leads for immunotherapy of infections and tumors. Curr Opin Immunol. 2006;18:539–46. doi: 10.1016/j.coi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Beetz S, Marischen L, Kabelitz D, Wesch D. Human γδ T cells: candidates for the development of immunotherapeutic strategies. Immunol Res. 2007;37:97–111. doi: 10.1007/BF02685893. [DOI] [PubMed] [Google Scholar]
- 59.Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, Salerno A. Differentiation of effector/memory Vδ2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–7. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Rosa SC, Andrus JP, Perfetto SP, Mantovani JJ, Herzenberg LA, Roederer M. Ontogeny of γδ T cells in humans. J Immunol. 2004;172:1637–45. doi: 10.4049/jimmunol.172.3.1637. [DOI] [PubMed] [Google Scholar]
- 61.Caccamo N, Meraviglia S, Ferlazzo V, et al. Differential requirements for antigen or homeostatic cytokines for proliferation and differentiation of human Vγ9Vδ2 naive, memory and effector T cell subsets. Eur J Immunol. 2005;35:1764–72. doi: 10.1002/eji.200525983. [DOI] [PubMed] [Google Scholar]
- 62.Shen Y, Zhou D, Qiu L, et al. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacterial infections. Science. 2002;295:2255–8. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nicol AJ, Tokuyama H, Mattarollo SR, Hagi T, Suzuki K, Yokokawa K, Nieda M. Clinical evaluation of autologous γδ T cell-based immunotherapy for metastatic solid tumours. Br J Cancer. 2011;105:778–86. doi: 10.1038/bjc.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alexander AA, Maniar A, Cummings JS, et al. Isopentenyl pyrophosphate-activated CD56+γδ T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res. 2008;14:4232–40. doi: 10.1158/1078-0432.CCR-07-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urban EM, Li H, Armstrong C, Focaccetti C, Cairo C, Pauza CD. Control of CD56 expression and tumor cell cytotoxicity in human Vγ2Vδ2 T cells. BMC Immunol. 2009;10:50. doi: 10.1186/1471-2172-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laggner U, Di Meglio P, Perera GK, et al. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J Immunol. 2011;187:2783–93. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wesch D, Glatzel A, Kabelitz D. Differentiation of resting human peripheral blood γδ T cells toward Th1- or Th2-phenotype. Cell Immunol. 2001;212:110–17. doi: 10.1006/cimm.2001.1850. [DOI] [PubMed] [Google Scholar]
- 69.Vermijlen D, Ellis P, Langford C, et al. Distinct cytokine-driven responses of activated blood γδ T cells: insights into unconventional T cell pleiotropy. J Immunol. 2007;178:4304–14. doi: 10.4049/jimmunol.178.7.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bansal RR, Mackay CR, Moser B, Eberl M. IL-21 enhances the potential of human γδ T cells to provide B-cell help. Eur J Immunol. 2012;42:110–19. doi: 10.1002/eji.201142017. [DOI] [PubMed] [Google Scholar]
- 71.Casetti R, Agrati C, Wallace M, Sacchi A, Martini F, Martino A, Rinaldi A, Malkovsky M. Cutting edge: TGF-β1 and IL-15 Induce FOXP3+γδ regulatory T cells in the presence of antigen stimulation. J Immunol. 2009;183:3574–7. doi: 10.4049/jimmunol.0901334. [DOI] [PubMed] [Google Scholar]
- 72.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human γδ T cells. Science. 2005;309:264–8. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 73.Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vγ2Vδ2 T cells. J Immunol. 2010;184:7268–80. doi: 10.4049/jimmunol.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moens E, Brouwer M, Dimova T, Goldman M, Willems F, Vermijlen D. IL-23R and TCR signaling drives the generation of neonatal Vγ9Vδ2 T cells expressing high levels of cytotoxic mediators and producing IFN-γ and IL-17. J Leukoc Biol. 2011;89:743–52. doi: 10.1189/jlb.0910501. [DOI] [PubMed] [Google Scholar]
- 75.Caccamo N, La Mendola C, Orlando V, et al. Differentiation, phenotype, and function of interleukin-17-producing human Vγ9Vδ2 T cells. Blood. 2011;118:129–38. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- 76.Deusch K, Luling F, Reich K, Classen M, Wagner H, Pfeffer K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the γδ T cell receptor, the CD8 accessory molecule and preferentially uses the Vδ1 gene segment. Eur J Immunol. 1991;21:1053–9. doi: 10.1002/eji.1830210429. [DOI] [PubMed] [Google Scholar]
- 77.Ebert LM, Meuter S, Moser B. Homing and function of human skin γδ T cells and NK cells: relevance for tumor surveillance. J Immunol. 2006;176:4331–6. doi: 10.4049/jimmunol.176.7.4331. [DOI] [PubMed] [Google Scholar]
- 78.Toulon A, Breton L, Taylor KR, et al. A role for human skin-resident T cells in wound healing. J Exp Med. 2009;206:743–50. doi: 10.1084/jem.20081787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maeurer MJ, Martin D, Walter W, et al. Human intestinal Vδ1+ lymphocytes recognize tumor cells of epithelial origin. J Exp Med. 1996;183:1681–96. doi: 10.1084/jem.183.4.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived γδ T cells of MICA and MICB. Proc Natl Acad Sci U S A. 1999;96:6879–84. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279:1737–40. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 82.Russano AM, Bassotti G, Agea E, Bistoni O, Mazzocchi A, Morelli A, Porcelli SA, Spinozzi F. CD1-restricted recognition of exogenous and self-lipid antigens by duodenal γδ+ T lymphocytes. J Immunol. 2007;178:3620–6. doi: 10.4049/jimmunol.178.6.3620. [DOI] [PubMed] [Google Scholar]
- 83.Dechanet J, Merville P, Lim A, et al. Implication of γδ T cells in the human immune response to cytomegalovirus. J Clin Invest. 1999;103:1437–49. doi: 10.1172/JCI5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vermijlen D, Brouwer M, Donner C, et al. Human cytomegalovirus elicits fetal γδ T cell responses in utero. J Exp Med. 2010;207:807–21. doi: 10.1084/jem.20090348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Maria A, Ferrazin A, Ferrini S, Ciccone E, Terragna A, Moretta L. Selective increase of a subset of T cell receptor γδ T lymphocytes in the peripheral blood of patients with human immunodeficiency virus type 1 infection. J Infect Dis. 1992;165:917–19. doi: 10.1093/infdis/165.5.917. [DOI] [PubMed] [Google Scholar]
- 86.Hviid L, Kurtzhals JA, Adabayeri V, et al. Perturbation and proinflammatory type activation of Vδ1(+)γδ T cells in African children with Plasmodium falciparum malaria. Infect Immun. 2001;69:3190–6. doi: 10.1128/IAI.69.5.3190-3196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Das H, Sugita M, Brenner MB. Mechanisms of Vδ1 γδ T cell activation by microbial components. J Immunol. 2004;172:6578–86. doi: 10.4049/jimmunol.172.11.6578. [DOI] [PubMed] [Google Scholar]
- 88.De Rosa SC, Mitra DK, Watanabe N, Herzenberg LA, Roederer M. Vδ1 and Vδ2 γδ T cells express distinct surface markers and might be developmentally distinct lineages. J Leukoc Biol. 2001;70:518–26. [PubMed] [Google Scholar]
- 89.Correia DV, Fogli M, Hudspeth K, Da Silva MG, Mavilio D, Silva-Santos B. Differentiation of human peripheral blood Vδ1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood. 2011;118:992–1001. doi: 10.1182/blood-2011-02-339135. [DOI] [PubMed] [Google Scholar]
- 90.Siegers GM, Dhamko H, Wang XH, et al. Human Vδ1 γδ T cells expanded from peripheral blood exhibit specific cytotoxicity against B-cell chronic lymphocytic leukemia-derived cells. Cytotherapy. 2011;13:753–64. doi: 10.3109/14653249.2011.553595. [DOI] [PubMed] [Google Scholar]
- 91.Gomes AQ, Martins DS, Silva-Santos B. Targeting γδ T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res. 2010;70:10024–7. doi: 10.1158/0008-5472.CAN-10-3236. [DOI] [PubMed] [Google Scholar]


