Abstract
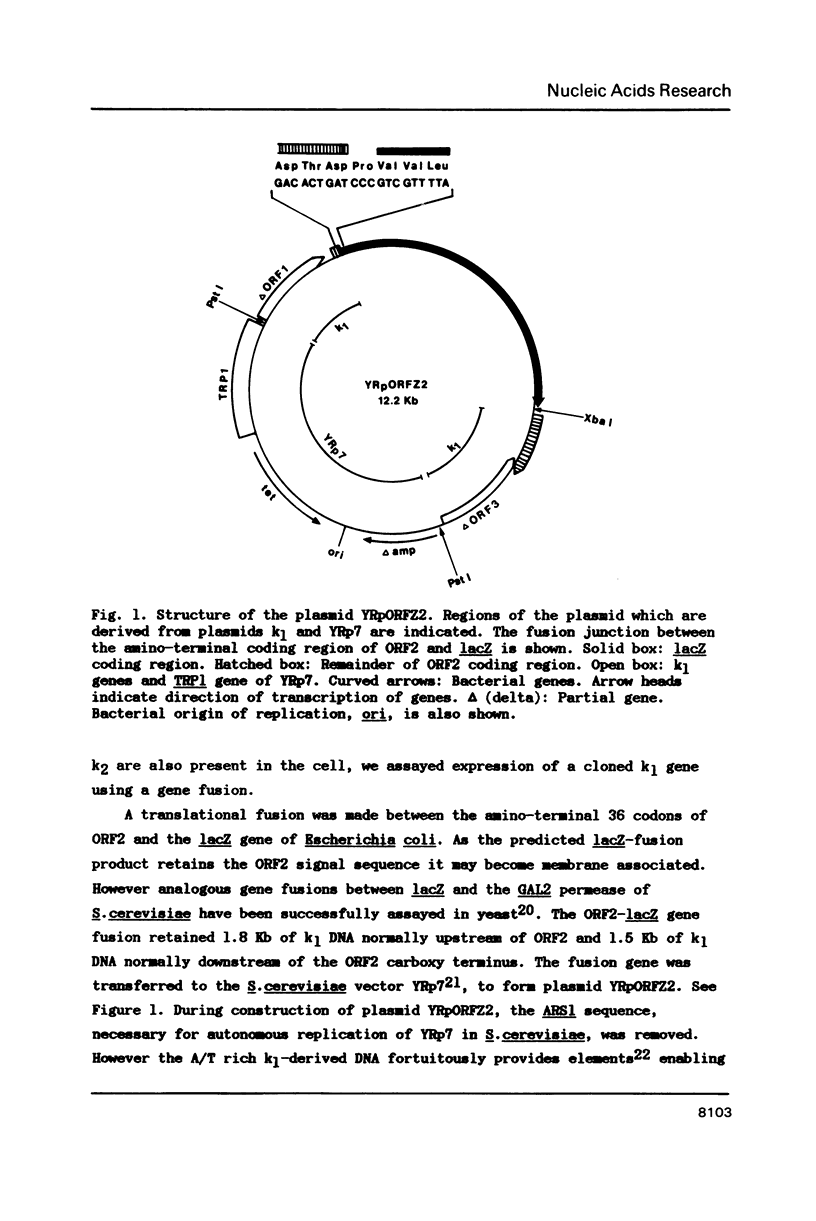
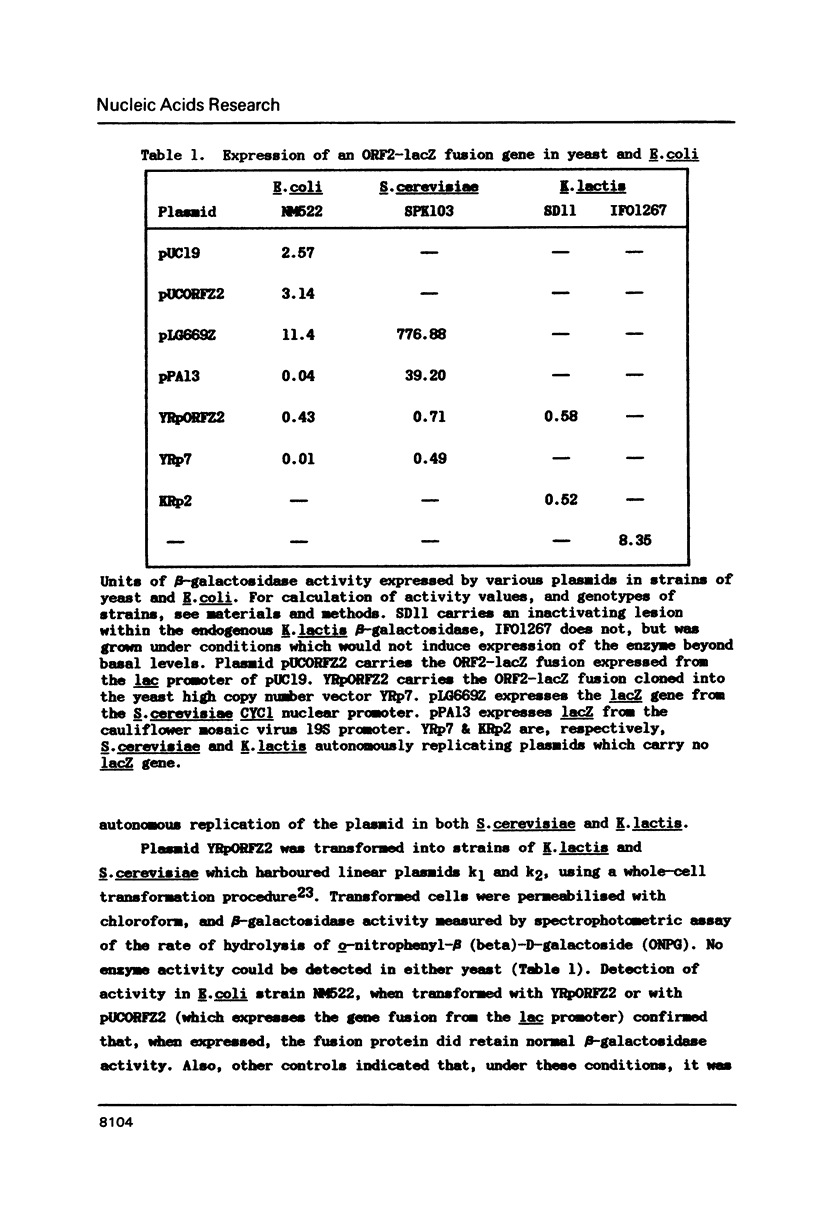
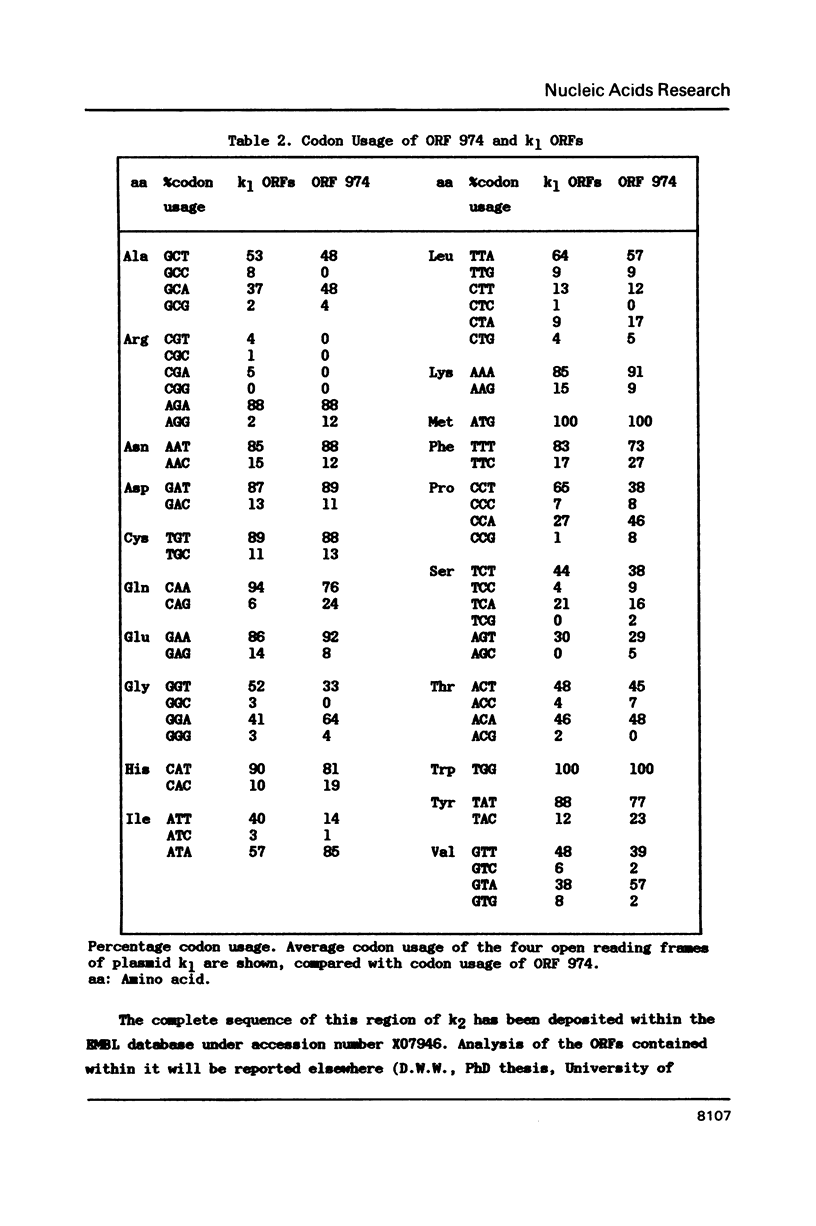
Strains of the yeast Kluyveromyces lactis that produce killer-toxin have been found to contain two linear dsDNA plasmids, k1 (8.9 Kb) and k2 (13.4 Kb). The four transcribed open reading frames of plasmid k1 contain no recognisable yeast nuclear expression signals. Moreover, a toxin subunit gene fused with the lacZ gene of Escherichia coli is not detectably expressed when introduced to K.lactis or Saccharomyces cerevisiae on a nuclear vector, even when native k1 and k2 are present in the cell. This and other evidence is consistent with the hypothesis that k1 and k2 reside in an extranuclear location, and do not utilise the nuclear RNA polymerases I, II or III for transcription of their genes. Sequencing of plasmid k2, which is thought to encode factors necessary for the maintenance or expression of k1, reveals an open reading frame predicted to encode a 974 amino acid polypeptide with homology to several DNA-directed RNA polymerases. We suggest that this is a component of a novel plasmid-specific extranuclear gene expression system.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison L. A., Moyle M., Shales M., Ingles C. J. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985 Sep;42(2):599–610. doi: 10.1016/0092-8674(85)90117-5. [DOI] [PubMed] [Google Scholar]
- Armstrong V. W., Sternbach H., Eckstein F. Affinity labeling of Escherichia coli DNA-dependent RNA polymerase with 5-formyl-l-(alpha-D-ribofuranosyl)uracil 5'-triphosphate. Biochemistry. 1976 May 18;15(10):2086–2091. doi: 10.1021/bi00655a009. [DOI] [PubMed] [Google Scholar]
- Biggs J., Searles L. L., Greenleaf A. L. Structure of the eukaryotic transcription apparatus: features of the gene for the largest subunit of Drosophila RNA polymerase II. Cell. 1985 Sep;42(2):611–621. doi: 10.1016/0092-8674(85)90118-7. [DOI] [PubMed] [Google Scholar]
- Broyles S. S., Moss B. Identification of the vaccinia virus gene encoding nucleoside triphosphate phosphohydrolase I, a DNA-dependent ATPase. J Virol. 1987 May;61(5):1738–1742. doi: 10.1128/jvi.61.5.1738-1742.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S., Moss B. Sedimentation of an RNA polymerase complex from vaccinia virus that specifically initiates and terminates transcription. Mol Cell Biol. 1987 Jan;7(1):7–14. doi: 10.1128/mcb.7.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro: origin and direction of daughter strand synthesis. J Mol Biol. 1979 Dec 25;135(4):999–1012. doi: 10.1016/0022-2836(79)90524-2. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R., Ishihama A. Subunits of RNA polymerase in function and structure; Maturation in vitro of core enzyme from Escherichia coli. J Mol Biol. 1974 Aug 15;87(3):523–540. doi: 10.1016/0022-2836(74)90102-8. [DOI] [PubMed] [Google Scholar]
- García J. A., Peñalva M. A., Blanco L., Salas M. Template requirements for initiation of phage phi 29 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Jan;81(1):80–84. doi: 10.1073/pnas.81.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough J. A., Murray N. E. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983 May 5;166(1):1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
- Gunge N., Murata K., Sakaguchi K. Transformation of Saccharomyces cerevisiae with linear DNA killer plasmids from Kluyveromyces lactis. J Bacteriol. 1982 Jul;151(1):462–464. doi: 10.1128/jb.151.1.462-464.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunge N., Sakaguchi K. Intergeneric transfer of deoxyribonucleic acid killer plasmids, pGKl1 and pGKl2, from Kluyveromyces lactis into Saccharomyces cerevisiae by cell fusion. J Bacteriol. 1981 Jul;147(1):155–160. doi: 10.1128/jb.147.1.155-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunge N., Tamaru A., Ozawa F., Sakaguchi K. Isolation and characterization of linear deoxyribonucleic acid plasmids from Kluyveromyces lactis and the plasmid-associated killer character. J Bacteriol. 1981 Jan;145(1):382–390. doi: 10.1128/jb.145.1.382-390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hirochika H., Sakaguchi K. Analysis of linear plasmids isolated from Streptomyces: association of protein with the ends of the plasmid DNA. Plasmid. 1982 Jan;7(1):59–65. doi: 10.1016/0147-619x(82)90027-0. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G. H., Leavitt M. C., Ito J. Yeast killer plasmid pGKL1 encodes a DNA polymerase belonging to the family B DNA polymerases. Nucleic Acids Res. 1987 Nov 11;15(21):9088–9088. doi: 10.1093/nar/15.21.9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble R. J., Thompson R. D. S1 and S2, the linear mitochondrial DNAs present in a male sterile line of maize, possess terminally attached proteins. Nucleic Acids Res. 1982 Dec 20;10(24):8181–8190. doi: 10.1093/nar/10.24.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Hirai K., Hishinuma F. The yeast linear DNA killer plasmids, pGKL1 and pGKL2, possess terminally attached proteins. Nucleic Acids Res. 1984 Jul 25;12(14):5685–5692. doi: 10.1093/nar/12.14.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin E. V., Levchenko I. V., Zaitseva G. N. S2 plasmid from cms-S-maize mitochondria potentially encodes a specific RNA polymerase. Nucleic Acids Res. 1988 May 11;16(9):4177–4177. doi: 10.1093/nar/16.9.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Masters B. S., Stohl L. L., Clayton D. A. Yeast mitochondrial RNA polymerase is homologous to those encoded by bacteriophages T3 and T7. Cell. 1987 Oct 9;51(1):89–99. doi: 10.1016/0092-8674(87)90013-4. [DOI] [PubMed] [Google Scholar]
- Niwa O., Sakaguchi K., Gunge N. Curing of the killer deoxyribonucleic acid plasmids of Kluyveromyces lactis. J Bacteriol. 1981 Dec;148(3):988–990. doi: 10.1128/jb.148.3.988-990.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Monastyrskaya G. S., Gubanov V. V., Guryev S. O., Salomatina I. S., Shuvaeva T. M., Lipkin V. M., Sverdlov E. D. The primary structure of E. coli RNA polymerase, Nucleotide sequence of the rpoC gene and amino acid sequence of the beta'-subunit. Nucleic Acids Res. 1982 Jul 10;10(13):4035–4044. doi: 10.1093/nar/10.13.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Monastyrskaya G. S., Gubanov V. V., Guryev S. O., Chertov OYu, Modyanov N. N., Grinkevich V. A., Makarova I. A., Marchenko T. V., Polovnikova I. N. The primary structure of Escherichia coli RNA polymerase. Nucleotide sequence of the rpoB gene and amino-acid sequence of the beta-subunit. Eur J Biochem. 1981 Jun 1;116(3):621–629. doi: 10.1111/j.1432-1033.1981.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sor F., Wésolowski M., Fukuhara H. Inverted terminal repetitions of the two linear DNA associated with the killer character of the yeast Kluyveromyces lactis. Nucleic Acids Res. 1983 Aug 11;11(15):5037–5044. doi: 10.1093/nar/11.15.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam J. C., Kwakman J., Meijer M., Stuitje A. R. Efficient isolation of the linear DNA killer plasmid of Kluyveromyces lactis: evidence for location and expression in the cytoplasm and characterization of their terminally bound proteins. Nucleic Acids Res. 1986 Sep 11;14(17):6871–6884. doi: 10.1093/nar/14.17.6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. J., Boyd A. The killer toxin of Kluyveromyces lactis: characterization of the toxin subunits and identification of the genes which encode them. EMBO J. 1986 Aug;5(8):1995–2002. doi: 10.1002/j.1460-2075.1986.tb04455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. J., Mileham A. J., Romanos M. A., Boyd A. Nucleotide sequence and transcription analysis of a linear DNA plasmid associated with the killer character of the yeast Kluyveromyces lactis. Nucleic Acids Res. 1984 Aug 10;12(15):6011–6030. doi: 10.1093/nar/12.15.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetser D., Nonet M., Young R. A. Prokaryotic and eukaryotic RNA polymerases have homologous core subunits. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1192–1196. doi: 10.1073/pnas.84.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A., Oliver S. G. Physical separation and functional interaction of Kluyveromyces lactis and Saccharomyces cerevisiae ARS elements derived from killer plasmid DNA. Yeast. 1986 Sep;2(3):179–191. doi: 10.1002/yea.320020306. [DOI] [PubMed] [Google Scholar]
- Tschopp J. F., Emr S. D., Field C., Schekman R. GAL2 codes for a membrane-bound subunit of the galactose permease in Saccharomyces cerevisiae. J Bacteriol. 1986 Apr;166(1):313–318. doi: 10.1128/jb.166.1.313-318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



