Abstract
Interleukin (IL)-6 family cytokine signaling through gp130 and signal transducer and activator of transcription (STAT) activation is believed important for early hematopoiesis. To determine whether gp130/STAT1/3 physical interaction is required, we compared hematopoietic repopulating activities of embryonic day (E)14.5 fetal liver cells from gp130FXXQ/FXXQ knock-in mice, which have four mutated STAT1/3 binding sites. In hematopoietic cells, failure to tyrosine phosphorylate STAT3 by gp130 did not cause any significant effects on myeloid progenitor colony forming units (CFU) in vitro and or on competitive multilineage hematopoietic reconstitution. Serial transplantation of fetal liver (FL) cells was unaffected throughout primary, secondary, and tertiary transplants indicating normal self-renewal capacity. Even gp130FXXQ/FXXQ on the background of STAT5 deficiency, with known hematopoietic stem cell (HSC) repopulating dysfunction, did not further impair HSCs beyond that of STAT5 alone. Overall, the defective gp130-mediated STAT1/3 signaling is surprisingly dispensable for HSC function. However, since these mice lack both STAT1/3 binding sites there are several possible explanations for this result and these are discussed.
Keywords: Signal transducer and activator of transcription, hematopoietic stem cell, cytokine signaling, fetal liver
Introduction
Members of the IL-6 family of cytokines commonly share the gp130 receptor to activate several downstream signaling pathways, including STAT3 which binds to four YXXQ motifs [1,2]. Notably, gp130-deficient mice die perinatally between E12.5 and term and the mutant embryos have reduced numbers of multipotent and committed hematopoietic progenitors in the liver [3]. Since serial transplant ability can be potentiated by IL-11 treatment [4] or transduction with an IL-11 overexpressing retrovirus [5] and gp130-mediated activation has been sufficient to minimally expand HSCs in culture [6], it has been considered likely that a threshold of gp130/STAT3-dependent signaling is important for hematopoiesis [7,8].
The potential for secondary effects has limited the analysis of hematopoietic stem cells (HSCs) in gp130Δ/Δ mice that lack hyperproliferation phenotypes [9]. Dominant-negative inhibition of STAT3 has been reported to suppress HSC repopulation from limiting numbers of HSCs [10,11]. Tie2-Cre conditional knockout mice for gp130 have recently provided evidence for endothelial cell-mediated extrinsic effects but not for an intrinsic role for gp130 in HSC function [12]. However these studies did not include competitive repopulation which might address HSC function under conditions of stress. Therefore, we have used the gp130FXXQ/FXXQ mutant mouse [13], which expresses a mutant human gp130 receptor that loses both STAT1/3 binding sites but retains SOCS binding sites and has no associated hyperproliferation or autoimmune phenotypes. These studies set out to determine whether the collective loss of both STAT1 and STAT3 binding would impair HSC function.
Materials and methods
Fetal liver (FL) cell collection and Western blot analysis
gp130FXXQ/FXXQ and gp130FXXQ/FXXQSTAT5abΔN/ΔN mice were generated by heterozygote crosses and PCR genotyping. E14.5 FL cells were collected as previously described from STAT5abΔN/ ΔN mice [14] and then stimulated with or without 50 ng/mL IL-6 for either 30 min. Western blot analysis was carried out as described [15].
FL cell transplantation and serial bone marrow transplants
FL cells were injected via the lateral tail-vein into lethally-irradiated primary recipients (1100 rads) either alone (non-competitive) or mixed 1:1 with CD45.1 FL cells (competitive) as described [14]. For non-competitive FL transplants, bone marrow was harvested from both hind limbs (tibias and femurs) of primary recipients and serially transplanted using at least 5 x 106 bone marrow cells (1 donor per 5 recipients). For limiting dilution competitive assays, FL cells were mixed with a radioprotective dose of 2×105 CD45.1 adult BM cells and injected via the lateral tail-vein into lethally-irradiated (1100 rads) adult Boy J mice.
Results
Loss of gp130-mediated STAT3 activation does not impair HSC multilineage repopulation or self-renewal
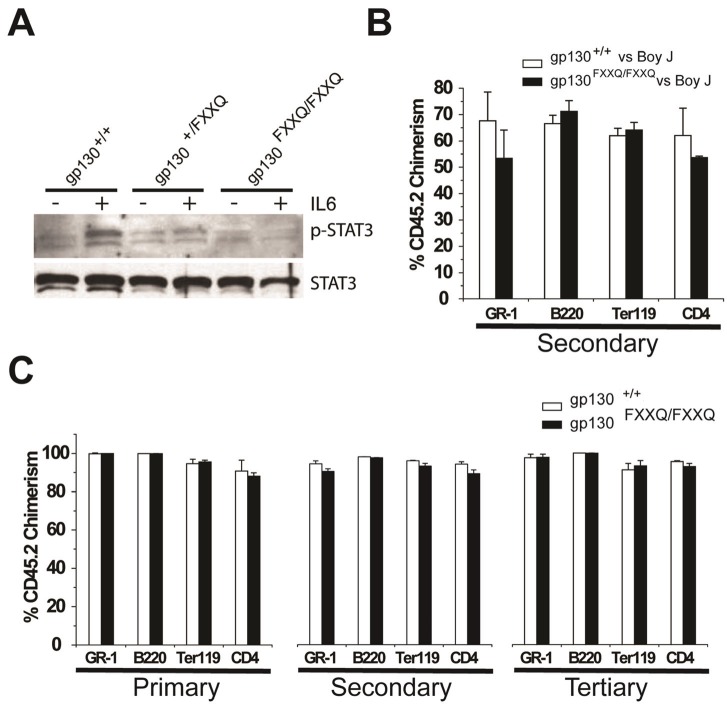
To confirm that gp130FXXQ/FXXQ mutant mice, in which Tyr-767, -814, -905, and -915 (corresponding to YXXQ motifs) were replaced by phenylalanines, lack gp130-mediated STAT3 activation, we performed Western analysis following IL-6 treatment for 30 min. Figure 1A shows that there was no induction of phospho-STAT3 at Tyr-705 in gp130FXXQ/FXXQ FL cells, but progressively increased phospho-STAT3 in gp130+/FXXQ FL and gp130+/+ FL cells. A similar result was also obtained when cells were treated with IL-6 for 4 hours (data not shown).
Figure 1.
Effect of gp130FXXQ/FXXQ mutations on STAT3 activation, HSC multilineage repopulation, and self-renewal. (A) Western blot analysis for phospho-STAT3 and STAT3. FL was harvested from E14.5 embryos of the indicated genotypes, starved for four hours in PBS with 2% FBS, and stimulated with or without 50 ng/ml IL-6 for 30 min. 20 μg of total protein was separated by SDS-PAGE and transferred to PDVF membrane. Phospho-STAT3 and STAT3 were detected by antibodies from Cell Signaling Technology. (B) A representative secondary competitive repopulation assay between gp130FXXQ/FXXQ and gp130+/+ FL grafts. FL cells were obtained from E14.5 fetuses from mating of gp130+/FXXQ (CD45.2) or wild type Boy J mice (CD45.1), genotyped by PCR, and then either gp130FXXQ/FXXQ or gp130+/+ cells were mixed with wild type Boy J FL cells at a 1:1 ratio. The cells were transplanted into lethally-irradiated adult Boy J recipient mice and analyzed 12-16 weeks later for the relative contribution of donor engraftment. Secondary competitive repopulation experiments were then set up using BM cells from primary recipients and transplanted into lethally-irradiated Boy J mice at one donor to five recipient mice, and analyzed 12-16 weeks later for CD45.2 cells co-staining for GR-1, B220, Ter119 or CD4. (C) Primary, secondary and tertiary FL repopulation assay between gp130FXXQ/FXXQ and gp130+/+ grafts. FL cells with either gp130FXXQ/FXXQ or gp130+/+ genotypes were transplanted into lethally-irradiated adult Boy J recipient mice and analyzed 12-16 weeks later for relative contribution of donor engraftment in each lineage. Secondary or tertiary transplantation were carried out using BM cells from primary or secondary recipient mice and then transplanted into lethally-irradiated Boy J mice respectively.
To perform competitive transplantation studies to assess the role of gp130-mediated STAT3 activation in HSC function, E14.5 embryos FL cells were harvested and genotyped. Competitive FL transplantations were done to determine whether a difference in relative stem cell activity could be observed between gp130FXXQ/FXXQ compared to its littermate control gp130+/+ FL cells. For these experiments, FL cells from either E14.5 gp130+/+ or gp130FXXQ/FXXQ embryos (CD45.2) were competed at a 1:1 ratio with a common pool of competitive E14.5 FL cells (CD45.1), and transplanted into lethally-irradiated (1100 rads) Boy J recipient mice (CD45.1 congenic). Peripheral blood chimerism was evaluated by flow cytometry. At 16 weeks after transplantation, secondary competitive repopulation experiments were then set up using BM cells collected from all gp130+/+ vs. Boy J or gp130FXXQ/FXXQ vs. Boy J chimeric primary recipients that had received transplants at a one donor to five recipient ratio. Two independent competitive repopulation experiments and their secondary transplantations were conducted with 5 recipients per cell group. Figure 1B shows the mean donor engraftment in cells expressing Gr-1, B220, Ter119 and CD4 markers from a representative experiment at 4 months following secondary transplantation. Almost identical results were obtained from the primary competitive transplantation (data not shown). These results showed no obvious defects in relative stem cell repopulating activity between gp130FXXQ/FXXQ FL cell and its littermate control. We also performed non-competitive transplantation using gp130FXXQ/FXXQ and its control gp130+/+ FL cells for 4 separate experiments (primary and secondary transplants) using 5 mice per group. Tertiary BM transplantation for one experiment assessed self-renewal and as shown in Figure 1C, there was no difference in serial transplant ability.
Normal HSC frequency at limiting dilution and no cooperation with STAT5 deficiency in HSC activity
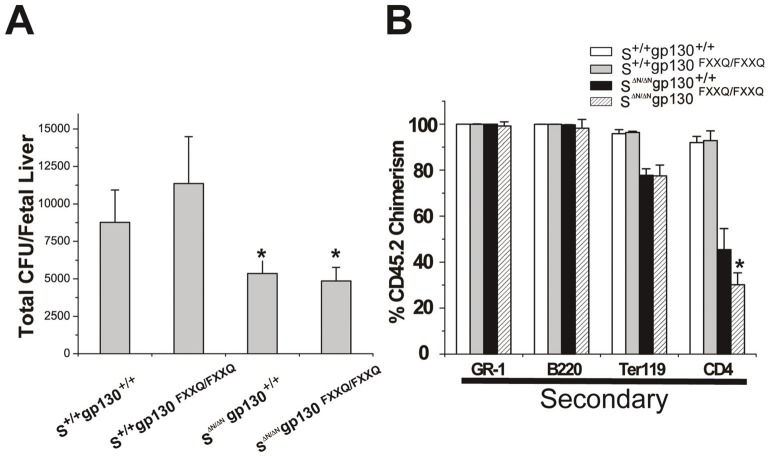
To measure the frequency of competitive repopulating units (CRU) in the gp130FXXQ/FXXQ and gp130+/+ FL cells, 100,000, 20,000, and 4000 FL testing cells (CD45.2) were intravenously injected into lethally-irradiated adult Boy J mice along with 2x105 radioprotective Boy J BM (CD45.1) cells. Donor contribution from test cells was determined by flow cytometry analysis of peripheral blood of transplanted mice 16 weeks after transplantation. Mice with at least 1% test cells positive for lymphoid (B220 or CD4 positive) and myeloid (Gr-1 or Ter119 positive) lineages were scored as positive while the other were considered as negative. The cell dose at which 37% of mice tested were negative for donor multilineage engraftment at 16 weeks was used to calculate the frequency of CRU based on Poisson statistics using L-Calc software (StemCell Technologies). The frequency of CRU for the gp130FXXQ/FXXQ FL cells (number of positive mice/injected mice at each dose: 5/5, 5/5, 1/5) was 1 in 7196 while the frequency of CRU for its control gp130+/+ (5/5, 5/5, 1/4) was 1 in 8219. Furthermore, we crossed gp130+/FXXQ mice with STAT5-deficient mice which already have severe HSC repopulation defects [14]. Colony forming unit assay was performed using STAT5ab+/+gp130+/+, STAT5ab+/+gp130FXXQ/FXXQ, STAT5abΔN/ΔNgp130+/+, and STAT5abΔN/ΔNgp130FXXQ/FXXQ FL cells stimulated with hematopoietic cytokines in vitro. As shown in Figure 2A, there was no significant difference in total myeloid progenitors per FL between gp130FXXQ/FXXQ and gp130+/+ (t-test, P=0.14) or between STAT5abΔN/ΔNgp130+/+ and STAT5abΔN/ΔNgp130FXXQ/FXXQ (t-test, P=0.46), whereas there was a significant decrease for the total myeloid progenitors per FL with the STAT5abΔN/ΔN genotype (t-test, P=0.02). We further carried out transplantation experiments using STAT5ab+/+gp130+/+, STAT5ab+/+gp130FXXQ/FXXQ, STAT5abΔN/ΔNgp130+/+, and STAT5abΔN/ΔNgp130FXXQ/FXXQ E14.5 FL and transplanted into lethally-irradiated adult Boy J mice (Figure 2B, one representative experiment of 2 independent experiments using 5-10 mice per group). Analysis 16 weeks later showed similar levels of engraftment for all genotypes with only a modest decline in STAT5abΔN/ΔNgp130FXXQ/FXXQ T-cells relative to STAT5abΔN/ΔNgp130+/+ (t-test, P=0.04).
Figure 2.
Cross of gp130FXXQ/FXXQ mutations onto a STAT5-deficient background and analysis of CFU and HSC activity from FL cells. (A). Colony-forming ability of STAT5ab (abbreviated as S for the figure) S+/+gp130+/+, S+/+gp130FXXQ/FXXQ, SΔN/ΔNgp130+/+, and SΔN/ΔNgp130FXXQ/FXXQ FL cells stimulated with hematopoietic cytokines in vitro. The frequency of CFU-Cs assayed in methylcellulose was multiplied by the total FL cellularity to derive the absolute number of FL CFU-Cs per FL. (B). A representative secondary transplantation for the repopulation assay among S+/+gp130+/+, S+/+gp130FXXQ/FXXQ, SΔN/ΔNgp130+/+, and SΔN/ΔNgp130FXXQ/FXXQ FL cells. FL cells with the above four genotypes were transplanted into lethally-irradiated adult Boy J recipient mice at the ratio of one donor to five recipients and analyzed 12-16 weeks later for relative contribution of donor engraftment in each lineage. Secondary transplantation was carried out using BM cells from primary recipient mice, transplanted into lethally-irradiated Boy J mice and analyzed for the donor contribution in each lineage. Results are presented as the mean ± SD (n=5-10 mice per transplant).
Discussion
We report that gp130 mediated STAT1/3 activation is dispensable in HSC expressing gp130FXXQ/FXXQ. Loss of gp130-mediated STAT1/STAT3 activation in these knock-in mice does not impact upon murine HSC multi-lineage competitive repopulation or self-renewal, even following tertiary bone marrow transplantation. Furthermore, the frequency of HSC in gp130FXXQ/FXXQ mice appears to be normal when compared to control mice by limiting dilution transplantation assay. STAT5-deficient mice have severe defects both in colony forming unit assay as well as HSC competitive repopulation No additional defects were observed at the level of the colony forming unit in culture (CFU-C) assay or fetal liver competitive repopulation ability when gp130FXXQ/FXXQ were crossed with STAT5-deficient mice. Collectively, these findings indicate that gp130 mediated STAT1/3 activation is not essential in normal hematopoiesis.
Gp130 is a ubiquitously expressed, shared signal transducing receptor that forms part of the receptor complex for several cytokines including IL-6, IL-11, IL-27, leukemia inhibitory factor (LIF), oncostatin M, ciliary neurotrophic factor, cardiotrophin 1 and cardiotrophin-like cytokine. Gp130 is present on hematopoietic and non-hematopoietic cells. As the gp130 subunit is shared by multiple cytokines, functional of redundancy of cytokine activities occurs. The IL-6 family of cytokines can activate STAT family proteins especially STAT3 and STAT1 depending on the phosphorylated YXXQ motif on four distinct tyrosines (Y767, Y814, Y905, Y915 in human or Y765, Y812, Y904, Y914 in mouse) of gp130 or activate SHP-2/ERK MAPK pathway depending on the phosphorylated YXXV motif on a single of tyrosine (Y759 in human or Y757 in mouse) of gp130. In the gp130FXXQ/FXXQ knock-in mouse model we have used, all four YXXQ motifs were mutated into FXXQ. Therefore, these mutations prevent IL-6 family cytokines mediated STAT1/3 activation. Our results indicate that gp130-dependent STAT1/3 activation is dispensable in HSC repopulation and self-renewal. This was initially unexpected as either gp130 or STAT3 can play an important role in HSC function. Gp130-deficient mice are embryonic lethal and the mutant embryos have greatly reduced numbers of pluripotent and committed hematopoietic progenitors in the liver and differentiated T and erythroid lineages [3]. Conditional deletion of gp130 by flanking the exon 16 encoding the transmembrane region of gp130 with two LoxP site induced with the interferon inducible Mx-Cre system shows that the number of hematopoietic precursors was reduced by 40% in the bone marrow and that a defect in resynthesis of erythrocytes and thrombocytes following treatment with 5-FU [16]. Since Mx1-Cre induced deletion mainly occurred in hematopoietic stem cells, endothelial cells and liver cells, mice with a specific deletion in endothelial cells were also analyzed [12]. Interestingly enough, these mice were normal at birth and did not display deficiency in hematopoiesis. However, the mice did develop bone marrow dysfunction that was accompanied by splenomegaly caused by extramedullary hematopoiesis. Transplanting wild-type bone marrow cells into irradiated gp130-deficient mice did not rescue the hematopoietic defects indicating that gp130 expression in the bone marrow microenvironment makes an important contribution to hematopoiesis [12].
Although prior studies indicate that gp130 may be involved in hematopoiesis, none of them actually checked the competitive repopulation activity of gp130 deleted mice. Mouse models with mutations of intracellular domain of gp130 were generated to better decipher the distinct functions of SHP-2/ERK and STAT1/3 pathways which could be downstream of gp130 signaling [17,13,18]. Transgenic mice with an Y759 mutation (gp130F759) which lack the SHP-2 and SOCS3 binding site have exaggerated gp130 mediated STAT1/3 signaling [13]. On the other hand, a gp130 knock-in mouse containing a COOH-terminal truncation mutation in gp130 (gp130ΔSTAT or gp130Δ) was generated by introducing the Y765F, Q768A and V769stop substitution at first YXXQ motif. Therefore the mice with all STAT binding sites deleted have an increase in the activation of the SHP-2/ERK pathway [17]. Studies based gp130Δ/Δ suggested that gp130-mediated STAT1/3 activation is required to maintain the normal balance of hematopoietic progenitors during fetal and adult hematopoiesis [9]. However, in our studies, gp130FXXQ/FXXQ mice [13], initially generated by Hirano, replacement of the last four tyrosines by phenylalanine (gp130FXXQ mutants) did not disturb other regions of gp130 that may contain putative binding sites for positive or negative regulators. Since the gp130 COOH-terminus remains intact rather than truncated as in the gp130Δ/Δ mice, we feel that this represents a cleaner system for STAT1/3 analysis. gp130FXXQ/FXXQ mice have a phenotype similar to gp130 complete knockout mice which are perinatal lethal while adult gp130Δ/Δ mice are clearly viable. Here we show that gp130FXXQ does not have defects in competitive repopulation and self-renewal of fetal liver HSC which is consistent with the previous finding that gp130 deletion had no intrinsic effects on hematopoiesis [12].
Gp130FXXQ disrupts the binding of STAT1/3 to gp130 receptor and prevents IL-6 family cytokine-mediated STAT1/3 activation. Murine embryonic stem cells require leukemia inhibitory factor for self-renewal. LIF belongs to the IL-6 family of cytokines. Targeted mutagenesis of STAT3 in ES cells demonstrated that a minimal dose of STAT3 is required for ES cell propagation and pluripotency [19] and that a dominant interfering mutant STAT3F specifically abrogated self-renewal and promoted differentiation [20]. Ablation of STAT3 produced early embryonic lethality [21]. Tissue specific disruption of STAT3 in bone marrow cells caused gradual lethality after birth and resulted in abnormalities in myeloid cells [22]. Overexpression of a dominant negative form of STAT3 selectively impairs hematopoietic stem cell activity and transduction of adult mouse bone marrow cells with a constitutively activated form of STAT3 promoted HSC self-renewal under stimulated conditions [11,10]. These studies suggest that STAT3 plays an important positive role in HSC function. One possible explanation to explain our results is that STAT3 may be activated by non-gp130 receptors and possibly Src through receptor tyrosine kinases. Therefore, even gp130 only mediated STAT3 activation is disrupted in gp130FXXQ mice, other pathways may utilize STAT3 more fully in HSC. The other possibility is that a negative function of STAT1 could exactly counteract that of STAT3, although there is no direct evidence to support this hypothesis. In gp130Y757F mice, hyperactivation of STAT1/3 results in hematopoietic proliferation and expansion in a STAT3-dependent manner, not requiring activation of STAT1 [8]. The comparison of the gene expression profiles from mouse embryonic, neural and HSC reveals that STAT3/4/6 is predominant in HSC but not STAT1 [23]. Although it has been reported that deletion of STAT1 resulted in overall reduction of erythroid progenitors and altered their distribution [24], the role of STAT1 in HSC self-renewal is still not clear. Most recently, it has been shown that type I interferon can act directly on HSCs to exit quiescent G0 and induce proliferation and exhaustion in HSCs. This effect is mediated through STAT1 or interferon receptor [25,26]. Interferon-γ also can induce expansion of KLS cells and this process was dependent on IFNγR1 signaling and the STAT1 pathway [27]. Mice lacking the IFN-inducible immunity-related p47 GTPase Irgm1 have hyperproliferation, self-renewal, and autophagy defects. Interestingly, these defects can be rescued in irgm1-/-STAT1-/- double-knockout mice [28]. All these studies indicate that STAT1 deficiency can prevent the adverse effect on HSCs from interferon but none of them show deficiency of STAT1 would improve competitive repopulation in the steady state. Further studies are needed to determine whether gp130-mediated positive signals from STAT3 are precisely counterbalanced by negative signals from STAT1.
Our study emphasizes the complexity of perturbations to JAK/STAT signaling pathways associated with deletion of shared binding sites (Figure 3). Furthermore, deletion of STATs in general can be accompanied by alternative STAT activation to compensate or lead to a gain of a new function [29]. We also cannot rule out that under conditions of stress such as inflammation (IFN or IL-6 stimulation) a difference might exist between STAT1 and STAT3 function in this model. In summary, we reported that STAT1/3 activation through gp130 is dispensable in murine HSC competitive repopulation and self-renewal. Further studies are still needed to further clarify whether STAT1 plays a role in HSC self-renewal and whether non-gp130 receptors can activate STAT3 and compensate for loss of gp130 mediated STAT3 function.
Figure 3.
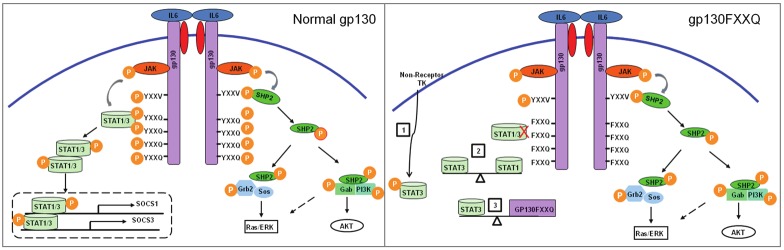
Gp130 mediated IL-6 family of cytokines signaling pathway. (Left) In the wild type gp130 mouse, IL-6 family of cytokines can activate STAT family proteins especially STAT3 and STAT1 depending on the YXXQ phosphorylation motif on four distinct tyrosines of gp130 or they can activate the SHP-2/ERK MAPK pathway depending on the YXXV phosphorylation motif on Y759 . (Right) In gp130FXXQ knockin mouse, tyrosines at the 767, 814, 905, and 915 YXXQ motifs was blocked by phenylalanine replacement thus preventing STAT family binding to gp130. Therefore, gp130FXXQ mice have lost gp130-mediated STAT1/3 activation. There are three possible explanations: (1) STAT3 is still activated in HSC via non-receptor tyrosine kinase (2) Function of STAT3 is perfectly counterbalanced by STAT1. (3) Function of STAT3 is exactly counterbalanced by unknown effectors that also dock with gp130FXXQ.
Acknowledgments
We thank Toshio Hirano for generously providing the C57BL/6 gp130+/FXXQ mice for our studies. This work was supported by NIH R01DK059380 (K.D. Bunting), the Flow Cytometry and Radiation Resources Core Facilities of the Case Comprehensive Cancer Center (P30CA43703), and the Aflac Cancer and Blood Disorders Center of Children’s Healthcare of Atlanta Flow Cytometry Core facility.
References
- 1.Hirano T, Matsuda T, Nakajima K. Signal transduction through gp130 that is shared among the receptors for the interleukin 6 related cytokine subfamily. Stem Cells. 1994;12:262–277. doi: 10.1002/stem.5530120303. [DOI] [PubMed] [Google Scholar]
- 2.Boulton TG, Stahl N, Yancopoulos GD. Ciliary neurotrophic factor/leukemia inhibitory factor/interleukin 6/oncostatin M family of cytokines induces tyrosine phosphorylation of a common set of proteins overlapping those induced by other cytokines and growth factors. J Biol Chem. 1994;269:11648–11655. [PubMed] [Google Scholar]
- 3.Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, Fujiwara H, Hirata M, Yamagami T, Nakahata T, Hirabayashi T, Yoneda Y, Tanaka K, Wang WZ, Mori C, Shiota K, Yoshida N, Kishimoto T. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci USA. 1996;93:407–411. doi: 10.1073/pnas.93.1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holyoake TL, Freshney MG, McNair L, Parker AN, McKay PJ, Steward WP, Fitzsimons E, Graham GJ, Pragnell IB. Ex vivo expansion with stem cell factor and interleukin-11 augments both short-term recovery posttransplant and the ability to serially transplant marrow. Blood. 1996;87:4589–4595. [PubMed] [Google Scholar]
- 5.Hawley RG, Hawley TS, Fong AZ, Quinto C, Collins M, Leonard JP, Goldman SJ. Thrombopoietic potential and serial repopulating ability of murine hematopoietic stem cells constitutively expressing interleukin 11. Proc Natl Acad Sci USA. 1996;93:10297–10302. doi: 10.1073/pnas.93.19.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller CL, Eaves CJ. Expansion in vitro of adult murine hematopoietic stem cells with transplantable lympho-myeloid reconstituting ability. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13648–13653. doi: 10.1073/pnas.94.25.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Audet J, Miller CL, Rose-John S, Piret JM, Eaves CJ. Distinct role of gp130 activation in promoting self-renewal divisions by mitogenically stimulated murine hematopoietic stem cells. Proc Natl Acad Sci USA. 2001;98:1757–1762. doi: 10.1073/pnas.98.4.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins BJ, Roberts AW, Najdovska M, Grail D, Ernst M. The threshold of gp130-dependent STAT3 signaling is critical for normal regulation of hematopoiesis. Blood. 2005;105:3512–3520. doi: 10.1182/blood-2004-09-3751. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins BJ, Quilici C, Roberts AW, Grail D, Dunn AR, Ernst M. Hematopoietic abnormalities in mice deficient in gp130-mediated STAT signaling. Exp Hematol. 2002;30:1248–1256. doi: 10.1016/s0301-472x(02)00929-3. [DOI] [PubMed] [Google Scholar]
- 10.Oh IH, Eaves CJ. Overexpression of a dominant negative form of STAT3 selectively impairs hematopoietic stem cell activity. Oncogene. 2002;21:4778–4787. doi: 10.1038/sj.onc.1205592. [DOI] [PubMed] [Google Scholar]
- 11.Chung YJ, Park BB, Kang YJ, Kim TM, Eaves CJ, Oh IH. Unique effects of STAT3 on the early phase of hematopoietic stem cell regeneration. Blood. 2006;108:1208–1215. doi: 10.1182/blood-2006-01-010199. [DOI] [PubMed] [Google Scholar]
- 12.Yao L, Yokota T, Xia L, Kincade PW, McEver RP. Bone marrow dysfunction in mice lacking the cytokine receptor gp130 in endothelial cells. Blood. 2005;106:4093–4101. doi: 10.1182/blood-2005-02-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohtani T, Ishihara K, Atsumi T, Nishida K, Kaneko Y, Miyata T, Itoh S, Narimatsu M, Maeda H, Fukada T, Itoh M, Okano H, Hibi M, Hirano T. Dissection of signaling cascades through gp130 in vivo: reciprocal roles for STAT3- and SHP2-mediated signals in immune responses. Immunity. 2000;12:95–105. doi: 10.1016/s1074-7613(00)80162-4. [DOI] [PubMed] [Google Scholar]
- 14.Bunting KD, Bradley HL, Hawley TS, Moriggl R, Sorrentino BP, Ihle JN. Reduced lymphomyeloid repopulating activity from adult bone marrow and fetal liver of mice lacking expression of STAT5. Blood. 2002;99:479–487. doi: 10.1182/blood.v99.2.479. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Malone MH, He H, McColl KS, Distelhorst CW. Microarray analysis uncovers the induction of the proapoptotic BH3-only protein Bim in multiple models of glucocorticoid-induced apoptosis. J Biol Chem. 2003;278:23861–23867. doi: 10.1074/jbc.M301843200. [DOI] [PubMed] [Google Scholar]
- 16.Betz UA, Bloch W, van den BM, Yoshida K, Taga T, Kishimoto T, Addicks K, Rajewsky K, Muller W. Postnatally induced inactivation of gp130 in mice results in neurological, cardiac, hematopoietic, immunological, hepatic, and pulmonary defects. J Exp Med. 1998;188:1955–1965. doi: 10.1084/jem.188.10.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernst M, Inglese M, Waring P, Campbell IK, Bao S, Clay FJ, Alexander WS, Wicks IP, Tarlinton DM, Novak U, Heath JK, Dunn AR. Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J Exp Med. 2001;194:189–203. doi: 10.1084/jem.194.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F, Heath JK, Ernst M. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- 19.Raz R, Lee CK, Cannizzaro LA, d'Eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci USA. 1999;96:2846–2851. doi: 10.1073/pnas.96.6.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welte T, Zhang SS, Wang T, Zhang Z, Hesslein DG, Yin Z, Kano A, Iwamoto Y, Li E, Craft JE, Bothwell AL, Fikrig E, Koni PA, Flavell RA, Fu XY. STAT3 deletion during hematopoiesis causes Crohn's disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci USA. 2003;100:1879–1884. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 24.Halupa A, Bailey ML, Huang K, Iscove NN, Levy DE, Barber DL. A novel role for STAT1 in regulating murine erythropoiesis: deletion of STAT1 results in overall reduction of erythroid progenitors and alters their distribution. Blood. 2005;105:552–561. doi: 10.1182/blood-2003-09-3237. [DOI] [PubMed] [Google Scholar]
- 25.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IF-Nalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 26.Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med. 2009;15:696–700. doi: 10.1038/nm.1973. [DOI] [PubMed] [Google Scholar]
- 27.Zhao X, Ren G, Liang L, Ai PZ, Zheng B, Tischfield JA, Shi Y, Shao C. Brief report: interferon-gamma induces expansion of Lin(-)Sca-1 (+)C-Kit(+) Cells. Stem Cells. 2010;28:122–126. doi: 10.1002/stem.252. [DOI] [PubMed] [Google Scholar]
- 28.King KY, Baldridge MT, Weksberg DC, Chambers SM, Lukov GL, Wu S, Boles NC, Jung SY, Qin J, Liu D, Songyang Z, Eissa NT, Taylor GA, Goodell MA. Irgm1 protects hematopoietic stem cells by negative regulation of IFN signaling. Blood. 2011;118:1525–1533. doi: 10.1182/blood-2011-01-328682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 2008;22:711–721. doi: 10.1101/gad.1643908. [DOI] [PMC free article] [PubMed] [Google Scholar]