Abstract
C-type natriuretic peptide (CNP) encoded by the NPPC (Natriuretic Peptide Precursor C) gene expressed in ovarian granulosa cells inhibits oocyte maturation by activating the natriuretic peptide receptor (NPR)B (NPRB) in cumulus cells. RT-PCR analyses indicated increased NPPC and NPRB expression during ovarian development and follicle growth, associated with increases in ovarian CNP peptides in mice. In cultured somatic cells from infantile ovaries and granulosa cells from prepubertal animals, treatment with CNP stimulated cGMP production. Also, treatment of cultured preantral follicles with CNP stimulated follicle growth whereas treatment of cultured ovarian explants from infantile mice with CNP, similar to FSH, increased ovarian weight gain that was associated with the development of primary and early secondary follicles to the late secondary stage. Of interest, treatment with FSH increased levels of NPPC, but not NPRB, transcripts in ovarian explants. In vivo studies further indicated that daily injections of infantile mice with CNP for 4 d promoted ovarian growth, allowing successful ovulation induction by gonadotropins. In prepubertal mice, CNP treatment alone also promoted early antral follicle growth to the preovulatory stage, leading to efficient ovulation induction by LH/human chorionic gonadotropin. Mature oocytes retrieved after CNP treatment could be fertilized in vitro and developed into blastocysts, allowing the delivery of viable offspring. Thus, CNP secreted by growing follicles is capable of stimulating preantral and antral follicle growth. In place of FSH, CNP treatment could provide an alternative therapy for female infertility.
Ovarian follicle development is regulated by FSH and LH secreted by the anterior pituitary. However, intraovarian paracrine mechanisms are also important to maintain optimal follicle growth and differentiation (1–3). Natriuretic peptides comprise a family of three structurally related molecules: atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP) (4). CNP is encoded by the NPPC (natriuretic peptide precursor C) gene, which is expressed in diverse cell types in which the precursor NPPC protein is cleaved into the 22-amino acid peptide CNP (5). CNP activates its cognate receptor guanylyl cyclase-B, also known as natriuretic peptide receptor-B (NPRB), whereas ANP and BNP stimulate guanylyl cyclase-A, also known as natriuretic peptide receptor-A (NPRA) (6, 7). Both receptors are membrane-anchored guanylyl cyclase enzymes that signal via the production of the second messenger cGMP and undergo both homologous and heterologous desensitization, reflected by dephosphorylation of specific sites in the kinase-homology domain (8). ANP and BNP act as endocrine hormones to regulate blood pressure and volume and inhibit cardiac hypertrophy (9). In contrast, CNP acts in an autocrine/paracrine fashion to induce vasorelaxation and vascular remodeling and to regulate bone growth (10).
Earlier studies have reported ovarian expression of NPPC and NPRB and their regulation by gonadotropins (11, 12). A recent study demonstrated the expression of NPPC mRNA in granulosa cells and the ability of CNP to stimulate cGMP production in cumulus cells to inhibit meiotic resumption of oocytes (13), consistent with earlier identification of a small molecular weight oocyte maturation inhibitor in follicular fluid and granulosa cell extracts (14). Subsequent studies indicated that the ovulatory LH surge decreased CNP levels in murine ovaries and human follicular fluid (15). Because earlier studies demonstrated the ability of cGMP analogs to promote the development of cultured preantral follicles in rats (16), we analyzed the expression of NPPC and NPRB genes during follicle development. We further demonstrated the ability of CNP to stimulate the growth of cultured preantral follicles and ovarian explants that is likely mediated through the cGMP pathway, distinct from the cAMP pathway activated by FSH. Based on in vivo studies, we demonstrated that CNP treatment promoted the development of preantral and early antral follicles to the preovulatory stage, leading to the generation of mature oocytes capable of undergoing fertilization to derive viable offspring.
Results
Expression of CNP and its receptor NPRB during follicle development
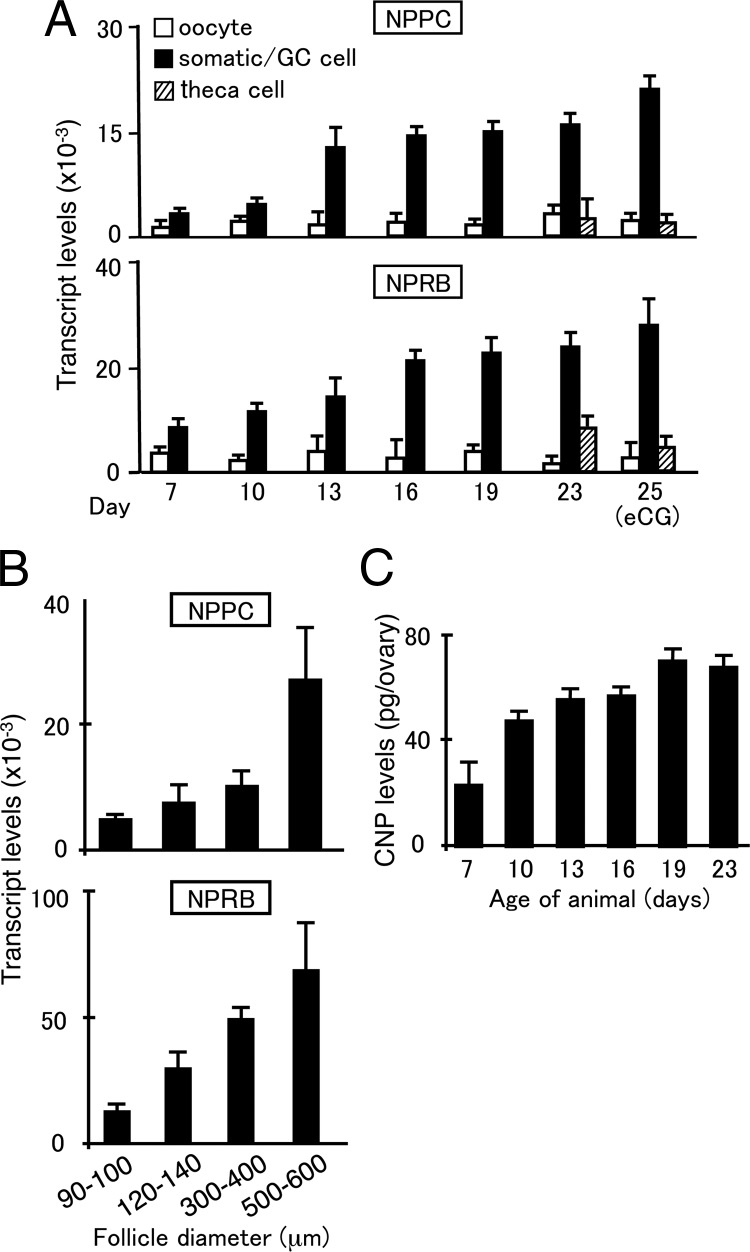
Real-time RT-PCR analyses indicated increased expression of NPPC and NPRB transcripts in somatic cells obtained from mice during prepubertal development (from d 7–d 19 of age) in an age-dependent manner with negligible levels in oocytes (Fig. 1A). Granulosa cells and theca shells were also obtained from early antral follicles in prepubertal mice at d 23 of age and from preovulatory follicles in mice treated with equine (e) chorionic gonadotropin (CG) for 2 d. As shown in Fig. 1A, the expression of both NPPC and NPRB transcripts was higher in granulosa cells than theca cells and oocytes. The purity of different cell types was confirmed using different cell markers [Supplemental Fig. 1 published on The Endocrine Society's Journals online web site at http://mend.endojournals.org: GDF9 for oocytes, FSH receptor for granulosa cells, and cytochrome P450 (CYP)17a1 for theca cells]. We further isolated follicles of different sizes (90–140 μm in diameter from mice at d 13 of age; 300–400 μm in diameter from prepubertal mice; 500–600 μm in diameter from eCG-treated mice). Increases in both NPPC and NPRB transcript levels were detected during follicle development, reaching highest levels in preovulatory follicles (500–600 μm in diameters) (Fig. 1B). To demonstrate the processing of mature CNP peptides from pro-CNP (17) in the ovary, ovarian extracts from mice during prepubertal development were analyzed using specific enzyme-linked immunoassay (EIA). Ovarian CNP content increased in an age-dependent manner during the first wave of follicle development, reaching highest levels in animals at d 19 of age (Fig. 1C).
Fig. 1.
Ovarian expression of NPPC and NPRB as well as ovarian CNP peptide content during development. Ovaries from immature mice at different ages were dissociated to obtain oocytes and somatic cells. In addition, granulosa cells, theca shell, and oocytes from immature mice at d 23 of age before and after eCG treatment for 2 d were isolated. Real-time RT-PCR was performed using specific primers. A, Expression of NPPC and NPRB in different ovarian cell types. GC, granulosa cells. B, Real-time RT-PCR of NPPC and NPRB transcripts in isolated follicles of different sizes. Follicles (one to five follicles per sample) of different diameters were isolated from juvenile (90–140 μm in diameter) and eCG-treated immature mice (300–600 μm in diameter) for analyses. C, Measurement of ovarian CNP content from pubertal mice at different ages. Mean ± se of six to 12 samples.
Stimulation of cGMP, but not cAMP, production by cultured ovarian somatic cells
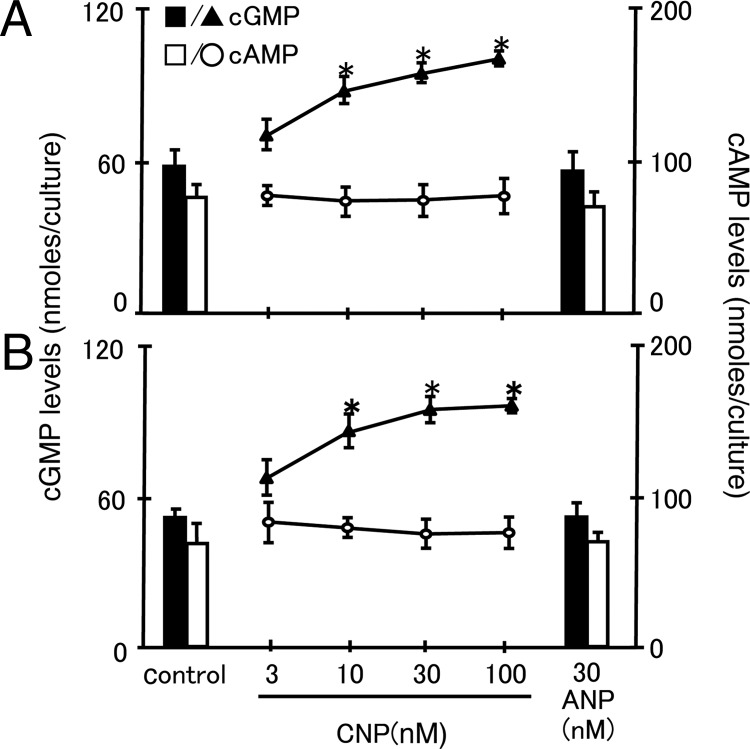
Because the NPRB receptors mediated cGMP production stimulated by the CNP ligand, we isolated somatic cells from ovaries of mice at d 13 of age and granulosa cells from mice at d 21 of age before treatment with increasing doses of CNP or a high dose (30 nm) of ANP for 2 h. Media content of both cGMP and cAMP were determined by RIA. As shown in Fig. 2, treatment with CNP, but not ANP, led to dose-dependent increases in media content of cGMP for both cell preparations. In contrast, no changes in cAMP production after CNP treatment were detected for both types of cells.
Fig. 2.
CNP stimulation of cGMP, but not cAMP, production by cultured somatic and granulosa cells. CNP stimulation of cGMP production by cultured somatic cells from mice at d 13 of age (A) and cultured granulosa cells from prepubertal mice at 21 d of age (B) were treated with CNP and ANP in media containing isobutylmethylxanthine. At 2 h after incubation, media content of cGMP and cAMP was measured. Mean ± se of eight samples. *, P < 0.05, significantly different from the control group.
CNP treatment promoted the growth of preantral follicles and cultured ovarian explants
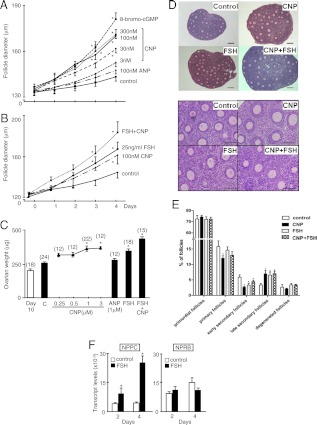
Preantral follicles (125–135 μm in diameter) were isolated from ovaries of mice at d 13 of age and treated with different hormones for 4 d with daily monitoring of follicle diameters. As shown in Fig. 3A, treatment with CNP led to dose- and time-dependent increases in follicle size, reaching levels comparable to those induced by a cGMP analog, 8-bromo-cGMP. In contrast, treatment with ANP was ineffective. Furthermore, combined treatment with CNP and FSH (25 ng/ml) led to further increases in follicle growth (Fig. 3B). Representative pictures of preantral follicles cultured with different hormones for 4 d indicated increased follicle sizes without a flattened morphology (Supplemental Fig. 2). These findings demonstrated the ability of CNP to promote preantral follicle growth.
Fig. 3.
CNP promotion of the growth of isolated preantral follicle and ovarian explants in culture. Preantral follicles (125–145 μm) were isolated from mice at d 13 of age and cultured for 4 d with media changes every 2 d. A, Follicles were treated with increasing doses of CNP (3–300 nm), 100 nm ANP, or 8-bromo-cGMP (5 mm), and follicle diameters were monitored daily (n =16–29 follicles). B, Additive effects of CNP and FSH on follicle growth. Preantral follicles were treated with FSH (25 ng/ml) and/or CNP (100 nm) (n =18–24 follicles). C–E, CNP promotion of the growth of ovarian explants. Individual ovaries from mice at d 10 of age were cultured with increasing doses (0.25–3 μm) of CNP, ANP (1 μm), or FSH (25 ng/ml) with or without CNP (1 μm) for 4 d with media changes every 2 d. Ovarian weights (C), ovarian histology (D, upper panels, lower magnification; lower panels, higher magnification; bars, 400 μm.), and follicle dynamics (E) were determined at the end of culture. Numbers of ovaries used are shown in parentheses. F, Ovarian explants from mice at d 10 of age were cultured for 2 and 4 d before measurement of NPPC and NPRB transcript levels (mean ± se) *, P < 0.05, significantly different from the control group.
We further used ovarian explant cultures to investigate CNP actions. Individual ovaries from mice at d 10 of age were cultured for 4 d with CNP, ANP, and/or FSH with media changes every 2 d. As shown in Fig. 3C, treatment with CNP, but not ANP, led to dose-dependent increases in ovarian weight. Similar to CNP, treatment with FSH also increased ovarian weight with additive increases when both CNP and FSH were included. Histological analyses indicated that treatment with CNP, like FSH, promoted the development of preantral follicles (Fig. 3D). Detailed counting of follicles of different sizes indicated that treatment with CNP decreased the percentages of primary and early secondary follicles, accompanied by 109% increases in late secondary follicles. Similarly, treatment with FSH promoted the development of early secondary follicles to the late secondary stage. Because FSH is the predominant stimulator of follicle development, we further tested whether FSH treatment could regulate NPPC gene expression during ovarian explant culture. As shown in Fig. 3F, real-time RT-PCR analyses showed increases in NPPC transcript levels at both 2 and 4 d after FSH treatment, suggesting the stimulatory effects of FSH are partially mediated via stimulating CNP expression. In contrast, no changes in NPRB transcript levels were detected.
In vivo treatment with CNP promoted follicle development for ovulation and subsequent pregnancy in juvenile and prepubertal mice
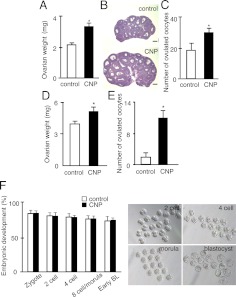
To test the ability of CNP to stimulate preantral follicle development in vivo, juvenile mice at d 13 of age were treated ip with CNP daily for 4 d. This was followed by a single ip injection of eCG for 2 more days before determination of ovarian weight. As shown in Fig. 4A, CNP pretreatment led to a 56% increase in ovarian weight. Histological analyses indicated increases in the development of antral follicles after CNP pretreatment followed by eCG (Fig. 4B). Some of these eCG-treated animals were further treated with an ovulatory dose of human (h) CG to check ovulation efficiency. Higher numbers of ovulated oocyte were found in the oviducts of CNP-pretreated animals as compared with controls (Fig. 4C). These findings suggest the ability of CNP to promote the development of secondary/preantral follicles to the early antral stage, thus allowing efficient induction of ovulation by the subsequent eCG-hCG treatment.
Fig. 4.
In vivo treatment with CNP promoted the development of preantral and antral follicles. To test the effects of CNP on the development of preantral follicle into early antral stage, juvenile mice at d 13 of age were treated ip with CNP (25 μg/kg body weight) or saline daily for 4 d to stimulate the development of preantral follicles. These animals were further treated ip with eCG (5 IU) for 48 h before determination of ovarian weight (A) and analyses of ovarian histology (B); bars, 500 μm. To estimate ovulation efficiency, some animals were then treated with an ovulatory dose of hCG (5 IU). At 16 h after hCG treatment, numbers of ovulated oocytes in oviducts were determined (C) (n = 20 animals). To test the effects of CNP on the growth of early antral follicles into the preovulatory stage for ovulation, prepubertal mice at d 21 of age were treated with CNP (50 μg/kg body weight) or saline daily for 4 d before ovulation induction with hCG (2.5 IU). At 16 h after hCG treatment, ovarian weights were determined and mature oocytes in oviducts were counted. D, Ovarian weight after CNP treatment. E, Ovulation efficiency after hCG treatment (n = 8). F, Early embryonic development. To study early embryonic development of oocytes derived from immature mice pretreated with CNP, mature oocytes were fertilized with viable sperm in vitro and cultured for different periods. Oocytes from the control group were derived from immature mice treated with 5 IU eCG for 2 d followed by an ovulatory dose of hCG for 16 h. Development of early embryos was determined based on morphology. Left panel, Ratios of embryos reaching different developmental stages. BL, Blastocyst. Right panel, Embryo morphology of CNP-pretreated oocytes. *, P 0.05.
To further test the ability of CNP to stimulate the development of early antral follicles to the preovulatory stage without exogenous eCG/FSH administration, prepubertal mice at 21 d of age were treated ip with CNP daily for 4 d followed by a single injection of an ovulatory dose of hCG. At 18 h after hCG treatment, the number of ovulated mature oocytes was determined. Pretreatment with CNP increased ovarian weight (Fig. 4D). As compared with saline-pretreated control animals, CNP pretreatment led to a major increase in the number of ovulated oocytes induced by hCG (Fig. 4E).
We further tested whether ovulated oocytes retrieved after CNP pretreatment are capable of being fertilized (Fig. 4F). Oocytes were obtained from oviducts of CNP-pretreated prepubertal mice after hCG treatment and used for in vitro fertilization. We used oocytes obtained from prepubertal mice treated sequentially with eCG (2 d) and hCG (16 h) as controls. Fertilized oocytes from CNP-pretreated animals developed to the blastocyst stage with the same efficiency as compared with control oocytes. Some prepubertal females pretreated with CNP followed by an ovulatory dose of hCG were mated with fertile males. Successful pregnancy was demonstrated followed by the delivery of healthy pups.
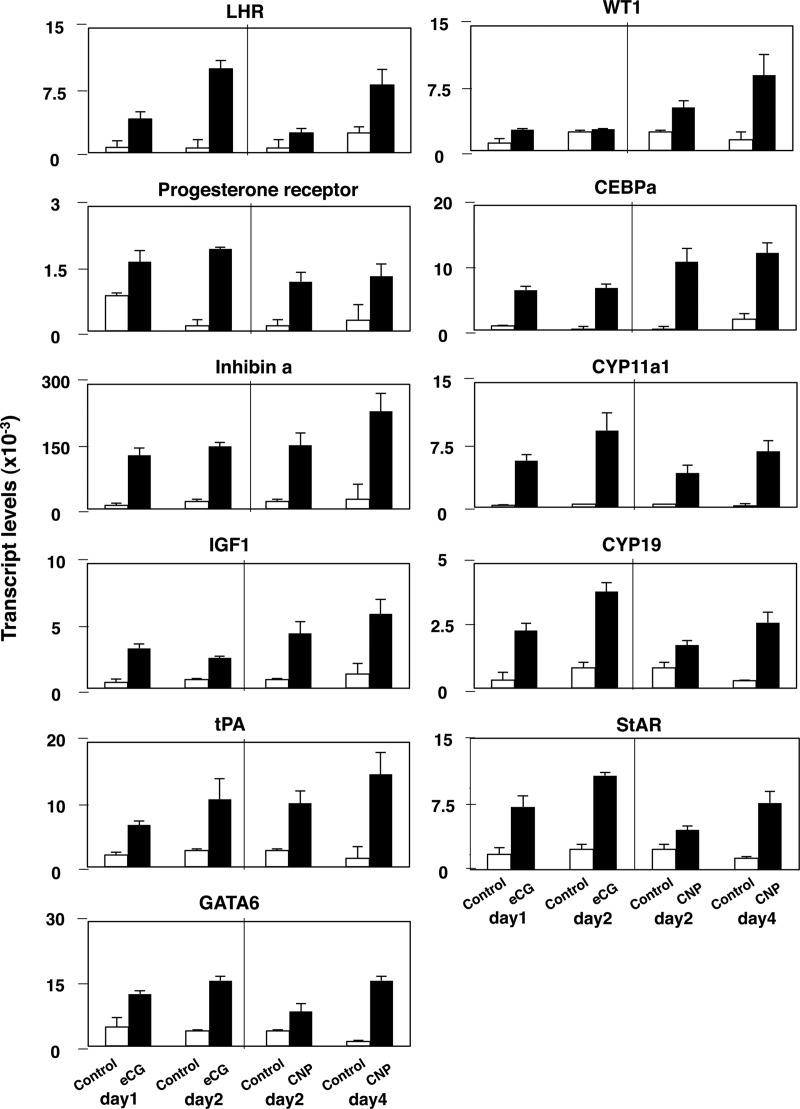
To further elucidate molecular mechanisms underlying CNP stimulation of the development of preovulatory follicles capable of responding to the preovulatory hCG stimulation, we analyzed the expression of diverse ovarian genes important for follicle maturation, steroidogenesis, and ovulation. Immature mice at d 21 of age were treated with CNP for 2 and 4 d without eCG treatment before quantitative analyses of transcript levels using real-time RT-PCR. As a control, mice at d 21 of age were treated for 1 and 2 d with eCG to induce known ovulatory genes. As shown in Fig. 5, treatment with CNP increased the expression of transcripts for LH receptor, progesterone receptor, inhibin-α (inhibin-a), IGF-I, tissue plasminogen activator, key transcriptional factors (GATA6, WT1, and Ccaat/enhancer-binding protein-α), and key steroidogenic enzymes (CYP11a1, CYP19, and steroidogenic acute regulatory protein) in a time-dependent manner. With the exception of WT1, eCG treatment also induced the expression of these ovarian genes, albeit in a shorter duration than CNP.
Fig. 5.
CNP stimulation of key ovarian genes important for follicle maturation, steroidogenesis, and ovulation in vivo. Prepubertal mice at d 21 of age were treated daily with CNP (50 μg/kg body weight) for 2 and 4 d without eCG treatment before real-time RT-PCR analyses of transcript levels for diverse ovarian genes. As a control, mice at d 21 of age were treated for 1 and 2 d with eCG before RT-PCR analyses. LHR, LH receptor; tPA, tissue plasminogen activator; CEBPA, Ccaat/enhancer-binding protein-α; StAR, steroidogenic acute regulatory protein (n = 5–7 samples).
Discussion
In addition to the role of CNP as an oocyte maturation inhibitor (13), our studies demonstrated the ability of CNP to promote preantral and antral follicle development. The NPPC gene is expressed in somatic/granulosa cells of preantral and antral follicles, and exogenous CNP is capable of promoting follicle growth by stimulating cGMP production mediated through the NPRB receptor. Furthermore, the paracrine hormone CNP, acting through the cGMP pathway, likely mediates some of the effects of the endocrine hormone FSH in the promotion of preantral follicle development because FSH increased NPPC expression in cultured ovarian explants. In explants of juvenile ovaries, CNP treatment promoted the development of primary/early secondary follicles to the late secondary stage whereas treatment of cultured preantral follicles with CNP, like FSH, stimulated follicle growth. Similar to the known follicle-stimulating effects of FSH on preantral follicles in juvenile rats (18), in vivo CNP treatment of juvenile mice stimulated preantral follicle development to the early antral stage to allow subsequent stimulation by FSH and LH/hCG. Furthermore, CNP treatment of immature mice containing early antral follicles led to the formation of preovulatory follicles without the need for exogenous FSH. These follicles are capable of responding to the preovulatory LH/hCG stimulation, resulting in successful ovulation, fertilization, and pregnancy. These findings suggest that CNP can promote follicle development ranging from primary to antral stages and could substitute for FSH in the penultimate phase of follicle development to the preovulatory stage.
CNP acts exclusively through the NPRB receptor to stimulate downstream cGMP signaling (6). Our findings are consistent with earlier studies using NPRB/guanylyl cyclase-B-null mice. In addition to the attenuation of longitudinal vertebra or limb-bone growth, female NPRB-null mice were infertile and acyclic with smaller ovaries that contained only primordial through secondary follicles (19). Because CNP acts exclusively through the NPRB receptor, the arrest of follicle development at the secondary stage found in NPRB-null mice underscores the essential role of CNP in the final stage of follicle development and the important role of CNP to mediate the actions of FSH. Although NPPC-null mice lacking CNP showed dwarfism and early death (20), hypomorphic NPPC mutant mice exhibited precocious resumption of meiosis in oocytes, also leading to an infertile phenotype (13). It is likely that the hypomorphic NPPC mutant still expresses sufficient CNP ligands to allow suboptimal antral follicle development, and future studies using mice with ovarian cell type-specific NPPC gene deletion could reveal the exact ovarian roles of CNP.
Detection of NPPC transcripts in ovarian follicles at different stages of development and ovarian CNP peptides in mice is consistent with an earlier study showing the presence of immunoreactive CNP in ovarian extracts during different phases of the estrous cycle in rats (11, 12). Although we investigated gene expression only in punctured granulosa cells without cumulus cells, recent studies showed that cumulus cells express higher levels of NPRB receptors than mural granulosa cells (13). Also, estradiol treatment stimulated NPRB expression (21). Our data further demonstrated increased expression of both NPPC and NPRB during follicle growth with preovulatory follicles expressing the highest levels of these genes. After the preovulatory LH surge, expression of NPPC and the ovarian content of CNP decreased in a time-dependent manner (15), concomitant with decreased levels of meiotic inhibitory activity associated with oocyte maturation (13). Coupled with our demonstration of the ability of CNP to promote follicle growth, it is becoming clear that CNP and its receptors in ovarian follicles play important roles in both somatic cell proliferation and the suppression of oocyte maturation during follicle development until the preovulatory LH surge. In humans, initiation of the preovulatory LH surge is also accompanied by decreased NPPC expression in granulosa cells and lower CNP secretion into the follicular fluid, coincident with cessation of follicle growth and resumption of meiotic maturation of oocytes (15).
Similar to the promoting effects of bromo-cGMP (a membrane-soluble cGMP analog) on the growth of cultured preantral follicles in rats (16), the present studies using cultured preantral follicles and ovarian explants demonstrated the ability of CNP to promote follicle development. This finding is consistent with the ability of CNP to stimulate cGMP, but not cAMP, production by cultured somatic cells from ovaries of juvenile mice and cultured granulosa cells from early antral follicles. Thus, the follicle-stimulating actions of CNP mediated by the cGMP pathway are distinct from the cAMP signaling pathway induced by FSH. Although higher levels of the NPRB receptors are present in granulosa cells from prepubertal mice as compared with somatic cells isolated from mice at d 13 of age, comparable levels of cGMP were generated in response to CNP, suggesting different signal transduction efficiency of the receptors. In cultured ovarian explants containing preantral and smaller follicles, treatment with FSH increased the expression of NPPC but not NPRB transcripts. These data suggested that intraovarian CNP induced by FSH in preantral follicles could partially mediate the ovarian actions of FSH. Because the circulating levels of CNP is approximately 1 pm (22) and CNP stimulated follicle growth at nanomolar levels, CNP is likely a local hormone in the ovary. The exact cross talks and overlapping actions of CNP-induced cGMP and FSH-stimulated cAMP pathways for ovarian follicle development require further analyses. Because activation of the NPRB receptor/enzyme by its ligand is a complex process requiring oligomerization, ligand binding, kinase homology domain phosphorylation, and ATP binding (23), future studies are needed to elucidate the exact cellular mechanisms underlying CNP actions in the ovary.
In vivo treatment using juvenile mice at d 13 of age demonstrated that CNP treatment promoted preantral follicle development and allowed subsequent eCG induction of preovulatory follicles capable of responding to an ovulatory surge of hCG, leading to ovulation. Furthermore, CNP treatment of prepubertal mice at d 21 of age facilitated the development of early antral follicles to the preovulatory stage, thus allowing the induction of ovulation by LH/hCG to generate fertilizable oocytes and successful pregnancy. Initial mechanistic analyses on the expression of key genes important for follicle maturation, steroidogenesis, and ovulation indicated similar increases after CNP treatment as compared with the traditional eCG treatment protocol. However, the duration of CNP treatment needed is longer than that for eCG. Of interest, mature oocytes retrieved after CNP treatment have similar developmental potential comparable to those obtained after the conventional eCG-hCG sequential priming of immature mice. Because the NPRB receptor is expressed in granulosa cells but lower in theca cells and both granulosa and theca cells are required for optimal estrogen production by preovulatory follicles (24), in vivo treatment with CNP could stimulate granulosa cell functions and sufficient endogenous LH could promote theca cell functions to allow the final maturation of follicles to the preovulatory stage. Also, exogenous CNP could act through NPRB receptors expressed in theca cells. The ability of CNP, like FSH, to stimulate the development of preovulatory follicles in prepubertal mice also suggested that CNP could substitute for FSH in preovulatory follicle formation, underscoring the importance of CNP as a follicle-stimulating paracrine hormone, consistent with the reported follicle arrest at the secondary follicle stage found in NPRB-null mice (19). Because NPRB receptor has been found in pituitary gonadotrophs (25), one cannot completely rule out pituitary actions of CNP in the present in vivo models.
CNP acts exclusively through the NPRB receptor whereas ANP and BNP act through NPRA. Although CNP has been shown to have vasodilating, hypotensive, and natriuretic activities (26), we did not observe abnormalities after CNP treatment using the present CNP dosages and injection protocols. The apparent lack of side effects using the present low doses of CNP is consistent with earlier reports showing minimal cardiac and renal actions using physiological concentrations of CNP (22, 27, 28). Indeed, short-term infusion of CNP in humans, achieving supraphysiological levels in plasma, are not vasodepressor or natriuretic (27).
FSH treatment has been used extensively for the stimulation of follicle development to generate mature oocytes for fertilization. Our findings demonstrated that CNP could also stimulate both preantral and antral follicles. CNP administration could provide future opportunities for the treatment of infertile women if no adverse side effects are found. It is possible that CNP treatment could benefit patients with low responses to the conventional FSH treatment (29). As compared with the longer half-life of the large glycoprotein FSH, treatment with the small shorter-acting CNP peptide (30) could also be useful to avoid ovarian hyperstimulation syndrome, a potentially life-threatening condition (31).
Materials and Methods
Animals
Female CD1 mice at different ages were obtained from Charles River Laboratories Inc. (Wilmington, MA) and housed at the animal facility of Stanford University with 12-h dark, 12-h light and free access to food and water. Mice were treated in accordance with the guidelines of the local Animal Research Committee.
Real-time RT-PCR analyses
Transcript levels for NPPC and NPRB in the ovary and different ovarian cell types were analyzed together with those for different cell markers. Ovaries from d 10 mice were treated with 0.25% trypsin, 0.1% collagenase I, 0.02% DNaseI for 15 min at 37 C. After addition of 1 mm EDTA, ovaries were incubated at 37 C for 30 min before oocytes and remaining somatic cells were collected. In addition, early antral follicles were isolated from prepubertal mice at d 23 of age and punctured to collect granulosa cells and oocytes, and for obtaining theca shells. Similar procedures were used to isolate different cells from preovulatory follicles at 48 h after treatment of prepubertal mice with eCG (5 IU). To investigate molecular mechanisms underlying CNP stimulation of preovulatory follicle formation in vivo, prepubertal mice at d 21 of age were treated daily with CNP (50 μg/kg body weight) for 2 and 4 d before real-time RT-PCR analyses of diverse ovarian genes important for follicle maturation, steroidogenesis, and ovulation. As a control, mice at d 21 of age were treated for 1 and 2 d with eCG before RT-PCR analyses. Total RNA was extracted using an RNeasy Micro Kit (QIAGEN Sciences, Valencia, CA), and cDNA was synthesized using a Sensicript RT Kit (QIAGEN) according to the manufacturer's protocol. Real-time PCR was performed using iTaq SYBR Green SuperMix (Bio-Rad Laboratories, Inc., Hercules, CA) on a Smart Cycler TD System (Cepheid, Sunnyvale, CA) as follows: 15 min at 95C and then 45 cycles of 15 sec at 95 C and 60 sec at 60 C. The primers used are shown in Supplemental Table 1. Data were analyzed by the cycle threshold method to determine the fold changes in expression. The relative abundance of specific genes was normalized to the relative abundance of β-actin levels.
Measurement of ovarian CNP levels
For EIA measurement of CNP peptide levels (15), ovaries from mice at different ages were obtained and boiled for 5 min in 5 volumes of water to inactivate intrinsic proteases. The solution was then adjusted to 1 m AcOH and 20 mm HCl. Ovaries were homogenized with a Polytron mixer (VWR International, West Chester, PA) before centrifugation at 20,000 × g for 30 min at 4C. The supernatant of extracts was subjected to precipitation at a concentration of 66% acetone. After removing the precipitates by centrifugation for 30 min at 3000 × g, acetone in the supernatant was evaporated, and extracted peptides were dissolved in water before EIA analyses using a CNP EIA kit (Phoenix Pharmaceuticals, Inc., Burlingame, CA) according to manufacturer's instructions.
Dissection of follicles of different sizes and culturing of preantral follicles
For RT-PCR analyses, early secondary follicles of 90–140 μm in diameter were isolated from mice at d 13 of age whereas antral follicles (300–400 μm in diameter) were isolated from prepubertal mice at d 23 of age. Preovulatory follicles (500–600 μm in diameter) were isolated from prepubertal mice after eCG (5 IU) treatment for 2 d. For follicle cultures, preantral follicles (125–145 μm in diameter) were isolated from mice at d 13 of age and cultured individually in 96-well plates containing α-MEM (100 μl/well) with penicillin and streptomycin together with insulin, transferring, and selenium (18). Explants were treated with or without CNP, ANP, 8-bromo-cGMP, and/or FSH. Media were changed every 2 d, and follicle growth was monitored daily by measuring follicle diameters.
Ovarian explant cultures and follicle counting
Ovaries from d 10 mice were placed on culture plate inserts (Millipore Corp., Bedford, MA) and cultured in 400 μl of DMEM/F12 containing 0.1% BSA (Sigma, St. Louis, MO), 0.1% Albumax II, insulin-transferrin-selenium, 0.05 mg/ml l-ascorbic acid and penicillin-streptomycin under the membrane insert to cover ovaries with a thin layer of medium (32). Ovaries were treated with CNP and/or FSH with media changes every 2 d for 4 d. At the end of culture, ovaries were fixed in Bouin's solution, paraffin embedded, and cut into continuous sections before staining with hematoxylin and eosin. Every third section from each ovary was used for counting. Follicles with one oocyte surrounded by a single layer of flattened granulosa cells were scored as primordial follicles, follicles with an oocyte surrounded by one layer of cubical granulosa cells were considered as primary, follicles with an oocyte surrounded by two layers of granulosa cells were considered as early secondary, whereas follicles with three or more layers of granulosa cells but without an antrum were considered as late secondary follicles. Only follicles with clearly stained oocyte nuclei were counted to prevent recounting of the same follicle.
In vivo treatment with CNP
Infantile mice at d 13 of age were treated with CNP (25 μg/kg body weight) ip daily for 4 d. This was followed by a single injection of eCG (5 IU) for 2 d to stimulate penultimate follicle maturation before collection of ovaries for weighting and histological analyses. Some animals were further treated with an ovulatory dose (5 IU) of hCG, and oocytes in oviducts were monitored 16 h later to evaluate ovulation efficiency. To test the effects of CNP on early antral follicle growth, prepubertal mice at d 21 of age were treated ip with CNP (50 μg/kg body weight) daily for 4 d to stimulate preovulatory follicle development, followed by the injection of an ovulatory dose (2.5 IU) of hCG. The number of ovulated oocytes was determined 16 h later. Mature oocytes obtained from CNP-pretreated animals were used for in vitro fertilization. Sperm from CD1 male mice (10–12 wk old) were collected into human tubal fluid media (Millipore) and incubated for 1 h at 37 C. Oocytes were fertilized with sperm (2–3 × 105/ml) for 6 h, and inseminated oocytes were transferred into KSOM-AA medium (Millipore) to allow development into blastocysts (33). Some of the CNP-pretreated females were mated with fertile males for the monitoring of pregnancy and pup delivery.
Statistical analyses
Results are presented as mean ± se of three or more independent determination. Statistical significance was determined by using the ANOVA test followed by Fisher's protected least significant difference with P < 0.05 being statistically significant.
Supplementary Material
Acknowledgements
This work was supported by funds from the National Institute of Child Health and Human Development (U54 HD068158) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research; K.K. was supported by a Grant-In-Aid for Scientific Research (Grant-In-Aid for Young Scientists B:21791539) and Grant-in-Aid for Scientific Research on Priority Areas (THE GERMLINE: Its Developmental Cycle and Epigenome Network: 23013004).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANP
- Atrial natriuretic peptide
- BNP
- brain natriuretic peptide
- CG
- chorionic gonadotropin
- CNP
- C-type natriuretic peptide
- CYP
- cytochrome P450
- EIA
- enzyme-linked immunoassay
- NPRB
- natriuretic peptide receptor-B.
References
- 1. Eppig JJ. 2001. Oocyte control of ovarian follicular development and function in mammals. Reproduction 122:829–838 [DOI] [PubMed] [Google Scholar]
- 2. Hsueh AJ, Adashi EY, Jones PB, Welsh TH., Jr 1984. Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocr Rev 5:76–127 [DOI] [PubMed] [Google Scholar]
- 3. Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, Lo YK, Sharma SC. 2002. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res 57:195–220 [DOI] [PubMed] [Google Scholar]
- 4. Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML. 1990. Diverse biological actions of atrial natriuretic peptide. Physiol Rev 70:665–699 [DOI] [PubMed] [Google Scholar]
- 5. Sudoh T, Minamino N, Kangawa K, Matsuo H. 1990. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun 168:863–870 [DOI] [PubMed] [Google Scholar]
- 6. Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, Matsuo H, Goeddel DV. 1991. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science 252:120–123 [DOI] [PubMed] [Google Scholar]
- 7. Tremblay J, Desjardins R, Hum D, Gutkowska J, Hamet P. 2002. Biochemistry and physiology of the natriuretic peptide receptor guanylyl cyclases. Mol Cell Biochem 230:31–47 [PubMed] [Google Scholar]
- 8. Schulz S. 2005. C-type natriuretic peptide and guanylyl cyclase B receptor. Peptides 26:1024–1034 [DOI] [PubMed] [Google Scholar]
- 9. Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM. 2000. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet 355:1126–1130 [DOI] [PubMed] [Google Scholar]
- 10. Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N, Imura H. 1992. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-β. Possible existence of “vascular natriuretic peptide system”. J Clin Invest 90:1145–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jankowski M, Reis AM, Mukaddam-Daher S, Dam TV, Farookhi R, Gutkowska J. 1997. C-type natriuretic peptide and the guanylyl cyclase receptors in the rat ovary are modulated by the estrous cycle. Biol Reprod 56:59–66 [DOI] [PubMed] [Google Scholar]
- 12. Gutkowska J, Jankowski M, Sairam MR, Fujio N, Reis AM, Mukaddam-Daher S, Tremblay J. 1999. Hormonal regulation of natriuretic peptide system during induced ovarian follicular development in the rat. Biol Reprod 61:162–170 [DOI] [PubMed] [Google Scholar]
- 13. Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. 2010. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 330:366–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsafriri A, Pomerantz SH. 1986. Oocyte maturation inhibitor. Clin Endocrinol Metab 15:157–170 [DOI] [PubMed] [Google Scholar]
- 15. Kawamura K, Cheng Y, Kawamura N, Takae S, Okada A, Kawagoe Y, Mulders S, Terada Y, Hsueh AJ. 2011. Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum Reprod 26:3094–3101 [DOI] [PubMed] [Google Scholar]
- 16. McGee E, Spears N, Minami S, Hsu SY, Chun SY, Billig H, Hsueh AJ. 1997. Preantral ovarian follicles in serum-free culture: suppression of apoptosis after activation of the cyclic guanosine 3′,5′-monophosphate pathway and stimulation of growth and differentiation by follicle-stimulating hormone. Endocrinology 138:2417–2424 [DOI] [PubMed] [Google Scholar]
- 17. Wu C, Wu F, Pan J, Morser J, Wu Q. 2003. Furin-mediated processing of Pro-C-type natriuretic peptide. J Biol Chem 278:25847–25852 [DOI] [PubMed] [Google Scholar]
- 18. McGee EA, Perlas E, LaPolt PS, Tsafriri A, Hsueh AJ. 1997. Follicle-stimulating hormone enhances the development of preantral follicles in juvenile rats. Biol Reprod 57:990–998 [DOI] [PubMed] [Google Scholar]
- 19. Tamura N, Doolittle LK, Hammer RE, Shelton JM, Richardson JA, Garbers DL. 2004. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc Natl Acad Sci USA 101:17300–17305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y, Itoh H, Tanaka K, Saito Y, Katsuki M, Nakao K. 2001. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci USA 98:4016–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang M, Su YQ, Sugiura K, Wigglesworth K, Xia G, Eppig JJ. 2011. Estradiol promotes and maintains cumulus cell expression of natriuretic peptide receptor 2 (NPR2) and meiotic arrest in mouse oocytes in vitro. Endocrinology 152:4377–4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barletta G, Lazzeri C, Vecchiarino S, Del Bene R, Messeri G, Dello Sbarba A, Mannelli M, La Villa G. 1998. Low-dose C-type natriuretic peptide does not affect cardiac and renal function in humans. Hypertension 31:802–808 [DOI] [PubMed] [Google Scholar]
- 23. Pandey KN. 2005. Biology of natriuretic peptides and their receptors. Peptides 26:901–932 [DOI] [PubMed] [Google Scholar]
- 24. Liu YX, Hsueh AJ. 1986. Synergism between granulosa and theca-interstitial cells in estrogen biosynthesis by gonadotropin-treated rat ovaries: studies on the two-cell, two-gonadotropin hypothesis using steroid antisera. Biol Reprod 35:27–36 [DOI] [PubMed] [Google Scholar]
- 25. McArdle CA, Olcese J, Schmidt C, Poch A, Kratzmeier M, Middendorff R. 1994. C-type natriuretic peptide (CNP) in the pituitary: is CNP an autocrine regulator of gonadotropes? Endocrinology 135:2794–2801 [DOI] [PubMed] [Google Scholar]
- 26. Stingo AJ, Clavell AL, Aarhus LL, Burnett JC., Jr 1992. Cardiovascular and renal actions of C-type natriuretic peptide. Am J Physiol 262:H308–H312 [DOI] [PubMed] [Google Scholar]
- 27. Hunt PJ, Richards AM, Espiner EA, Nicholls MG, Yandle TG. 1994. Bioactivity and metabolism of C-type natriuretic peptide in normal man. J Clin Endocrinol Metab 78:1428–1435 [DOI] [PubMed] [Google Scholar]
- 28. Potter LR. 2004. CNP, cardiac natriuretic peptide? Endocrinology 145:2129–2130 [DOI] [PubMed] [Google Scholar]
- 29. Tarlatzis BC, Zepiridis L, Grimbizis G, Bontis J. 2003. Clinical management of low ovarian response to stimulation for IVF: a systematic review. Hum Reprod Update 9:61–76 [DOI] [PubMed] [Google Scholar]
- 30. Charles CJ, Espiner EA, Richards AM, Nicholls MG, Yandle TG. 1996. Comparative bioactivity of atrial, brain, and C-type natriuretic peptides in conscious sheep. Am J Physiol 270:R1324–R1331 [DOI] [PubMed] [Google Scholar]
- 31. Broer SL, Dólleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJ. 2011. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update 17:46–54 [DOI] [PubMed] [Google Scholar]
- 32. Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, Duan EK, Hsueh AJ. 2010. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci USA 107:10280–10284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kawamura K, Kawamura N, Mulders SM, Sollewijn Gelpke MD, Hsueh AJ. 2005. Ovarian brain-derived neurotrophic factor (BDNF) promotes the development of oocytes into preimplantation embryos. Proc Natl Acad Sci USA 102:9206–9211 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.