Abstract
The skin epidermis is a stratified epithelium that forms a barrier that protects animals from dehydration, mechanical stress, and infections. The epidermis encompasses different appendages, such as the hair follicle (HF), the sebaceous gland (SG), the sweat gland, and the touch dome, that are essential for thermoregulation, sensing the environment, and influencing social behavior. The epidermis undergoes a constant turnover and distinct stem cells (SCs) are responsible for the homeostasis of the different epidermal compartments. Deregulation of the signaling pathways controlling the balance between renewal and differentiation often leads to cancer formation.
In the skin, at least three types of stem cells are responsible for maintaining different epidermal compartments. Distinct signaling pathways control their functions; deregulation of these pathways may lead to cancer.
1. FUNCTIONAL ANATOMY OF THE SKIN EPIDERMIS
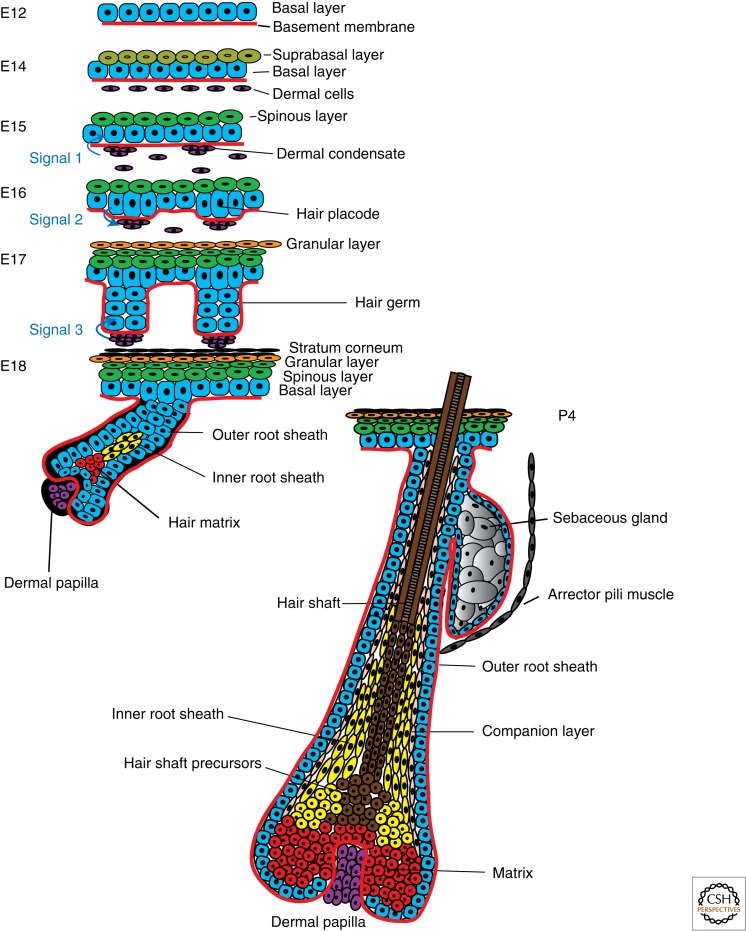
The skin consists of the epidermis and its underlying dermis. The epidermis is a stratified epithelium containing one layer of proliferative cells and several layers of differentiated cells. The formation of the different layers of the epidermis (stratification) occurs during embryonic development, so that animals already present a functional barrier at birth. In mice, stratification begins around embryonic day 15 (E15) and coincides with the rotation of the plane of cell division from parallel to the basal membrane (symmetric cell division), which results in tissue expansion, to perpendicular (asymmetric cell division), which results in one basal proliferative and one suprabasal committed cell (Smart 1970). The asymmetric division is driven by the polarized localization of the proteins LGN and NuMA to the apical cortex of the dividing basal cells (Lechler and Fuchs 2005; Poulson and Lechler 2010). Serum response factor is required to establish a rigid cortical actomyosin network, allowing the critical shape changes associated with mitosis (Luxenburg et al. 2011). Using in vivo skin-specific RNA interference, it has been recently shown that Numa1, LGN, and dynactin promote asymmetric divisions by reorienting the mitotic spindle, and that Notch may be involved in this process (Williams et al. 2011), thus connecting stratification with induction of differentiation.
Hair follicle (HF) morphogenesis relies on a series of cross talks between the epithelium and its underlying mesenchyme (Fig. 1). Pioneering reconstitution experiments showed that the dermis instructs the generation of HFs from hairless regions, demonstrating the critical role of the mesenchyme in specifying the location and number of HFs (Hardy 1992). The morphogenesis of the mouse HF requires at least three signals, the first coming from the mesenchyme at E15 and resulting in the formation of the hair placode; the second at E16 from the newly formed placode to the dermal mesenchymal cells, instructing the formation of the dermal papilla (DP); and the third at E18 from the DP, stimulating the proliferation and differentiation of the HF cells (Fig. 1) (Blanpain and Fuchs 2006). Mature HFs consist of one layer of basal cells called the outer root sheath (ORS) that expresses Keratin-14 (K14) and K5 and contacts the basement membrane. HF progenitors residing in the lower part of the HF, called the matrix cells, proliferate and differentiate into the six concentric cell layers of differentiated cells (Fig. 1) (Blanpain and Fuchs 2006). When HF morphogenesis is completed, the lower two-thirds of the HF degenerates by apoptosis in a process called catagen, and the permanent portion enters a resting stage (telogen) until a new cycle of hair growth takes place (Blanpain and Fuchs 2006).
Figure 1.
Skin morphogenesis and generation of epidermal cell lineages. During embryonic development the single-layered epidermis stratifies, resulting in layers of differentiated cells forming the mature epidermis and its appendages.
The sensory function of the skin is mediated by the activity of specific receptors on distinct cells residing in the epidermis such as keratinocytes, Merkel cells (MCs), and free nerve endings (Lumpkin and Caterina 2007). MCs are neuroendocrine cells that can be found in hairy and glabrous skin. They are clustered in touch-sensitive areas called touch domes (Boulais and Misery 2007). MCs express intermediate filaments of simple epithelia, such as K8, K18, and K20, as well as neuropeptides, proneural transcription factors, and components of presynaptic machinery, suggesting that MCs arise from neural crest cells. However, lineage-tracing experiments using epidermal and neural crest–specific promoters unambiguously show that MCs arise from epidermal cells by a mechanism that requires Atoh1 expression (Morrison et al. 2009; Van Keymeulen et al. 2009).
Thermoregulation in the epidermis is controlled mainly by the sweat glands (Shibasaki et al. 2006). In humans, sweat glands are formed during embryonic development and reach the morphology, size, and final number of the adult appendages by the eighth month of gestation (Saga 2002). It is still unclear how homeostasis of sweat glands is regulated and how their specification occurs. Lineage-tracing studies during embryonic development and adult life will clarify the mechanisms that regulate their specification and homeostasis.
2. STEM CELLS WITH DIFFERENT PROLIFERATION DYNAMICS REGULATE EPIDERMAL HOMEOSTASIS
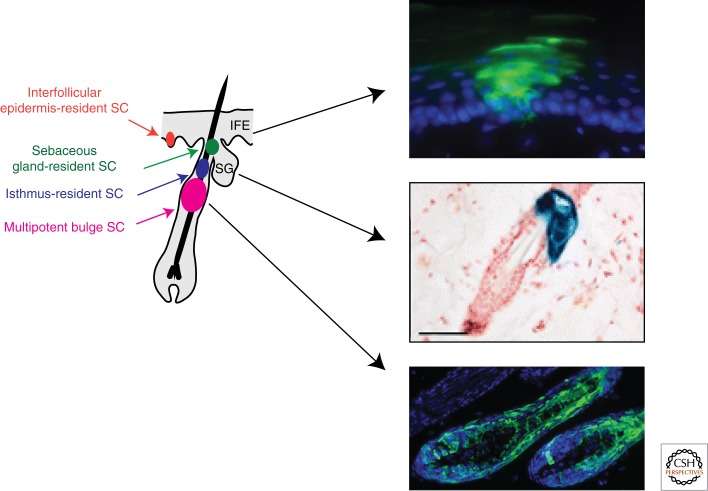
Homeostasis of the different compartments of the skin epidermis is accomplished by the activity of three different classes of stem cells (SCs) that reside in specific niches and exhibit distinct turnover rates (Fig. 2).
Figure 2.
Different types of SCs regulate epidermal homeostasis. Lineage-tracing analysis provided evidence for the contribution of each type of SC of the epidermis during normal homeostasis. (Middle boxed image reproduced, with permission, from Snippert et al. 2010 © Science.)
2.1. Bulge Stem Cells
The first functional characteristic of bulge SCs was their relative quiescence (Cotsarelis et al. 1990). Nucleotide analog pulse-chase experiments showed the presence of label-retaining cells (Bickenbach 1981) in the bulge region of the HF (Cotsarelis et al. 1990), indicating that bulge cells are more quiescent than the other epidermal cells. HF lineage-tracing experiments using tetracycline-regulated green fluorescent protein (GFP)-tagged histone H2B (Tet-o-H2B-GFP) (Tumbar et al. 2004) or the expression of reporter genes under the control of HF-specific promoters such as K15 (Morris et al. 2004), Lgr5 (Jaks et al. 2008), or K19 (Youssef et al. 2010) showed that the cells residing in the bulge and upper germ are responsible for HF regeneration during adult homeostasis. Bulge SCs isolated by microdissection (Rochat et al. 1994; Oshima et al. 2001) or by flow cytometry based on the expression of CD34 (Trempus et al. 2003; Blanpain et al. 2004) or K15-GFP (Morris et al. 2004) and cultured in vitro produced large proliferative colonies that could be propagated for a long time. Transplantation of the progeny of a single bulge SC can generate all HF lineages (Blanpain et al. 2004; Claudinot et al. 2005). During anagen, bulge SCs migrate along the ORS to the matrix, where they proliferate, differentiate, and produce the inner root sheath (IRS) and the hair shaft (Oshima et al. 2001; Ito et al. 2004; Tumbar et al. 2004).
Quantitative H2B-GFP label-retention studies during normal hair homeostasis showed that almost all bulge SCs accomplish at least one division (94%) over a hair cycle, whereas the majority of bulge SCs accomplish three or more divisions (Waghmare et al. 2008). Bromodeoxyuridine pulse during the early stage of HF regeneration indicates that the first cells to incorporate bromodeoxyuridine are the cells of the hair germ (HG), followed by bulge SCs, suggesting a two-step mechanism of HF regeneration in which the first cells to be activated are the HG cells, followed by bulge SCs (Greco et al. 2009). After an initial burst of proliferation that accompanies the early stage of HF regeneration, bulge SCs continue to divide at a lower rate during all of the growing stages of the hair cycle and then stop dividing during catagen (Sotiropoulou et al. 2008; Waghmare et al. 2008). Lineage tracing of single bulge SCs shows their ability to migrate from the bulge to the HG and their multipotency at a clonal level (Zhang et al. 2009b). Although the vast majority (>90%) of Lgr5+ cells express CD34 and are quiescent like all other bulge cells, Lgr5+ cells in the HG have been proposed to cycle frequently (Jaks et al. 2008). However, H2B-GFP label-retention studies show that both bulge and HG contain cells that did not cycle extensively during the previous hair cycle (Nowak et al. 2008; Zhang et al. 2009b; Hsu et al. 2011). Defining more precisely the respective contribution of HG versus bulge SCs during HF regeneration will require the establishment of new inducible CRE mice because all the current models are expressed in both the bulge and HG areas.
Interestingly, although the existence of a morphologically distinct bulge region (Fig. 2) and the expression of bulge SC markers begin during the first hair cycle in 3-week-old mice (Blanpain et al. 2004), the slow-cycling properties of the prospective bulge cells are acquired relatively early during HF morphogenesis. H2B-GFP pulse-chase experiments starting at E18.5 show that the slow-cycling property of some cells of the upper part of the HF is acquired during the first days of postnatal life, and at the end of morphogenesis when the bulge SC niche is formed, and that all label-retaining cells are located in bulge and HG regions (Nowak et al. 2008). The specification of the prospective slow-cycling bulge SC depends on Sox9 expression, as early follicle progenitors fail to sustain hair growth and to establish the adult bulge SC niche in the absence of Sox9 (Vidal et al. 2005; Nowak et al. 2008).
During HF regeneration, some bulge SCs migrate out and home back to their niche after accomplishing several cell divisions. Some of these cells can directly contribute to the next round of HF regeneration as normal resident bulge SCs, whereas others that pass a certain threshold of commitment are still able to home back to their niche but are unable to proliferate anymore. In contrast, these cells contribute to the establishment of their niche by providing a physical anchor between the HF SC and the old club hair and secrete high levels of bone morphogenetic protein-6 (BMP6) and fibroblast growth factor-18 (FGF18) (Hsu et al. 2011) that regulate SC quiescence (Blanpain et al. 2004).
2.2. Stem Cells of the Infundibulum and the Sebaceous Gland
The homeostasis of the upper part of the HF and the sebaceous gland (SG) is maintained independently of bulge SCs and relies on the presence of unipotent SCs (Fig. 2) characterized by the expression of the cell surface markers MTS24, Lrig1, Blimp1, or Lgr6, which are able to differentiate into all epidermal lineages upon transplantation into immunodeficient mice (Horsley et al. 2006; Nijhof et al. 2006; Jensen et al. 2008, 2009; Snippert et al. 2010). Lgr6 lineage-tracing experiments showed that these cells contribute to SG homeostasis but also mark some cells in the interfollicular epidermis (IFE) (Snippert et al. 2010). It is still not clear whether Lgr6 marks bipotent SCs or two different SC populations (Blanpain 2010).
2.3. Stem Cells of the Interfollicular Epidermis
The IFE was initially believed to be maintained by small units of proliferation containing one quiescent SC and several transit-amplifying cells (Potten 1974, 1981) that would amplify the number of differentiated cells and thereby limit the number of cell divisions that a SC needs to accomplish to maintain epidermal homeostasis (Jones et al. 2007). Retroviral marking of basal IFE cells and their progeny revealed the presence of columns of marked cells from the basal layer to the top of the cornified layer, which supports the notion that epidermal proliferative units (EPUs) do exist in vivo (Fig. 2) (Kolodka et al. 1998; Ghazizadeh and Taichman 2001, 2005). Subsequent studies using mutagens to restore the open reading frame of the stop–enhanced GFP reporter suggested that EPUs do not form the hexagonal shape predicted by histological studies and that SCs can migrate between EPUs (Ro and Rannala 2004). Using clonal analysis of the tail epidermis coupled with mathematical modeling, Clayton and colleagues showed that the number of labeled clones decreases with time together with an ever-increasing size of the labeled clones. The absence of steady state in the number and size of labeled clones is not expected based on the SC–transit-amplifying cell model of EPU. These data are instead compatible with a model in which the basal epidermis contains only one type of progenitors, without the involvement of transit-amplifying cells, which choose randomly to renew or differentiate with a defined probability (Clayton et al. 2007).
To maintain the tissue with a constant cell number, the fate of each cell depends on the division rate and the proportion of cells that differentiate thereafter, and there should be an exquisite balance between the number of cells lost by desquamation and cells that proliferate at a given time point. According to this model, epidermal homeostasis is maintained by a combination of symmetric and asymmetric cell divisions; the majority of divisions are asymmetric and progenitors divide every 6–7 d. Using the same strategy of clonal analysis, it has been proposed that the ear epidermis is also maintained by one type of progenitor that randomly chooses between two fates, although the parameters of the cell cycle and the probability to self-renew and differentiate were different (Doupe et al. 2010). Although these data challenge our current view of hierarchical homeostasis, it remains to be shown that the cells targeted in these studies are not a particular subpopulation of epidermal cells.
In conclusion, these data indicate that under physiological conditions, the skin epidermis is maintained by different SC populations (Fig. 2). New tools such as H2B-GFP label retention and clonal analysis have been instrumental in challenging our current view of SC dynamics in the skin epidermis. A lot of questions still remain unanswered. What is the exact contribution of bulge SC versus HG cell in the initiation of HF regeneration? What is the proportion of multipotent SCs in the bulge and the HG? Do HF SCs participate in IFE homeostasis? Do bulge SCs divide randomly and compete for the niche? Does IFE contain slow-cycling cells?
3. MULTIPOTENCY AND PLASTICITY OF EPIDERMAL STEM CELLS
The notion that the skin epidermis contains multipotent SCs with the ability to differentiate into all epidermal cell lineages including the IFE, the SG, and all HF lineages came from transplantation studies, in which different types of epidermal cells were transplanted together with embryonic skin mesenchyme onto immunodeficient mice. Pioneering studies were performed by the group of Barrandon, in which different regions of the epidermis were microdissected and transplanted into immunodeficient mice. These studies demonstrated the ability of bulge SCs to re-form all epidermal lineages (Rochat et al. 1994; Oshima et al. 2001) and suggested the existence of multipotent bulge SCs. Similarly, transplantation of fluorescence-activated cell sorting (FACS)-isolated bulge SCs together with embryonic mesenchyme into immunodeficient mice also resulted in epidermal regeneration and differentiation into the complete repertoire of epidermal cell types (Morris et al. 2004). Transplantation of the progeny of a single cultured bulge SC also led to differentiation into all epidermal lineages including bulge SCs that were able to renew and cycle for more than a year after transplantation (Blanpain et al. 2004) and to be serially transplanted (Claudinot et al. 2005), confirming the enormous renewal and differentiation potential of bulge SCs.
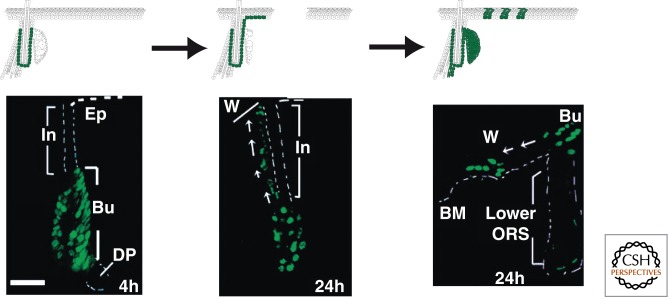
Lineage tracing using inducible CRE specific for adult bulge SCs or constitutive Sonic hedgehog (Shh)-CRE, which marked embryonic HF progenitors and all future adult hair cells, showed that during homeostasis the IFE is maintained independently of the HF SCs and during homeostasis bulge SCs essentially contribute to HF regeneration (Morris et al. 2004; Levy et al. 2005). However, following wounding, bulge SCs rapidly migrate toward the injured region (Tumbar et al. 2004) and actively participate in the repair of the skin barrier (Fig. 3) (Ito et al. 2005; Levy et al. 2007). However, there are conflicting results regarding the long-term fate of the bulge SCs that migrated into the IFE. Some studies suggest that they are only transiently present, whereas others report that they can be maintained long term in the regenerated epidermis (Ito et al. 2005; Levy et al. 2007; Nowak et al. 2008).
Figure 3.
Multipotency of bulge SCs during wound healing. Upon IFE wounding, HF bulge SCs migrate to the site of wounding and contribute to the regeneration of IFE. (Bottom images reproduced, with permission, from Tumbar et al. 2004, © AAAS.)
The contribution of bulge SCs to SG homeostasis is not entirely clear. Although SG arises during morphogenesis from HF progenitors that may be common to bulge SCs, it is unclear whether bulge SCs participate in the normal renewal of the gland in the absence of pathological conditions. Lineage tracing using K15CREPR mice showed labeling of rare SG cells (Morris et al. 2004), whereas Lgr5 (Jaks et al. 2008) and K19CREER failed to label the SG during homeostasis (Youssef et al. 2010; Lapouge et al. 2011). In contrast, during pathological conditions that stimulate SG proliferation such as oncogenic Ras expression (Lapouge et al. 2011) or Blimp1 deletion (Horsley et al. 2006), bulge SCs can contribute to the expansion of SG. Under physiological conditions, the SG contains its own unipotent progenitors that ensure its high cellular turnover. One population of cells expressing MTS24, Lrig1, and Lgr6 located in the upper isthmus and the lower infundibulum has extensive renewal capacities in culture and is capable of differentiating into all epidermal lineages upon transplantation (Nijhof et al. 2006; Jensen et al. 2008, 2009; Snippert et al. 2010). Lineage tracing using Lgr6 promoter confirmed the existence of unipotent SG progenitor cells in the isthmus region that ensure SG homeostasis and that are also capable of migrating toward the IFE to participate in wound repair (Snippert et al. 2010).
Although the IFE during homeostasis clearly contains unipotent progenitors giving rise to suprabasal differentiated cells, overexpression of a constitutively active form of β-catenin in adult IFE progenitors induces de novo HF morphogenesis that recapitulates all aspects of normal embryonic HF development including the formation of DP (Gat et al. 1998). Not all epidermal compartments are equally sensitive to β-catenin. Infundibulum and sebaceous progenitors are the most sensitive epidermal cells and bulge SCs are the most resistant (Baker et al. 2010). De novo HF morphogenesis has been reported to occur during wound healing, and K15CREPR lineage tracing suggested that new follicles arise from IFE, isthmus, or infundibulum cells rather than bulge SCs. Interestingly, β-catenin is also required for HF morphogenesis during wound healing, and increasing the level of Wnt ligands promotes this process (Ito et al. 2007).
In conclusion, upon wounding, epidermal SCs rapidly respond to changes in their microenvironment and adopt a different fate than during normal homeostasis (Fig. 3). These data also indicate that transplantation experiments into immunodeficient mice, although very informative about the differentiation potential of SCs, mimic a wounding environment and extrapolation of the results of such experiments to what happens during physiological conditions can be misleading. They also stress the importance of the underlying mesenchyme in dictating the differentiation potential of epithelial SCs. Nonhairy epithelia including central corneal cells (Ferraris et al. 2000) and epithelial thymic cells (Bonfanti et al. 2010) can adopt HF fate when grafted together with embryonic backskin dermis. More studies will be needed to identify the inductive factors secreted by the mesenchyme and to determine the molecular mechanisms of their cellular memory and epithelial plasticity.
4. SIGNALING PATHWAYS AND GENE NETWORKS CONTROL TISSUE DIFFERENTIATION IN THE SKIN
4.1. Epidermal Stratification, Renewal, and Differentiation
Although the precise mechanisms controlling epidermal stratification, homeostasis, and renewal upon injury are still unfolding, a number of molecules crucial for these processes have been identified. The first transcription factor specifically expressed in the epidermis is p63, a member of p53 family that consists of two major isoforms, TAp63 and ΔNp63, which express and lack the N-terminal transactivation domain, respectively. Mice lacking p63 exhibit major defects in all stratified epithelia including the epidermis, and die immediately after birth because of the absence of skin barrier (Mills et al. 1999; Yang et al. 1999; Koster and Roop 2007). It is a matter of intense debate whether p63 regulates the specification of stratified epidermis (Mills et al. 1999), or controls the renewal of epidermal progenitors (Yang et al. 1999), or a combination of both mechanisms. Forced expression of TAp63 in the lung epithelium induces stratification and keratinization, supporting its role in the induction of stratification (Koster et al. 2004). A more recent study suggests that p63 is essential for the maintenance of the proliferative ability of SCs, whereas it is dispensable for their initial commitment and differentiation (Senoo et al. 2007). Several p63 target genes have been identified, including Perp (Ihrie et al. 2005), Fras1 (Koster and Roop 2007), and the basal integrins β1 and β4 (Carroll et al. 2006).
The mitogen-activated protein kinase (MAPK) cascade is a critical regulator of the balance between epidermal proliferation and differentiation. Deletion of Mek1/2 results in decreased proliferation, leading to epidermal hypoplasia (Scholl et al. 2007). Simultaneous deletion of Erk1/2 also results in proliferation defects and hypoplasia (Dumesic et al. 2009). Conversely, activation of Ras, Raf, or the downstream Mek1 in the skin results in hyperproliferation accompanied by epidermal hyperplasia and down-regulation of differentiation markers (Tarutani et al. 2003; Scholl et al. 2004). Activation of the MAPK pathway during normal homeostasis is mediated by tyrosine kinase receptors such as epidermal growth factor receptor (EGFR) and by basal integrins, and is negatively regulated by adhesion molecules. Accordingly, reduced EGF signaling leads to a decrease in IFE proliferation, whereas constitutive activation of EGFR results in hyperproliferation and epidermal tumors (Janes and Watt 2006). Human IFE SCs express high levels of β1-integrins (Jones and Watt 1993; Jones et al. 1995), whose overexpression leads to their proliferation and expansion (Zhu et al. 1999; Haase et al. 2001). On the contrary, deletion of the cytoplasmic proteins associated with cadherin cell–cell adhesions, α- or p120-catenin, leads to hyperproliferative epidermis because of sustained activation of the Ras-MAPK pathway (Vasioukhin et al. 2001; Perez-Moreno et al. 2006). Consistent with its role as a regulator of proliferation versus differentiation in the epidermis, MAPK signaling is crucial for epidermal tumor formation, and indeed most squamous cell carcinomas (SCCs) are characterized by activation of the Ras pathway and up-regulation of MAPK activity (Khavari and Rinn 2007).
The canonical Notch pathway is a major regulator of IFE differentiation. Notch receptors are expressed in the suprabasal committed cells of the IFE, whereas their ligand Jagged2 is expressed in the basal layer (Watt et al. 2008). Monitoring the activation of the Notch pathway using an antibody against the active form of Notch, lineage-tracing experiments using Cre recombinase triggered by the activation of Notch1, or expression of Notch target gene Hes1 revealed that the Notch pathway is active mainly in suprabasal cells (Blanpain et al. 2006; Vooijs et al. 2007; Moriyama et al. 2008). Complete inactivation of the Notch pathway in mice deficient for γ-secretase 1 and 2 or multiple Notch receptors (Pan et al. 2004) or the transcription factor that relays Notch signaling (RBP-Jκ) (Blanpain et al. 2006) or Hes1 (Moriyama et al. 2008) leads to a lack of spinous differentiation and loss of skin barrier formation. Conversely, overexpression of Notch intracellular domain in the epidermis leads to spinous layer expansion (Uyttendaele et al. 2004; Blanpain et al. 2006). Collectively, those studies show that the Notch pathway regulates the early steps of IFE commitment and differentiation. Notch acts synergistically with the AP2 protein family to regulate the expression of C/EBP transcription factors, which in turn influences terminal differentiation and skin barrier formation (Wang et al. 2008). Accordingly, AP2- (Wang et al. 2008) or C/EBP-deficient (Lopez et al. 2009) mice exhibit defects in epidermal differentiation.
Whereas Notch represents a proto-oncogene in many mammalian tissues, in the skin epidermis, consistent with its role in promoting terminal differentiation, Notch signaling acts as a tumor suppressor, and several mouse models deficient for factors of the Notch pathway develop tumors and are more susceptible to cancer development upon Ras activation (Nicolas et al. 2003; Proweller et al. 2006). In vitro studies using human keratinocytes have indicated that Notch1 acts as a tumor suppressor gene by up-regulating p21 (Rangarajan et al. 2001). However, in vivo studies indicated that Notch1 promotes tumorigenesis in a non-cell-autonomous manner by creating a woundlike microenvironment because of persistent skin barrier defects (Blanpain et al. 2006; Demehri et al. 2009). In addition to cancer, Notch signaling controls inflammatory responses. Notch deficiency during embryonic development leads to non-cell-autonomous B-cell lymphoproliferative disorder, whereas in adult mice it results in severe atopic dermatitis because of elevated levels of thymic stromal lymphopoietin, possibly because of impaired barrier formation, consistent with the finding that patients suffering from atopic dermatitis have reduced Notch expression in the skin (Dumortier et al. 2010). The elevated levels of thymic stromal lymphopoietin may subsequently act systemically and lead to allergic asthma development, explaining the elevated cases of asthma within atopic dermatitis patients (Demehri et al. 2009).
In addition to these signaling pathways, other genes control later steps of IFE differentiation. Mice deficient for Irf6 exhibit hyperproliferative epidermis with concomitant lack of differentiation (Ingraham et al. 2006; Richardson et al. 2006). Mice lacking Klf4 die perinatally by dehydration because of skin barrier loss, associated with alterations in late-stage differentiation markers, such as the cornified envelope gene Sprr2α (Segre et al. 1999). Conversely, ectopic expression of Klf4 in the basal layer leads to a premature barrier formation, accelerated differentiation, and reduced proliferation (Jaubert et al. 2003). Grhl3, one of the mammalian homologs of the Drosophila transcription factor grainyhead, is important for the establishment of the skin barrier function. Grhl3 is expressed in the embryonic epidermis from E8.5 to E17.5 and is maintained at low levels thereafter (Auden et al. 2006). Grhl3-null mice exhibit defective skin barrier and wound repair because of defective protein cross-linking in the suprabasal layers of the epidermis mediated by the reduced transglutaminase-1 expression (Ting et al. 2005; Boglev et al. 2011). These data indicate that different signaling pathways and transcription factors orchestrate the balance between renewal and differentiation of the skin epidermis.
4.2. HF Morphogenesis and Cycling
Wnt/β-catenin signaling is the earliest signaling pathway controlling HF specification. Deletion of β-catenin in the skin epidermis or overexpression of Dkk1, a soluble inhibitor of Wnt signaling, results in the absence of HF with no sign of HF or DP formation (Huelsken et al. 2001; Andl et al. 2002). Conditional deletion of β-catenin in adult mouse epidermis leads to HF degeneration and cyst formation, demonstrating the essential function of β-catenin in maintaining the identity of adult HF SCs (Lowry et al. 2005). Increased levels of β-catenin in adult HF SCs results in the shortening of telogen and the acceleration of telogen to anagen transition (Van Mater et al. 2003; Lo Celso et al. 2004; Lowry et al. 2005), with a concomitant increase in bulge SC proliferation (Lowry et al. 2005). Transcriptional profiling of bulge SCs during telogen and anagen, as well as following β-catenin gain-of-function expression, identified a set of genes modulated by Wnt/β-catenin signaling and associated with anagen reentry. Chromatin immunoprecipitation and functional studies identified among them CyclinD1, TIMP3, and biglycan as direct β-catenin target genes (Lowry et al. 2005). Tcf3 and Tcf4 are also expressed in early undifferentiated embryonic epidermal progenitors and in adult HF SCs and their progeny, and seem to act at least partially as transcriptional repressors in a Wnt-dependent and -independent manner. Their simultaneous deletion leads to rapid HF disappearance (Nguyen et al. 2009). β-Catenin signaling acts upstream of the Eda-A1/Edar/NF-κB signaling pathway, which controls HF morphogenesis during the early stage of HF specification, but EDA/EDAR/NF-κB activity helps to sustain Wnt activity at latter stages (Zhang et al. 2009a). Although overexpression of β-catenin during adult homeostasis leads to de novo HF formation from the IFE and SG and to a lesser extent from HF, high levels of β-catenin signaling during embryonic development lead to expansion of HF placodes, which fail to down-grow and differentiate. Interestingly, increasing β-catenin during embryonic development enhances innervations and pigmentation, as a consequence of higher expression of proteins such as KitL and axonal guidance molecules (Zhang et al. 2008). FACS-isolated DP cells rapidly lose their inductive properties upon in vitro culture, which can be rescued by addition of Wnt3a (Kishimoto et al. 2000). β-Catenin deletion specifically in DP cells results in a strong decrease in HF progenitor proliferation and HF terminal differentiation, leading to a premature entry in catagen and preventing bulge SCs from reinitiating a novel cycle of HF regeneration (Enshell-Seijffers et al. 2010). In adult epidermis, β-catenin regulates the expression of nephronectin by HF SCs, which in turn regulates the attachment of the arrector pili muscle to the top of the bulge (Fujiwara et al. 2011). These data nicely illustrate that similar signals regulate epithelial SCs and the underlying inductive mesenchymal compartments, and that Wnt/β-catenin signaling acts as key regulator of the HF fate specification intrinsically and through the regulation of SC niche. Moreover, deregulation of Wnt/β-catenin signaling leads to HF tumors in mice (Gat et al. 1998) and is activated in human HF tumors (Chan et al. 1999), suggesting that β-catenin acts as an oncogene in the skin.
Shh is expressed in embryonic epidermis in HF down-growths just after placode progression. Shh is genetically downstream of Wnt signaling because Shh is not expressed in the absence of β-catenin (Huelsken et al. 2001; Andl et al. 2002), whereas Lef1 and β-catenin are present in the HG of Shh-null mice (St-Jacques et al. 1998). Shh-CRE marks all HF lineages including the SG, showing that all HF cells derive from the initial HG (Levy et al. 2005). Shh-null mice develop placodes and HG, which fail to invade the dermis and to expand into the different HF lineages (St-Jacques et al. 1998), demonstrating the essential role of Shh for HF progenitor expansion and differentiation. Shh is also expressed in a cyclic manner during the hair cycle. Shh is expressed in adult HG and then in the prospective IRS cells and finally in one polarized side of the matrix called the lateral disk, which gives rise preferentially to IRS (Panteleyev et al. 2001). In addition, Shh is expressed in the mesenchymal part of the hair (Oro and Higgins 2003), suggesting that Shh signaling plays a role in the epithelial–mesenchymal interaction regulating hair cycle. Ectopic administration of Shh or Shh agonists stimulates the proportion of hair in its growing stage (Sato et al. 1999; Paladini et al. 2005), and administration of Shh inhibitor blocks anagen progression (Wang et al. 2000; Silva-Vargas et al. 2005), showing that Shh exhibits a role in mediating anagen progression during the hair cycle. Gli2-deficient mice present an arrest in HF development similar to Shh-null mice, which is rescued by overexpression of a constitutively active but not wild-type form of Gli2 in the epidermis (Mill et al. 2003), showing that Gli2 expression in the epidermis is essential to mediate Shh signaling during HF morphogenesis. Constitutive activation of Shh leads to the formation of familial and sporadic basal cell carcinomas (BCCs) (Epstein 2008), and Shh inhibitors are currently administered in clinical trials for advanced and metastatic BCC (Von Hoff et al. 2009).
Normal HF development and maintenance requires a balance between BMP ligands (e.g., BMP2 and BMP4) and BMP antagonists (e.g., Noggin). Noggin is expressed by the underlying mesenchyme, and its deletion results in a reduction of HF specification and a decrease in HF maturation, partly by a failure to induce Lef1 expression and amplify the Wnt response (Botchkarev et al. 1999; Jamora et al. 2003). Epidermal cells express Bmpr1a, whose conditional deletion results in the formation of large placodelike structures expressing high levels of Lef1, which continue to grow as a mass of undifferentiated ORS-like cells, fail to terminally differentiate, and finally degenerate into hyperproliferative cystic structures leading to HF tumors (Kobielak et al. 2003; Andl et al. 2004; Ming Kwan et al. 2004; Yuhki et al. 2004). In adult mice, BMP signaling regulates bulge SC quiescence. During the quiescent stage of the hair cycle, BMP2 and BMP4 are highly expressed by different dermal cells including fibrobasts, DP cells, and adipocytes (Plikus et al. 2008). The expression of dermal BMP progressively decreases during the resting stage, promoting a switch from quiescence to activation of bulge SCs and the initiation of HF regeneration (Plikus et al. 2008). Bulge SCs secrete high levels of BMP6, which promotes their quiescence in an autocrine or paracrine manner (Blanpain et al. 2004). BMP signaling controls HF SC quiescence in part by regulating the expression of Nfatc1, which is abolished in BMPR1a-null epidermis. Loss of Nfatc1 stimulates bulge SC proliferation and anagen progression through loss of CDK4 repression (Horsley et al. 2008). Transcriptional profiling showed that DP cells express a number of BMP receptors and several BMPs, the most potent being BMP6, promoting the maintenance of the DP-inductive properties upon in vitro culture (Rendl et al. 2005, 2008). Accordingly, conditional ablation of BMPR1a specifically in DP cells in vivo results in loss of the DP-inductive capacity in HF reconstitution assays (Rendl et al. 2008).
During embryonic development, Runx1 is expressed in both the embryonic HF and its underlying mesenchyme (Osorio et al. 2008, 2011). In adult mice, Runx1 is expressed in the bulge, HG, and HF progenitors. Deletion of Runx1 in the skin epidermis during embryonic development results in bulge SCs that fail to actively proliferate during telogen-to-anagen transition, leading to delayed HF regeneration (Osorio et al. 2008). In contrast, Runx1 expression in the embryonic mesenchyme is essential for HF differentiation and long-term maintenance of bulge SCs (Osorio et al. 2011).
4.3. Fine-Tuning of Epidermal Differentiation and Homeostasis by Epigenetic Controls
Ablation of microRNA in the epidermis by conditional deletion of Dicer or DGDR8 leads to defects in epidermal differentiation, such as evaginating HF, increased apoptosis, impaired barrier function, and neonatal lethality (Andl et al. 2006; Yi et al. 2006, 2009). The expression of microRNA is spatiotemporally regulated in the epidermis. For instance, the miR-200 and miR-19/miR-20 families are preferentially expressed in the IFE, whereas the miR-199 family is present exclusively in the HF and miR-125b is highly expressed in HF SCs (Yi et al. 2006; Zhang et al. 2011). Moreover, miR-125b is expressed in HF SCs but dramatically down-regulated in their early progeny, whereas sustained miR-125b expression resulted in a reversible delay in the transition from SCs to committed progenitors, concomitant with thickened IFE, enlarged SG, and failure to form hair (Zhang et al. 2011). Furthermore, miR-203 promotes differentiation and suppresses stemness in the IFE through direct repression of p63. In vitro and in vivo experiments showed that loss of function of miR-203 induces proliferation in the suprabasal cells, whereas gain of function resulted in exit of the cell cycle and loss of colony-forming potential (Lena et al. 2008; Yi et al. 2008).
Histone methylation is crucial for the epidermal differentiation and stratification. Histone deacetylase (HDAC) 1/2 directly mediate the repressive functions of p63, partially by deacetylating p53, which opposes p63 functions. Double conditional knockout mice for HDAC1/2 have thin, smooth skin and failure for limb-digit separation and eyelid fusion (LeBoeuf et al. 2010). Small interfering RNA–mediated deletion of JMJD3, an H3K27me3 demethylase in organotypic human epidermal cultures, prevented epidermal differentiation, whereas conversely, JMJD3 overexpression led to premature differentiation (Sen et al. 2008). Whereas conditional deletion in the epidermis of the polycomb complex protein Ezh2 alone leads to rather mild phenotype (Ezhkova et al. 2009), conditional deletion of both EZH1/2 differentially affects HF and IFE. HF morphogenesis and maintenance are compromised, exhibiting low proliferation and increased apoptosis that is at least partially mediated by ectopic activation of the Ink4a/Inkb/Arf locus. Conversely, upon EZH1/2 deletion, IFE exhibits hyperproliferation (Ezhkova et al. 2011). DNA methyltransferase 1 (DNMT1) and ubiquitinlike, containing PHD and RING finger domains-1 (UHRF1) are expressed in the basal layer of the epidermis and are down-regulated during differentiation. DNMT1 depletion using short hairpin RNA led to premature differentiation of the progenitors and progressive tissue loss in human skin regeneration studies, demonstrating the essential role of DNMT1 for epidermal self-renewal (Sen et al. 2010).
5. GENOMIC MAINTENANCE IN EPIDERMAL STEM CELLS
The DNA integrity of all cells, including SCs, is continuously endangered by genotoxic assaults coming from the surrounding environment, such as UV radiation and by-products of the cellular metabolism such as radical oxygen species (Sancar et al. 2004). It has been estimated that each cell undergoes as many as 105 spontaneous DNA lesions per day (Lindahl 1993). As SCs reside and self-renew in the adult tissues over a lifetime, unrepaired DNA damage may be transmitted to their progeny, leading to precancerous mutations or compromising SC fitness and leading to aging (Blanpain et al. 2011; Mandal et al. 2011). Mutations in components of DNA repair pathways can lead to premature aging syndromes, such as xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy, which are characterized by premature aging, neurodegeneration, and increased incidence of skin cancer (Hoeijmakers 2009). Because of the high cellular turnover of the skin, epidermal SCs have to divide many times throughout life, and thus are at risk of accumulating errors during DNA replication. It has been proposed that SCs may undergo asymmetric divisions during which the SC retains the older (“immortal”) DNA strand and the committed daughter cell inherits the newest synthesized DNA strand (Cairns 1975). Double-labeling pulse-chase experiments (Sotiropoulou et al. 2008) and chromatin labeling with single uridine labeling (Waghmare et al. 2008) provided evidence that in vivo HF SCs segregate their chromosomes randomly. Consistent with the skin barrier function, epidermal SCs are continuously assaulted by UV irradiation. Studies in human and mouse have shown that the proliferating basal cells of the IFE are more sensitive to UV irradiation compared with the more differentiated suprabasal cells (reviewed in Blanpain et al. 2011). Upon UV irradiation the IFE basal cells exhibit sustained p53 accumulation and higher apoptosis rates, concomitant with an inverse gradient of Nrf2 expression, which controls the expression of oxidative stress regulators (Schafer et al. 2010).
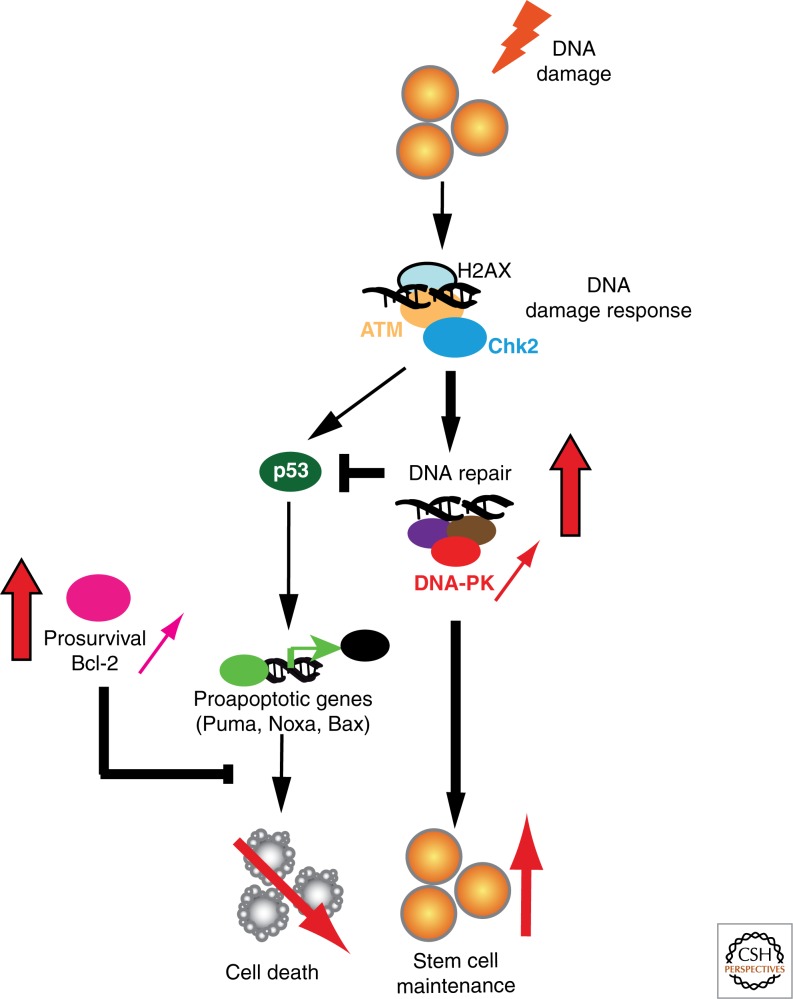
Ex vivo studies of human epidermal SCs have shown that they are more resistant to ionizing radiation, possibly because of an autocrine/paracrine FGF2-mediated increase in DNA repair activity (Rachidi et al. 2007; Harfouche et al. 2010). HF SCs are also more resistant to DNA damage–induced cell death compared with their more mature counterparts (Sotiropoulou et al. 2010). Higher expression of the antiapoptotic factor Bcl-2 and the shorter duration of DNA damage response in HFs because of accelerated nonhomologous end-joining (NHEJ) DNA repair activity contribute to the increased resistance of HF SCs to DNA damage–induced cell death (Fig. 4). The importance of controlled DNA damage response in HF SCs is illustrated by the SC exhaustion and progressive alopecia in mice lacking Atr (Ruzankina et al. 2007) and the SC senescence and premature skin aging in mice lacking Mdm2, a negative regulator of p53 (Gannon et al. 2011). Because NHEJ is an error-prone DNA repair mechanism, this mechanism could be a double-edged sword promoting immediate cell survival at the expense of long-term genomic integrity, potentially leading to the accumulation of precancerous mutations. Indeed, mice deficient for NHEJ and the antiapoptotic factor Bcl-XL are more resistant to chemical-induced carcinogenesis because of increased apoptosis and elimination of mutated SCs (Kemp et al. 1999; Kim et al. 2009b).
Figure 4.
Mechanisms ensuring genomic integrity in skin stem cells. Upon DNA damage, hair follicle bulge stem cells initiate the DNA damage response pathway, but because of higher levels of antiapoptotic Bcl-2 and DNA-PK (red arrows), they exhibit lower DNA damage–induced cell death, accelerated DNA repair, faster attenuation of p53 stabilization, and inhibition of apoptosis.
6. EPIDERMAL STEM CELLS AND CANCER INITIATION
BCC and SCC are the most frequent skin cancers and the most common neoplasias in humans, arising from sun-exposed regions of the skin. Because SCs exist and proliferate for extended periods in adult tissue, they present, in theory, a higher chance of accumulating the necessary mutations to induce cancer formation (Pardal et al. 2003; Clarke and Fuller 2006). However, for most cancers, the precise identification of the tumor-initiating cells remains elusive.
6.1. Basal Cell Carcinoma
BCC is a slow-growing and locally invasive cancer, with more than a million new cases each year worldwide, and is believed to arise from the HF because of its histological and biochemical resemblances to HFs (Owens and Watt 2003; Perez-Losada and Balmain 2003). All BCCs present constitutive hedgehog (HH) activation, loss of heterozygosity of Patched being the most common genetic alteration, followed by constitutively active mutations in Smoothened (Hahn et al. 1996; Johnson et al. 1996; Xie et al. 1998; Epstein 2008). Mouse models for BCC, either by Patched loss of function or by gain-of-function mutations of Shh, SmoM2, and their downstream transcription factors (Gli1 and Gli2) (Dahmane et al. 1997; Fan et al. 1997; Grachtchouk et al. 2000, 2003; Mao et al. 2006), give rise to skin lesions that closely resemble human BCC.
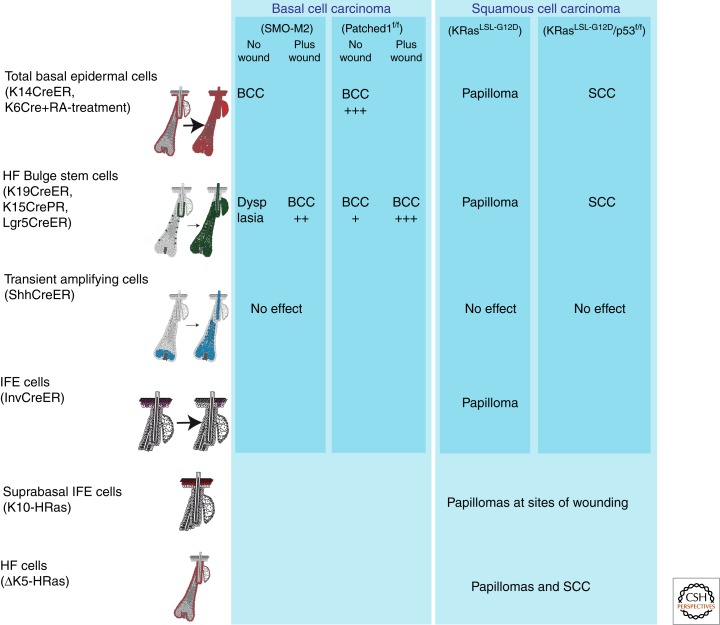
Conditional expression of the SmoM2 oncogene showed that during physiological conditions, bulge SCs and their progeny are highly resistant to SmoM2-induced BCC development (Fig. 5) (Youssef et al. 2010; Wong and Reiter 2011). Using clonal analysis of oncogene-targeted cells, Kass Youssef and colleagues showed that more than 90% of SmoM2-induced BCCs arise from long-lived progenitors residing in the IFE, the rest arising from the upper infundibulum (Youssef et al. 2010). Bulge SCs generate hyperplastic or dysplastic lesions upon SmoM2 expression, which do not progress into invasive cancer (Fig. 5) (Youssef et al. 2010). However, when SmoM2-expressing bulge SCs are stimulated to migrate into the IFE following wounding, bulge SC progeny are competent to progress into invasive BCC (Kasper et al. 2011; Wong and Reiter 2011). Using a pharmacological trick to specifically delete Patched-1 in the IFE and the infundibulum, these cells were also shown to be competent to initiate BCC formation (Adolphe et al. 2006). Patched-1 deletion in bulge SC and its progeny using Lgr5CREER led to BCC at low frequency, indicating that bulge SCs are not completely resistant to HH-mediated BCC development, and their competence is greatly enhanced upon wounding. This indicates that either the microenvironment of IFE is more permissive or that following migration to the IFE (Kasper et al. 2011) bulge SCs are reprogrammed into an IFE fate, which is required to allow the oncogenic program dictated by HH signaling. Genetic lineage-tracing experiments followed by ionizing radiation suggested that bulge SCs could be the cellular origin of ionizing radiation–induced BCC (Wang et al. 2011). However, the infundibulum and IFE-ectopic activity of the K15CREPR, the K15 expression in BCC (Youssef et al. 2010), and the leakiness of K15CREPR (Lapouge et al. 2011) may also explain the apparent bulge SC origin of BCC in this model.
Figure 5.
Cells at the origin of the most common types of skin cancer. Activation of Hedgehog signaling in different cellular compartments of the epidermis using mice conditionally expressing constitutively active Smoothened mutant (SmoM2) showed that BCC does not arise by hair follicle stem cells as previously believed, but from long-term resident progenitor cells of the interfollicular epidermis and the upper infundibulum. Likewise, using the same approach and mice conditionally expressing a constitutively active KRas mutant (G12D), it was shown that squamous tumors can be generated by both interfollicular epidermis and hair follicle stem cells, but not their immediate progeny, whereas additional mutations have to occur to progress to the malignant state.
The primary cilium represents a small membrane organelle that plays an important role in regulating HH signaling (Goetz and Anderson 2010). Deletion of Kif3a or Itf88, critical for cilia formation, results in hyperproliferative skin lesions and excessive migration of bulge SCs to the IFE (Croyle et al. 2011). Human BCCs are also ciliated, and deletion of Kif3a or Itf88 blocked SmoM2-induced tumorigenesis but enhanced Gli2-mediated BCC formation (Wong et al. 2009), suggesting that cilia can either potentiate or inhibit tumorigenesis depending on the oncogenic context.
Different HH target genes such as Sox9 and Wnt ligands and receptors are expressed in mouse and human BCC (Vidal et al. 2005; Yang et al. 2008), and blocking Wnt signaling by Dkk1 overexpression inhibits SmoM2-induced tumorigenesis during skin morphogenesis (Yang et al. 2008). The exact role of Wnt signaling and Sox9 expression during BCC development and whether these signaling pathways control similar functions during HF morphogenesis and tumor development remain to be determined.
6.2. Squamous Cell Carcinoma
SCC is the second-most-frequent skin cancer, with more than 500,000 patients per year worldwide (Alam and Ratner 2001). The most extensively used mouse model for skin SCC is a multistage, chemically induced carcinogenesis (Kemp 2005), in which mice are treated first with a mutagen (the 9,10-dimethyl-1,2-benzanthracene, DMBA) and then with a drug that stimulates epidermal proliferation, such as 12-O-tetradecanoyl phorbol-13-acetate (TPA). During TPA treatment, benign tumors (papillomas) arise, probably as a consequence of additional mutations, some of which progress into invasive SCC. This model selects cells with active mutations in the Ras pathway. Although the most common form of Ras mutations found in papillomas occurred in the HRas gene (Quintanilla et al. 1986), mutations in the KRas gene have also been reported (van der Schroeff et al. 1990; Sutter et al. 1993; Spencer et al. 1995).
SCC often present signs of squamous differentiation, suggesting that SCC may originate from cells that naturally undergo squamous differentiation such as the IFE (Owens and Watt 2003). TPA administration stimulates papilloma formation even when started a year after DMBA treatment, suggesting that the initial mutation arises in long-lived SCs (Morris et al. 1986, 1997; Morris 2000). The demonstration that tumors can form, although at lower frequency, when the IFE is removed by dermabrasion after DMBA and repaired by HF cells suggests that cancer-initiating cells may reside in the IFE as well as in HFs (Argyris and Slaga 1981). However, some studies suggest that oncogenic mutations could also occur in differentiated cells of the epidermis (Owens et al. 2003). Suprabasal expression of α6β4 integrins increases papilloma formation following DMBA/TPA treatment and expression of active MEK in suprabasal cells enhances papilloma formation following wounding. The promotion of tumorigenesis by differentiated cells has been proposed to be mediated through an increase in inflammation (Arwert et al. 2010). In addition, transgenic mice expressing a mutated form of HRas in IFE suprabasal cells under the promoter K10 develop papillomas at sites of wounding (Bailleul et al. 1990). Transgenic mice expressing HRas under the control of a truncated form of the K5 promoter are preferentially expressed in the HF of adult mice, and develop papillomas and SCC (Brown et al. 1998). Mosaic activation of a constitutively active mutant of KRas (KRasLSL-G12D) mimicking the natural occurrence of sporadic tumors in different compartments of the skin epidermis revealed the SCC-initiating cells (Fig. 5) (Lapouge et al. 2011; White et al. 2011). Papilloma formation was observed upon KRasG12D expression in bulge SCs and their progeny, but not in HF transit-amplifying cells. KRasG12D expression in the IFE induced hyperproliferation and hyperplasia, sometimes progressing into papillomas, indicating that cells of the IFE are competent to develop papillomas (Lapouge et al. 2011). The cellular origin of DMBA/TPA-induced papilloma and SCC has not been determined yet, but the absence of tumors in DMBA/TPA-treated CD34-null mice (Trempus et al. 2007) and the resistance to DMBA/TPA-induced tumor formation in mice with Stat3 deletion in bulge SCs and their progeny (Chan et al. 2004; Kim et al. 2009a) indicate that bulge SCs probably participate in papilloma formation.
No sign of malignant transformation was observed upon oncogenic KRas expression alone, suggesting that other genetic events are required to induce the development of invasive SCC (Fig. 5). Combined p53 deletion and KRasG12D expression in the basal layer of the epidermis and in bulge SCs rapidly induced SCC, suggesting that at least two genetic hits are required to mediate SCC initiation in the context of oncogenic Ras (Fig. 5) (Lapouge et al. 2011; White et al. 2011). Whereas p53 loss of function has been associated with malignant progression (Owens and Watt 2003; Perez-Losada and Balmain 2003), p53 gain-of-function mutation has been shown to increase tumor initiation and progression (Caulin et al. 2007). More studies are needed to better understand SCC initiation, which genes control the renewal and malignant progression of oncogene-targeted cells, what is the role of the tumor microenvironment, and how p53 controls malignant progression.
7. THERAPEUTIC APPLICATIONS OF SKIN STEM/PROGENITOR CELLS
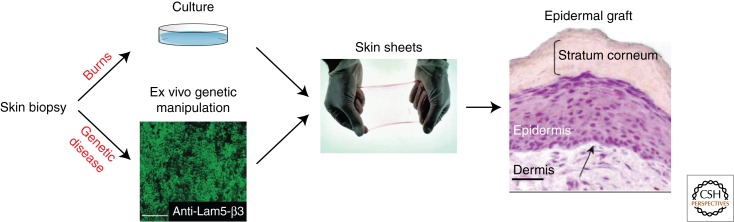
Skin stem cells are already being used for patients with severe burns, as well as several skin disorders (Fig. 6). Since the first demonstration by Green and colleagues in 1975 that skin keratinocytes can be grown in vitro to form cohesive sheets of cells (Rheinwald and Green 1975), numerous improvements have been proposed, and culture of a small skin biopsy can now provide sufficient epidermal cells for extensive autograft. Currently, the use of cultured epidermal grafts is hampered by the length of the culture time, the type of burn surface influencing the graft adherence, and the high cost. Bioengineered skin equivalents have been proposed, such as the use of collagen-based matrices or human plasma as dermal scaffold, but their use in clinic is still limited (Alonso and Fuchs 2003). The use of cryopreserved allogeneic skin grafts, which are replaced by the epithelial autografts when ready, provides the best results in terms of functional and esthetic results, but unfortunately is more expensive and requires special facilities (Atiyeh and Costagliola 2007).
Figure 6.
Clinical use of skin stem cells. Autologous keratinocytes from skin biopsies can be cultured in vitro to generate skin sheets that will be used in patients with severe burns or, upon genetic manipulation, in patients with genetic skin disorders. (Portions reproduced, with permission, from Mavilio et al. 2006 © Nat Med.)
Cultured skin SCs also hold great promise for the treatment of genetic skin diseases (Fig. 6). Preclinical studies have suggested that skin SCs can be used for the treatment of disorders such as epidermolysis bullosa, an inherited deficiency of components of hemidesmosomes. Keratinocytes have been isolated from epidermolysis bullosa patients, the deficiency in laminin-5 has been corrected in vitro, and the cells have been grafted back to the patients, resulting in stable correction of the disease in the transplanted site (Mavilio et al. 2006; Siprashvili et al. 2010).
In conclusion, although cell therapy based on skin SCs is already currently used in clinical practice, there are specific hurdles that need to be overcome before it is more generally applied, such as improving the culture conditions so as to obtain larger numbers of cells in less time, as well as understanding why a fraction of skin transplants do not engraft. It will be a great improvement for the comfort of patients to be able to reconstruct the skin appendages, in particular the sweat glands, but also the hair follicles. Skin SCs may also hold promise to treat additional disorders, and preclinical studies should evaluate the types of disease and the safety of these therapeutic approaches.
Footnotes
Editors: Patrick P.L. Tam, W. James Nelson, and Janet Rossant
Additional Perspectives on Mammalian Development available at www.cshperspectives.org
REFERENCES
- Adolphe C, Hetherington R, Ellis T, Wainwright B 2006. Patched1 functions as a gatekeeper by promoting cell cycle progression. Cancer Res 66: 2081–2088 [DOI] [PubMed] [Google Scholar]
- Alam M, Ratner D 2001. Cutaneous squamous-cell carcinoma. N Engl J Med 344: 975–983 [DOI] [PubMed] [Google Scholar]
- Alonso L, Fuchs E 2003. Stem cells of the skin epithelium. Proc Natl Acad Sci 100: 11830–11835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE 2002. WNT signals are required for the initiation of hair follicle development. Dev Cell 2: 643–653 [DOI] [PubMed] [Google Scholar]
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, et al. 2004. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development 131: 2257–2268 [DOI] [PubMed] [Google Scholar]
- Andl T, Murchison EP, Liu F, Zhang Y, Yunta-Gonzalez M, Tobias JW, Andl CD, Seykora JT, Hannon GJ, Millar SE 2006. The miRNA-processing enzyme dicer is essential for the morphogenesis and maintenance of hair follicles. Curr Biol 16: 1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyris TS, Slaga TJ 1981. Promotion of carcinomas by repeated abrasion in initiated skin of mice. Cancer Res 41: 5193–5195 [PubMed] [Google Scholar]
- Arwert EN, Lal R, Quist S, Rosewell I, van Rooijen N, Watt FM 2010. Tumor formation initiated by nondividing epidermal cells via an inflammatory infiltrate. Proc Natl Acad Sci 107: 19903–19908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiyeh BS, Costagliola M 2007. Cultured epithelial autograft (CEA) in burn treatment: Three decades later. Burns 33: 405–413 [DOI] [PubMed] [Google Scholar]
- Auden A, Caddy J, Wilanowski T, Ting SB, Cunningham JM, Jane SM 2006. Spatial and temporal expression of the Grainyhead-like transcription factor family during murine development. Gene Expr Patterns 6: 964–970 [DOI] [PubMed] [Google Scholar]
- Bailleul B, Surani MA, White S, Barton SC, Brown K, Blessing M, Jorcano J, Balmain A 1990. Skin hyperkeratosis and papilloma formation in transgenic mice expressing a ras oncogene from a suprabasal keratin promoter. Cell 62: 697–708 [DOI] [PubMed] [Google Scholar]
- Baker CM, Verstuyf A, Jensen KB, Watt FM 2010. Differential sensitivity of epidermal cell subpopulations to β-catenin-induced ectopic hair follicle formation. Dev Biol 343: 40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickenbach JR 1981. Identification and behavior of label-retaining cells in oral mucosa and skin. J Dent Res 60: 1611–1620 [DOI] [PubMed] [Google Scholar]
- Blanpain C 2010. Stem cells: Skin regeneration and repair. Nature 464: 686–687 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E 2006. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol 22: 339–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E 2004. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118: 635–648 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Pasolli HA, Fuchs E 2006. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev 20: 3022–3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Mohrin M, Sotiropoulou PA, Passegue E 2011. DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell 8: 16–29 [DOI] [PubMed] [Google Scholar]
- Boglev Y, Wilanowski T, Caddy J, Parekh V, Auden A, Darido C, Hislop NR, Cangkrama M, Ting SB, Jane SM 2011. The unique and cooperative roles of the Grainy head-like transcription factors in epidermal development reflect unexpected target gene specificity. Dev Biol 349: 512–522 [DOI] [PubMed] [Google Scholar]
- Bonfanti P, Claudinot S, Amici AW, Farley A, Blackburn CC, Barrandon Y 2010. Microenvironmental reprogramming of thymic epithelial cells to skin multipotent stem cells. Nature 466: 978–982 [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, et al. 1999. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol 1: 158–164 [DOI] [PubMed] [Google Scholar]
- Boulais N, Misery L 2007. Merkel cells. J Am Acad Dermatol 57: 147–165 [DOI] [PubMed] [Google Scholar]
- Brown K, Strathdee D, Bryson S, Lambie W, Balmain A 1998. The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Curr Biol 8: 516–524 [DOI] [PubMed] [Google Scholar]
- Cairns J 1975. Mutation selection and the natural history of cancer. Nature 255: 197–200 [DOI] [PubMed] [Google Scholar]
- Carroll DK, Carroll JS, Leong CO, Cheng F, Brown M, Mills AA, Brugge JS, Ellisen LW 2006. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol 8: 551–561 [DOI] [PubMed] [Google Scholar]
- Caulin C, Nguyen T, Lang GA, Goepfert TM, Brinkley BR, Cai WW, Lozano G, Roop DR 2007. An inducible mouse model for skin cancer reveals distinct roles for gain- and loss-of-function p53 mutations. J Clin Invest 117: 1893–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EF, Gat U, McNiff JM, Fuchs E 1999. A common human skin tumour is caused by activating mutations in β-catenin. Nat Genet 21: 410–413 [DOI] [PubMed] [Google Scholar]
- Chan KS, Carbajal S, Kiguchi K, Clifford J, Sano S, DiGiovanni J 2004. Epidermal growth factor receptor-mediated activation of Stat3 during multistage skin carcinogenesis. Cancer Res 64: 2382–2389 [DOI] [PubMed] [Google Scholar]
- Clarke MF, Fuller M 2006. Stem cells and cancer: Two faces of Eve. Cell 124: 1111–1115 [DOI] [PubMed] [Google Scholar]
- Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y 2005. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci 102: 14677–14682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH 2007. A single type of progenitor cell maintains normal epidermis. Nature 446: 185–189 [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM 1990. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61: 1329–1337 [DOI] [PubMed] [Google Scholar]
- Croyle MJ, Lehman JM, O’Connor AK, Wong SY, Malarkey EB, Iribarne D, Dowdle WE, Schoeb TR, Verney ZM, Athar M, et al. 2011. Role of epidermal primary cilia in the homeostasis of skin and hair follicles. Development 138: 1675–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A 1997. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature 389: 876–881 [DOI] [PubMed] [Google Scholar]
- Demehri S, Turkoz A, Kopan R 2009. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell 16: 55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe DP, Klein AM, Simons BD, Jones PH 2010. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev Cell 18: 317–323 [DOI] [PubMed] [Google Scholar]
- Dumesic PA, Scholl FA, Barragan DI, Khavari PA 2009. Erk1/2 MAP kinases are required for epidermal G2/M progression. J Cell Biol 185: 409–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumortier A, Durham AD, Di Piazza M, Vauclair S, Koch U, Ferrand G, Ferrero I, Demehri S, Song LL, Farr AG, et al. 2010. Atopic dermatitis-like disease and associated lethal myeloproliferative disorder arise from loss of Notch signaling in the murine skin. PLoS ONE 5: e9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA 2010. β-Catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell 18: 633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein EH 2008. Basal cell carcinomas: Attack of the hedgehog. Nat Rev Cancer 8: 743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su IH, Hannon G, Tarakhovsky A, Fuchs E 2009. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell 136: 1122–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhkova E, Lien WH, Stokes N, Pasolli HA, Silva JM, Fuchs E 2011. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev 25: 485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Oro AE, Scott MP, Khavari PA 1997. Induction of basal cell carcinoma features in transgenic human skin expressing Sonic Hedgehog. Nat Med 3: 788–792 [DOI] [PubMed] [Google Scholar]
- Ferraris C, Chevalier G, Favier B, Jahoda CA, Dhouailly D 2000. Adult corneal epithelium basal cells possess the capacity to activate epidermal, pilosebaceous and sweat gland genetic programs in response to embryonic dermal stimuli. Development 127: 5487–5495 [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, Hartner A, Sekiguchi K, Reichardt LF, Watt FM 2011. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144: 577–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon HS, Donehower LA, Lyle S, Jones SN 2011. Mdm2-p53 signaling regulates epidermal stem cell senescence and premature aging phenotypes in mouse skin. Dev Biol 353: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E 1998. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell 95: 605–614 [DOI] [PubMed] [Google Scholar]
- Ghazizadeh S, Taichman LB 2001. Multiple classes of stem cells in cutaneous epithelium: A lineage analysis of adult mouse skin. EMBO J 20: 1215–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazizadeh S, Taichman LB 2005. Organization of stem cells and their progeny in human epidermis. J Invest Dermatol 124: 367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV 2010. The primary cilium: A signalling centre during vertebrate development. Nat Rev Genet 11: 331–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC, Dlugosz AA 2000. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet 24: 216–217 [DOI] [PubMed] [Google Scholar]
- Grachtchouk V, Grachtchouk M, Lowe L, Johnson T, Wei L, Wang A, de Sauvage F, Dlugosz AA 2003. The magnitude of hedgehog signaling activity defines skin tumor phenotype. EMBO J 22: 2741–2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E 2009. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4: 155–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase I, Hobbs RM, Romero MR, Broad S, Watt FM 2001. A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J Clin Invest 108: 527–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S, et al. 1996. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 85: 841–851 [DOI] [PubMed] [Google Scholar]
- Hardy MH 1992. The secret life of the hair follicle. Trends Genet 8: 55–61 [DOI] [PubMed] [Google Scholar]
- Harfouche G, Vaigot P, Rachidi W, Rigaud O, Moratille S, Marie M, Lemaitre G, Fortunel NO, Martin MT 2010. Fibroblast growth factor type 2 signaling is critical for DNA repair in human keratinocyte stem cells. Stem Cells 28: 1639–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers JH 2009. DNA damage, aging, and cancer. New Engl J Med 361: 1475–1485 [DOI] [PubMed] [Google Scholar]
- Horsley V, O’Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, Nussenzweig M, Tarakhovsky A, Fuchs E 2006. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell 126: 597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E 2008. NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132: 299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Pasolli HA, Fuchs E 2011. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 144: 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W 2001. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105: 533–545 [DOI] [PubMed] [Google Scholar]
- Ihrie RA, Marques MR, Nguyen BT, Horner JS, Papazoglu C, Bronson RT, Mills AA, Attardi LD 2005. Perp is a p63-regulated gene essential for epithelial integrity. Cell 120: 843–856 [DOI] [PubMed] [Google Scholar]
- Ingraham CR, Kinoshita A, Kondo S, Yang B, Sajan S, Trout KJ, Malik MI, Dunnwald M, Goudy SL, Lovett M, et al. 2006. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6). Nat Genet 38: 1335–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Kizawa K, Hamada K, Cotsarelis G 2004. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation 72: 548–557 [DOI] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G 2005. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11: 1351–1354 [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G 2007. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447: 316–320 [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R 2008. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 40: 1291–1299 [DOI] [PubMed] [Google Scholar]
- Jamora C, DasGupta R, Kocieniewski P, Fuchs E 2003. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature 422: 317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes SM, Watt FM 2006. New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer 6: 175–183 [DOI] [PubMed] [Google Scholar]
- Jaubert J, Cheng J, Segre JA 2003. Ectopic expression of kruppel like factor 4 (Klf4) accelerates formation of the epidermal permeability barrier. Development 130: 2767–2777 [DOI] [PubMed] [Google Scholar]
- Jensen UB, Yan X, Triel C, Woo SH, Christensen R, Owens DM 2008. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J Cell Sci 121: 609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM 2009. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 4: 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH Jr, et al. 1996. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 272: 1668–1671 [DOI] [PubMed] [Google Scholar]
- Jones PH, Watt FM 1993. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73: 713–724 [DOI] [PubMed] [Google Scholar]
- Jones PH, Harper S, Watt FM 1995. Stem cell patterning and fate in human epidermis. Cell 80: 83–93 [DOI] [PubMed] [Google Scholar]
- Jones PH, Simons BD, Watt FM 2007. Sic transit gloria: Farewell to the epidermal transit amplifying cell? Cell Stem Cell 1: 371–381 [DOI] [PubMed] [Google Scholar]
- Kasper M, Jaks V, Are A, Bergstrom A, Schwager A, Barker N, Toftgard R 2011. Wounding enhances epidermal tumorigenesis by recruiting hair follicle keratinocytes. Proc Natl Acad Sci 108: 4099–4104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp CJ 2005. Multistep skin cancer in mice as a model to study the evolution of cancer cells. Semin Cancer Biol 15: 460–473 [DOI] [PubMed] [Google Scholar]
- Kemp CJ, Vo K, Gurley KE 1999. Resistance to skin tumorigenesis in DNAPK-deficient SCID mice is not due to immunodeficiency but results from hypersensitivity to TPA-induced apoptosis. Carcinogenesis 20: 2051–2056 [DOI] [PubMed] [Google Scholar]
- Khavari TA, Rinn J 2007. Ras/Erk MAPK signaling in epidermal homeostasis and neoplasia. Cell Cycle 6: 2928–2931 [DOI] [PubMed] [Google Scholar]
- Kim DJ, Kataoka K, Rao D, Kiguchi K, Cotsarelis G, Digiovanni J 2009a. Targeted disruption of Stat3 reveals a major role for follicular stem cells in skin tumor initiation. Cancer Res 69: 7587–7594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Kataoka K, Sano S, Connolly K, Kiguchi K, DiGiovanni J 2009b. Targeted disruption of Bcl-xL in mouse keratinocytes inhibits both UVB- and chemically induced skin carcinogenesis. Mol Carcinog 48: 873–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto J, Burgeson RE, Morgan BA 2000. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev 14: 1181–1185 [PMC free article] [PubMed] [Google Scholar]
- Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E 2003. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol 163: 609–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodka TM, Garlick JA, Taichman LB 1998. Evidence for keratinocyte stem cells in vitro: Long term engraftment and persistence of transgene expression from retrovirus-transduced keratinocytes. Proc Natl Acad Sci 95: 4356–4361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster MI, Roop DR 2007. Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol 23: 93–113 [DOI] [PubMed] [Google Scholar]
- Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR 2004. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev 18: 126–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapouge G, Youssef KK, Vokaer B, Achouri Y, Michaux C, Sotiropoulou PA, Blanpain C 2011. Identifying the cellular origin of squamous skin tumors. Proc Natl Acad Sci 108: 7431–7436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBoeuf M, Terrell A, Trivedi S, Sinha S, Epstein JA, Olson EN, Morrisey EE, Millar SE 2010. Hdac1 and Hdac2 act redundantly to control p63 and p53 functions in epidermal progenitor cells. Dev Cell 19: 807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E 2005. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437: 275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena AM, Shalom-Feuerstein R, di Val Cervo PR, Aberdam D, Knight RA, Melino G, Candi E 2008. miR-203 represses “stemness” by repressing ΔNp63. Cell Death Differ 15: 1187–1195 [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Harfe BD, Morgan BA 2005. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell 9: 855–861 [DOI] [PubMed] [Google Scholar]
- Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA 2007. Epidermal stem cells arise from the hair follicle after wounding. FASEB J 21: 1358–1366 [DOI] [PubMed] [Google Scholar]
- Lindahl T 1993. Instability and decay of the primary structure of DNA. Nature 362: 709–715 [DOI] [PubMed] [Google Scholar]
- Lo Celso C, Prowse DM, Watt FM 2004. Transient activation of β-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development 131: 1787–1799 [DOI] [PubMed] [Google Scholar]
- Lopez RG, Garcia-Silva S, Moore SJ, Bereshchenko O, Martinez-Cruz AB, Ermakova O, Kurz E, Paramio JM, Nerlov C 2009. C/EBPα and β couple interfollicular keratinocyte proliferation arrest to commitment and terminal differentiation. Nat Cell Biol 11: 1181–1190 [DOI] [PubMed] [Google Scholar]
- Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E 2005. Defining the impact of β-catenin/Tcf transactivation on epithelial stem cells. Genes Dev 19: 1596–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ 2007. Mechanisms of sensory transduction in the skin. Nature 445: 858–865 [DOI] [PubMed] [Google Scholar]
- Luxenburg C, Amalia Pasolli H, Williams SE, Fuchs E 2011. Developmental roles for Srf, cortical cytoskeleton and cell shape in epidermal spindle orientation. Nat Cell Biol 13: 203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal PK, Blanpain C, Rossi DJ 2011. DNA damage response in adult stem cells: Pathways and consequences. Nat Rev Mol Cell Biol 12: 198–202 [DOI] [PubMed] [Google Scholar]
- Mao J, Ligon KL, Rakhlin EY, Thayer SP, Bronson RT, Rowitch D, McMahon AP 2006. A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer Res 66: 10171–10178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavilio F, Pellegrini G, Ferrari S, Di Nunzio F, Di Iorio E, Recchia A, Maruggi G, Ferrari G, Provasi E, Bonini C, et al. 2006. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med 12: 1397–1402 [DOI] [PubMed] [Google Scholar]
- Mill P, Mo R, Fu H, Grachtchouk M, Kim PC, Dlugosz AA, Hui CC 2003. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev 17: 282–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A 1999. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398: 708–713 [DOI] [PubMed] [Google Scholar]
- Ming Kwan K, Li AG, Wang XJ, Wurst W, Behringer RR 2004. Essential roles of BMPR-IA signaling in differentiation and growth of hair follicles and in skin tumorigenesis. Genesis 39: 10–25 [DOI] [PubMed] [Google Scholar]
- Moriyama M, Durham AD, Moriyama H, Hasegawa K, Nishikawa S, Radtke F, Osawa M 2008. Multiple roles of Notch signaling in the regulation of epidermal development. Dev Cell 14: 594–604 [DOI] [PubMed] [Google Scholar]
- Morris RJ 2000. Keratinocyte stem cells: Targets for cutaneous carcinogens. J Clin Invest 106: 3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Fischer SM, Slaga TJ 1986. Evidence that a slowly cycling subpopulation of adult murine epidermal cells retains carcinogen. Cancer Res 46: 3061–3066 [PubMed] [Google Scholar]
- Morris RJ, Coulter K, Tryson K, Steinberg SR 1997. Evidence that cutaneous carcinogen-initiated epithelial cells from mice are quiescent rather than actively cycling. Cancer Res 57: 3436–3443 [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G 2004. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22: 411–417 [DOI] [PubMed] [Google Scholar]
- Morrison KM, Miesegaes GR, Lumpkin EA, Maricich SM 2009. Mammalian Merkel cells are descended from the epidermal lineage. Dev Biol 336: 76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Merrill BJ, Polak L, Nikolova M, Rendl M, Shaver TM, Pasolli HA, Fuchs E 2009. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nat Genet 41: 1068–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, Hui CC, Clevers H, Dotto GP, Radtke F 2003. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet 33: 416–421 [DOI] [PubMed] [Google Scholar]
- Nijhof JG, Braun KM, Giangreco A, van Pelt C, Kawamoto H, Boyd RL, Willemze R, Mullenders LH, Watt FM, de Gruijl FR, et al. 2006. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development 133: 3027–3037 [DOI] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E 2008. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 3: 33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro AE, Higgins K 2003. Hair cycle regulation of Hedgehog signal reception. Dev Biol 255: 238–248 [DOI] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y 2001. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104: 233–245 [DOI] [PubMed] [Google Scholar]
- Osorio KM, Lee SE, McDermitt DJ, Waghmare SK, Zhang YV, Woo HN, Tumbar T 2008. Runx1 modulates developmental, but not injury-driven, hair follicle stem cell activation. Development 135: 1059–1068 [DOI] [PubMed] [Google Scholar]
- Osorio KM, Lilja KC, Tumbar T 2011. Runx1 modulates adult hair follicle stem cell emergence and maintenance from distinct embryonic skin compartments. J Cell Biol 193: 235–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DM, Watt FM 2003. Contribution of stem cells and differentiated cells to epidermal tumours. Nat Rev Cancer 3: 444–451 [DOI] [PubMed] [Google Scholar]
- Owens DM, Romero MR, Gardner C, Watt FM 2003. Suprabasal α6β4 integrin expression in epidermis results in enhanced tumourigenesis and disruption of TGFβ signalling. J Cell Sci 116: 3783–3791 [DOI] [PubMed] [Google Scholar]
- Paladini RD, Saleh J, Qian C, Xu GX, Rubin LL 2005. Modulation of hair growth with small molecule agonists of the hedgehog signaling pathway. J Invest Dermatol 125: 638–646 [DOI] [PubMed] [Google Scholar]
- Pan Y, Lin MH, Tian X, Cheng HT, Gridley T, Shen J, Kopan R 2004. γ-Secretase functions through Notch signaling to maintain skin appendages but is not required for their patterning or initial morphogenesis. Dev Cell 7: 731–743 [DOI] [PubMed] [Google Scholar]
- Panteleyev AA, Jahoda CA, Christiano AM 2001. Hair follicle predetermination. J Cell Sci 114: 3419–3431 [DOI] [PubMed] [Google Scholar]
- Pardal R, Clarke MF, Morrison SJ 2003. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer 3: 895–902 [DOI] [PubMed] [Google Scholar]
- Perez-Losada J, Balmain A 2003. Stem-cell hierarchy in skin cancer. Nat Rev Cancer 3: 434–443 [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E 2006. p120-catenin mediates inflammatory responses in the skin. Cell 124: 631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM 2008. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 451: 340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS 1974. The epidermal proliferative unit: The possible role of the central basal cell. Cell Tissue Kinet 7: 77–88 [DOI] [PubMed] [Google Scholar]
- Potten CS 1981. Cell replacement in epidermis (keratopoiesis) via discrete units of proliferation. Int Rev Cytol 69: 271–318 [DOI] [PubMed] [Google Scholar]
- Poulson ND, Lechler T 2010. Robust control of mitotic spindle orientation in the developing epidermis. J Cell Biol 191: 915–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proweller A, Tu L, Lepore JJ, Cheng L, Lu MM, Seykora J, Millar SE, Pear WS, Parmacek MS 2006. Impaired Notch signaling promotes de novo squamous cell carcinoma formation. Cancer Res 66: 7438–7444 [DOI] [PubMed] [Google Scholar]
- Quintanilla M, Brown K, Ramsden M, Balmain A 1986. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature 322: 78–80 [DOI] [PubMed] [Google Scholar]
- Rachidi W, Harfourche G, Lemaitre G, Amiot F, Vaigot P, Martin MT 2007. Sensing radiosensitivity of human epidermal stem cells. Radiother Oncol 83: 267–276 [DOI] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster JC, Krishna S, Metzger D, Chambon P, et al. 2001. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J 20: 3427–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendl M, Lewis L, Fuchs E 2005. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol 3: e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendl M, Polak L, Fuchs E 2008. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev 22: 543–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald JG, Green H 1975. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell 6: 331–343 [DOI] [PubMed] [Google Scholar]
- Richardson RJ, Dixon J, Malhotra S, Hardman MJ, Knowles L, Boot-Handford RP, Shore P, Whitmarsh A, Dixon MJ 2006. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat Genet 38: 1329–1334 [DOI] [PubMed] [Google Scholar]
- Ro S, Rannala B 2004. A stop-EGFP transgenic mouse to detect clonal cell lineages generated by mutation. EMBO Rep 5: 914–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat A, Kobayashi K, Barrandon Y 1994. Location of stem cells of human hair follicles by clonal analysis. Cell 76: 1063–1073 [DOI] [PubMed] [Google Scholar]
- Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ 2007. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1: 113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga K 2002. Structure and function of human sweat glands studied with histochemistry and cytochemistry. Prog Histochem Cytochem 37: 323–386 [DOI] [PubMed] [Google Scholar]
- Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S 2004. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 73: 39–85 [DOI] [PubMed] [Google Scholar]
- Sato N, Leopold PL, Crystal RG 1999. Induction of the hair growth phase in postnatal mice by localized transient expression of Sonic hedgehog. J Clin Invest 104: 855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M, Dutsch S, auf dem Keller U, Navid F, Schwarz A, Johnson DA, Johnson JA, Werner S 2010. Nrf2 establishes a glutathione-mediated gradient of UVB cytoprotection in the epidermis. Genes Dev 24: 1045–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl FA, Dumesic PA, Khavari PA 2004. Mek1 alters epidermal growth and differentiation. Cancer Res 64: 6035–6040 [DOI] [PubMed] [Google Scholar]
- Scholl FA, Dumesic PA, Barragan DI, Harada K, Bissonauth V, Charron J, Khavari PA 2007. Mek1/2 MAPK kinases are essential for mammalian development, homeostasis, and Raf-induced hyperplasia. Dev Cell 12: 615–629 [DOI] [PubMed] [Google Scholar]
- Segre JA, Bauer C, Fuchs E 1999. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet 22: 356–360 [DOI] [PubMed] [Google Scholar]
- Sen GL, Webster DE, Barragan DI, Chang HY, Khavari PA 2008. Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes Dev 22: 1865–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA 2010. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature 463: 563–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, McKeon F 2007. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell 129: 523–536 [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Crandall CG 2006. Neural control and mechanisms of eccrine sweating during heat stress and exercise. J Appl Physiol 100: 1692–1701 [DOI] [PubMed] [Google Scholar]
- Silva-Vargas V, Lo Celso C, Giangreco A, Ofstad T, Prowse DM, Braun KM, Watt FM 2005. β-Catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev Cell 9: 121–131 [DOI] [PubMed] [Google Scholar]
- Siprashvili Z, Nguyen NT, Bezchinsky MY, Marinkovich MP, Lane AT, Khavari PA 2010. Long-term type VII collagen restoration to human epidermolysis bullosa skin tissue. Hum Gene Ther 21: 1299–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart IH 1970. Variation in the plane of cell cleavage during the process of stratification in the mouse epidermis. Br J Dermatol 82: 276–282 [DOI] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, et al. 2010. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327: 1385–1389 [DOI] [PubMed] [Google Scholar]
- Sotiropoulou PA, Candi A, Blanpain C 2008. The majority of multipotent epidermal stem cells do not protect their genome by asymmetrical chromosome segregation. Stem Cells 26: 2964–2973 [DOI] [PubMed] [Google Scholar]
- Sotiropoulou PA, Candi A, Mascre G, De Clercq S, Youssef KK, Lapouge G, Dahl E, Semeraro C, Denecker G, Marine JC, et al. 2010. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat Cell Biol 12: 572–582 [DOI] [PubMed] [Google Scholar]
- Spencer JM, Kahn SM, Jiang W, DeLeo VA, Weinstein IB 1995. Activated ras genes occur in human actinic keratoses, premalignant precursors to squamous cell carcinomas. Arch Dermatol 131: 796–800 [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, McMahon JA, Lewis PM, Paus R, McMahon AP 1998. Sonic hedgehog signaling is essential for hair development. Curr Biol 8: 1058–1068 [DOI] [PubMed] [Google Scholar]
- Sutter C, Strickland PT, Mukhtar H, Agarwal R, Winter H, Schweizer J 1993. ras gene activation and aberrant expression of keratin K13 in ultraviolet B radiation-induced epidermal neoplasias of mouse skin. Mol Carcinog 8: 13–19 [DOI] [PubMed] [Google Scholar]
- Tarutani M, Cai T, Dajee M, Khavari PA 2003. Inducible activation of Ras and Raf in adult epidermis. Cancer Res 63: 319–323 [PubMed] [Google Scholar]
- Ting SB, Caddy J, Hislop N, Wilanowski T, Auden A, Zhao LL, Ellis S, Kaur P, Uchida Y, Holleran WM, et al. 2005. A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science 308: 411–413 [DOI] [PubMed] [Google Scholar]
- Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW 2003. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol 120: 501–511 [DOI] [PubMed] [Google Scholar]
- Trempus CS, Morris RJ, Ehinger M, Elmore A, Bortner CD, Ito M, Cotsarelis G, Nijhof JG, Peckham J, Flagler N, et al. 2007. CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res 67: 4173–4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E 2004. Defining the epithelial stem cell niche in skin. Science 303: 359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyttendaele H, Panteleyev AA, de Berker D, Tobin DT, Christiano AM 2004. Activation of Notch1 in the hair follicle leads to cell-fate switch and Mohawk alopecia. Differentiation 72: 396–409 [DOI] [PubMed] [Google Scholar]
- van der Schroeff JG, Evers LM, Boot AJ, Bos JL 1990. Ras oncogene mutations in basal cell carcinomas and squamous cell carcinomas of human skin. J Invest Dermatol 94: 423–425 [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A, Mascre G, Youseff KK, Harel I, Michaux C, De Geest N, Szpalski C, Achouri Y, Bloch W, Hassan BA, et al. 2009. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol 187: 91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER 2003. Transient activation of β-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev 17: 1219–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E 2001. Hyperproliferation and defects in epithelial polarity upon conditional ablation of α-catenin in skin. Cell 104: 605–617 [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, Lutzkendorf S, Cotsarelis G, Mill P, Hui CC, Ortonne N, Ortonne JP, Schedl A 2005. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol 15: 1340–1351 [DOI] [PubMed] [Google Scholar]
- Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, et al. 2009. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med 361: 1164–1172 [DOI] [PubMed] [Google Scholar]
- Vooijs M, Ong CT, Hadland B, Huppert S, Liu Z, Korving J, van den Born M, Stappenbeck T, Wu Y, Clevers H, et al. 2007. Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development 134: 535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghmare SK, Bansal R, Lee J, Zhang YV, McDermitt DJ, Tumbar T 2008. Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. EMBO J 27: 1309–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Liu ZY, Gambardella L, Delacour A, Shapiro R, Yang J, Sizing I, Rayhorn P, Garber EA, Benjamin CD, et al. 2000. Regular articles: Conditional disruption of hedgehog signaling pathway defines its critical role in hair development and regeneration. J Invest Dermatol 114: 901–908 [DOI] [PubMed] [Google Scholar]
- Wang X, Pasolli HA, Williams T, Fuchs E 2008. AP-2 factors act in concert with Notch to orchestrate terminal differentiation in skin epidermis. J Cell Biol 183: 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GY, Wang J, Mancianti ML, Epstein EH Jr 2011. Basal cell carcinomas arise from hair follicle stem cells in Ptch1+/− mice. Cancer Cell 19: 114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Estrach S, Ambler CA 2008. Epidermal Notch signalling: Differentiation, cancer and adhesion. Curr Opin Cell Biol 20: 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AC, Tran K, Khuu J, Dang C, Cui Y, Binder SW, Lowry WE 2011. Defining the origins of Ras/p53-mediated squamous cell carcinoma. Proc Natl Acad Sci 108: 7425–7430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SE, Beronja S, Pasolli HA, Fuchs E 2011. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature 470: 353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SY, Reiter JF 2011. Wounding mobilizes hair follicle stem cells to form tumors. Proc Natl Acad Sci 108: 4093–4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH Jr, Dlugosz AA, Reiter JF 2009. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med 15: 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, et al. 1998. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 391: 90–92 [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, et al. 1999. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398: 714–718 [DOI] [PubMed] [Google Scholar]
- Yang SH, Andl T, Grachtchouk V, Wang A, Liu J, Syu LJ, Ferris J, Wang TS, Glick AB, Millar SE, et al. 2008. Pathological responses to oncogenic Hedgehog signaling in skin are dependent on canonical Wnt/β3-catenin signaling. Nat Genet 40: 1130–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, O’Carroll D, Pasolli HA, Zhang Z, Dietrich FS, Tarakhovsky A, Fuchs E 2006. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat Genet 38: 356–362 [DOI] [PubMed] [Google Scholar]
- Yi R, Poy MN, Stoffel M, Fuchs E 2008. A skin microRNA promotes differentiation by repressing “stemness.” Nature 452: 225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Pasolli HA, Landthaler M, Hafner M, Ojo T, Sheridan R, Sander C, O’Carroll D, Stoffel M, Tuschl T, et al. 2009. DGCR8-dependent microRNA biogenesis is essential for skin development. Proc Natl Acad Sci 106: 498–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef KK, Van Keymeulen A, Lapouge G, Beck B, Michaux C, Achouri Y, Sotiropoulou PA, Blanpain C 2010. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol 12: 299–305 [DOI] [PubMed] [Google Scholar]
- Yuhki M, Yamada M, Kawano M, Iwasato T, Itohara S, Yoshida H, Ogawa M, Mishina Y 2004. BMPR1A signaling is necessary for hair follicle cycling and hair shaft differentiation in mice. Development 131: 1825–1833 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, Tobias JW, Piccolo S, Schmidt-Ullrich R, Nagy A, et al. 2008. Activation of β-catenin signaling programs embryonic epidermis to hair follicle fate. Development 135: 2161–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tomann P, Andl T, Gallant NM, Huelsken J, Jerchow B, Birchmeier W, Paus R, Piccolo S, Mikkola ML, et al. 2009a. Reciprocal requirements for EDA/EDAR/NF-κB and Wnt/β-catenin signaling pathways in hair follicle induction. Dev Cell 17: 49–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T 2009b. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell 5: 267–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Stokes N, Polak L, Fuchs E 2011. Specific microRNAs are preferentially expressed by skin stem cells to balance self-renewal and early lineage commitment. Cell Stem Cell 8: 294–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AJ, Haase I, Watt FM 1999. Signaling via β1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc Natl Acad Sci 96: 6728–6733 [DOI] [PMC free article] [PubMed] [Google Scholar]