Abstract
Activated macrophages are described as classically activated or M1 type and alternatively activated or M2 type, depending on their response to proinflammatory stimuli and the expression of genetic markers including iNOS, arginase1, Ym1, and Fizz1. Here we report that Akt kinases differentially contribute to macrophage polarization, with Akt1 ablation giving rise to an M1 and Akt2 ablation resulting in an M2 phenotype. Accordingly, Akt2−/− mice were more resistant to LPS-induced endotoxin shock and to dextran sulfate sodium (DSS)-induced colitis than wild-type mice, whereas Akt1−/− mice were more sensitive. Cell depletion and reconstitution experiments in a DSS-induced colitis model confirmed that the effect was macrophage-dependent. Gene-silencing studies showed that the M2 phenotype of Akt2−/− macrophages was cell autonomous. The microRNA miR-155, whose expression was repressed in naive and in LPS-stimulated Akt2−/− macrophages, and its target C/EBPβ appear to play a key role in this process. C/EBPβ, a hallmark of M2 macrophages that regulates Arg1, was up-regulated upon Akt2 ablation or silencing. Overexpression or silencing of miR-155 confirmed its central role in Akt isoform-dependent M1/M2 polarization of macrophages.
Keywords: inflammation, peritonitis, sepsis, inflammatory bowel disease
Activated macrophages express proinflammatory factors and are known as classically activated or M1-type macrophages. Toll-like receptor (TLR) stimulation may induce the M1 phenotype through the activation of several signaling cascades, which regulate the induction of proinflammatory mediators such as TNF-α, IL-6, and iNOS. However, macrophages can also undergo alternative activation to become alternatively activated or M2-type macrophages. M2 macrophages are characterized by reduced responsiveness to TLR ligands, which results in the induction of low levels of proinflammatory cytokines and in the up-regulation of arginase 1 (Arg1), IL-10, found in inflammatory zone 1 (Fizz1), and chitinase 3-like-3 (YM1/CHI3l3) (1). Although the molecular mechanisms that regulate M2 macrophage polarization are not well understood, it appears that STAT6 activation and the induction of C/EBPβ play a central role in this process (2–4). C/EBPβ regulates the expression of Arg1 (3), the gene that encodes the inducible arginase, and selective inhibition of C/EBPβ in macrophages blocks M2 polarization (4).
Akt (also known as PKB) is a family of three serine/threonine protein kinases (Akt1, Akt2, and Akt3) that regulate a host of cellular functions, including cell survival, proliferation, differentiation, and intermediary metabolism (5–7). Even though the majority of the literature does not make a distinction between different Akt isoforms, there is a growing list of differences between them. Akt1 appears not to be dispensable for eNOS induction and endothelial cell function (8, 9), whereas Akt2 is not dispensable for insulin signaling (10). Deletion of Akt1 resulted in enhanced atherosclerosis in the APOE−/− mouse model (5), and Akt1−/− mice do not develop endotoxin tolerance (11). Akt1 ablation protects and Akt2 ablation promotes MMTV-PyMT and MMTVErbB2-induced mammary tumors (12) and Akt1−/− tumors, although very few, are more invasive as the result of microRNA expression differences (7). Moreover, Akt2-expressing tumor cells are more resistant to hypoxia than Akt1−/− ones (13). Akt1 and Akt2 share common upstream activators but frequently result in targeting distinct downstream molecules (14).
In the present study we show that Akt2 −/− macrophages are hypo-responsive to LPS, exhibiting the opposite phenotype than Akt1−/− macrophages both in culture and in vivo. Moreover, we show that Akt2−/− macrophages display an M2 phenotype attributed to reduced expression of miR-155, which targets C/EBPβ, a key regulator of Arg1 expression and M2 polarization.
Results
Akt2−/− Macrophages Are Hypo-Responsive, Whereas Akt1−/− Macrophages Are Hyper-Responsive to LPS.
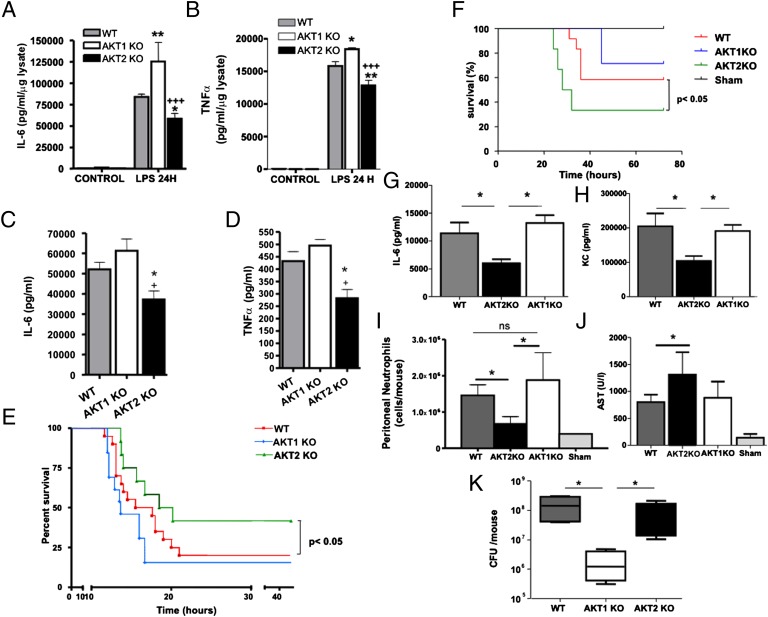
LPS activates the PI3K/Akt-signaling cascade (15). Here we show that, upon stimulation with LPS, peritoneal macrophages from Akt2−/− mice produce lower levels of IL-6 and TNFα than wild-type (WT) macrophages, whereas macrophages from Akt1−/− mice produce higher levels of both proinflammatory mediators (Fig. 1 A and B). In agreement with the cell culture experiments, Akt2−/− mice inoculated with a marginally lethal dose of LPS produce lower levels of IL-6 and TNFα (Fig. 1 C and D) and exhibit partial resistance to endotoxin shock compared with similarly treated WT and Akt1−/− mice (Fig. 1E).
Fig. 1.
Depletion of Akt1 or Akt2 leads to distinct responses to LPS-induced septic shock or CLP-induced peritonitis. (A and B) IL-6 and TNFα were measured by ELISA in the supernatants of peritoneal macrophages from WT, Akt1−/−, and Akt2−/− mice stimulated 24 h with LPS. Results expressed as the mean ± SEM of data obtained from five independent experiments. (C and D) WT (n = 8), Akt1−/− (n = 8), and Akt2−/− mice (n = 8) were treated with 1.5 mg/25 g LPS. IL-6 and TNF-α levels were measured in serum at 6 h. (E) Survival of the mice was monitored. (F) WT (n = 9), Akt1−/− (n = 7), and Akt2−/− (n = 6) mice were subjected to CLP or sham operation (n = 4), and survival was monitored. (G and H) Serum IL-6 or KC was determined at 6 h following CLP. (I) Peritoneal lavage was collected 24 h following CLP (n = 5/group), and the number of neutrophils was measured. (J) AST was measured in the sera of mice subjected to CLP. (K) Akt1\x{2212}/\x{2212} mice had reduced bacterial burden in the peritoneal lavage fluid compared with WT or Akt2−/− ones, 24 h following CLP (n = 5/group). Results are expressed as the mean ± SEM. Comparison was made using an ANOVA test (A–D and G–K) and log-rank analysis (E and F).
In the active infection model of cecal ligation and puncture (CLP), Akt2−/− mice were more susceptible as determined by their shorter survival (Fig. 1F). These mice also produced lower levels of IL-6 and the murine IL-8 homolog KC than WT and Akt1−/− mice (Fig. 1 G and H) and had lower number of neutrophils infiltrating the peritoneum (Fig. 1I). A robust proinflammatory response is essential for containing the infection. Indeed, aspartic transaminase (AST) levels in the serum, which increase upon liver and muscle damage, were elevated in Akt2−/− mice relative to WT or Akt1−/− mice, following CLP-induced peritonitis (Fig. 1J). Bacterial counts in the peritoneum of Akt2−/− mice did not differ from those in WT mice, but they were lower in Akt1−/− mice (Fig. 1K), indicating that the robust proinflammatory response of Akt1−/− mice was more effective in clearing pathogens.
To elucidate whether the differential response of Akt isoforms upon LPS stimulation was due to the ability of LPS to selectively activate different isoforms or due to differences in signaling output between isoforms, we stimulated peritoneal macrophages from Akt1−/−, Akt2−/−, and WT mice with LPS and found equally robust phosphorylation of Akt in macrophages of all genotypes. Moreover, the ablation of Akt1 or Akt2 did not induce compensatory changes in the expression of the remaining Akt isoform (Fig. S1 A and B) and did not affect TLR4 and CD14 expression (Fig. S1 C and D). Moreover, the observed differences in LPS responses were not due to differences in LPS-induced NFkB activation (Fig. S1E) (11).
Akt1−/− Macrophages Undergo M1 Polarization, Whereas Akt2−/− Macrophages Undergo M2 Polarization.
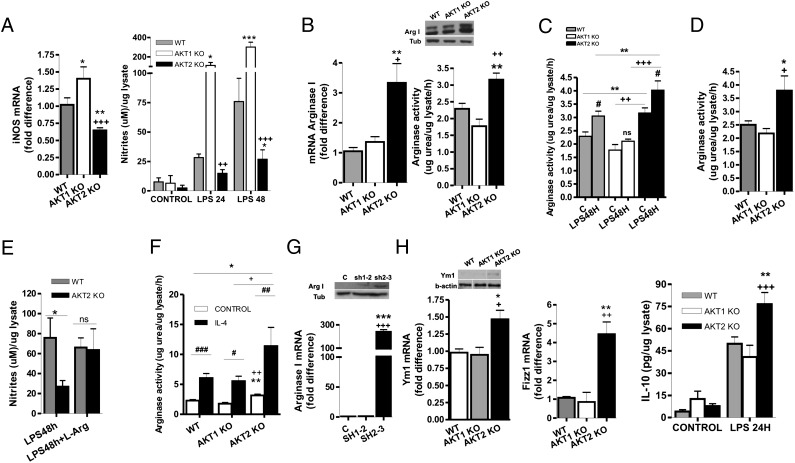
M1 macrophages stimulated with LPS express high levels of TNF-α, IL-6, and iNOS and produce high levels of the iNOS catalytic product, NO (1), whereas LPS-stimulated M2 macrophages produce reduced levels of all these molecules. Data presented here show that LPS-stimulated Akt1−/− macrophages also express high levels of iNOS and produce high levels of NO, TNFα, and IL-6, whereas LPS-stimulated Akt2−/− macrophages produce low levels of these proinflammatory mediators (Fig. 1 A and B; Fig. 2A). These data suggest that the ablation of Akt1 may give rise to an M1, whereas the ablation of Akt2 may give rise to an M2 macrophage phenotype.
Fig. 2.
Akt1−/− macrophages exhibit enhanced M1 response whereas Akt2−/− macrophages possess M2 phenotype. (A) iNOS mRNA levels and NO production in thioglycollate-elicited peritoneal macrophages stimulated for 24 or 48 h with LPS. (B) Arg1 mRNA and protein levels and activity were evaluated in thioglycollate-elicited peritoneal macrophages. (C and D) Arginase activity was measured after LPS stimulation for 48 h in peritoneal macrophages (C) and in primary non thioglycollate-elicited peritoneal macrophages (D). (E) Thioglycollate-elicited peritoneal macrophages were stimulated for 48 h with LPS in the presence or absence of 2 mM l-arginine, and NO production was determined. (F) Peritoneal macrophages were cultured in the presence or absence of IL-4 for 24 h and arginase activity was measured. (G) Raw264.7 cells were infected with a lentivirus-expressing empty vector, shAkt1 (sh1-2), or shAkt2 (sh2-3). mRNA and protein levels of Arg1 were analyzed. (H) Protein levels of Ym1, mRNA levels of Ym1 and Fizz1, and IL-10 production after 24 h of LPS stimulation were measured in thioglycollate-elicited peritoneal macrophages. Results are expressed as the mean ± SEM of three (D, E, G) or four (A–C, F, H) independent experiments. Western blots are representative of at least three independent experiments. Comparisons were made using an ANOVA test.
Expression of high levels of Arg1 and increased arginase activity is one of the hallmarks of M2 macrophages. Thioglycollate-elicited Akt2−/− peritoneal macrophages expressed high levels of Arg1 at both the mRNA and protein levels and exhibit high Arg1 enzymatic activity (Fig. 2B). Moreover, LPS treatment induced Arg1 activity in WT and Akt2−/−, but not in Akt1−/− macrophages (Fig. 2C). Increased Arg1 activity was also observed in primary, not thioglycollate-elicited peritoneal macrophages from Akt2−/− mice (Fig. 2D). Similarly, alveolar macrophages from Akt2−/− mice express increased mRNA levels of Arg1 (Fig. S2). These data combined support the hypothesis that Akt2 ablation gives rise to an M2 macrophage phenotype.
Arg1 converts l-arginine to urea and ornithine, competing with iNOS, which converts it into NO. By inhibiting NO production, Arg1 promotes the M2 phenotype and contributes to the suppression of the M1 phenotype. The defect of LPS-stimulated Akt2−/− macrophages in NO production was reversed following supplementation of the culture media with l-arginine (Fig. 2E), suggesting that the low levels of iNOS in Akt2−/− cells cannot compete with the high levels of Arg1 for l-arginine.
Arginase activity can also be induced by IL-4, which promotes M2 differentiation of macrophages. Akt2−/− macrophages treated with IL-4 up-regulated their arginase activity to significantly higher levels than IL-4–treated WT and Akt1−/− macrophages (Fig. 2F), supporting the propensity of macrophages to polarize to M2 phenotype in the absence of Akt2. Basal IL-4 levels in the serum of WT and Akt2−/− mice did not differ (Fig. S3A), suggesting that the M2 phenotype observed in macrophages from Akt2−/− mice was not due to circulating IL-4.
Leptin and adiponectin also regulate arginase expression and macrophage polarization to the M2 phenotype (16). Previous studies had shown that 6-mo-old Akt2−/− mice have reduced levels of circulating leptin (17). Because of this finding, we measured the levels of leptin and adiponectin in the serum of WT, Akt1−/−, and Akt2−/− mice at the age of 6–8 wk, the age of the mice used in our experiments, and found that they do not vary between mice of these genotypes (Fig. S3 B and C). This suggested that the M2 polarization observed in Akt2−/− macrophages is leptin- and adiponectin-independent and may be cell autonomous, which is consistent with the observation that the knockdown of Akt2, but not Akt1, increased the expression of Arg1 (Fig. 2G). shAkt1 did not alter Akt2 expression nor did shAkt2 affect Akt1 expression (Fig. S4 A–D).
Additional features of the M2 macrophages are the expression of YM1 and Fizz1 and the elevated production of IL-10 upon LPS stimulation. Akt2−/− macrophages expressed elevated levels of Fizz1 and YM1 and produced more IL-10 upon LPS stimulation (Fig. 2H). IL-10 was undetectable at basal levels both in WT and Akt2−/− macrophages and in the serum of both strains.
As ablation of Akt2 results in an M2 macrophage phenotype, we investigated whether it can result in M2-related pathologies such as fibrosis in the lung, heart, or liver. WT and Akt2−/− mice at the age of 42 wk were histologically examined and showed no signs of fibrosis or other tissue abnormalities (Fig. S5A). Moreover, total lung capacity, a measure of lung compliance that decreases in fibrosis, and serum levels of AST, a measure of liver or muscle damage, did not differ between the two strains (Fig. S5 B and C).
Akt1 Ablation Promotes, Whereas Akt2 Ablation Protects from, Dextran Sulfate Sodium (DSS)-Induced Inflammatory Bowel Disease in Mice.
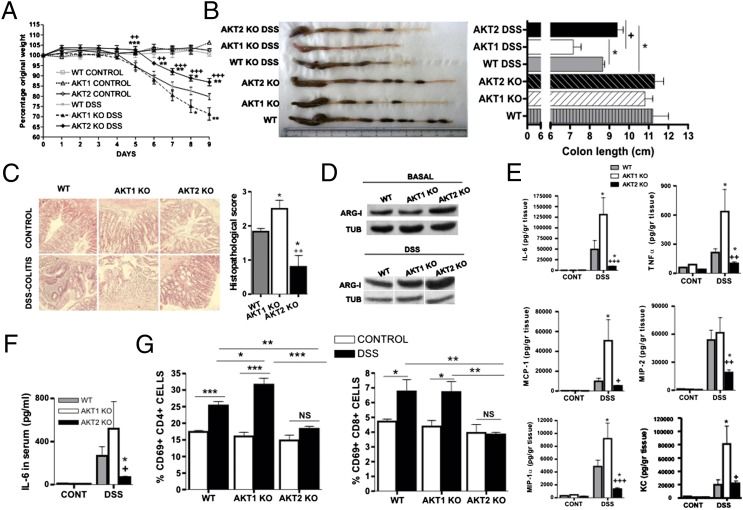
Macrophage polarization plays a critical role in the pathogenesis of DSS-induced colitis and other forms of inflammatory bowel disease, and transfer of M2 macrophages to DSS-treated animals is protective (18). We therefore hypothesized that the ablation of Akt1 will promote DSS-induced colitis, whereas the ablation of Akt2 will inhibit it. Monitoring the body weight (Fig. 3A), measuring the colon length (Fig. 3B), and histological analyses of the colon of DSS-treated mice (Fig. 3C) indeed confirmed that Akt1 ablation exacerbates the disease, whereas Akt2 ablation reduces its severity. The expression of Arg1 was also higher in the colon of both DSS-treated and untreated Akt2−/− mice (Fig. 3D), suggesting that Akt2−/− macrophages accumulating in the intestine express higher levels of Arg1. Moreover, the expression of inflammatory cytokines and chemokines was lower in the colon (Fig. 3E) and in the serum (Fig. 3F) of Akt2−/− DSS-treated mice. Finally, CD4+ and CD8+ T cells from mesenteric lymph nodes of the same Akt2−/− mice were found to express reduced levels of the T-cell activation marker CD69 (Fig. 3G), an effect that is likely secondary to reduced macrophage activation because replacement of Akt2−/− macrophages with WT macrophages rendered mice susceptible to the disease (Fig. 4) and T-cell activation in culture was not affected by a single Akt isoform depletion (Fig. S6).
Fig. 3.
Depletion of Akt2 results in reduced severity of DSS-induced colitis, whereas depletion of Akt1 results in exacerbation of the disease. (A) WT (n = 9), Akt1−/− (n = 9), and Akt2−/− (n = 9) mice were subjected to a DSS-colitis model. Results are expressed as percentage of initial weight. (B) Mice were euthanized at day 9, and colon length was measured. (C) Histopathological analysis and scoring was performed using colon samples. (D) Protein levels of Arg1 and (E) IL-6, TNFα, MCP1, MIP1α, MIP2, and KC were determined in colon samples. (F) Serum IL-6 and (G) the percentage of CD69+ cells from the CD4+ or the CD8+ populations in mesenteric lymph nodes were evaluated. Results are expressed as the mean ± SEM and as representative images and Western blot. Comparisons were made using ANOVA (A, C, E) or Student’s t (B, F, G) tests.
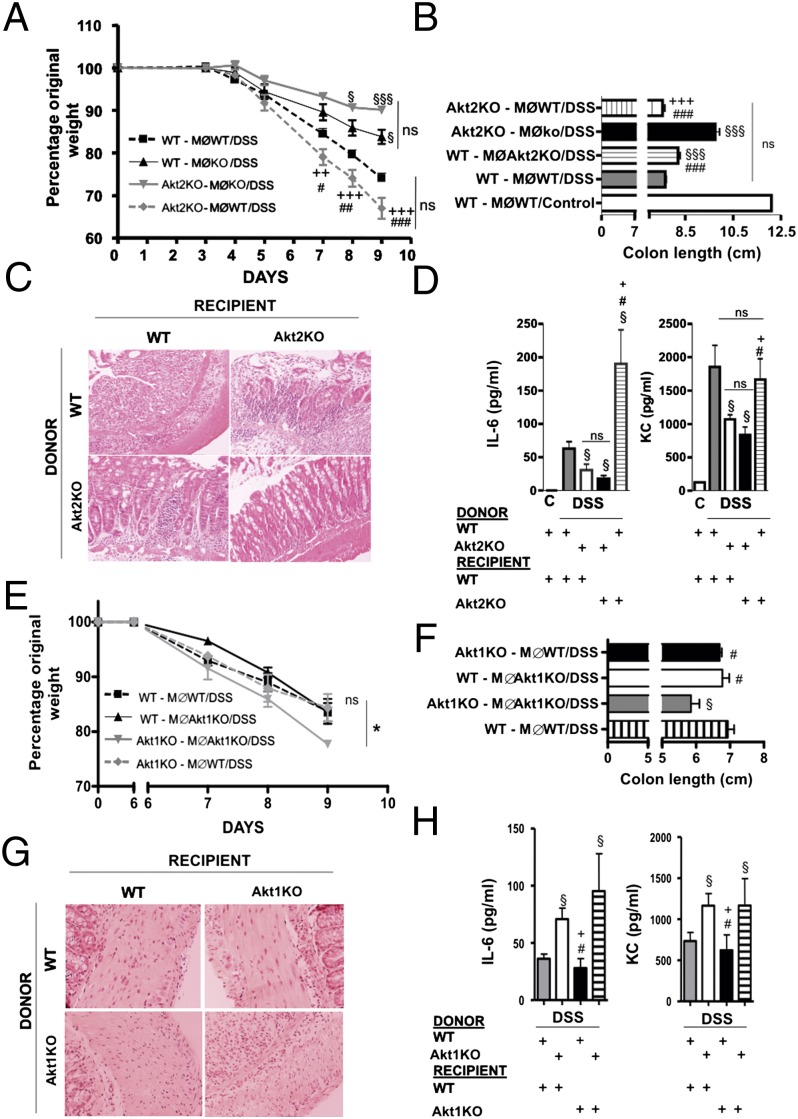
Fig. 4.
Protection from DSS colitis in Akt2−/− mice is due to the M2 phenotype of Akt2−/− macrophages whereas Akt1−/− macrophages are only partly responsible for the enhanced susceptibility of Akt1−/− mice to DSS colitis. Macrophages were depleted from WT (n = 8) and Akt2−/− mice (n = 8) and then reconstituted with WT macrophages or with Akt2−/− macrophages (four mice per group). (A) Body weight loss was monitored daily and following euthanasia at day 9 colon length (B), histological analysis (C), and IL-6 and KC levels in serum (D) were evaluated. In an independent experiment macrophages were depleted from WT (n = 10) and Akt1−/− (n = 10) and reconstituted with WT or Akt1−/− macrophages in all possible combinations (five mice per group). Weight loss (E), colon length (F), histology (G) and serum IL-6 and KC (H) were evaluated. Results are expressed as the mean ± SEM. Comparisons were made with ANOVA analysis (A) or with a Student’s t test (B–D). MØ: macrophages. §P < 0.05 and §§§P <0.001 vs. WT animals reconstituted with WT macrophages. #P < 0.05, ##P <0.01, and ###P <0.001 vs. KO mice reconstituted with KO macrophages. +P < 0.05, ++P <0.01, and +++P <0.001 vs. WT mice reconstituted with KO macrophages.
To confirm that the protection against DSS-induced inflammatory bowel disease observed in Akt2−/− mice was due to the M2 phenotype of macrophages, we depleted macrophages from WT Akt1−/− and Akt2−/− mice using liposome-encapsulated clodronate and transferred WT Akt1−/− or Akt2−/− macrophages. The results showed that transfer of Akt2−/− macrophages into WT animals reduced the severity of DSS-induced inflammatory bowel disease whereas transfer of WT macrophages into Akt2−/− mice exacerbated the disease (Fig. 4 A–D). Transfer of WT macrophages into Akt1−/− mice reduced the severity of the disease whereas transfer of Akt1−/− macrophages into WT mice did not worsen it, suggesting that additional cell types deficient in Akt1 may contribute to the increased susceptibility of Akt1−/− mice to the disease (Fig. 4 E–H), such as intestinal epithelial cells in which Akt protects from cytokine-induced apoptosis (19).
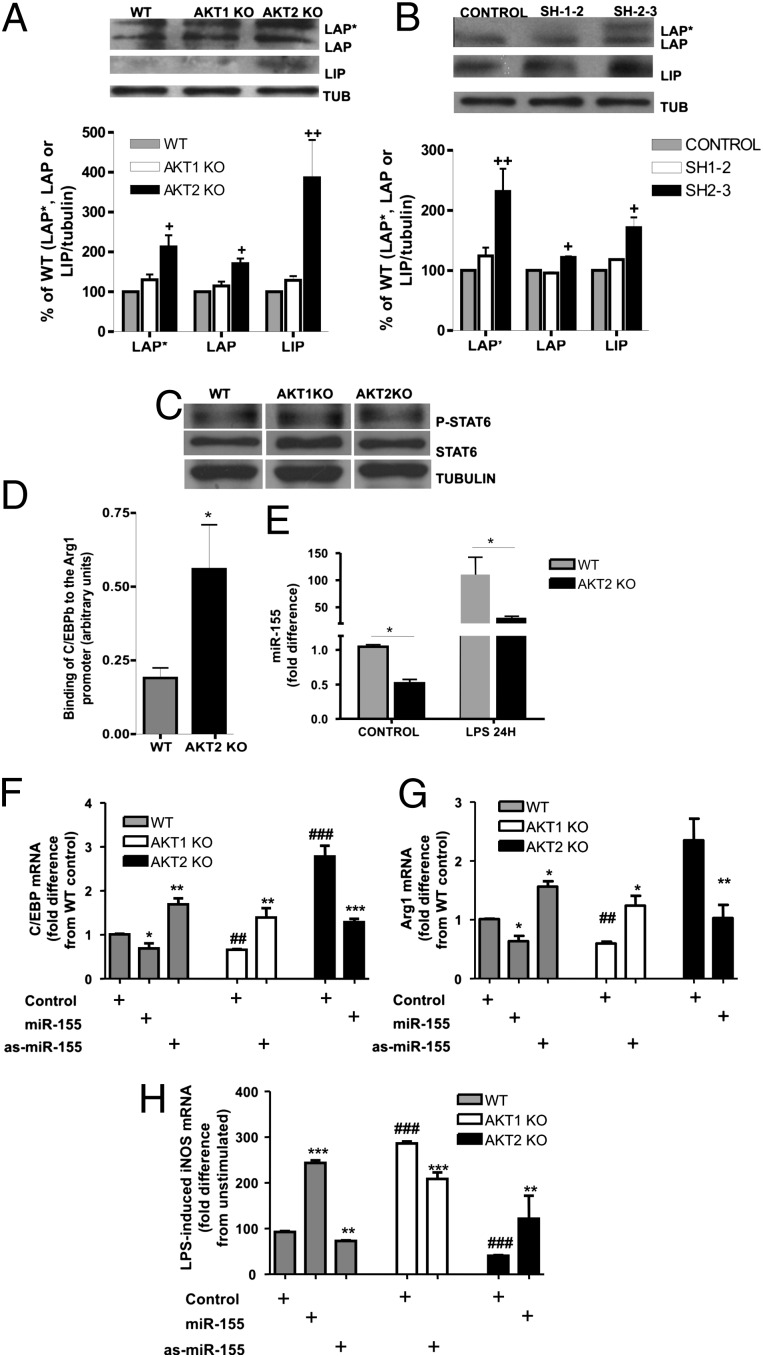
Akt2 Ablation Results in Reduced Levels of miR-155 and Increased Expression of Its Target C/EBPβ, a Key Regulator of M2 Polarization.
The expression of Arg1 and the M2 polarization of macrophages are regulated primarily by C/EBPβ and STAT6 (3, 4). Akt2−/− macrophages and Raw264.7 cells in which Akt2 expression is silenced express elevated levels of C/EBPβ compared with their respective WT controls or Akt1-deficient cells (Fig. 5 A and B). On the other hand, STAT6 phosphorylation was not affected by the ablation of either Akt1 or Akt2 in macrophages (Fig. 5C). These results suggest that the M2 phenotype promoted by Akt2 ablation or knocking down was due to altered C/EBPβ expression. Indeed, chromatin immunoprecipitation (ChIP) experiments showed increased binding of C/EBPβ on Arg1 promoter in Akt2−/− macrophages (Fig. 5D).
Fig. 5.
The M2 phenotype in Akt2−/− macrophages was due to increased C/EBPβ expression, mediated by down-regulation of miR-155. (A) C/EBPβ (LAP*, LAP, and LIP isoforms) expression was analyzed by Western blot in peritoneal macrophages and (B) Raw264.7 cells transduced with empty vector, shAkt1 (sh1-2), or shAkt2 (sh2-3) constructs. (C) phospho-STAT6 (Tyr641) and total STAT6 expression levels were analyzed in peritoneal macrophages. (D) ChIP of C/EBPβ in WT and Akt2−/− macrophages and PCR quantification using primers flanking the C/EBPβ-binding element of the Arg1 promoter were performed. Results are shown as the ratio of target to input. (E) miR-155 expression was evaluated in LPS-stimulated (24 h) and unstimulated WT and Akt2−/− macrophages. (F and G) Expression of C/EBPβ (F) or Arg1 (G) was measured by real-time RT-PCR in macrophages transfected with miR-155 or as-miR-155 or scrambled RNA (control). (H) iNOS expression was measured by real-time RT-PCR in macrophages transfected with scrambled RNA (control), miR-155, or as-miR-155 and stimulated with LPS for 6 h. Results are expressed as the mean ± SEM of data obtained from at least three independent experiments. Western blots are representative of at least three independent experiments. Comparisons were made using Student’s t test. In (F–H) *P < 0.05, **P < 0.01, ***P < 0.001 and #P < 0.05, ##P < 0.01, and ###P < 0.001 compared with the control of the same strain or the WT control, respectively.
C/EBPβ expression is regulated posttranscriptionally by the microRNA miR-155, which targets its 3′ UTR (4, 17). Earlier work from our group had shown that Akt1 ablation promotes miR-155 expression in LPS-stimulated macrophages (11). Measuring miR-155 in Akt2−/− macrophages revealed that Akt2 ablation had the opposite effect, reducing miR-155 expression in both resting and LPS-activated macrophages (Fig. 5E). These results suggest that the down-regulation of miR-155 in Akt2−/− macrophages results in the up-regulation of its target C/EBPβ and, consequently, the induction of Arg1, a hallmark of M2 macrophage polarization. Introduction of miR-155 into Akt2−/− macrophages suppressed C/EBPβ and Arg1 expression and resulted in increased LPS-induced iNOS expression (Fig. 5 F–H), suggesting that miR-155 up-regulation can restore the M1 phenotype in these cells. Suppression of miR-155 with antisense-miR-155 augmented C/EBPβ and Arg1 levels in Akt1−/− macrophages and suppressed LPS-induced iNOS expression. Induction or suppression of miR-155 modulated C/EBPβ, Arg1, and LPS-induced iNOS expression in WT macrophages. Overall, these results indicated that miR-155 was, at least partly, responsible for the differential phenotype observed in Akt1−/− and Akt2−/− macrophages.
Discussion
Macrophages are central mediators of the inflammatory response, contributing both to the initiation and the resolution of inflammation. Our earlier studies had shown that the ablation of Akt1 gives rise to a proinflammatory phenotype (11). In the present report we show that the ablation of Akt2 gives rise to an anti-inflammatory phenotype that is due to the hypo-responsiveness of macrophages to LPS signals, evidence of the opposite roles of Akt isoforms.
Activated macrophages can be M1- or M2-polarized. M2-polarized macrophages express high levels of Arg1 that compete with iNOS for l-arginine, the common substrate of both, suppressing the production of NO and converting l-arginine into urea (20). Data presented in this report showed that the ablation of Akt2 resulted in M2 polarization because they express elevated Arg1, Ym1, and Fizz1, markers of M2 polarization. LPS-induced IL-10 expression, but not the basal expression of IL-10, was also elevated in Akt2−/− macrophages.
Modulating the M1/M2 polarization status of macrophages can affect the severity of inflammatory diseases such as inflammatory bowel disease (18). Data presented in this report show that the ablation of Akt2 partially protects mice from DSS-induced inflammatory bowel disease. Ablation of Akt1, on the other hand, increased the severity of the disease. Transfer of WT macrophages into Akt2−/− mice resulted in increased disease severity compared with WT mice that received WT macrophages, due to loss of the protective effect of Akt2−/− macrophages. Transfer of Akt2−/− macrophages into Akt2−/− mice protected them from the disease. Increased susceptibility of the Akt2−/− mice that receive WT macrophages may be due to the already compromised health status of the Akt2−/− mice, which develop insulin-resistant diabetes mellitus (10).
Opposite contribution of the two Akt kinases was also observed in LPS-induced endotoxin shock, with the ablation of Akt1 enhancing and the ablation of Akt2 reducing the severity of the disease. In a polymicrobial sepsis model, defective M1 response rendered Akt2 mice more susceptible to the disease whereas the robust M1 response of Akt1−/− mice resulted in improved bacterial clearance. Our results are further supported by an earlier study showing that deletion of Akt2 renders mice more susceptible to Salmonella enterica infection (21). These findings support the role of macrophage polarization in the development of inflammatory diseases, and they show that Akt1 and Akt2 play central but opposite roles in the control of macrophage responsiveness and inflammation.
Arg1 is primarily regulated by the transcription factors C/EBPβ and STAT6 (3). Ablation or knockdown of C/EBPβ abrogates Arg1 expression and M2 differentiation (4). Ablation or knockdown of Akt2 enhances C/EBPβ expression and its binding to the Arg1 promoter, suggesting that the M2 phenotype observed in Akt2−/− macrophages is mediated by C/EBPβ. C/EBPβ is regulated by miR-155, which is differentially regulated by Akt1 and Akt2 in macrophages. The ablation or knockdown of Akt2 inhibits the expression of miR-155, suggesting that the up-regulation of C/EBPβ and Arg1 in Akt2−/− macrophages is, at least partly, due to miR-155 suppression.
In conclusion, the present report demonstrates that Akt2 ablation results in the M2 polarization of macrophages whereas Akt1 ablation promotes their M1 polarization. The studies presented here show that, by inhibiting individual Akt isoforms, we may be able to modulate innate immunity and inflammation.
Materials and Methods
Mice.
WT, Akt1−/−, and Akt2−/− mice (22) were housed at the University of Crete School of Medicine, and at the Association for Assessment and Accreditation of Laboratory Animal Care International-accredited animal facility of the Tufts University Health Sciences campus. All procedures described below were approved by the Veterinary Department of the Heraklion Prefecture (Heraklion, Crete, Greece) or by the Institutional Animal Care and Use Committee of Tufts University School of Medicine.
Cell Culture.
Primary murine peritoneal macrophages and the murine macrophage cell line RAW264.7 were cultured as previously described (23). Escherichia coli-derived LPS (1 μg/mL; O111:B4; catalog no. L2630; Sigma-Aldrich), IL-4 (10 ng/mL; R&D Systems), or l-arginine (2 mM; Sigma-Aldrich) were used when indicated.
LPS-Induced Endotoxin Shock.
A minimal lethal dose of LPS (1.5 mg/25 g of body weight) was used as described previously (11). Injected animals were monitored for a 24-h period and euthanized accordingly with the established end points. Serum was collected at 6 h after LPS stimulation and stored at −80 until ELISAs were performed (SI Materials and Methods).
Nitric Oxide Determination.
NO production was determined indirectly by measuring the accumulation of the stable end product NO in the cell culture supernatant using the Griess reaction, as described previously (24) and in SI Materials and Methods.
Isolation of Total RNA and Real-Time RT-PCR.
Total cellular RNA was isolated and Real Time PCR was performed as described in SI Materials and Methods.
Arginase Activity Determination.
Arginase activity was assessed as described previously (25) and in SI Materials and Methods.
Silencing Experiments.
Silencing Experiments are described in SI Materials and Methods.
DSS-Induced Colitis Model.
An acute model of DSS-induced colitis was established as described previously (26) and in SI Materials and Methods.
Chemokine/Cytokine Determination in Colon Extracts.
Chemokine/cytokine determination in colon extracts is described in SI Materials and Methods.
Flow Cytometry.
Mesenteric lymph nodes were aseptically removed, and cell suspensions were prepared by mechanical dissociation. Nonspecific binding was blocked with Fc block (BD Biosciences). Cells were stained with phycoerythrin (PE)-conjugated anti-CD8 antibody, allophycocyanin (APC)-conjugated anti-CD4 antibody, and FITC-conjugated anti-CD69 antibody (all from eBioscience). Stained cells were analyzed by flow cytometry (FACSCalibur, BD Biosciences).
Histology and Histopathological Score.
Tissues were prepared and evaluated as described previously (27) and in SI Materials and Methods.
Immunoblot Analysis.
Western blot analyses were performed as described previously (28) and in SI Materials and Methods.
Detection of miR-155 Expression.
Detection of miR-155 expression is described in SI Materials and Methods.
Chromatin Immunoprecipitation.
ChIP was performed according to the protocol described previously (29). The C/EBPβ antibody described above was used in a concentration of 1:100. ChIP data were normalized to input. Primers used for PCR and quantitative-PCR of the Arg1 promoter that contains the C/EBPβ binding site were the following: 5′GGAGGGTGGTAGCCGACGAGA 3′; 5′ TAGCCCAGCACCCTCAACCCAA 3′.
Statistical Analysis.
All values are expressed as the mean ± SEM of data obtained from at least three experiments. Comparison between groups was made with the Student’s t test and ANOVA test. Log-rank analysis was used for survival experiments. P < 0.05 was the significance level. Unless otherwise specified, *P < 0.05, **P < 0.01, ***P < 0.001, compared with WT (Fig. 2 B, D, and H), WT LPS (Figs. 1 A–D and 2A), WT DSS (Fig. 3) or empty vector (Fig. 2G), and +P < 0.05, ++P < 0.01, +++P < 0.001, compared with Akt1−/− (Fig. 2 B, D, and H), Akt1−/− LPS (Figs. 1 A–D and 2A), Akt1−/− DSS (Fig. 3) or shAkt1 (Fig. 2G). “ns” indicates “nonsignificant,” P > 0.05. #, ##, and ### indicate P < 0.05, P < 0.01, and P < 0.001 compared with “control” (no LPS or no IL-4 in Fig. 2 C and F, respectively).
Supplementary Material
Acknowledgments
This work was supported by the European Commission within the 7th Framework Programme (FP7-PIAP-GA-2008-230725-TACIT), the Association for International Cancer Research (AICR07-0072 and AICR11-0505) (to C.T.), and from the National Institutes of Health (R01-CA057436, to P.N.T.). A. Arranz was supported by a grant from the Spanish Foundation for Research and Technology (Fundación Española para la Ciencia y la Tecnología) and the Spanish Ministry of Science and Innovation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119038109/-/DCSupplemental.
References
- 1.Gordon S, Martinez FO. Alternative activation of macrophages: Mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 3.Gray MJ, Poljakovic M, Kepka-Lenhart D, Morris SM., Jr Induction of arginase I transcription by IL-4 requires a composite DNA response element for STAT6 and C/EBPbeta. Gene. 2005;353:98–106. doi: 10.1016/j.gene.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Ruffell D, et al. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci USA. 2009;106:17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernández-Hernando C, et al. Loss of Akt1 leads to severe atherosclerosis and occlusive coronary artery disease. Cell Metab. 2007;6:446–457. doi: 10.1016/j.cmet.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franke TF, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 7.Iliopoulos D, et al. MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci Signal. 2009;2:ra62. doi: 10.1126/scisignal.2000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang ZZ, et al. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 9.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 10.Cho H, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 11.Androulidaki A, et al. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity. 2009;31:220–231. doi: 10.1016/j.immuni.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maroulakou IG, Oemler W, Naber SP, Tsichlis PN. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007;67:167–177. doi: 10.1158/0008-5472.CAN-06-3782. [DOI] [PubMed] [Google Scholar]
- 13.Polytarchou C, et al. Akt2 regulates all Akt isoforms and promotes resistance to hypoxia through induction of miR-21 upon oxygen deprivation. Cancer Res. 2011;71:4720–4731. doi: 10.1158/0008-5472.CAN-11-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou GL, et al. Opposing roles for Akt1 and Akt2 in Rac/Pak signaling and cell migration. J Biol Chem. 2006;281:36443–36453. doi: 10.1074/jbc.M600788200. [DOI] [PubMed] [Google Scholar]
- 15.Monick MM, et al. Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of beta-catenin. J Immunol. 2001;166:4713–4720. doi: 10.4049/jimmunol.166.7.4713. [DOI] [PubMed] [Google Scholar]
- 16.Ohashi K, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen WS, et al. Leptin deficiency and beta-cell dysfunction underlie type 2 diabetes in compound Akt knockout mice. Mol Cell Biol. 2009;29:3151–3162. doi: 10.1128/MCB.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter MM, et al. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138:1395–1405. doi: 10.1053/j.gastro.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 19.Yan F, et al. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J Clin Invest. 2011;121:2242–2253. doi: 10.1172/JCI44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kum WW, Lo BC, Yu HB, Finlay BB. Protective role of Akt2 in Salmonella enterica serovar typhimurium-induced gastroenterocolitis. Infect Immun. 2011;79:2554–2566. doi: 10.1128/IAI.01235-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao C, et al. Unequal contribution of Akt isoforms in the double-negative to double-positive thymocyte transition. J Immunol. 2007;178:5443–5453. doi: 10.4049/jimmunol.178.9.5443. [DOI] [PubMed] [Google Scholar]
- 23.Arranz A, et al. Vasoactive intestinal peptide suppresses toll-like receptor 4 expression in macrophages via Akt1 reducing their responsiveness to lipopolysaccharide. Mol Immunol. 2008;45:2970–2980. doi: 10.1016/j.molimm.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 24.Kleinbongard P, Rassaf T, Dejam A, Kerber S, Kelm M. Griess method for nitrite measurement of aqueous and protein-containing samples. Methods Enzymol. 2002;359:158–168. doi: 10.1016/s0076-6879(02)59180-1. [DOI] [PubMed] [Google Scholar]
- 25.Morrison AC, Correll PH. Activation of the stem cell-derived tyrosine kinase/RON receptor tyrosine kinase by macrophage-stimulating protein results in the induction of arginase activity in murine peritoneal macrophages. J Immunol. 2002;168:853–860. doi: 10.4049/jimmunol.168.2.853. [DOI] [PubMed] [Google Scholar]
- 26.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 27.Jiang HR, et al. Influence of Slc11a1 (formerly Nramp1) on DSS-induced colitis in mice. J Leukoc Biol. 2009;85:703–710. doi: 10.1189/jlb.0708397. [DOI] [PubMed] [Google Scholar]
- 28.Zacharioudaki V, et al. Adiponectin promotes endotoxin tolerance in macrophages by inducing IRAK-M expression. J Immunol. 2009;182:6444–6451. doi: 10.4049/jimmunol.0803694. [DOI] [PubMed] [Google Scholar]
- 29.Nissen RM, Yamamoto KR. The glucocorticoid receptor inhibits NFkappaB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 2000;14:2314–2329. doi: 10.1101/gad.827900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.