Abstract
Community differentiation is a fundamental topic of the social sciences, and its prehistoric origins in Europe are typically assumed to lie among the complex, densely populated societies that developed millennia after their Neolithic predecessors. Here we present the earliest, statistically significant evidence for such differentiation among the first farmers of Neolithic Europe. By using strontium isotopic data from more than 300 early Neolithic human skeletons, we find significantly less variance in geographic signatures among males than we find among females, and less variance among burials with ground stone adzes than burials without such adzes. From this, in context with other available evidence, we infer differential land use in early Neolithic central Europe within a patrilocal kinship system.
The dispersal of farming fundamentally altered social organization in Europe, providing the basis for millennia of population growth (1) and underlying the modern distribution of European languages, genotypes, and some communicable diseases (2–6). Farming spread within 5 centuries with the Linearbandkeramik (LBK) Neolithic cultural assemblage, from the Hungarian Plain, beginning at ca. 5500 cal B.C., through to the Paris Basin and the Ukraine, ending just after 5000 cal B.C. (7, 8).
These early farmers built their settlements of timber longhouses mostly on the low-lying loess soils along the river valleys of central Europe (8–10). Because they are easy to till and drain surface water well, while retaining adequate moisture, the areas of loess provided fertile and productive soils for the early crop species (e.g., barley, emmer, einkorn, pea, lentil, and flax), which were grown on small garden-like plots using intensive cultivation methods (10, 11).
Intensified agriculture may also have provided the basis for differentiation of resource access, transferred along kinship lines (8, 12, 13). Architecture, grave good assemblages, and the circulation of exotic goods, such as Spondylus shells from the North Aegean and Adriatic (14), suggest that social differentiation was present among LBK communities, but the models proposed for the social organization of these early farmers vary from egalitarian to highly stratified, often extrapolating from one specific case study. Wider studies of mortuary evidence have suggested the presence of a developing hierarchy throughout the 500- to 600-y span of the LBK, moving from a generally equalitarian, but gerontocratic, society toward greater differentiation within burial communities (8).
Status and wealth differences, which correlate with reproductive advantages (12, 13, 15, 16), could well be crucial for modeling and understanding the genetic consequences of prehistoric human dispersals (17). Because genetic modeling approaches also begin to incorporate sex-biased mobility differences (18, 19), there is increasing need for explicit evidence concerning these phenomena more directly.
Here, we present evidence concerning forms of social organization and differentiation at the population scale from across the LBK distribution. The evidence is derived from isotopic analysis of human skeletons, which provides indicators concerning diet, health, and place of origin that can be compared with the differing LBK burial contexts.
Among these indicators are strontium isotopes, which are conveyed from weathering rocks, waters, and soils into the food chain, and ultimately into the skeleton of local animals, where strontium substitutes for calcium, retaining the 87Sr/86Sr ratio of the mixed geologic source materials (20–26). The 87Sr/86Sr ratio in archaeological human tooth enamel thus serves as an averaged geographic signature from childhood (when the enamel mineral was mineralizing).
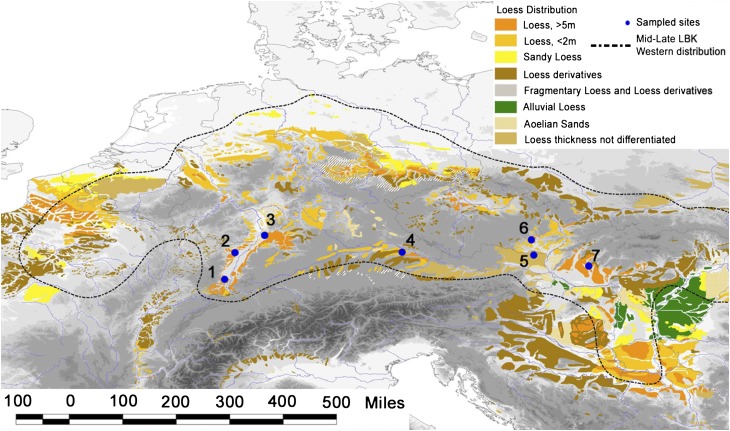
As part of a fresh wider study of more than 550 samples from eastern France to northern Hungary, we obtained 87Sr/86Sr ratios in new samples of tooth enamel from more than 300 human individuals interred in burial grounds, including the earliest known LBK cemetery of Vedrovice ( ; 5400–5250 cal B.C.) in the Czech Republic (27), as well as six additional LBK cemeteries (Fig. 1) of Aiterhofen (
; 5400–5250 cal B.C.) in the Czech Republic (27), as well as six additional LBK cemeteries (Fig. 1) of Aiterhofen (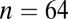 ; 5300–5000 cal B.C.) and Schwetzingen (
; 5300–5000 cal B.C.) and Schwetzingen (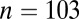 ; 5100–5000 cal B.C.) in Germany; Nitra (
; 5100–5000 cal B.C.) in Germany; Nitra (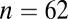 ; 5100–5000 cal B.C.) in Slovakia; Kleinhadersdorf (
; 5100–5000 cal B.C.) in Slovakia; Kleinhadersdorf (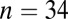 ; 5300–4900 cal B.C.) in Austria; and Ensisheim (
; 5300–4900 cal B.C.) in Austria; and Ensisheim (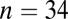 ; 5200–5000 cal B.C.) and Souffelweyersheim (
; 5200–5000 cal B.C.) and Souffelweyersheim (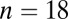 ; 5200–5000 cal B.C.) in France. For convenience we refer to these latter six sites, all with similar dates and expected 87Sr/86Sr ranges, as SNAKES (Souffelweyersheim, Nitra, Aiterhofen, Kleinhadersdorf, Ensisheim, and Schwetzingen). To these data we took the opportunity to add an extra 18 individuals from Vedrovice analyzed by Richards et al. (28) and calibrated to the same Sr isotope reference standard (Dataset S1). Vedrovice has yielded the oldest radiocarbon dates so far from a cemetery context in the central European LBK (27).
; 5200–5000 cal B.C.) in France. For convenience we refer to these latter six sites, all with similar dates and expected 87Sr/86Sr ranges, as SNAKES (Souffelweyersheim, Nitra, Aiterhofen, Kleinhadersdorf, Ensisheim, and Schwetzingen). To these data we took the opportunity to add an extra 18 individuals from Vedrovice analyzed by Richards et al. (28) and calibrated to the same Sr isotope reference standard (Dataset S1). Vedrovice has yielded the oldest radiocarbon dates so far from a cemetery context in the central European LBK (27).
Fig. 1.
Geographical distribution of loess soils in central and western Europe. Map indicates the mid to late western distribution of the LBK and the sampled cemeteries: 1, Ensisheim; 2, Souffelweyersheim; 3, Schweztingen; 4, Aiterhofen; 5, Kleinhadersdorf; 6, Vedrovice; 7, Nitra. After Loess distribution from Haase et al. (51).
As shown in Fig. 1, the SNAKES sites are all located within the belt of European loess, the widespread aeolian sediment favored by LBK farmers (2, 7, 9, 10). Certain uplands of central Europe are underlain by granitic formations of higher 87Sr/86Sr ratios than the more homogeneous lowlands often covered in loess (22–24, 29). Although measuring 87Sr/86Sr in loess deposits across this continental scale is not currently feasible (30, 31), it is known that the main sources of loess along the Danubian corridor are Alpine carbonates, with low 87Sr/86Sr ratios (29, 32, 33). For this reason, these similar loess soils are the basis for biologically available 87Sr/86Sr ratios, which range between 0.7085 and 0.7104, as characterized in LBK tooth enamel from loess-underlain sites of Alsace, Germany, Czech Republic, and Austria (22–24, 26, 29, 34–38). Situated slightly differently in this respect, Vedrovice lies at the base of the Bohemian Massif, where Precambrian contributions to the loess raise the resulting 87Sr/86Sr ratios in human skeletons to between 0.7108 and 0.7115 (28).
At each site, higher than expected biologically available 87Sr/86Sr ratios should generally indicate subsistence off the favored soils, such as (but not limited to) uplands underlain by high-87Sr/86Sr granitic rocks and sandstones derived from their erosion (22–24, 26, 34–38). These peripheral soils could be as close as several kilometers from the settlement (34); our inference is not that they were distant but that they were relegated to certain groups within the society.
Geology is only a coarse guide, however, to expected dietary 87Sr/86Sr ratios, and therefore not the crux of how we interpret our results. The 87Sr/86Sr ratios we measure in human enamel ultimately reflect a complex mixture of weathered sediments, streamwaters, and prehistoric anthropogenic inputs in agricultural soils (21). As a result, the 87Sr/86Sr in Neolithic human enamel will reflect cultural regularities of subsistence rather than an exact geologic location (20, 21, 26).
In fact, early agricultural communities often yield a range of 87Sr/86Sr ratios much narrower than their environmental surroundings. This is due to biopurification, whereby mammals average a variety of strontium inputs (39). Human agriculturalists typically reduce the 87Sr/86Sr variance even further through cultural practices altering those sources, such as the manuring and recycling of agricultural soils, and subsistence based on intergenerational cultivation of specific plots of land or favored soil types (11, 20, 25, 26).
The bio-averaging effect thus provides an opportunity for characterizing prehistoric social differentiation, by comparing the means and variances of 87Sr/86Sr between human groups (20, 21, 25, 26). By using the modal 87Sr/86Sr ratio among humans at each site as our reference points, we bypass much of the uncertainty in trying to connect geology with prehistoric behavior. By focusing on statistically significant differences in 87Sr/86Sr between human groups (20, 21, 26), we seek to determine whether some individuals or groups used different soils than others for their subsistence.
For this reason, in addition to the raw 87Sr/86Sr data, we also present these data normalized to maximize the visibility of group patterns (Dataset S1). This is done by normalizing the 87Sr/86Sr ratio, 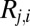 for each human individual
for each human individual 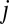 from site i, by subtracting mean 87Sr/86Sr ratio at the site,
from site i, by subtracting mean 87Sr/86Sr ratio at the site, 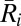 , and dividing by the site SD,
, and dividing by the site SD, 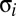 . We refer to the normalized 87Sr/86Sr ratio for individual
. We refer to the normalized 87Sr/86Sr ratio for individual 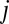 as
as 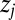 :
:
 |
By measuring, in units of SD, the distance of each individual’s Sr isotope ratio from the site mean, we can then pool all seven sites together to examine patterns of variance among individuals across all of the sampled LBK sites.
With our interest in varied land use, and possible correlates to these differences in material culture, we focus on males buried with ground stone adzes. Found more often with males, this labor-intensive artifact is one of the most distinctive of the LBK. Fashioned from raw stone often exchanged over hundreds of kilometers and requiring a long preparation process, LBK adzes seem to have conveyed social, or even status, differences (7, 8).
Sex is the other obvious potential differentiator: it has been suggested that early Neolithic society was patrilineal (6, 24, 40), but there are counter-suggestions (41) and compromises (42). As a result, and in context with previous archaeological studies (11, 43), our hypotheses are that the distributions of 87Sr/86Sr signatures will be (i) more variable among females than among males and (ii) less variable for males buried with a distinctive Neolithic ground stone adze.
Our main purpose is to present the strontium isotopic data set—the largest of its kind—and to demonstrate the remarkable patterns concerning sex and adzes. Results show the robust statistical support we find for these patterns in the data. Our aim lies in demonstrating the differences between groups (21, 26) and “to consider first and foremost the isotope data in a wider archaeological context, and be prepared to look for… something which is different rather than looking ab initio for a specific postcode of origin” (20). In Discussion and Conclusions, we therefore interpret the 87Sr/86Sr patterns in context with archeology (2, 3, 13), archaeobotany (11), and cross-cultural anthropology (12, 44–46), which generally support the premise that “as agricultural input intensifies, rights become more permanent and more exclusive” (13).
Results
Our results are given in Dataset S1 and summarized in Table 1 and Figs. 1 and 2. Among 382 individuals, 310 have determinations of sex, including 153 females and 147 males.
Table 1.
Summary of results from each site
| Site | Start cal B.C. | n (all, F, M) | Site median 87Sr/86Sr | Females mean 87Sr/86Sr | Females σ 87Sr/86Sr | Males mean 87Sr/86Sr | Males σ 87Sr/86Sr | F test sex P value | F test adze P value |
| Vedrovice | 5450 | 64, 32, 19 | 0.71111 | 0.71073 | 0.00102 | 0.71098 | 0.00070 | 0.05 | <0.01 |
| Aiterhofen | 5300 | 65, 24, 35 | 0.70951 | 0.70958 | 0.00044 | 0.70947 | 0.00036 | 0.13 | 0.42 |
| Ensisheim | 5200 | 34, 12, 15 | 0.70925 | 0.70932 | 0.00067 | 0.70936 | 0.00075 | 0.36 | <0.01 |
| Souffelweyersheim | 5200 | 18, 3, 9 | 0.70886 | 0.70969 | 0.00155 | 0.70890 | 0.00024 | <0.01 | 0.13 |
| Kleinhadersdorf | 5200 | 33, 9, 11 | 0.70991 | 0.71011 | 0.00121 | 0.70980 | 0.00055 | 0.01 | 0.42 |
| Schwetzingen | 5100 | 103, 48, 41 | 0.70979 | 0.71014 | 0.00086 | 0.70987 | 0.00053 | <0.01 | 0.04 |
| Nitra | 5100 | 63, 25, 17 | 0.70947 | 0.70953 | 0.00035 | 0.70946 | 0.00013 | <0.01 | 0.18 |
The first F test column is a one-tailed test that the variance in 87Sr/86Sr ratios is greater among females than males; the second is the one-tailed test that variance in 87Sr/86Sr ratios among males without adze is greater than among males with adze. The symbol σ denotes SD, and the column showing sample size n shows all individuals, identified females (F), and identified males (M).
Fig. 2.
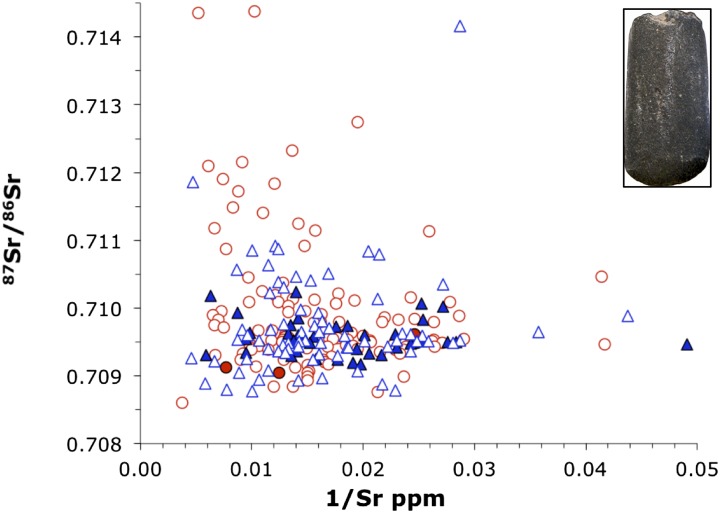
The 87Sr/86Sr vs. 1/Sr from adult individuals of identifiable (definite or probable) sex. Circles show adult females, triangles show adult males, and filled symbols denote individuals buried with an adze. Inset: Typical LBK adze.
We begin by looking at differences between the sexes. When the normalized data from all seven sites are pooled together, the variance in 87Sr/86Sr is significantly larger for females (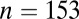 ) than for males (
) than for males (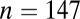 ), by an F test (
), by an F test (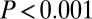 without outliers;
without outliers; 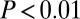 adding the four outliers) and by the more robust Levene’s test (
adding the four outliers) and by the more robust Levene’s test (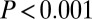 with or without outliers).
with or without outliers).
In addition to measures of variance, we look at patterns above a certain threshold 87Sr/86Sr ratio. Pooling the data from SNAKES (Table 1 and Dataset S1), females and males show similar Gaussian distributions of 87Sr/86Sr ratios up to a cutoff of 0.711 (Fig. 3A), but above 0.711 there are 15 females and only three males, a significant difference (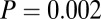 ,
,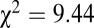 , df = 1).
, df = 1).
Fig. 3.
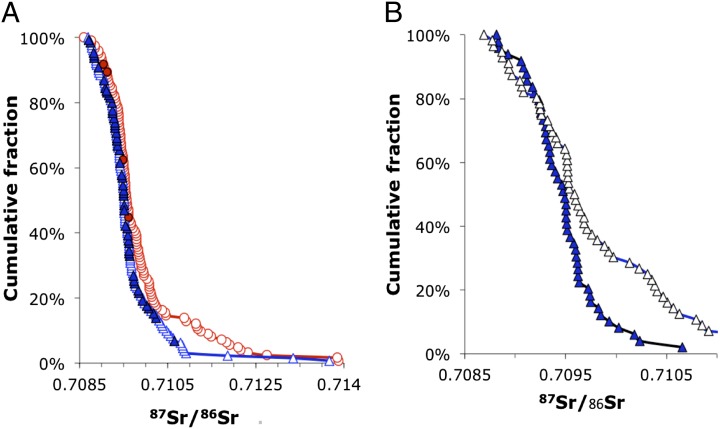
Cumulative distribution plots of 87Sr/86Sr in human enamel from sampled LBK individuals at SNAKES. (A) Individuals sexed either definitely or probably; (B) adze vs. no adze among males sexed definitely. Circles show adult females, triangles show adult males, and filled symbols denote individuals buried with an adze.
This is evident also at five of the seven sites considered individually, where we find significantly (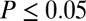 ) greater variance in 87Sr/86Sr among females; the two exceptions were Aiterhofen, which showed the same pattern but not quite significantly (
) greater variance in 87Sr/86Sr among females; the two exceptions were Aiterhofen, which showed the same pattern but not quite significantly (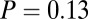 ) and Ensisheim, the only site where the variance was not larger among females (Table 1). This statistical significance is clearest from the larger cemetery samples of Nitra, Schwetzingen, and Vedrovice (Table 1). At Vedrovice the pattern is the most significant, even with the more robust Levene’s test (
) and Ensisheim, the only site where the variance was not larger among females (Table 1). This statistical significance is clearest from the larger cemetery samples of Nitra, Schwetzingen, and Vedrovice (Table 1). At Vedrovice the pattern is the most significant, even with the more robust Levene’s test (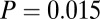 ). At Vedrovice, adult females make up the majority (14 of 16) of individuals with 87Sr/86Sr ratios above 0.712 or below 0.7104, which is significant even when accounting for the larger number of females in the overall sample (
). At Vedrovice, adult females make up the majority (14 of 16) of individuals with 87Sr/86Sr ratios above 0.712 or below 0.7104, which is significant even when accounting for the larger number of females in the overall sample (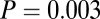 ,
, 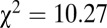 , df = 1). At Nitra, all six of the sampled individuals with 87Sr/86Sr above 0.7097 were females (
, df = 1). At Nitra, all six of the sampled individuals with 87Sr/86Sr above 0.7097 were females (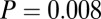 ,
, 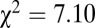 , df = 1). At Schwetzingen, all eight individuals of identified sex with 87Sr/86Sr above 0.711 are female (one was of unidentified sex).
, df = 1). At Schwetzingen, all eight individuals of identified sex with 87Sr/86Sr above 0.711 are female (one was of unidentified sex).
Taken together, these patterns indicate that women were more likely than men to have originated from, or obtained their subsistence from, areas outside the preferred loess of these LBK settlements.
We now turn to patterns regarding adzes. Among 311 sampled individuals pooled together from SNAKES, which included 62 adze burials from these six sites, only one of the 41 individuals with 87Sr/86Sr above 0.7103 is an adze burial (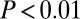 ,
, 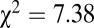 ).
).
Because LBK adzes are disproportionately found with males, we test this also within the set of males. When the normalized data from all seven sites are pooled together, the variance in 87Sr/86Sr is significantly smaller for males with adzes (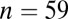 ) than for males without adzes (
) than for males without adzes (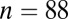 ), by an F test (
), by an F test (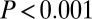 , with or without outliers) and by the more robust Levene’s test (
, with or without outliers) and by the more robust Levene’s test (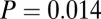 without outliers;
without outliers; 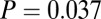 adding the three male outliers).
adding the three male outliers).
When the sex of a skeleton cannot be determined definitively but is suggested by certain skeletal traits, the individual is identified as a “probable” male or female (47). If we restrict ourselves just to the males sexed definitely (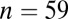 with adzes,
with adzes, 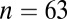 without) the difference is still significant by Levene’s test (
without) the difference is still significant by Levene’s test (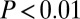 without outliers;
without outliers; 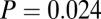 adding the three male outliers) and by F test (
adding the three male outliers) and by F test (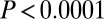 , with or without outliers). Alternatively, if we look at just the raw 87Sr/86Sr data from 128 males sexed most definitely from the six sites of SNAKES, the 87Sr/86Sr from both males with adzes and males without adzes follow a similar normal distribution below 0.7103 (Fig. 3B), but above this value only one of the 15 definite males has an adze (
, with or without outliers). Alternatively, if we look at just the raw 87Sr/86Sr data from 128 males sexed most definitely from the six sites of SNAKES, the 87Sr/86Sr from both males with adzes and males without adzes follow a similar normal distribution below 0.7103 (Fig. 3B), but above this value only one of the 15 definite males has an adze (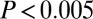 ,
, 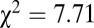 ).
).
With smaller sample size at the individual sites, the adze pattern is still significant among the larger samples from Vedrovice and Nitra. At Vedrovice, we find among the 19 identified males that the 10 adze burials had significantly smaller variance in 87Sr/86Sr than the nine without adzes (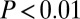 , F test;
, F test; 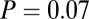 , Levene’s test). Among five highest and five lowest 87Sr/86Sr ratios among Vedrovice males, only two are adze burials (
, Levene’s test). Among five highest and five lowest 87Sr/86Sr ratios among Vedrovice males, only two are adze burials (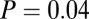 ,
, 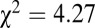 , df = 1). At Nitra, seven of the 17 identified males had adzes, four of which make up the lowest four 87Sr/86Sr ratios of all Nitra males (
, df = 1). At Nitra, seven of the 17 identified males had adzes, four of which make up the lowest four 87Sr/86Sr ratios of all Nitra males (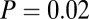 ,
, 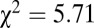 , df = 1).
, df = 1).
Discussion and Conclusions
The results confirm that 87Sr/86Sr signatures are more variable among females than among males and less variable for males buried with adzes. We interpret these results as consistent with patrilocality, and with differential use of—or access to—preferred loess soils for males with adzes. Alternative interpretations for the patterns could be proposed—the former as indicating marginal land access for women, and the latter as indicating that males without adzes moved for marriage, whereas males with adzes did not—but such explanations are not well supported by archaeological or genetic evidence.
The difference in strontium isotope signatures between adze and nonadze burials is either due to different geographic origins of the food (plots of land), or possibly to different combination of foods (from a variety of locations) among the adze and non-adze groups. In either case, this demonstrates a difference of lifeways between the adze and non-adze groups.
The males buried with adzes, with their narrow range of 87Sr/86Sr signatures compared with others, apparently derived their subsistence from a remarkably consistent source material. This was the case across hundreds of kilometers of Central Europe and several different regional mortuary/cultural traditions. Loess soil, being widespread across central Europe, consistent with these 87Sr/86Sr ratios, and preferred for LBK settlement (2, 8–10), is by far the best candidate for these signatures, especially if cultivated for generations toward homogeneity.
For the SNAKES sites, considering that we expect loess areas not generally to yield 87Sr/86Sr above 0.7103, this indicates that males buried with adzes derived more of their subsistence from loess areas compared with those buried without an adze. The same is true at Vedrovice, with shift in the expected 87Sr/86Sr range for loess at the base of the Bohemian Massif.
This suggests that, by the developed phase of the LBK, males buried with adzes had the most consistent access to preferred loess soils (with 87Sr/86Sr ratios below 0.7103). This gains support from independent archaeological evidence from cereal husbandry practices that independently indicates the differential, intergenerational transfer of access to the most productive growing areas (11). Although these differences may reflect the colonization process, because the earliest groups control the best land, another possible explanation is that these differences took hold through transhumance, with male stockherders living much of the year outside the loess (and not needing adzes). In any case, these differences are reflected across the LBK distribution in time and space, which suggests they were foundational to more pronounced inequality later in prehistoric Europe.
The pattern among the sexes is quite consistent with marriage within a patrilocal kinship system, which is also consistent with independent archaeological (11, 43), genetic (6, 18, 19, 48), anthropological (16, 44–46), and even new linguistic (49) evidence concerning Neolithic Europe. These results have implications for genetic modeling of Neolithic expansion, for which sex-biased mobility patterns and status differences are increasingly seen as crucial (17–19, 40). Generally speaking, “male inheritance of land means that males tend to live where they were born, while females marry and move elsewhere” (40). Because patrilocality, intergenerational wealth transfer, and agriculture tend to correlate in small-scale societies (12, 13, 40, 44, 46), a simple explanation is that unequal and inherited land access developed in time among the early farmers in Central Europe (cf. ref. 50).
Two decades ago, Bogucki (9) considered it fairly likely that “individual residential units were responsible for the cultivation of particular plots” during the LBK, that “the household had exclusive rights to the crops produced by this plot,” and that “fields were located as close to the houses of their cultivators as possible” (p. 119). Our evidence, along with archaeobotanical evidence (11), supports this, and suggests that the origins of differential access can be traced back to an early part of the Neolithic era (13) rather than only to later prehistory when inequality and intergenerational wealth transfers are more clearly evidenced in burials and material culture (8, 50).
Materials and Methods
We analyzed samples of enamel from the molars or premolars of more than 300 LBK human skeletons. Based upon preservation and availability, the molar selected was typically M1 or M2, but occasionally M3, and premolars for Schwetzingen. Although these molars mineralize at different times of life, there is no statistical correlation between the molar sampled and the patterns of sex or adze described. Using an established procedure, approximately 5 mg of tooth enamel from each individual was mechanically cleaned and dentine removed with a surgical steel scalpel, and soaked for 1 h in weak [5% (vol/vol)] acetic acid. Each sample was then dissolved in 3 N HNO3, purified by extraction chromatography in polyethelyne columns with Sr-spec Resin. With the purified Sr in 3% (vol/vol) HNO3 acid, 87Sr/86Sr analyses were carried out on a Thermo Electron Neptune Multi Collector Mass Spectrometer in the Department Earth Sciences, Durham University. Over all of the separate analytical sessions, the average composition and reproducibility of the 212 analyses of NBS 987 Sr isotope reference material (0.71024) was 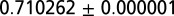 (1 SE). Blanks were typically below 10 pg Sr and always below 30 pg Sr for all runs.
(1 SE). Blanks were typically below 10 pg Sr and always below 30 pg Sr for all runs.
From 87Sr/86Sr ratios in 311 LBK human individuals, five outliers were identified. One (Nitra burial 17/64) had 483 ppm Sr, which suggested possible postburial contamination. The four others (Schwetzingen female burial 54 and male burial 132, and Aiterhofen female burial 92 and male burial 57) had 87Sr/86Sr 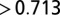 , several SDs from the means at their respective sites. These outliers were excluded in calculating the mean 87Sr/86Sr at each site.
, several SDs from the means at their respective sites. These outliers were excluded in calculating the mean 87Sr/86Sr at each site.
Supplementary Material
Acknowledgments
We thank Britta Ramminger for the photograph of the LBK adze shown in Fig. 2.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.J.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113710109/-/DCSupplemental.
References
- 1.Bocquet-Appel JP. When the world’s population took off: The springboard of the Neolithic Demographic Transition. Science. 2011;333:560–561. doi: 10.1126/science.1208880. [DOI] [PubMed] [Google Scholar]
- 2.Harris DR, editor. The Origins and Spread of Agriculture and Pastoralism in Eurasia. London: Univ of London Press; 1996. [Google Scholar]
- 3.Renfrew C. Archaeology and Language: The Puzzle of Indo-European Origins. London: Cape; 1987. [Google Scholar]
- 4.Bramanti B, et al. Genetic discontinuity between local hunter-gatherers and central Europe’s first farmers. Science. 2009;326:137–140. doi: 10.1126/science.1176869. [DOI] [PubMed] [Google Scholar]
- 5.Haak W, et al. Members of the Genographic Consortium Ancient DNA from European early neolithic farmers reveals their near eastern affinities. PLoS Biol. 2010;8:e1000536. doi: 10.1371/journal.pbio.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacan M, et al. Ancient DNA reveals male diffusion through the Neolithic Mediterranean route. Proc Natl Acad Sci USA. 2011;108:9788–9791. doi: 10.1073/pnas.1100723108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whittle A. Europe in the Neolithic: The Creation of New Worlds. Cambridge, UK: Cambridge Univ Press; 1996. [Google Scholar]
- 8.Jeunesse C. Pratiques Funéraires au Néolithique Ancien: Sépultures et Nécropoles Danubiennes 5500 – 4900 av. J. C. Paris: Éditions Errance; 1997. [Google Scholar]
- 9.Bogucki P. Forest Farmers and Stockherders. Cambridge, UK: Cambridge Univ Press; 1988. [Google Scholar]
- 10.Bakels CC. The Western European Loess Belt: Agrarian History, 5300 BC – AD 1000. New York: Springer; 2009. [Google Scholar]
- 11.Bogaard A, Krause R, Strien H-C. Towards a social geography of cultivation and plant use in an early farming community. Antiquity. 2011;85:395–416. [Google Scholar]
- 12.Borgerhoff Mulder M, et al. Intergenerational wealth transmission and the dynamics of inequality in small-scale societies. Science. 2009;326:682–688. doi: 10.1126/science.1178336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shennan SJ. Property and wealth inequality as cultural niche construction. Philos Trans R Soc Lond B Biol Sci. 2011;366:918–926. doi: 10.1098/rstb.2010.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zvelebil M, Pettitt P. Human condition, life, and death at an early Neolithic settlement. Anthropologie. 2008;46:195–218. [Google Scholar]
- 15.Mace R. The coevolution of human fertility and wealth inheritance strategies. Philos Trans R Soc Lond B Biol Sci. 1998;353:389–397. doi: 10.1098/rstb.1998.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mace R, Alvergne A. Female reproductive competition within families in rural Gambia. Proc R Soc B. 2012;279:2219–2227. doi: 10.1098/rspb.2011.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas MG, Stumpf MPH, Härke H. Evidence for an apartheid-like social structure in early Anglo-Saxon England. Proc Biol Sci. 2006;273:2651–2657. doi: 10.1098/rspb.2006.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasteiro R, Bouttier PA, Sousa VC, Chikhi L. Investigating sex-biased migration during the Neolithic transition in Europe, using an explicit spatial simulation framework. Proc Biol Sci. 2012 doi: 10.1098/rspb.2011.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavalli-Sforza LL, Minch E. Paleolithic and Neolithic lineages in the European mitochondrial gene pool. Am J Hum Genet. 1997;61:247–254. doi: 10.1016/S0002-9297(07)64303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollard AM. Isotopes and impact: A cautionary tale. Antiquity. 2011;85:631–638. [Google Scholar]
- 21.Bentley RA. Strontium isotopes from the earth to the archaeological skeleton: A review. J ArchaeolMethod Theory. 2006;13:135–187. [Google Scholar]
- 22.Bentley RA, Knipper C. Geographical patterns in biologically available Sr, C and O isotope signatures in prehistoric SW Germany. Archaeometry. 2005;47:629–644. [Google Scholar]
- 23.Grupe G, et al. Mobility of Bell Beaker people revealed by strontium isotope ratios of tooth and bone. Appl Geochem. 1997;12:517–525. [Google Scholar]
- 24.Bentley RA, et al. Prehistoric migration in Europe: Strontium isotope analysis of Early Neolithic skeletons. Curr Anthropol. 2002;43:799–804. [Google Scholar]
- 25.Montgomery J. Passports from the past: Investigating human dispersals using strontium isotope analysis of tooth enamel. Ann Hum Biol. 2010;37:325–346. doi: 10.3109/03014461003649297. [DOI] [PubMed] [Google Scholar]
- 26.Bentley RA, Price TD, Stephan E. Determining the ‘local’ 87Sr/86Sr range for archaeological skeletons: A case study from Neolithic Europe. J Archaeol Sci. 2004;31:365–375. [Google Scholar]
- 27.Pettit P, Hedges REM. The age of the Vedrovice cemetery: The AMS radiocarbon dating programme. Anthropologie. 2008;46:125–134. [Google Scholar]
- 28.Richards MP, Montgomery J, Nehlich O, Grimes V. Isotopic analysis of humans and animals from Vedrovice. Anthropologie. 2008;46:169–177. [Google Scholar]
- 29.Tricca A, et al. Rare earth elements and Sr and Nd isotopic compositions of dissolved and suspended loads from small river systems in the Vosges mountains (France), the river Rhine and groundwater. Chem Geol. 1999;160:139–158. [Google Scholar]
- 30.Gallet S, Jahn BM, Lanoe BV, Dia A, Rossello E. Loess geochemistry and its implications for particle origin and composition of the upper continental crust. Earth Planet Sci Lett. 1998;156:157–172. [Google Scholar]
- 31.Zoeller L. New approaches to European loess: A stratigraphic and methodical review of the past decade. Cent Eur J Geosci. 2010;2:19–31. [Google Scholar]
- 32.Schnetger B. Chemical composition of loess from a local and worldwide view. Neues Jahrb Miner Monatsh. 1992;1:29–47. [Google Scholar]
- 33.Újvári G, et al. Evaluating the use of clay mineralogy, SrNd isotopes and zircon UPb ages in tracking dust provenance: An example from loess of the Carpathian Basin. Chem Geol. 2012;304–305:83–96. [Google Scholar]
- 34.Knipper C. Die Räumliche Organisation der Linearbandkeramischen Rinderhaltung. Oxford: British Archaeological Reports; 2012. [Google Scholar]
- 35.Bentley RA, Krause R, Price TD, Kaufmann B. Human mobility at the early Neolithic settlement of Vaihingen, Germany: Evidence from strontium isotope analysis. Archaeometry. 2003;45:481–496. [Google Scholar]
- 36.Nehlich O, et al. Mobility or migration: A case study from the Neolithic settlement of Nieder-Mörlen (Hessen, Germany) J Archaeol Sci. 2009;36:1791–1799. [Google Scholar]
- 37.Price TD, Knipper C, Grupe G, Smrcka V. Strontium isotopes and prehistoric human migration: The Bell Beaker period in Central Europe. Eur J Archaeol. 2004;7:9–40. [Google Scholar]
- 38.Schweissing MM, Grupe G. Stable strontium isotopes in human teeth and bone: A key to migration events of the late Roman period in Bavaria. J Archaeol Sci. 2003;30:1373–1383. [Google Scholar]
- 39.Burton JH, Price TD, Cahue L, Wright LE. The use of barium and strontium abundances in human skeletal tissues to determine their geographic origins. Int J Osteoarchaeol. 2003;13:88–95. [Google Scholar]
- 40.Wilkins JF, Marlowe FW. Sex-biased migration in humans: What should we expect from genetic data? Bioessays. 2006;28:290–300. doi: 10.1002/bies.20378. [DOI] [PubMed] [Google Scholar]
- 41.Podborský V. Vedrovická pohřebiště ve starším moravském a středoevropském neolitu. In: Podborský V, editor. Dvě Pohřebivstě Neolitického Lidu s Lineárníkeramikou ve Vedrovicích na Moravě. Brno, Czech Republic: Ustav archeologie a muzeologie; 2002. pp. 293–338. [Google Scholar]
- 42.van de Velde P. The social anthropology of a Neolithic cemetery in the Netherlands. Curr Anth. 1979;20:37–58. [Google Scholar]
- 43.Eisenhauer U. Matrilokalität in der Bandkeramik? Archäologische Informationen. 2003;26:321–331. [Google Scholar]
- 44.Holden CJ, Sear R, Mace R. Matriliny as daughter-biased investment. Evol Hum Behav. 2003;24:99–112. [Google Scholar]
- 45.Holden CJ, Mace R. Spread of cattle led to the loss of matrilineal descent in Africa: A coevolutionary analysis. Proc Biol Sci. 2003;270:2425–2433. doi: 10.1098/rspb.2003.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hrdy SB. Mothers and Others. Cambridge, MA: Harvard Univ Press; 2009. [Google Scholar]
- 47.Buikstra JE, Ubelaker DH. Standards for Data Collection from Human Skeletal Remains: Proceedings of a Seminar at the Field Museum of Natural History (Arkansas Archeological Survey Research Series) Fayetteville, AR: Arkansas Archeological Survey; 1994. [Google Scholar]
- 48.Seielstad MT, Minch E, Cavalli-Sforza LL. Genetic evidence for a higher female migration rate in humans. Nat Genet. 1998;20:278–280. doi: 10.1038/3088. [DOI] [PubMed] [Google Scholar]
- 49.Fortunato L, Jordan F. Your place or mine? A phylogenetic comparative analysis of marital residence in Indo-European and Austronesian societies. Philos Trans R Soc Lond B Biol Sci. 2010;365:3913–3922. doi: 10.1098/rstb.2010.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherratt A. Economy and Society in Prehistoric Europe. Edinburgh: Edinburgh Univ Press; 1997. [Google Scholar]
- 51.Haase D, et al. Loess in Europe—its spatial distribution based on a European Loess Map, scale 1:2,500,000. Quat Sci Rev. 2007;26:1301–1312. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.