Abstract
The seed-borne mycoflora of sorghum and foxtail millet collected from different growing areas in South Korea were isolated and taxonomically identified using dry inspection, standard blotter and the agar plate method. We investigated the in vitro and in vivo germination rates of disinfected and non-disinfected seeds of sorghum and foxtail millet using sterilized and unsterilized soil. The percent recovery of seed-borne mycoflora from the seed components of sorghum and foxtail millet seeds was determined and an infection experiment using the dominant species was evaluated for seedling emergence and mortality. A higher number of seed-borne fungi was observed in sorghum compared to that of foxtail millet. Eighteen fungal genera with 34 fungal species were identified from the seeds of sorghum and 13 genera with 22 species were identified from the seeds of foxtail millet. Five dominant species such as Alternaria alternata, Aspergillus flavus, Curvularia lunata, Fusarium moniliforme and Phoma sp. were recorded as seed-borne mycoflora in sorghum and 4 dominant species (Alternaria alternata, Aspergillus flavus, Curvularia lunata, Fusarium moniliforme) were observed in foxtail millet. The in vitro and in vivo germination rates were higher using disinfected seeds and sterilized soil. More seed-borne fungi were recovered from the pericarp compared to the endosperm and seed embryo. The percent recovery of seed-borne fungi ranged from 2.22% to 60.0%, and Alternaria alternata, Curvularia lunata and 4 species of Fusarium were isolated from the endosperm and embryo of sorghum and foxtail millet. Inoculation of the dominant seed-borne fungi showed considerable mortality of seedlings. All the transmitted seed-borne fungi might well be a primary source of infection of sorghum and foxtail millet crops.
Keywords: Foxtail millet, Mycoflora, Seed-borne, Seed germination, Seed health testing
Sorghum [Sorghum bicolor (L.) Moench] and foxtail millet [Setaria italica (L.) P. Beauv.] are commonly cultivated cereal crops and they form a substantial part of the farming system for people living in Asia [1]. Foxtail millet ranks second in the world's total production of millets [2] and it is used as human food in Korea, which produced about 1,851 t of this in 2001 [3]. It is also cultivated for emergency purposes and it is widely consumed due to its ability to compensate for the nutrient deficiencies of rice such as the lack of vitamins and minerals [1]. In Korea, functional products derived from millets and sorghums have a great potential as therapeutic agents [4, 5]. In fact, there are some reports on the antimicrobial [6, 7] and anti-carcinogenic [4, 8] effects of sorghum, whereas millets have an anti-diabetes action by improving the cholesterol metabolism of the body [9, 10]. In Asia and Africa, the annual economic loss due to grain mold is more than US$130 million [11]. Grain yields are relatively low due to insect pests and diseases [12-15]. The Korean climate have distinct four seasons and the harvesting time of sorghum and millet is usually during the late part of September or the early part of October (the fall season). At this time, seeds are vulnerable to attack by mold fungi. Several reports about seed-borne mycoflora on sorghum [16, 17] and foxtail millet have been published. Post-harvest fungal infection, according to farmers, has been one of the constraints for mass production of these grains. Most of the literature on fungal pathogens of millets and sorghum is derived from India and eastern Africa. There is a lack of quantitative information on the disease prevalence and on the level of disease severity in the grain producing regions of Southern Korea. Preliminary research on the constraints of millet and sorghum production and the subsequent reviews do not provide quantitative values or information on the disease prevalence or severity, nor have there been any studies that have examined the relationship between disease severity and the different agro-ecological locations in Southern Korea. Such knowledge gaps have hindered efforts to assess the true economic importance of diseases of millet and sorghum in Southern Korea. An accurate assessment of the relative importance of millet and sorghum diseases is needed in order to help target research priorities and justify the use of resources. We report here on the most recent assessment of the seed-borne mycoflora from sorghum and foxtail millet that was collected from five different growing areas in Southern Korea. We also investigated and compared the in vitro and in vivo germination rates of disinfected and non-disinfected seeds of sorghum and foxtail millet, with using sterilized and unsterilized soil, to determine the recovery of seed-borne mycoflora from different seed components of sorghum and foxtail millet seeds. We also evaluated the effect of seed-borne inoculums of the main fungi on seedling emergence, seedlings mortality and transmission.
Materials and Methods
The experiment was conducted at the Plant Pathology Laboratory of National Institute of Crop Science (NICS), Rural Development Administration (RDA), Miryang, Korea. The fungi associated with sorghum and foxtail millet seeds were detected by using seed health testing methods. The seeds that were characteristic of five different seed sources derived from sorghum and foxtail millet growing areas in Southern Korea were used in this study (Table 1). The seeds were collected in September 2010 and they were stored in a standard storage room at the Plant Breeding Division of NICS, RDA, Korea. After one year of storage, seed viability tests were conducted under both in in vitro and in vivo conditions by comparing the germination rates of disinfected and non-disinfected seeds of sorghum and foxtail millet and we investigated the effect of different mycoflora associated with the seeds prior to seed sowing.
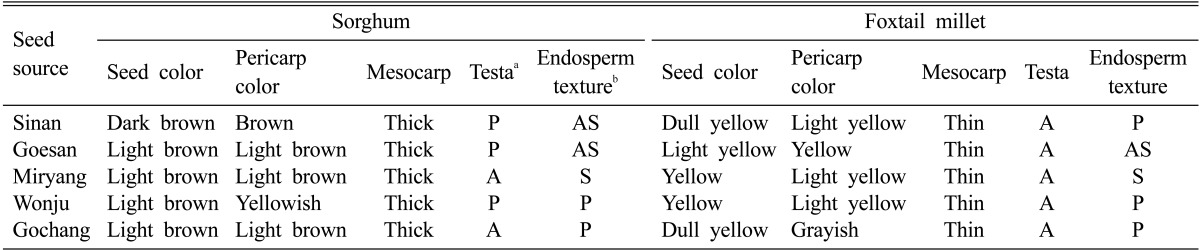
Table 1.
Phenotypic characteristics of the five sorghum germplasms collected from different locations of South Korea
aA, absence of a pigmented testa layer; P, presence of a pigmented testa.
bP, partly corneous; AS, almost starchy; S, completely starchy.
Seed health testing methods
Three conventional seed health testing methods as reported by Mathur and Kongsdal [18] were used to detect the mycoflora associated with sorghum and foxtail millet seeds. Dry inspection methods were used in the study to detect sclerotia of ergot in the seed samples. One hundred grams each of the foxtail millet and sorghum seeds were used for visual inspection using a stereo microscope. The standard blotter method was used to detect a wide range of fungi that are able to easily arise from seeds in the presence of humidity. Four hundred untreated pure seeds from each samples were plated on moisten blotters (Whatman No. 1) in a 9 cm diameter plastic Petri dish at the rate of 300 seeds per dish and the seeds were incubated for 7 days at 20~25℃ under alternating cycles of 12 hr near ultraviolet light and 12 hr darkness. Individual seeds were examined under a stereomicroscope for the presence and absence of fungi. Identification was confirmed by examining for the presence of mycelium and/or conidia under a compound microscope. The fungal species present on each of the seeds were recorded and the percent incidence of each fungus per sample was computed. The washing test was used to detect teliospores of smut and oospores that were present on the surface of the seeds. Sorghum seeds (5 g) and foxtail millet seeds (10 g) were shaken in 25 mL of distilled water. The suspension was centrifuged at 3,000 rpm for 5 min. The sediment was suspended in 1 mL of distilled water and then it was examined under a compound microscope. The agar plate method was used to further characterize the fungal growth that was observed in the plate. A mix culture was re-isolated into pure cultures using potato dextrose agar as a medium. After seven days of incubation, detailed examination was done by preparing semi-permanent slides and examining them under a compound microscope.
Germination test
In vitro germination rate tests for the disinfected and non-disinfected seeds were conducted in order to determine the effect of mycoflora associated with the seeds. Disinfected seeds were treated with 1% sodium hypochlorite (NaOCl) solution and no treatment was applied for the non-disinfected seeds. There were two separate methods of testing the germination of sorghum and foxtail millet. The 'between paper' method was used for testing the viability of sorghum seeds. The seeds were germinated between two layers of moist paper towels. The seeds were arranged in rows at regular intervals 4 cm from the top edge and with leaving a 3~4 cm gap on the sides. The seeds were covered with another sheet of dry paper towel. The paper was loosely rolled and a paper clip was used to hold the rolled paper towels from falling apart. The tray containing the rolls was incubated at 20℃ for 4 days. Foxtail millet seeds were germinated on a top moist paper (Whatman Grade 181) in a 9 cm diameter petri dish. Each plate was moistened with 4 mL of distilled water. Fifty seeds in each plate were spread at a regular distance on the surface of the paper. The petri dishes were covered with a plastic bag to prevent drying and they were incubated at 20℃ for 4 days. Germination was considered present when the radical protrudes by 2~4 mm. The percent of germination of sorghum and foxtail millet was calculated and recorded after 24 hr. The effect of seed-borne mycoflora on sorghum and foxtail millet seeds was also tested in vivo. A completely randomized design with three replications was used in this study. The germination rate was tested using disinfected and non-disinfected sorghum seeds and foxtail millet seeds. Two types of soil were used in the study: sterilized and unsterilized cocopeat soil. Five different seed sources were used in the experiment. Five hundred seeds of sorghum and foxtail millet per replicate were sown in plastic trays and the percent germination was recorded 7 days after sowing. Three consecutive trials were conducted to determine the effects of disinfection of seeds and sterilization of soil.
Component plating test
Forty five seeds per sample were used for detecting the mycoflora associated with different components of the sorghum and foxtail millet seeds. Seed samples were soaked in sterile distilled water in 9 cm diameter Petri dishes for 24 hr. Prior to separation of different seed components, the seeds were disinfected with 1% sodium hypochlorite (NaOCl) for 5 min and then they were washed three times with distilled water. The seeds were allowed to air dry for 1 hr under a laminar flowhood. The soaked seeds were dissected using a sterile scalpel under a stereomicroscope and they were separated into three parts; pericarp, endosperm and embryo. Potato carrot agar was used as a medium for initiating fungal growth from the different seed components. The petri dishes were incubated for 7 days in the same way as the blotter method using alternating cycles of 12 hr NUV light and 12 hr darkness. The fungi on the different seed components were observed under a stereo microscope and a compound microscope for identification. Identification of fungi was carried out based on the morphological characteristics described by previous studies [18-24].
Seedling emergence and mortality and the evaluation of seed-to-seedling transmission The effects of the fungi isolated from the incubated seeds of sorghum and foxtail millet were tested on the seedling emergence and seedling mortality. One hundred fifty seeds of the sorghum and foxtail millet that were previously disinfected in 70% ethanol for 5 min were used. The seeds were inoculated by immersing the seeds in a standardized solution containing spores at a concentration of 1 × 106 spores/mL of the five dominant species isolated from the blotter test and the agar plate method. A haemacytometer was used for determining the quantity of spores per mL. The seeds were sown in a plastic pot (25 seeds per pot) containing sterilized soil and by following the procedure used by Mathur et al. [25]. The pots were kept in a growing room for 10 days under 12 hr fluorescent light/12 hr darkness at 25~ 29℃. Untreated seeds, seeds disinfected with ethanol and seeds treated with a chemical called calthio (20% Lindane, 25% Thirame) were used as controls. A completely randomized design was used with three replications. Ten days after sowing, the seedlings' emergence and the seedlings' mortality were evaluated and the percent of emerged seedlings and the percent of dead seedling were calculated. To evaluate seed-to-seedling transmission of the fungi, ten seedlings from each treatment were cut at the level of the coleoptiles, disinfected in 70% ethanol for 2 min and plated on moistened blotter papers in a plastic box for 5 days. The plants infected by the target fungus were counted using a stereomicroscope and the result was expressed as a percent. The severity of infection (the capacity of the fungus to propagate inside the seedling) was estimated for the incubated seedlings by assigning a score based on the presence or absence of the fungus in the plant parts: score 1 is for healthy plants, score 2 is for slightly infected plants (fungus present on the plant stem) and score 3 is for highly infected plants (fungus present on the plant stem and/or leaves). An index of severity was calculated following the formula used by Williams and Singh [26] and the result was expressed as a percent:
S (%) = {Σ(xi - 1)/[E(xi) - 1] × N} × 100
Where:
Xi = Note attributed to each plant from class I
Ni = Number of plants from class I
E(x) = Range of the scale of notation (3)
N = Total number of observed seedlings (10)
S = Severity of infection or the capacity of the fungus to invade the plant (%). The percents of the severity of infection were transformed into Arcsine values before performing the statistical analysis
Data analysis
The main seed-borne fungi of sorghum and foxtail millet, the effect of fungi on seedlings, seedling emergence and seedling mortality and the main pathogenic and seed-transmitted fungi were determined by analysis of variance (ANOVA) using a completely randomized design. The significance (p < 0.05) of differences between treatments were determined using the Duncan's multiple ranged test of SAS ver. 8 (SAS Institute Inc., Cary, NC, USA).
Results
Seed-borne mycoflora of the sorghum and foxtail millet
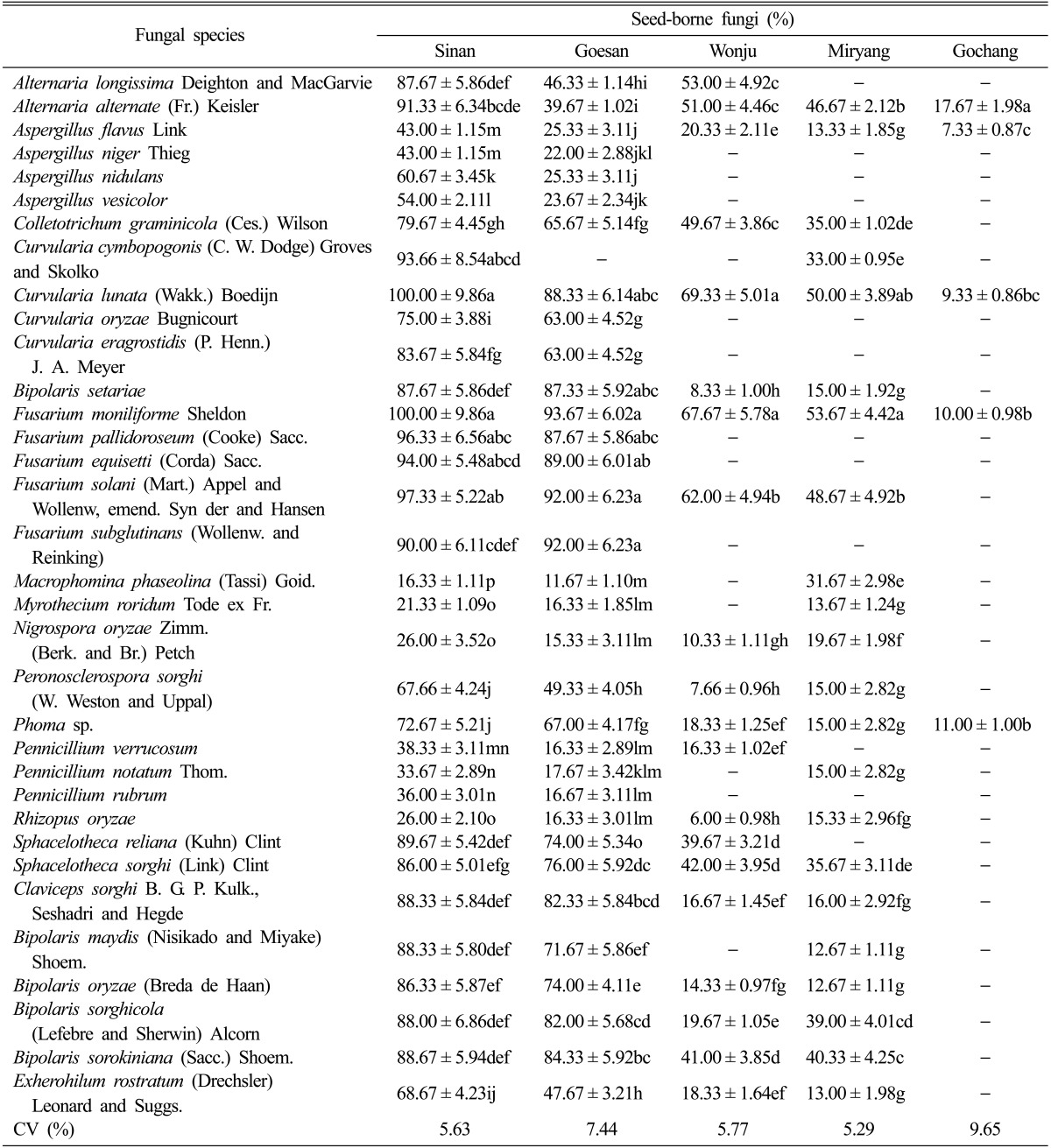
The assessment of seed-borne mycoflora revealed that more seed-borne fungi were recovered from sorghum as compared to that of foxtail millet. The occurrence of seed-borne mycoflora also varied between locations where out of the five growing areas of sorghum, Sinan, Jeonnam Province (34 fungal species) and Goesan, Chungbuk Province (33 fungal species) recorded the highest number of seed-borne mycoflora followed by Miryang, Gyeongnam Province, Wonju, Gangwon Province and Gochang, Jeonbuk Province (22, 20, and 5 fungal species, respectively) (Table 2). The seed-borne mycoflora isolated in sorghum were Alternaria longissima, Alternaria alternata, Aspergillus flavus, Aspergillus niger, Aspergillus nidulans, Aspergillus vesicolor, Colletotrichum graminicola, Curvularia cymbopogonis, Curvularia lunata, Curvularia oryzae, Curvularia eragrostidis, Bipolaris setariae, Fusarium moniliforme, Fusarium pallidoroseum, Fusarium equisetti, Fusarium solani, Fusarium subglutinans, Macrophomina phaseolina, Myrothecium roridum, Nigrospora oryzae, Peronosclerospora sorghi, Phoma sp., Pennicillium verrucosum, Pennicillium notatum, Pennicillium rubrum, Rhizopus oryzae, Sphacelotheca reiliana, Sphacelotheca sorghi, Claviceps sorghi, Bipolaris maydis, Bipolaris oryzae, Bipolaris sorghicola, Bipolaris sorokiniana and Exherohilum rostratum. F. moniliforme, C. lunata, F. pallidoroseum, F. solani, and Fusarium equisetti were recorded as the five dominant seed-borne mycoflora isolated from Sinan, Jeonnam Province. The percent of seed-borne infection ranged from 94% to 100%. Fusarium species such F. moniliforme, F. solani, F. subglutinans, F equisetti, and F. pallidoroseum dominated the seeds collected from Goesan, Chungbuk Province. The percent of seeds with mycofloral infection ranged from 87.67% to 93.67%. The five dominant seed-borne mycoflora isolated from Wonju were C. lunata, F. moniliforme, F. solani, and A. longgisima. Out of twenty two fungal species isolated from Miryang, Gyeongnam Province, the five most dominant seed-borne mycoflora were F. moniliforme, C. lunata, F. solani, A. alternata, and B. sorokiniana. The percent infection of these fungi ranged from 40.33% to 53.67%. Five fungal species only were isolated from the seeds collected from Gochang, Jeonbuk Province. The percent of infections of seeds were lower at 7.33% to 17.67% and the fungal species isolated were A. alternata, Phoma sp., F. moniliforme, C. lunata, and A. flavus.
Table 2.
Seed-borne fungi associated with the sorghum seed samples collected from five different locations in Southern Koreaa
aA total number of 900 seeds were studied for the presence of seed-borne fungi, and this was replicated three times. Three trials were conducted and the average percent occurrence of fungi was counted and recorded. Each value represents the mean ± SD. Means followed by the same letter(s) in a column did not differ significantly at the 1% level by Duncan's multiple ranged test.
CV, coefficient of variation.
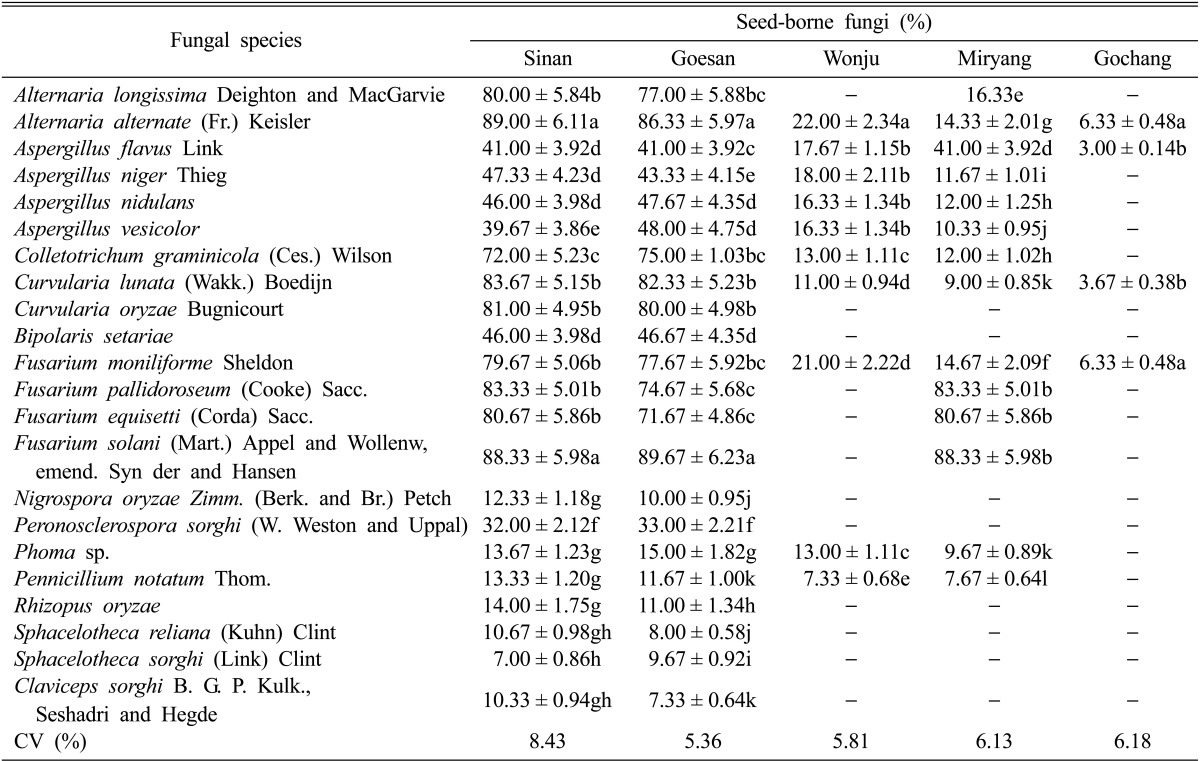
On the other hand, only twenty four fungal species were isolated from the foxtail millet derived from Sinan, Jeonnam Province and Goesan, Chungbuk Province, 14 seed-borne fungi were isolated from the seeds collected from Miryang, Gyeongnam Province, 10 fungal species were isolated from Wonju, Gangwon Province and only 4 fungal species were isolated from Gochang, Jeonbuk Province (Table 3). The seed-borne fungi isolated from foxtail millet were A. longissima, A. alternata, A. flavus, A. niger, A. nidulans, A. vesicolor, C. graminicola, C. lunata, C. oryzae, B. setariae, F. moniliforme, F. pallidoroseum, F. equisetti, F. solani, N. oryzae, P. sorghi, Phoma sp., P. notatum, R. oryzae, S. reliana, S. sorghi, and C. sorghi. The percent of seeds infected by mycoflora from the seeds collected from Sinan, Jeonnam Province ranged from 80.67% to 89.00%. The five dominant seedborne fungi were A. alternata, F. solani, C. lunata, F. pallidoroseum, and F. equisetti. In Goesan, Chungbuk Province, the range of percent infection was 77.67% to 89.67% and the five dominant seed-borne fungi were F. solani, A. alternata, C. lunata, F. pallidoroseum, and C. oryzae. A low percent of seed-borne infection was observed for Wonju, Gangwon Province. The highest value of infection ranged from 16.33% to only 22.00%. A. alternata recorded the highest percent infection followed by A. niger, A. flavus, and A. vesicolor. The top 4 most dominant seed-borne fungi in Miryang, Gyeongnam Province were F. solani, F. pallidoroseum, A. flavus and A. nidulans. The percent of infected seeds was 12.00% to 83.33%, which was lower compared to the percent of infected seeds observed for seeds from Sinan, Jeonnam and Goesan, Chungbuk provinces. About 3.00% to 6.33% of the seeds were infected with seed-borne mycoflora from the seed from Gochang, Jeonbuk Province and there were 4 fungal seed-borne mycoflora: A. alternata, F. moniliforme, C. lunata and A. flavus.
Table 3.
Seed-borne fungi associated with the foxtail millet seed samples collected from five different locations in Southern Koreaa
aA total number of 900 seeds were studied for the presence of seed-borne fungi, and this was replicated three times. Three trials were conducted and the average percent occurrence of fungi was counted and recorded. Each value represents the mean ± SD. Means followed by the same letter(s) in a column did not differ significantly at the 1% level by Duncan's multiple ranged test.
CV, coefficient of variation.
Comparison of the in vitro percent germination of the disinfected and non-disinfected seeds of sorghum and foxtail millet
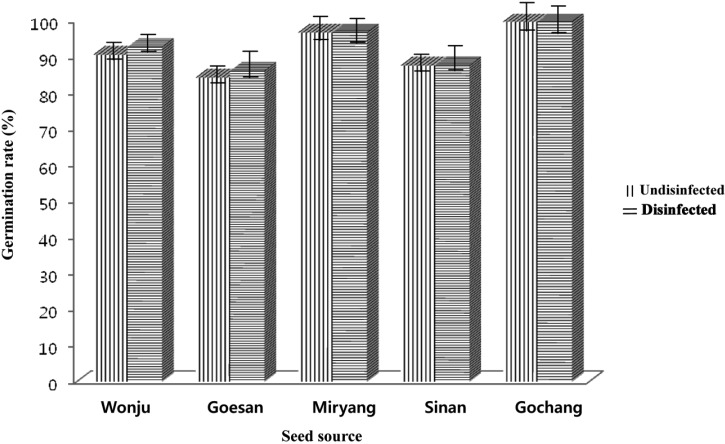
Significant differences were observed depending on the types of treatment the seeds received prior to germination as well as the percent germination of the sorghum and foxtail millet collected from different growing areas of Southern Korea (Figs. 1 and 2). Sorghum and foxtail millet seeds disinfected with 1% NaOCl had higher germination rates compared to the non-disinfected seeds. The percent of germination of the collected seeds from different growing areas in Southern Korea showed significant differences. Sorghum seeds collected from Gochang, Jeonbuk Province had the highest percent germination (93.17%) followed by the seeds collected from Miryang, Gyeongnam Province (84.77%), Wonju, Gangwon Province (84.17%) and Goesan, Chungbuk Province (76.67%), and the seeds from Sinan, Jeonnam Province (39.83%) had the lowest germination rate. The germination rate of foxtail millet seeds from Gochang, Jeonbuk Province had the highest germination value (99.84%) followed by Miryang, Gyeongnam Province, Wonju, Gangwon Province, Sinan, Jeonnam Province and Goesan, Chungbuk Province with germination rates of 96.84%, 92.84%, 87.67%, and 86.17%, respectively.
Fig. 1.
Comparison of the germination rate (%), using the paper towel method, between nondisinfected and disinfected sorghum seeds collected from different locations in South Korea.
Fig. 2.
Comparison of the germination rate (%), using top of paper method, between non-disinfected and disinfected foxtail millet seeds collected from different locations in South Korea.
Comparison of the in vivo percent germination of the disinfected and undisinfected seeds of sorghum and foxtail millet sown in sterilized and unsterilized soil
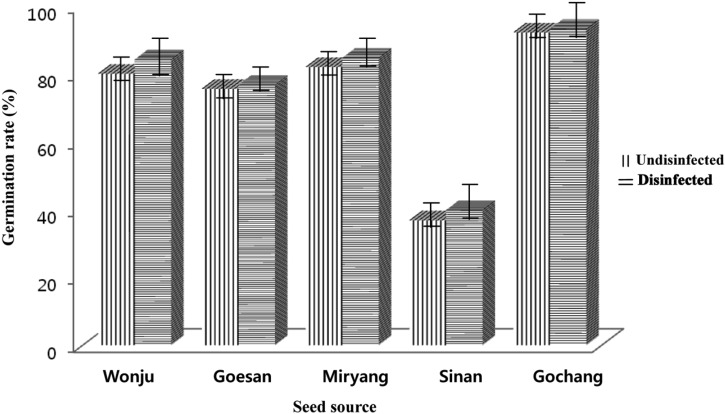
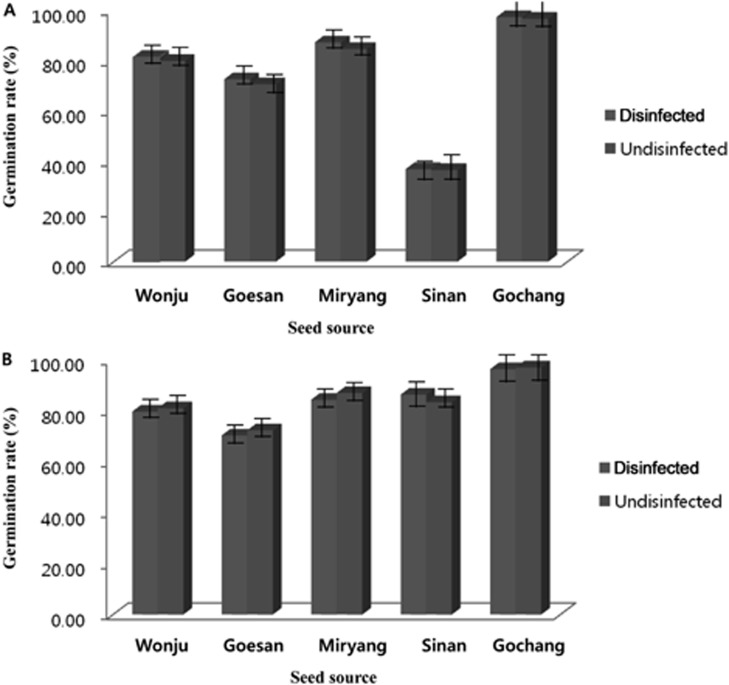
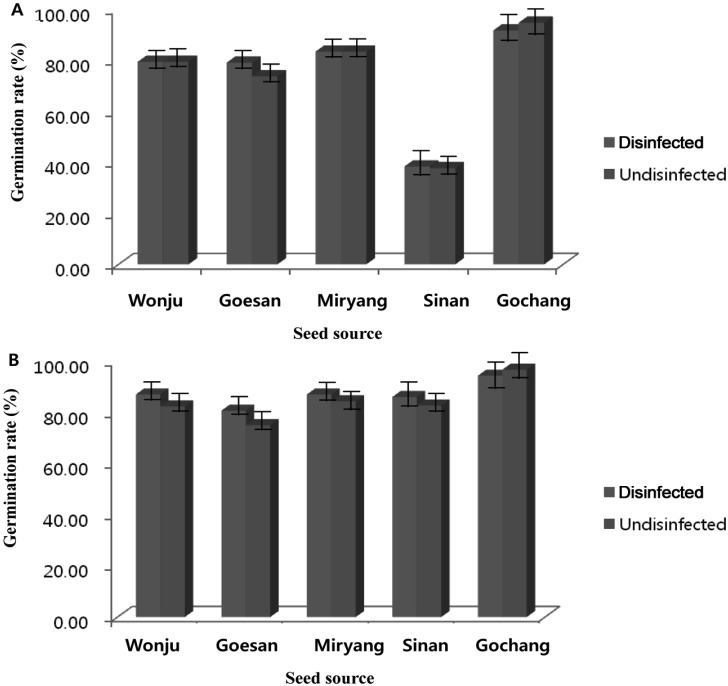
In vivo germination was conducted to compare the effect of treating the seeds prior to seed sowing and the effect of using sterilized and unsterilized soil. The results of the experiment showed that significant differences of the germination rate of sorghum and foxtail millet were observed by treating the seeds and by using 2 types of soil (sterilized or unsterilized). Using 2 types of soil and treating the seeds prior to seed sowing had a direct effect on the germination rate. The germination rates of the sorghum and foxtail millet seeds were significantly higher for the disinfected seeds and using sterilized soil compared to that of non-disinfected seeds and using unsterilized soil (Figs. 3 and 4). Disinfected or undisinfected sorghum seeds sown in sterilized soil and that were collected from Gochang, Jeonbuk Province had the highest germination rate (96.33% to 97.00%) and the germination rate was significantly reduced when disinfected or non-disinfected sorghum seeds were sown in unsterilized soil (91.67% to 94.67% respectively). Comparable germination rates were observed for the disinfected or undisinfected sorghum seeds from Miryang, Gyeongnam Province, Wonju, Gangwon Province and Goesan, Chungbuk Province with of values of 83.33% to 87.00%, 79.30% to 81.00%, and 70.33% to 79%, respectively. There was an abrupt decrease of the germination rate of the sorghum collected from Sinan, Jeonnam Province. The germination rate ranged from 36.33% to 38.33% using either disinfected or non-disinfected seeds sown in sterilized or unsterilized soil (Fig. 3A and 3B). The results indicated that the germination rate of foxtail millet using disinfected seeds and sterilized soil was better compared to that of non-disinfected seeds sown in unsterilized soil, yet a however higher percent of germination was observed for foxtail millet compared to the germination rate derived from sorghum. The range of germination of foxtail millet was 70.33% to 97%. The germination performance of the seeds collected from Gochang, Jeonbuk Province had the highest value (94.33% to 97%) followed by Miryang, Gyeongnam, Sinan, Jeonnam, Wonju, Gangwon and Goesan, Chungbuk provinces with values of 84.33% to 87.00%, 82.67% to 86.00%, 79.67% to 87%, and 70.33% to 80.67%, respectively.
Fig. 3.
Comparison of in vivo germination of disinfected and undisinfected seeds of sorghum sown in (A) sterilized soil and (B) unsterilized soil.
Fig. 4.
Comparison of in vivo germination of disinfected and nondisinfected seeds of foxtail millet sown in (A) sterilized soil and (B) unsterilized soil.
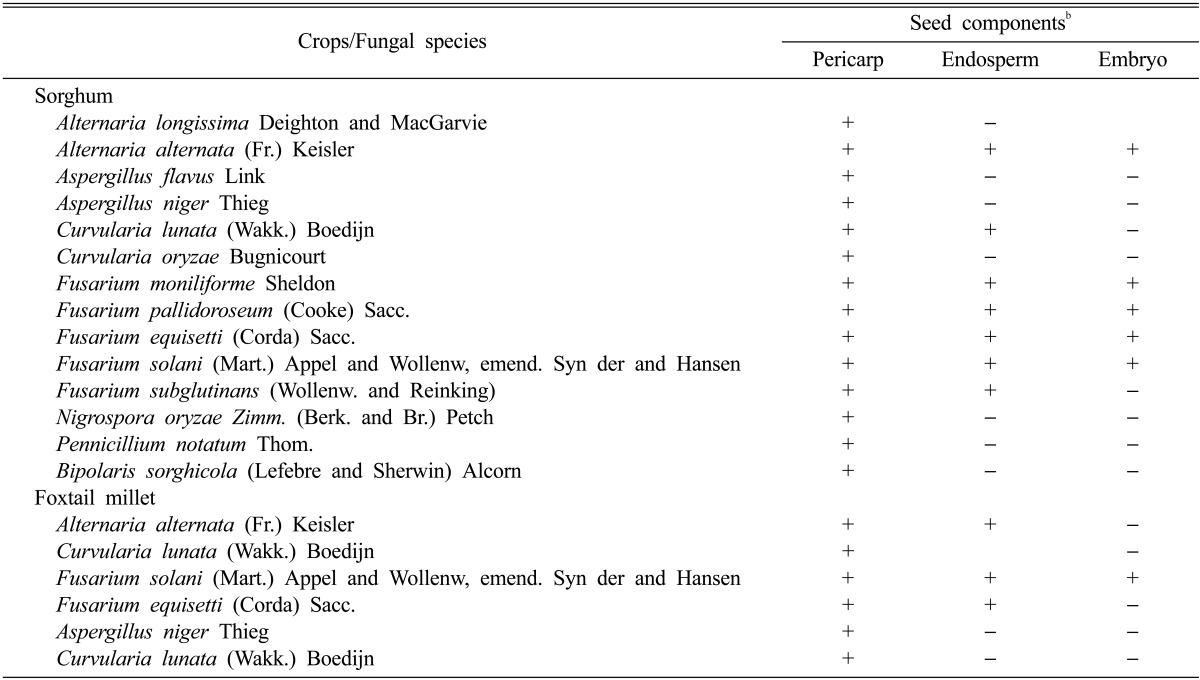
Percent recovery of seed-borne mycoflora from sorghum and foxtail millet from the different seed components
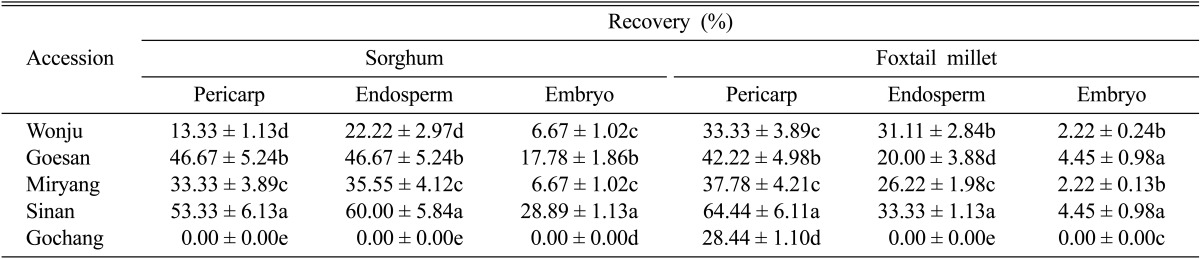
Significant variation was observed in terms of seed-borne fungal recovery from the different seed components. There was a significant difference of percent of fungi recovered between the locations where the seeds were collected. Generally, more fungal species were recovered from the pericarp of both sorghum and foxtail millet than from the endosperm and embryo (Table 4). Seed samples collected from Sinan, Jeonnam Province exhibited the greatest seed-borne recovered fungi (66.67%) followed by Goesan, Chungbuk (46.67%), Miryang, Gyeongnam (37.78%), Wonju, Gangwon (33.33%) and Gochang, Jeonbuk provinces (26.67%). The fungal growth isolated from the endosperm ranged from 0% to 60%, while that from the sorghum embryos ranged from 0% to 28.89%. The fungal growth from foxtail millet ranged was from 28.44% to 64.44%, while that from the foxtail millet endosperm and embryo ranged from 0% to 33.33% and 0% to 4.45%, respectively. Fifteen fungal species were recovered and identified from the pericarp of sorghum seeds (Table 5). The identified species were A. longissima, A. alternata, A. flavus, A. niger, C. lunata, C. oryzae, F. moniliforme, F. pallidoroseum, F. equisetti, F. solani, F. subglutinans, Nigrospora oryzae, P. notatum, and B. sorghicola. From the fifteen fungal species isolated from the pericarp, seven species were further colonized from the endosperm and these were A. alternata, C. lunata, F. moniliforme, F. pallidoroseum, F. equisetti, F. solani, and F. subglutinans. The five fungal species from the embryo were A. alternata, F. moniliforme, F. pallidoroseum, F. equisetti, F. solani, and F. subglutinans (Table 4). Only five fungal species were recovered from the pericarp of foxtail millet (A. alternata, C. lunata, F. solani, F. equisetti, and A. niger), three species were isolated from the endosperm of foxtail millet (A. alternata, F. solani, and F. equisetti) and F. solani was the only species isolated from the seed embryo of foxtail millet.
Table 4.
Percent recovery of seed-borne fungi from different seed components of sorghum and foxtail milleta
aA total number of 135 seeds were observed for the presence of seed-borne fungi, and this was replicated three times. Three trials were conducted and the average percent occurrence of fungi was counted and recorded. Each value represents the mean ± SD. Means followed by the same letter(s) in a column did not differ significantly at the 1% level by Duncan's multiple ranged test.
Table 5.
Summary of seed-borne fungi recovered from the seed components of sorghum and foxtail milleta
aA total number of 900 seeds were observed for the presence of seed-borne fungi, and this was replicated three times. Three trials were conducted and the average percent occurrence of fungi was counted and recorded.
b+, present; -, absent.
Seedling emergence and mortality and evaluation of seed-to-seedling transmission
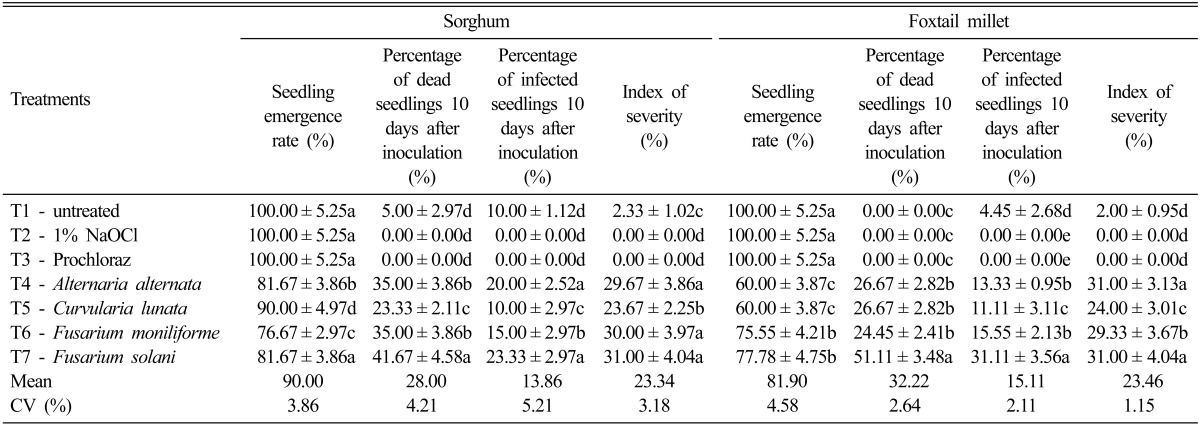
Significant differences were observed for seedling emergence of sorghum as affected by inoculation of different seed-borne fungal organisms (Table 6). Untreated seeds (T1), seeds disinfected with 1% NaOCl (T2) and seeds disinfected with Prochloraz (T3) showed 100% germination. The percent of seedling emergence was significantly affected by the inoculation of the four dominant seed-borne fungi. Inoculation of F. moniliforme (T6) exhibited the highest reduction of seedling emergence (76.67%) followed by F. solani (T7) and A. alternata (T4) (81.67% respectively) and that for the seedling emergence of C. lunata was 90.0% (T5). The percentage of dead seedlings 10 days after inoculation was also significantly differently different according to the four dominant seed-borne fungi that were inoculated. The percent of dead seedlings ranged from 23.33% to 41.67%. Inoculation with F. solani (T7) had the highest percentage of dead seedlings 10 days after inoculation (41.67%) followed by the plants inoculated with F. moniliforme (T6) and A. alternata (T4) at 35.0%, respectively, and the lowest was C. lunata (T5) at 23.33%. The percent of dead seedlings from the untreated seeds (T1), the 1% NaOCl treated seeds and the Prochloraz treated seeds were comparable with each other and the ranged of dead seedlings were from 0~5%. The percentage of infected seedlings 10 days after inoculation was also significantly different. All the plants inoculated with seed-borne fungi exhibited variable degrees of infection. The value of infection ranged from 10 to 23.33% in which F. solani (T7) and A. alternata (T5) recorded the highest rate of infected seedlings at 20.0 to 23.33%, respectively. Zero percent of infected seedlings were observed in T2 and T3. The percent index of severity ranged from 23.67% to 31.0% with the plants inoculated with F. solani having the highest percentage of severity index. Zero index of severity was observed on seeds in T2 and T3 respectively.
Table 6.
Seedling emergence, disease transmission and severity of infection as affected by different fungal speciesa
aA total number of 1,500 germinated seeds were observed and this was replicated three times. Three trials were conducted and the average percent occurrence of fungi was counted and recorded. Each value represents the mean ± SD. Means followed by the same letter(s) in a column did not differ significantly at the 1% level by Duncan's multiple ranged test.
CV, coefficient of variation.
The percent of seedling emergence was significantly lower for foxtail millet as compared to that of sorghum. The percentage of seedling emergence ranged from 60% to 77.78% and the plants treated with F. solani exhibited the highest seedling emergence rate. A comparable effect was observed for the plants treated with A. alternata (T4) and C. lunata (T5) with 60% seedling emergence, respectively. Untreated seeds (T1), seeds treated with 1% NaOCl (T2) and seeds treated with chemical control (T3) had 100% seedling emergence. The percentage of dead seedlings 10 days after inoculation ranged from 24.45% to 51.11% and the plants inoculated with F. solani (T7) had the highest percentage of dead seedlings. The percent of infected seedlings 10 days after inoculation was also significantly different. The plant inoculated with F. solani (T7) had 31.11% infected seedlings followed by comparable effects of F. moniliforme (T6) and A. alternata (T4) at 15.55% and 13.33% respectively. The index of severity ranged from 24.0% to 31.0%. The plants inoculated with F. solani (T7) and A. alternata (T4) had the highest index of severity (31.0%, respectively). A comparable effect was observed for the untreated seeds (T1), the seeds treated with 1% NaOCl (T2) and the chemical control (T3). The index of severity from these treatments ranged from 0 to 2%.
Discussion
Good seed must have a high germination rate and be free of seed-borne pathogens and good seeds are recognized as an important input in any agricultural production system [27]. The presence of seed-borne mycoflora and the effect of seed-borne fungi on germination and disease transmission are important factors in determining the reaction of sorghum and foxtail millet to grain mold [28-32]. This study is the most updated assessment of seed-borne mycoflora on stored sorghum and foxtail millet in different growing areas of the southern part of Korea. The assessment of seed-borne mycoflora revealed that more seed-borne fungi were recovered from sorghum compared to that of foxtail millet (Tables 1 and 2). Mycological analysis of seeds naturally infected with grain mold showed that 34 fungal species of seed-borne fungi were observed on sorghum and only 24 fungal species were isolated from foxtail millet. The most dominant fungal species that were isolated from five growing areas of sorghum were ,A. alternata A. flavus, C. lunata, F. moniliforme, and Phoma sp. These fungi heavily infected both sorghum and foxtail millet. The findings of our study coincided with the data obtained from India, [17, 33-35], Bangladesh [36], Burkina Faso [27], Nigeria [37], Pakistan [38], and the USA [16]. The high frequency of occurrence of the above organisms was also observed in the previous surveys done by Mathur et al. [39] and Tarp et al. [40]. These five dominant species were aggressive in causing grain mold on sorghum and foxtail millet. Hepperly et al. [41] identified F. moniliforme, C. lunata, and Alternaria spp. as the most frequently recovered fungal species from natural grain mold-infected seed. F. moniliforme has been shown to be comprised of a number of Fusarium species. This would suggest that the five dominant species would be the most important fungal species for evaluating sorghum and foxtail millet germplasm for resistance to grain mold in South Korea. The occurrence of all the major fungi in both sorghum and foxtail millet seeds from South Korea could be related to the large number of seed samples coming from this agro-ecological zone. In addition, the total production is higher (data not shown) and the climate is more favorable for fungal colonization in Sinan and Goesan provinces. The high infection of seed-borne fungi in sorghum could be attributed to the starch component of the seeds compared to the starch component of millet. The average starch content of sorghum is 69.5%, while that of millet is from 56.3% to 63.7% [42]. The grain hardness is other factor that may contribute to fungal infection as foxtail millet is harder compared to sorghum. Grain hardness has been implicated in reducing mold infestation [43-47]. Harder grains have lower mold infection compared to softer grains [48]. Many of the same factors that contribute to varietal differences of resistance to fungal infection are responsible for the varietal differences of resistance [49].
The in vitro germination rate was comparable between the disinfected and non-disinfected seeds. This study indicated that disinfection with hypochlorite could not be a guarantee that all microorganisms will be killed. The use of hypochlorite serves as surface disinfection where advance penetration of fungus to deeper layer of the cells prior to germination would not be suppressed, which was also confirmed by Melchers [50]. The low germination rate was due to the presence of microorganisms that affect the growth of newly emerging shoots. It was observed that germination occurred, but the fungal growth was faster. It means that there was an existing source of inoculums in the seeds, which will inhibit germination. The use of sodium hypochlorite helped in minimizing the incidence of superficial and fast growing fungi as well as common seed borne fungi like Aspergillus spp., Chaetomium spp., Cladosporium spp., Rhizopus spp., and Cephalosporium spp. Similar results were also obtained by Dawar and Ghaffar [51] for sunflower seeds. Surface disinfection of seed with 1% Na(OCl)2 reduced the incidence of Aspergillus spp. However, other slow growing deep seated seed borne fungi like Curvularia spp., Drechslera spp., Fusarium spp., Botryodiplodia theobromae, and Macrophomina phaseolina were detected at greater frequencies. These results are similar with the findings of Limonard [52]. Mycological analysis of disinfected and non-disinfected seeds gave only general information about inner seed infection, with assuming that fungi is present in non-disinfected seeds and absent in disinfected seeds and that the fungi were contaminated their surface and they did not penetrate the inner tissues. This information, although not very precise, can be a starting point to determine proper strategies of seed treatment.
In vivo experiment study proved that disinfected seeds using sterilized soil had a higher germination rate. This could be due to the effect of disinfection and sterilization of the soil substrate used in the nursery. There are fungi that are only saprophytic in nature and that could easily be removed by disinfection, yet there also seed-borne fungi that could penetrate the inner layer of the seeds. Hence, fungal organism that infect the endosperm and embryo usually do this during the seedling stage. The sterilized seeds showed a lesser population of seed-borne fungi than did the unsterilized seeds and this is agreement with the data of Limonard [53], who reported that disinfection effectively reduced the microbial contamination. Surface sterilization also has the advantage of minimizing competition among fungi on the seed [54]. Seed surface disinfection with HgCl2 usually suppresses the growth of saprophytic fungi and other superficial fast growing fungi [51, 55]. It was also observed by Ramakrishna et al. [56] that surface sterilization with 0.1 or 2.0 (w/v) HgCl2 for 3 min significantly decreased A. alternata, Fusarium sp., and Epicoccum purpurascens infection, but Niaz and Dawar [57] observed that surface disinfection of seed with 1% Na(OCl)2 reduced the incidence of Aspergillus spp., Chaetomium spp., Rhizopus spp., and Cephalosporium spp. Reduction of the frequency of fungi from sterilized sunflower seeds was also found by Sharfun-Nahar and Hashmi [58] and Bhutta [55]. At the beginning of storage, some of the fungi that infected the seeds were classified as field fungi and their population decreased with the increase of the storage duration [59, 60]. A clear result of the current study indicated that there was an increase germination rate in sterilized soil. However, if the seeds were infected with seed-borne fungi, then there is still a possibility of increasing the source of inoculum from the seeds and this eventually multiplied in the soil. Seeds infected with pathogens could play a role of transferring the pathogens to a new place and be a primary inoculum source in the field [61]. A drastic reduction in soil microbial activity may result in rapid re-infestation of the sterilized soil by a contaminating inoculum with this ultimately leading to the incidence of disease, which could even be higher than that in the non-treated soil due to a "biological vacuum" in the sterilized soil [62].
Significant variation was observed in terms of seed-borne fungal recovery from different seed components. More fungal species were recovered from the pericarp of both sorghum and foxtail millet than from the endosperm and embryo. The results derived from the current study found out that species of Alternaria, Curvularia, and Fusarium penetrated the endosperm layer of the seeds and further colonization of Alternaria and Fusarium was observed in the embryo. The percent recovery ranged from 2.22% to 64.44%. The same species of fungi were also observed in the penetrating endosperm and embryo in carrot seeds [61] and eggplant seeds [63]. It has been reported that A. alternata has many hosts and it mostly causes leaf blight and spots on a variety of plants [64, 65]. However, the fungus is also known as weak and opportunistic pathogen or a saprophyte in many plants. There has been no previous report that the fungus penetrates the endosperm and embryo of sorghum.
According to the infection experiments carried out with the inoculation of the most dominant recorded fungi (A. alternata, C. lunata, F. solani and F. moniliforme), it has a significant effect on the emergence of sorghum and foxtail millet seedlings. There were an increased percentage of dead seedlings and infected seedlings 10 days after inoculation and the index of severity was increased. It means that these tested isolates were pathogenic during the seedling stage. The same findings were also observed by Zida et al. [27] in sorghum and pearl millet, but our findings are not in accordance with the findings made by Mathur et al. [25]. However, considering the high infection level encountered in the seeds, further studies are necessary to elucidate the exact role of this fungus in seeds.
The results of our study indicated that seed-borne fungi could be the main fungal pathogen involved in sorghum and foxtail millet plant diseases in South Korea. The presence of many seed-borne pathogenic fungi at high levels from various geographical areas indicates a clear need for field surveys for these fungi and other pathogens. There is also a need to increase public awareness on the aspects related to seed health and to develop suitable management for improving the quality of seeds. Testing the seed health of major crops should be introduced as a national seed quality control system.
Acknowledgements
This study was carried out with the support of National Institute of Crop Science (NICS) (Project No. PJP071252011), Rural Development Administration, Miryang, Republic of Korea.
References
- 1.Soh HS, Lee SP, Ha YD. Total lipid content and fatty acid composition in Setaria italica, Panicum miliaceum and Sorghum bicolor. J East Asian Soc Diet Life. 2002;12:123–128. [Google Scholar]
- 2.Marathee JP. Structure and characteristics of world millet economy. In: Riley KW, Gupta SC, Seetharam A, Mushonga JN, editors. Advances in small millets. New Delhi: Oxford and IBH Publishing Co.; 1993. pp. 159–178. [Google Scholar]
- 3.Korea National Crop Experiment Station. Millet Production. Suwon: KNCES; 1999. p. 18. [Google Scholar]
- 4.Awika JM, Rooney LW. Sorghum phytochemical and their potential impact on human health. Phytochemistry. 2004;65:1199–1221. doi: 10.1016/j.phytochem.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Kayodé AP, Linnemann AR, Nout MJ, Van Boekel MA. Impact of sorghum processing on phytate, phenolic compounds and in vitro solubility of iron and zinc in thick porridges. J Sci Food Agric. 2007;87:832–838. [Google Scholar]
- 6.Soetan KO, Oyekunle MA, Aiyelaagbe OO, Fafunso MA. Evaluation of the antimicrobial activity of saponins extract of Sorghum bicolor. L. Moench. Afr J Biotechnol. 2006;5:2405–2407. [Google Scholar]
- 7.Kil HY, Seong ES, Ghimire BK, Chung IM, Kwon SS, Goh EJ, Heo K, Kim MJ, Lim JD, Lee D, et al. Antioxidant and antimicrobial activities of crude sorghum extract. Food Chem. 2009;115:1234–1239. [Google Scholar]
- 8.Kwak CS, Lim SJ, Kim SA, Park SC, Lee MS. Antioxidative and antimutagenic effects of Korean buckwheat, sorghum, millet and job's tears. J Korean Soc Food Sci Nutr. 2004;33:921–929. [Google Scholar]
- 9.Nishizawa N, Fudamoto Y. The elevation of plasma concentration of high-density lipoprotein cholesterol in mice fed with protein from proso millet. Biosci Biotechnol Biochem. 1995;59:333–335. doi: 10.1271/bbb.59.333. [DOI] [PubMed] [Google Scholar]
- 10.Choi YY, Osada K, Ito Y, Nagasawa T, Choi MR, Nishizawa N. Effects of dietary protein of Korean foxtail millet on plasma adiponectin, HDL-cholesterol, and insulin levels in genetically type 2 diabetic mice. Biosci Biotechnol Biochem. 2005;69:31–37. doi: 10.1271/bbb.69.31. [DOI] [PubMed] [Google Scholar]
- 11.Chandrashhekar A, Bandyopadhyay R, Hall AJ. Technical and institutional options for sorghum grain mold management; Proceedings of an International Consultation; 2000 May 18-19; Patancheru, India. Andhra Pradesh: International Crops Research Institute for the Semi-Arid Tropics; 2000. p. 229. [Google Scholar]
- 12.Guiragossian V. Sorghum production constraints and research needs in eastern Arica; Proceedings of 5th Regional Workshop Sorghum Millet Improvement in Eastern Africa; 1986 Jul 5-12; Bujumbura, Brundi. Nairobi: Institut des Sciences Agronomiques du Burundi; 1986. pp. 28–46. [Google Scholar]
- 13.Hulluka M, Esele JP. Sorghum production constraints and research needs in eastern Arica. In: de Milliano WA, Frederiksen RA, Bengston GD, editors. Sorghum and millet diseases, a second world review. Patencheru: ICRISAT; 1992. pp. 21–24. [Google Scholar]
- 14.M'Ragwa LR, Kanyenji BM. Strategies for improvement of sorghum and millet in Kenya. In: Meyonga JM, Bezuneh T, Youdeowei A, editors. Food grain production in semi-arid Africa. Ouagadougou: OAU/STRC-SAFGRAD; 1987. pp. 173–190. [Google Scholar]
- 15.Ngugi HK. Epedemiology and management of sorghum anthrachnose and leaf blight in Kenya [dissertation] Reading: University of Reading; 1998. [Google Scholar]
- 16.Erpelding JE, Prom LK. Variation for anthracnose resistance within the sorghum germplasm collection from Mozambique, Africa. Plant Pathol J. 2006;5:28–34. [Google Scholar]
- 17.Girish AG, Rao VP, Thakur RP. Diversity of grain mold fungi on selected sorghum genotypes. Indian Phytopathol. 2004;57:84–87. [Google Scholar]
- 18.Mathur SK, Kongsdal O. Common laboratory seed health testing methods for detecting fungi. Basserdorf: International Seed Testing Association; 2003. [Google Scholar]
- 19.Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. 4th ed. St. Paul: American Phytopathological Society Press; 1998. [Google Scholar]
- 20.David JC. Alternaria dauci. CMI Descriptions of pathogenic fungi and bacteria. No. 952. Kew: Commonwealth Mycological Institute; 1999. [Google Scholar]
- 21.Ellis EB. Dematiaceous Hyphomycetes. Kew: Commonwealth Mycological Institute; 1971. [Google Scholar]
- 22.Ellis EB. More dematiaceous hyphomycetes. Kew: Commonwealth Mycological Institute; 1976. [Google Scholar]
- 23.Ellis MB, Holiday P. Drechslera ciccans. Descriptions of pathogenic fungi and bacteria. Kew: Commonwealth Mycological Institute; 1972. [Google Scholar]
- 24.Chidambaran P, Mathur SB, Neergaard P. Identification of seed-borne Dreschslera species. Friesia. 1973;10:165–207. [Google Scholar]
- 25.Mathur SK, Ram N, Mathur SB. Seed-borne fungi of pearl millet (Pennisetum thypoides) and their significance. Seed Sci Tecnol. 1973;1:811–820. [Google Scholar]
- 26.Williams RJ, Singh SD. Control of pearl millet downy mildew by seed treatment with metalaxyl. Ann Appl Biol. 1981;79:263–268. [Google Scholar]
- 27.Zida PE, Sérémé P, Leth V, Sankara P, Somda I, Néya A. Importance of seed-borne fungi of sorghum and pearl millet in Burkina Faso and their control using plant extracts. Pak J Biol Sci. 2008;11:321–331. doi: 10.3923/pjbs.2008.321.331. [DOI] [PubMed] [Google Scholar]
- 28.Bandyopadhyay R, Chandrashekar A. Biology and management of sorghum grain mold; Proceedings of Consultative Group Meeting on Technical and Institutional Options for Sorghum Grain Mold Management; 2000 May 18-19; Patancheru, India. Patancheru: International Crop Research Institute for the Semi-Arid Tropics; 2000. p. 2. [Google Scholar]
- 29.Singh SD, Banyopadhyay R. Grain mold. In: Frederiksen RA, Odvody GN, editors. Compendium of sorghum diseases. St. Paul: American Phytopathological Society Press; 2000. pp. 38–40. [Google Scholar]
- 30.Esele JP, Frederiksen RA, Miller FR. Importance of plant color and modifier genes in grain mold resistance in sorghum. East Afr Agric For J. 1995;61:31–37. [Google Scholar]
- 31.Bandyopadhyay R, Mughogho LK. Evaluation of field screening techniques for resistance to sorghum grain molds. Plant Dis. 1988;72:500–503. [Google Scholar]
- 32.Ratnadass A, Butler DR, Marley PS, Bandyopadhyay R, Hess DE, Akintayo I. Sorghum head-bugs and grain molds in West and Central Africa: II. Relationship between weather, head-bug and mold damage on sorghum grains. Crop Prot. 2003;22:853–858. [Google Scholar]
- 33.Narasimhan K, Rangaswami G. Influenece of mould isolate from sorghum grain on viability of seed. Curr Sci. 1969;38:389–390. [Google Scholar]
- 34.Sundaram NV. Pathological research in India; International Sorghum Workshop; 1977 Mar 6-13; Hyderabad, India. Patancheru: International Crops Research Institute for the Semi-Arid Tropics; 1977. pp. 16–17. [Google Scholar]
- 35.Sreenivasa MY, Dass RS, Janardhana GR. Survey of postharvest fungi associated with sorghum grains produced in Karnataka (India) J Plant Prot Res. 2010;50:335–339. [Google Scholar]
- 36.Islam SM, Masum MM, Fakir MG. Prevalence of seed-borne fungi in sorghum of different locations of Bangladesh. Sci Res Essay. 2009;4:175–179. [Google Scholar]
- 37.Amusa NA, Falola O. Pre-harvest fungal infection of sorghum (Sorghum bicolor (L.) Moench) cultivars in the humid forest agroecological zones of Nigeria. Acta Fytotec Zootech. 2004;7:10. [Google Scholar]
- 38.Hussain A, Anwar SA, Sahi GM, Abbas Q, Imran Seed borne fungal pathogens associated with pearl millet (Pennisetum typhoides) and their impact on seed germination. Pak J Phytopathol. 2009;21:55–60. [Google Scholar]
- 39.Mathur SK, Mathur SB, Neergaard P. Detection of seed-borne fungi in sorghum and location of Fusarium moniliforme in seed. Seed Sci Technol. 1975;3:683–690. [Google Scholar]
- 40.Tarp G, Lange L, Kongsdal O. Seed-borne pathogens of major food crops in Mozambique. Seed Sci Technol. 1987;15:793–810. [Google Scholar]
- 41.Hepperly PR, Feliciano C, Sotomayor A. Chemical control of seedborne fungi of sorghum and their association with seed quality and germination in Puerto Rico. Plant Dis. 1982;66:902–904. [Google Scholar]
- 42.Jambunathan R, Subramanian V. Grain quality and utilization of sorghum and pearl millet; Biotechnology in tropical crop improvement: Proceedings of the International Biotechnology Workshop; 1987 Jan 12-15; Patancheru, India. Patancheru: International Crops Research Institute for the Semi-Arid Tropics; 1987. pp. 133–139. [Google Scholar]
- 43.Audilakshmi S, Stenhouse JW, Reddy TP, Prasad MV. Grain mold resistance and associated characters of sorghum genotypes. Euphytica. 1999;107:91–103. [Google Scholar]
- 44.Esele JP, Frederiksen RA, Miller FR. The association of genes controlling caryopsis traits with grain mold resistance in sorghum. Phytopathology. 1993;83:490–495. [Google Scholar]
- 45.Jambunathan R, Kherdekar MS, Stenhouse JW. Sorghum grain hardness and its relationship to mold susceptibility and mold resistance. J Agric Food Chem. 1992;40:1403–1408. [Google Scholar]
- 46.Kumari SR, Chandrashekar A, Shetty HS. Proteins in developing sorghum endosperm that may be involved in resistance to grain moulds. J Sci Food Agric. 1992;60:275–282. [Google Scholar]
- 47.Kumari SR, Chandrashekar A. Relationships between grain hardness and the contents of prolamin and three anti-fungal proteins in sorghum. J Cereal Sci. 1994;20:93–99. [Google Scholar]
- 48.Glueck JA, Rooney LW. Chemistry and structure of grain in relation to mold resistance; Sorghum diseases, a world review: Proceedings of the International Workshop on Sorghum Diseases; 1978 Dec 11-15; Hyderabad, India. Patancheru: International Crops Research Institute for the Semi-Arid Tropics; 1980. pp. 119–140. [Google Scholar]
- 49.Chandrashekar A, Satyanarayana KV. Disease and pest resistance in grains of sorghum and millets. J Cereal Sci. 2006;44:287–304. [Google Scholar]
- 50.Melchers LE. Sorghum kernel smut infection in relation to seed germination. Trans Kansas Acad Sci. 1956;59:320–326. [Google Scholar]
- 51.Dawar S, Ghaffar A. Detection of the seed-borne mycoflora of sunflower. Pak J Bot. 1991;23:173–178. [Google Scholar]
- 52.Limonard T. Ecological aspect of seed health testing. Proc Int Seed Test Assoc. 1968;33:343–513. [Google Scholar]
- 53.Limonard T. Ecological aspects of seed health testing. Wageningen: International Seed Testing Association; 1968. [Google Scholar]
- 54.Kaur B. Development and evaluation of methods for the detection of seed borne fungi in rice. Int J Educ Adm. 2010;2:123–130. [Google Scholar]
- 55.Bhutta AR. Comparison of cotton seed health testing method and their economics. Pak Cotton. 1988;32:146–153. [Google Scholar]
- 56.Ramakrishna N, Lacey J, Smith JE. Effect of surface sterilization, fumigation and gamma irradiation on the microflora and germination of barley seeds. Int J Food Microbiol. 1991;13:47–54. doi: 10.1016/0168-1605(91)90135-c. [DOI] [PubMed] [Google Scholar]
- 57.Niaz I, Dawar S. Detection of seed borne mycoflora in maize (Zea mays L.) Pak J Bot. 2009;41:443–451. [Google Scholar]
- 58.Sharfun-Nahar MM, Hashmi MH. Seed-borne mycoflora of sunflower (Heliathus annuus L.) Pak J Bot. 2005;37:451–457. [Google Scholar]
- 59.Worang RL, Dharmaputra OS, Syarief R, Miftahudin The quality of physic nut (Jatropha curcas L.) seeds packed in plastic material during storage. Biotropia. 2008;15:25–36. [Google Scholar]
- 60.Dharmaputra OS, Worang RL, Syarief R, Miftahudin The quality of physic nut (Jatropha curcas) seeds affected by water activity and duration of storage. Microbiol Indones. 2009;3:139–145. [Google Scholar]
- 61.Kim WG, Mathur SB. Detection of Alternaria spp. in carrot seeds and effect of the fungi on seed germination and seedling growth of carrot. Plant Pathol J. 2006;22:11–15. [Google Scholar]
- 62.Baker KF. Principles of heat treatment of soil and planting material. J Aust Inst Agric Sci. 1962;28:118–126. [Google Scholar]
- 63.Habib A, Sahi ST, Ghazanfar MU, Ali S. Location of seedborne mycoflora of eggplant (Solanum melongena L.) in different seed components and impact on seed germinability. Int J Agric Biol. 2007;9:514–516. [Google Scholar]
- 64.Farr DF, Bills GF, Chamuris GP, Rossman AY. Fungi on plants and plant products in the United States. St. Paul: American Phytopathological Society Press; 1989. [Google Scholar]
- 65.Rotem J. The genus Alternaria: biology, epidemiology, and pathogenicity. St. Paul: American Phytopathological Society Press; 1994. [Google Scholar]