Abstract
Tau hyperphosphorylation is one hallmark of Alzheimer's disease (AD) pathology. Pharmaceutical companies have thus developed kinase inhibitors aiming to reduce tau hyperphosphorylation. One obstacle in screening for tau kinase inhibitors is the low phosphorylation levels of AD-related phospho-epitopes in normal adult mice and cultured cells. We have shown that hypothermia induces tau hyperphosphorylation in vitro and in vivo. Here, we hypothesized that hypothermia could be used to assess tau kinase inhibitors efficacy. Hypothermia applied to models of biological gradual complexity such as neuronal-like cells, ex vivo brain slices and adult non-transgenic mice leads to tau hyperphosphorylation at multiple AD-related phospho-epitopes. We show that Glycogen Synthase Kinase-3 inhibitors LiCl and AR-A014418, as well as roscovitine, a cyclin-dependent kinase 5 inhibitor, decrease hypothermia-induced tau hyperphosphorylation, leading to different tau phosphorylation profiles. Therefore, we propose hypothermia-induced hyperphosphorylation as a reliable, fast, convenient and inexpensive tool to screen for tau kinase inhibitors.
Alzheimer's disease is a neurological disease marked by progressive neuronal loss, as well as memory deficits1. AD is characterized by two specific histological lesions: amyloid plaques, composed of amyloid-β peptides deposits2, and neurofibrillary tangles, composed of hyperphosphorylated and aggregated protein tau3,4 Tau hyperphosphorylation can induce tau aggregation in vitro5, and decrease its solubility in vivo6. Tau phosphorylation is a highly regulated process resulting from the balance between kinase and phosphatase activities7. During AD development, increased tau phosphorylation is thought to result from a deregulation of these activities8. For example, protein phosphatase 2A (PP2A) activity and expression are decreased in AD brains9,10. Tau kinases such as GSK-3β11,12 and Cdk513 have also been implicated. Many laboratories and pharmaceutical companies have thus focused on the development of specific inhibitors for these kinases. For instance, GSK-3β inhibitors were shown to reduce tau pathology in a mouse model of neurodegeneration14, and are currently in clinical trials15. One of the main roadblocks for drug screening and testing is that normal adult mice and cells in culture have low basal tau phosphorylation levels at many AD-related phospho-epitopes. Previously, we demonstrated that hypothermia induced by either glucose metabolism deregulation16 or anesthesia17,18,19 leads to tau hyperphosphorylation at multiple epitopes in the mouse brain. Here, we tested the applicability of hypothermia as a model to screen for specific kinase inhibitors, based on hypothermia's capability to reliably induce tau hyperphosphorylation. As expected, hypothermic conditions led to rapid tau hyperphosphorylation at major phospho-epitopes deregulated in AD in neuroblastoma cells, metabolically active brain slices, as well as in non-transgenic mice. Treatment in various biological models with known GSK-3 or Cdk5 kinase inhibitors prevented partially or totally hypothermia-induced tau hyperphosphorylation. Based on these observations, we propose hypothermia as a reliable, easy and inexpensive model to screen for and characterize pharmacological modulators of tau phosphorylation.
Results
Anesthesia-induced tau hyperphosphorylation is partially prevented by LiCl administration in vivo
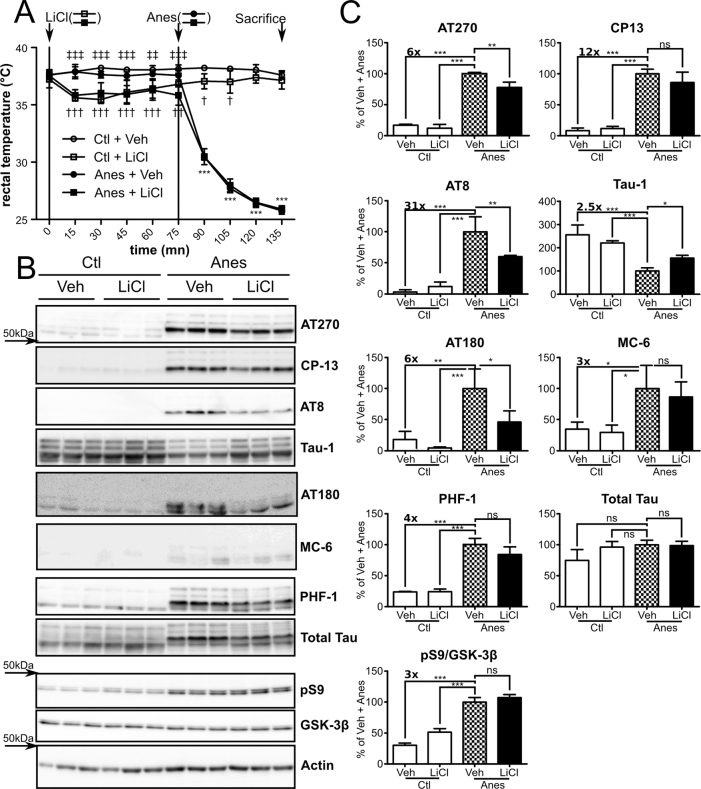
We have shown previously that anesthesia-induced hypothermia causes tau hyperphosphorylation in vivo17. In this study, we used ketamine/xylazine anesthesia to induce tau hyperphosphorylation. Adult mice were pre-treated for two days with LiCl, a well-known GSK-3 inhibitor14,20. On the third day, the mice received a final LiCl injection 75 minutes before anesthesia (Anes). Rectal temperature analysis showed that LiCl injection per se induced a significant drop in body temperature after 15 minutes (Figure 1A: 37.9°C Ctl+Veh vs 35.6°C Ctl+LiCl, 37.8°C and Anes+Veh vs 35.8°C Anes+LiCl) and remained constant (∼36°C) until anesthesia. Body temperatures of non-treated mice remained unchanged until anesthesia (Figure 1A). Anesthesia induced a progressive and drastic drop in temperature reaching ∼26°C after 60 minutes of anesthesia.
Figure 1. Anesthesia-induced tau hyperphosphorylation is prevented by LiCl administration in vivo.
A. Mice rectal temperature curve following LiCl and anesthetic injections. 2-way ANOVA followed by bonferonni's post test was performed. ***p<0.001 Ctl+LiCl vs Anes+LiCl or Ctl+Veh vs Anes+Veh, respectively. †††p<0.001, ††p<0.01, †p<0.05 Ctl+Veh vs Ctl+LiCl. ‡‡‡p<0.001, ‡‡p<0.01 Anes+Veh vs Anes+LiCl. B. Immunoblots of cortical homogenates extracted protein using using several phospho-tau antibodies (AT270, AT8, CP13, Tau-1, pS262 and PHF-1). Total tau was probed using a pan tau antibody. GSK-3β inhibition was monitored by assessing both GSK-3β pS9 levels and total levels of GSK-3β. Actin probe was used as a loading control. C. Immunoblot quantifications. Ratios of phospho-epitope levels over total tau protein ±SD are represented as a percentage of vehicle+Anes group condition (checkerboard bar). N = 3 per condition.
As expected, tau phosphorylation significantly increased in anesthetized animals at all phospho-epitopes analyzed (Figure 1B, C: Ctl+Veh vs Anes+Veh, AT270:∼+6x, CP13:∼+12x; AT8:∼+31x; Tau-1:∼−2.5x, AT180:∼+6x, MC-6:∼+3x and PHF-1:∼+4x). Treating anesthetized mice with LiCl, but not vehicle, reduced tau phosphorylation at AT270 (∼−22%), AT8 (∼−41%), Tau-1 (∼+55%) and AT180 (∼−53%) phospho-epitopes (Figure 1B, C: Anes+LiCl vs Anes+ Veh). Other phospho-epitopes, such as CP13, MC-6 and PHF-1, were also decreased to a lesser extent in LiCl-treated mice but did not reach statistical significance. No significant changes in tau phosphorylation were observed between control groups (Figure 1B, C: Ctl+Veh vs Ctl+LiCl). Likewise, no significant changes were observed in total tau levels in all groups. Notably, GSK-3β serine 9 phosphorylation (pS9), indicating GSK-3β inhibition, was significantly increased in the anesthetized groups compared to non-anesthetized mice (Figure 1B, C). A significant increase in GSK-3β pS9 was observed between control groups (Figure 1B, C: Ctl+Veh vs Ctl+LiCl p<0.001 Bonferroni's post hoc test) but not between anesthetized groups (Anes+Veh vs Anes+LiCl). Taken together, these results demonstrate that anesthesia-induced hypothermia leads to in vivo tau hyperphosphorylation that can be attenuated by LiCl administration.
Hypothermia-induced tau hyperphosphorylation is prevented by LiCl treatment in mouse brain slices
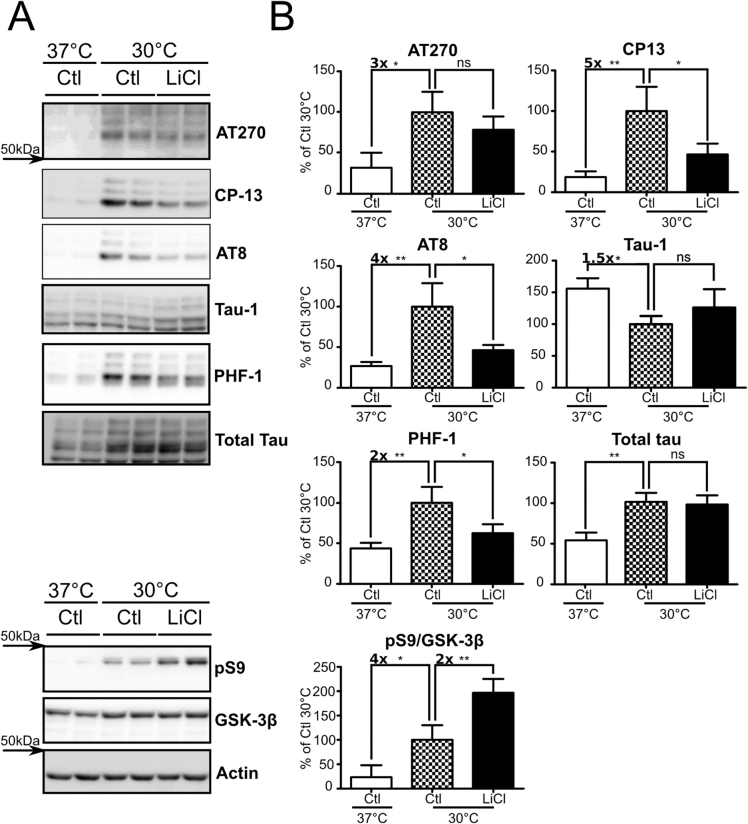
As LiCl prevents hypothermia-induced tau hyperphosphorylation in vivo, we intended to transpose these results in an ex vivo model. To this end, we performed hypothermia experiments using mouse metabolically active brain slices21. After 2h under hypothermia, tau phosphorylation levels were significantly increased at all phospho-epitopes analyzed, including AT270 (∼+3x), CP13 (∼+5x), AT8 (∼+4x), Tau-1 (∼−1.5x) and PHF-1 (∼+2x) (Figure 2A, B: Ctl 37°C vs Ctl 30°C). On the other hand, slices exposed to hypothermia while treated with LiCl for 2h showed reduced tau phosphorylation levels (CP13:∼54%, AT8:∼54% and PHF-1:∼38% (Figure 2A, B Ctl 30°C vs LiCl 30°C). The same trend was observed on the AT270 and Tau-1 phospho-epitopes even though it did not reach statistical significance. AT180 and MC-6 signals were below the detection threshold (Data not shown). In these experiments, we used an optimized 20 mM LiCl dose (supplementary Figure S1 online), which is consistent with previous findings22. Total tau protein levels were significantly changed by hypothermia but not by LiCl treatment. Hypothermia also induced a ∼4-fold GSK-3β pS9 increase (Figure 2A, B: Ctl 37°C vs Ctl 30°C), while LiCl treatment under hypothermic condition raised GSK-3β pS9 levels up to ∼8-fold (Figure 2A, B). Finally, total GSK-3β levels were significantly increased (∼+20%) with hypothermia. In summary, and as seen in vivo, LiCl treatment partially prevented tau hyperphosphorylation induced by hypothermia in ex vivo brain slices, through GSK-3β inhibition.
Figure 2. Hypothermia-induced tau hyperphosphorylation is prevented by LiCl treatment in mouse brain slices.
Mouse brain slices were subjected to hypothermia for 2h and treated with either LiCl or medium alone. A. Immunoblots of mouse brain slice proteins using several phospho-tau antibodies (AT270, CP13, AT8 and PHF-1). Total tau was probed using a pan tau antibody. GSK-3β inhibition was monitored by assessing both GSK-3β pS9 levels and total GSK-3β. B. Immunoblot quantifications. Ratios of phospho-epitope levels over total protein levels ±SD are represented as a percentage of hypothermic non-treated condition Ctl 30°C (Checkerboard bar). N = 3 per condition.
Hypothermia-induced tau hyperphosphorylation is prevented by LiCl treatment in wild-type SH-SY5Y cells or SH-SY5Y 3R-tau
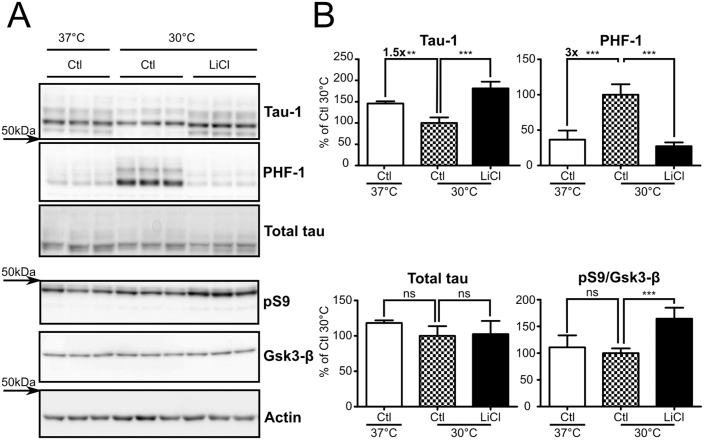
To further test our experimental paradigm in a cell system more suitable for drug screening, we performed hypothermia experiments in native neuroblastoma SH-SY5Y cells from human origin. Cells exposed to hypothermia (30°C) for 2h showed a significant tau phosphorylation increase at PHF-1 (∼+3x) and Tau-1 (∼−1.5x) phospho-epitopes (Figure 3 A, B: Ctl 37°C vs Ctl 30°C). Treating cells with LiCl during hypothermia reduced tau phosphorylation to control levels for PHF-1 and Tau-1 phospho-epitopes (Figure 3 A, B: Ctl 30°C vs LiCl 30°C). Total tau levels were not changed in all groups tested. While no increase in GSK-3β pS9 was observed in hypothermic cells, this epitope was increased (∼+50%) in LiCl treated cells. Other tau phospho-epitopes, including, AT8, AT180, CP13 and AT270, were also assessed but were below detection limits, probably because of low endogenous tau expression and low physiological levels of these phospho-epitopes in these cells.
Figure 3. Hypothermia-induced tau hyperphosphorylation is prevented by LiCl treatment in natives SH-SY5Y cells.
Natives SH-SY5Y cells were subjected to 2h of hypothermia and treated with either LiCl or medium alone. A. Immunoblots of cell extracted proteins using phospho-tau antibodies Tau-1 and PHF-1. Total tau was probed using a pan tau antibody. GSK-3β inhibition was assessed by monitoring both GSK-3β pS9 levels and total levels. B. Immunoblot quantifications. Ratios of phospho-epitope levels over total protein levels ±SD are represented as a percentage of hypothermic non-treated condition Ctl 30°C (Checkerboard bar). N = 6 per condition.
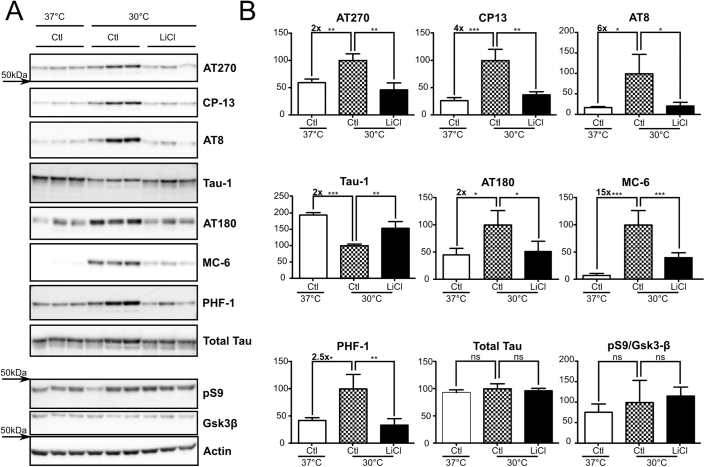
To overcome these detection limitations, we performed similar experiments in SH-SY5Y cells stably expressing human 3R-tau without any mutation (SH-SY5Y 3R-tau). Here, hypothermic cells exhibited hyperphosphorylation at several phospho-epitopes (AT270:∼+2x, CP13:∼+4x, AT8:∼+6x, Tau-1:∼−2x, AT180:∼+2x, MC-6:∼+15x and PHF-1:∼+2.5x (Figure 4 A, B: Ctl 37°C vs Ctl 30°C). As seen previously, LiCl treatment restored tau phosphorylation to control levels at AT270, CP13, AT8, Tau-1, AT180, MC-6 and PHF-1 phospho-epitopes (Figure 4A, B: Ctl 30°C vs LiCl 30°C). Again, total tau protein levels were not affected in these experimental conditions. Finally, while an increase in GSK-3β pS9 was observed in hypothermic cells with or without LiCl treatment, this effect did not reach statistical significance. In sum, these results show that GSK-3 inhibition by LiCl leads to reduced hypothermia-induced tau hyperphosphorylation in these cells.
Figure 4. Hypothermia-induced tau hyperphosphorylation is prevented by LiCl treatment in SH-SY5Y 3R-tau.
SH-SY5Y 3R-tau were subjected to hypothermia for 2h and treated with either LiCl or medium alone. A. Immunoblots of cell extracted proteins using several phospho-tau antibodies (AT270, CP13, AT8 Tau-1 and PHF-1). Total tau was probed using a pan tau antibody. GSK-3β inhibition was monitored by assessing both GSK-3β pS9 levels and total levels. B. Immunoblot quantifications. Ratios of phospho-epitope levels over total protein levels ±SD are represented as a percentage of hypothermic non-treated condition Ctl 30°C (Checkerboard bar). N = 3 per condition.
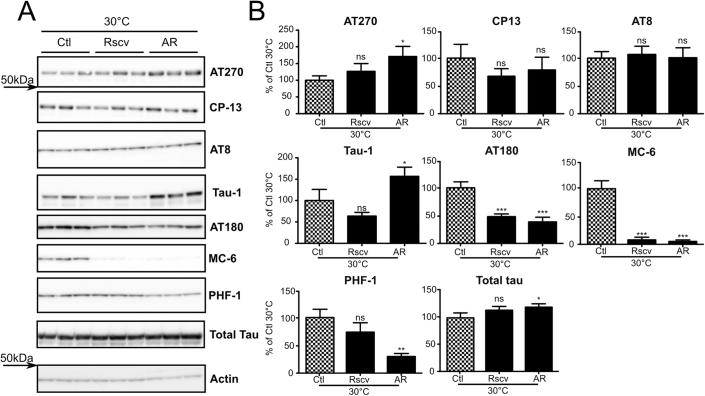
Hypothermia-induced tau hyperphosphorylation is differentially prevented by Cdk5 and GSK-3β inhibitors treatment in SH-SY5Y 3R-tau
To extend our experimental paradigm to other kinase inhibitors, we performed hypothermia experiments using SH-SY5Y 3R-tau cells treated with the GSK-3β inhibitor AR-A014418 (AR) or the Cdk5 inhibitor roscovitine. Two hours of AR treatment caused a significant decrease of phosphorylation at Tau-1, AT180, MC-6 and PHF-1 phospho-epitopes (+57%, −62%, −95% and −70% respectively) (Figure 5A, B: AR 30°C vs Ctl 30°C). Tau phosphorylation was also decreased to a lesser extent at CP13 phospho-epitope but did not reach statistical significance. AT270 and AT8 phosphorylation were not decreased by AR treatment with an unexplained slight significant increase of phosphorylation at AT270 phospho-epitope. Cells treated with roscovitine showed significant decreased tau phosphorylation levels at AT180 (−52%) and MC-6 (−92%) phospho-epitopes when compared to untreated conditions (Figure 5A, B: Rscv 30°C vs Ctl 30°C). Notably, roscovitine had no significant effect at AT270, CP13, AT8, Tau-1 and PHF-1 phospho-epitopes. Total tau protein levels remained constant in all experimental conditions. In summary, treatments with Cdk5 inhibitor roscovitine and GSK-3β inhibitor AR-A014418 leads to different reduced tau phosphorylation patterns reflecting the specificity of inhibitors used.
Figure 5. Hypothermia-induced tau hyperphosphorylation is differentially prevented by Cdk5 and GSK-3β inhibitor treatment in SH-SY5Y 3R-Tau.
SH-SY5Y 3R-tau cells were subjected to hypothermia for 2 h and treated with medium alone or medium containing either Cdk5 inhibitor roscovitine (Rscv) or GSK-3β inhibitor AR-A014418. A. Immunoblots of SH-SY5Y 3R-tau cell extracted proteins using several phospho-tau antibodies (AT8, AT180, MC-6 and PHF-1). Total tau was probed using a pan tau antibody. B. Immunoblot quantifications. Ratios of phospho-epitope levels over total protein levels ±SD are represented as a percentage of hypothermic non-treated condition Ctl 30°C (Checkerboard bar). N = 3 per condition.
Discussion
Here, we used hypothermia to induce tau hyperphosphorylation in various biological models ranging from immortal cultured cells, to mice and ex vivo metabolically active slices, to test the effects of tau kinase inhibitors. We used the GSK-3 inhibitor LiCl as a proof of concept to demonstrate the feasibility of this approach. As expected, hypothermia induced tau phosphorylation at multiple epitopes in all our models, and treatment with LiCl reduced hypothermia-induced tau hyperphosphorylation. We also extended this study to two other kinase inhibitors, AR-A014418 and roscovitine targeting GSK-3 or Cdk5, respectively, and were able to observe a specific pattern of tau phosphorylation reduction indicative of kinase inhibitor specificity.
All general anesthetics induce hypothermia through the deregulation of both non- shivering and shivering thermogenesis regulation23. This hypothermia directly results in an in vivo increase of AD-related tau phospho-epitopes including AT270 (Thr181), Tau-1 (Ser195/Ser198/Ser199/Ser202), Ser199, CP13 (Ser202), AT8 (Ser202/Thr205), TG-3 (Thr231 conformational), MC-6 (Ser235), Ser262, PHF-1 (Ser396/Ser404) and Ser42217,24 at comparable levels to those observed in AD brains18; reviewed in25. This hypothermia-induced tau hyperphosphorylation is also reversible since we demonstrated earlier that tau phosphorylation levels recover to normal after 1 week of post-anesthesia recovery19. In the present work, we confirmed these results and showed that ketamine/xylazine led to an hypothermia-induced increase of tau phosphorylation from ∼1.5x to ∼31x, depending of the phospho-epitope. Among all the phospho-epitopes studied here, Thr231 and Ser262 are accepted to be early tau modifications in neurodegeneration while other tau phosphorylation sites such as Thr181, Ser202/Thr205 and Ser396/Ser404 appear later during disease progression26. Thr231 and Thr181 are used as AD biomarkers27,28, and Ser202/Thr205 is used to stage AD pathology29. Thus, the profiles and levels of tau hyperphosphorylation generated by our hypothermia models are highly relevant to tau pathology observed in AD.
The exact mechanisms underlying tau hyperphosphorylation in AD are not well understood. However, it is commonly accepted that tau hyperphosphorylation results from the imbalance between kinase and phosphatase activities8. Among all the phosphatases, PP2A is the major enzyme that dephosphorylates tau30, accounting for more than 70% of the total tau phosphatase activity in the brain, regulating nearly all tau phosphorylation sites31. Moreover, its inhibition is associated with AD evolution32. Indeed, both PP2A expression10,33 and activity9,34 are decreased in AD brains. Tau phosphorylation is exquisitely sensitive to temperature, increasing by 80% for each degree below 37°C, due to exponential decrease in PP2A activity during direct hypothermia16, or anesthesia-induced hypothermia17. Therefore, the hypothermia-mediated inhibition of PP2A is a highly relevant model to study modulators of tau phosphorylation targeting tau pathology in AD.
Among tau kinases, GSK-3β12,35 and Cdk536 are considered to be the main tau kinases inducing tau hyperphosphorylation in AD. This statement does not conflict with the aforementioned role of PP2A inhibition in tau hyperphosphorylation, because phosphatases merely remove phosphate from tau while kinases are still needed for hyperphosphorylation to take place. In fact, PP2A inhibition is considered to be the functional equivalent of activating all the tau kinases together because PP2A dephosphorylates all known tau epitopes while each kinase is specific for only a set of given sites37. For example, GSK-3β has been shown in vitro and/or in vivo to target phosphorylation at sites such as Thr181, Ser202/Thr205, Thr231 and Ser396/Ser404. On the other hand, Cdk5 can phosphorylate about 10 phosphorylation sites in vitro including Ser202, Thr231, Ser235, Ser396/Ser4047.
In all our biological systems, we observed that LiCl reduced hypothermia-induced tau hyperphosphorylation at almost every epitope analyzed. We also observed that the effects of LiCl was more pronounced in cell culture than in ex vivo and in vivo models. This could be attributed, in part, to a lower bio-availability of compounds and for the low phosphorylation of some epitopes such as AT180 and MC-6 in ex vivo and in vivo models compared to cell cultures. Essentially, we observed the same results with another GSK-3β inhibitor (AR) at many phospho-epitopes. However, phosphorylation on CP13 (Ser202), AT8 (Ser202/Thr205) and AT270 (Thr181) were not significantly decreased at the dose used. These discrepancies could be explained by both the high specificity of AR to GSK-3β and the fact that LiCl is also known to inhibit other kinases38. Therefore, the significant decrease of phosphorylation at those phospho-epitopes observed in LiCl treated samples could be the result of a cumulative effect of LiCl on GSK-3β and other kinases. The use of roscovitine in hypothermic SH-SY5Y 3R-tau, reduced hyperphosphorylation at Thr231 and Ser235 epitopes. However, AT270, (Thr181), CP13 (Ser202), AT8 (Ser202/Thr205), Tau-1 (non-phosphorylated Ser195/Ser198/Ser199/Ser202) and PHF-1 (Ser396/Ser404) epitopes were not significantly decreased. These results could be explained by a lesser activity of Cdk5 at CP13, AT8 and Tau-1 phosphorylation sites7 since the activation of Cdk5 in neuroblastoma cells leads to a smaller increase of phosphorylation at Ser202/Thr205 than at Thr231 and Ser235 and does not increase tau phosphorylation at Ser396/Ser40439. Moreover, increased activity of Cdk5 observed in amyloid precursor protein knockout mice does not result in increased tau phosphorylation at Thr18140. Therefore, inhibiting Cdk5 by roscovitine in our hypothermia paradigm reduced tau hyperphosphorylation at preferred Cdk5 sites such as Thr231 and Ser235. Altogether, our results show that hypothermia is a suitable model to assess the specificity of tau kinase inhibitors on tau phosphorylation.
One of the main roadblocks for screening and drug testing of tau phosphorylation inhibitors is that immortal cell lines and adult mice show low levels of tau phosphorylation. To override this problem, a large number of both cellular and animal models have been based on the over-expression of mutated human tau to achieve tau hyperphosphorylation41. However, the use of high expression levels of aggressive tau mutants could lead to pathophysiological mechanisms irrelevant to AD, since only non-mutant tau proteins are implicated in the disease. Other studies have induced both hyperphosphorylated42 and abnormally phosphorylated tau43 through PP2A inhibition with okadaic acid. However, the high toxicity of okadaic acid, associated to its pro-apoptotic side effects, limits the relevance of such models44. Here, we showed that hypothermia led to easily detectable tau phosphorylation levels on non-mutated tau expressed at endogenous levels, which is compatible with tau kinase inhibitors testing.
In conclusion, we have shown in cells, brain slices and mice, that hypothermia is a reliable system to model AD-like tau hyperphosphorylation with endogenous wild-type tau protein levels. Moreover, we demonstrated that hypothermia could be used as a transverse paradigm to evaluate the potency and specificity of tau kinase inhibitors. This is of particular importance as recent evidences implicate pre-fibrillar hyperphosphorylated tau as the toxic species in AD45,46, and therefore fuel a regain of interest in tau kinase inhibitor development and clinical trials15. Overall, we propose that hypothermia and anesthesia-induced hypothermia represent a fast and useful tool to help in the development and characterization of new tau phosphorylation modulators from cells to animals.
Methods
Kinase Inhibitors
We used Lithium chloride (LiCl) (Sigma-Aldrich, St-Louis, MO, USA), AR-A014418 (Enzo life sciences, Farmingdale, NY, USA), and roscovitine (New England Biolabs, Ipswich, MA, USA).
Animal treatments
4-month-old C57BL/6 mice of either sex were injected intra-peritoneally with either sterile water (control group) or sterile water containing therapeutically relevant dose of 0.6M LiCl for 2 days (once daily at 10 ml/kg), as previously published14,47. On the third day, the mice received a last injection and 75 minutes later, half the mice of each group were anesthetized by injection of ketamine/xylazine (100/10 mg/kg) in sterile water. The other half received a vehicle injection (sterile water). Rectal temperature was monitored every 15 minutes after the first injection until sacrifice, using a rectal probe (Thermalert TH-5; Physitemp, Clifton, NJ, USA). Mice were sacrificed by decapitation 135 minutes after the last LiCl injection (i.e. 60 minutes after anesthesia). Brains were removed and dissected on ice, and frozen on dry ice. Tissues were kept at −80°C until processing.
Brain slices
4-month-old C57BL/6 mice were sacrificed by decapitation. Slices were prepared as previously published48. Slices were divided into 2 hemibrain slices and placed into permeable inserts (Transwell Permeable Supports, Corning, Corning, NY, USA). After 30 minutes of recovery, transwells were transferred into new 24-well plates containing pre-oxygenated DMEM or DMEM with 20 mM LiCl. Half the slices and their contra-lateral counterparts were placed in a 5% CO2 humidified incubator at 30°C for hypothermic conditions or 37°C. After 2 h, the medium was removed and the slices were harvested directly in a modified RIPA buffer (see below). Slice homogenates were kept at −80°C until processing. All animals were handled according to procedures approved by the Comité de Protection des Animaux under the guidelines of the Canadian Council on Animal Care.
Cell cultures
Native SH-SY5Y were purchased from American Type Culture Collection (ATCC #CRL-2266, Manassas, VA, USA) and grown as recommended by the manufacturer. SH-SY5Y stably overexpressing human 3-repeats (SH-SY5Y 3R-tau) tau cells were a kind gift from Dr Luc Buée (Inserm UMR837, Lille, France) and subcultured at 37°C, as previously described49. Cells were treated or not with either 20 mM LiCl, or 20 µM AR-A014418 as previously published22, or 20 µM roscovitine50 for 2h. For the duration of the treatment, cells were transferred in a 5% CO2 humidified incubator at 30°C for hypothermic conditions.
Western blots
Samples were prepared as previously described19. Proteins were quantified, separated on SDS-PAGE gels, blotted onto nitrocellulose membranes and blocked. Membranes were probed with antibodies diluted at the concentration indicated in Table 1. All antibodies were purchased directly from the provider as mentioned in Table 1 except for CP1351, TG-3 and MC-652, and PHF-153 incubated with a Horseradish Peroxidase-conjugated secondary antibody (Jackson Immunoresearch laboratories, West Grove, PA, USA), and revealed by chemiluminescence in a Fujifilm LAS4000 imaging system (Fujifilm Life Science USA, Stamford, CT), as previously described54. Of note, contrary to other antibodies, Tau-1 is directed against non-phosphorylated multiple tau epitopes, and therefore the signal decreases when tau is hyperphosphorylated55. Immunoblot quantifications were performed by Fujifilm Multigauge software 3.0.
Table 1. Antibodies used and their respective dilutions.
| Antibody | Phospho-epitope | Dilution | Provider |
|---|---|---|---|
| AT100 | pS212/T214 | 1/1000 | Pierce |
| AT180 | pT231 | 1/1000 | Pierce |
| AT270 | pT181 | 1/1000 | Pierce |
| AT8 | pS202/T205 | 1/1000 | Pierce |
| CP13 | pS202 | 1/1000 | Gift of Peter Davies |
| MC-6 | pS235 | 1/1000 | Gift of Peter Davies |
| PHF-1 | pS396/S404 | 1/1000 | Gift of Peter Davies |
| Tau-1 | 1/10000 | Millipore | |
| Tau A0024 | 1/10000 | Dako Cytomation | |
| TG-3 | pT231 | 1/1000 | Gift of Peter Davies |
| GSK-3β | 1/1000 | BD biosciences | |
| Gsk3 S9 | GSK-3β pS9 | 1/2000 | Cell signaling Technology |
| Actin-β | 1/3000 | Sigma | |
| pS262 | pS262 | 1/5000 | Life Technologies |
Statistics/Miscellaneous
Statistical analysis was performed with one-way ANOVA followed by the Dunnet's post hoc test. For multiple comparisons, one-way ANOVA followed by the Bonferroni's post hoc test was performed. *p<0.05, **p<0.01, ***p<0.001, ns: non significant, except otherwise mentioned. GraphPad Prism 5.0 (GraphPad, La Jolla, CA). LibreOffice suite, The GIMP and Mendeley free softwares were used for data processing, manuscript editing and referencing.
Author Contributions
Conceived and designed the experiments: AB, EP. Performed the experiments: AB, FM, CJ. Analyzed the Data: AB, EP. Contributed reagents/material/analysis tools: DM, GL. Performed revision experiments: NBEK, FRP, IP. Wrote the manuscript: AB, DM, GL, SSH, EP. All authors reviewed the manuscript.
Supplementary Material
Figure S1: LiCl treatment dose establishment in SH-SY5Y 3R tau cells. SH-SY5Y 3R tau cells were subjected to indicated LiCl dose in hypothermic conditions for 2 hours
Acknowledgments
We would like to thank Dr Peter Davies (Albert Einstein College of Medicine, Bronx, NY, USA) for the kind gift of monoclonal tau antibodies, and Ms Mélaine Henry and Ms Chantal Godin for technical help. We would like to thank also Dr Luc Buée for the gift of SH-SY5Y 3R tau cells. This work was supported by Doctoral Award from Alzheimer Society of Canada (NBEK) and Postdoctoral Awards from the Alzheimer Society of Canada and the Alzheimer Society of Saskatchewan (C.J.) and grants to E.P. from the Canadian Institute of Health Research (MOP-106423, PCN-102993), Fonds de Recherche en Santé du Québec (16205, 20048) and the Natural Sciences and Engineering Research Council of Canada (354722).
References
- Alzheimer A., Stelzmann R. A., Schnitzlein H. N. & Murtagh F. R. An English translation of Alzheimer's 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin Anat 8, 429–31 (1995). [DOI] [PubMed] [Google Scholar]
- Glenner G. G. & Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120, 885–890 (1984). [DOI] [PubMed] [Google Scholar]
- Iqbal K., Grundke-Iqbal I., Zaidi T., Ali N. & Wisniewski H. M. Are Alzheimer neurofibrillary tangles insoluble polymers? Life Sci 38, 1695–700 (1986). [DOI] [PubMed] [Google Scholar]
- Brion J. P., Couck A. M., Passareiro E. & Flament-Durand J. Neurofibrillary tangles of Alzheimer's disease: an immunohistochemical study. J Submicrosc Cytol 17, 89–96 (1985). [PubMed] [Google Scholar]
- Alonso A., Zaidi T., Novak M., Grundke-Iqbal I. & Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci U S A 98, 6923–8 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundelt M. et al. Altered phosphorylation but no neurodegeneration in a mouse model of tau hyperphosphorylation. Neurobiol Aging 32, 991–1006 (2011). [DOI] [PubMed] [Google Scholar]
- Hanger D. P., Anderton B. H. & Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol Med 15, 112–9 (2009). [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q. & Lee V. M. Phosphorylation of paired helical filament tau in Alzheimer's disease neurofibrillary lesions: focusing on phosphatases. FASEB J 9, 1570–6 (1995). [DOI] [PubMed] [Google Scholar]
- Gong C. X., Singh T. J., Grundke-Iqbal I. & Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem 61, 921–7 (1993). [DOI] [PubMed] [Google Scholar]
- Sontag E. et al. Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol 63, 287–301 (2004). [DOI] [PubMed] [Google Scholar]
- Pei J. J. et al. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol 58, 1010–9 (1999). [DOI] [PubMed] [Google Scholar]
- Planel E., Sun X. & Takashima A. Role of GSK-3 beta in Alzheimer's disease pathology. Drug Development Research 56, 491–510 (2002). [Google Scholar]
- Pei J.-J. et al. Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer's disease neurofibrillary degeneration. Brain Research 797, 267–277 (1998). [DOI] [PubMed] [Google Scholar]
- Noble W. et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci U S A 102, 6990–5 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravitz L. Drugs: a tangled web of targets. Nature 475, S9–11 (2011). [DOI] [PubMed] [Google Scholar]
- Planel E. et al. Alterations in glucose metabolism induce hypothermia leading to tau hyperphosphorylation through differential inhibition of kinase and phosphatase activities: implications for Alzheimer's disease. J Neurosci 24, 2401–11 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planel E. et al. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J Neurosci 27, 3090–3097 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planel E. et al. Anesthesia-induced hyperphosphorylation detaches 3-repeat tau from microtubules without affecting their stability in vivo. J Neurosci 28, 12798–807 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planel E. et al. Acceleration and persistence of neurofibrillary pathology in a mouse model of tauopathy following anesthesia. FASEB J 23, 2595–604 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R. V., Budd Haeberlein S. L. & Avila J. Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem 89, 1313–1317 (2004). [DOI] [PubMed] [Google Scholar]
- Gong C. X., Lidsky T., Wegiel J., Grundke-Iqbal I. & Iqbal K. Metabolically active rat brain slices as a model to study the regulation of protein phosphorylation in mammalian brain. Brain Res Brain Res Protoc 6, 134–40 (2001). [DOI] [PubMed] [Google Scholar]
- Bhat R. et al. Structural insights and biological effects of glycogen synthase kinase 3-specific inhibitor AR-A014418. J Biol Chem 278, 45937–45945 (2003). [DOI] [PubMed] [Google Scholar]
- Sessler D. I. Perianesthetic thermoregulation and heat balance in humans. FASEB J 7, 638–44 (1993). [DOI] [PubMed] [Google Scholar]
- Run X., Liang Z. & Gong C.-X. Anesthetics and tau protein: animal model studies. J Alzheimers Dis 22 Suppl 3, 49–55 (2010). [DOI] [PubMed] [Google Scholar]
- Papon M.-A., Whittington R. A., El-Khoury N. B. & Planel E. Alzheimer's disease and anesthesia. Front Neurosci 4, 272 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustinack J., Schneider A., Mandelkow E.-M. & Hyman B. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathologica 103, 26–35 (2002). [DOI] [PubMed] [Google Scholar]
- Buerger K. et al. CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology 59, 627–629 (2002). [DOI] [PubMed] [Google Scholar]
- Hampel H. et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch Gen Psychiatry 61, 95–102 (2004). [DOI] [PubMed] [Google Scholar]
- Braak H., Alafuzoff I., Arzberger T., Kretzschmar H. & Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112, 389–404 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Jakes R., Qi Z., Wang J. H. & Cohen P. Protein phosphatase 2A is the major enzyme in brain that dephosphorylates tau protein phosphorylated by proline-directed protein kinases or cyclic AMP-dependent protein kinase. J. Neurochem. 65, 2804–2807 (1995). [DOI] [PubMed] [Google Scholar]
- Liu F., Grundke-Iqbal I., Iqbal K. & Gong C.-X. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci 22, 1942–50 (2005). [DOI] [PubMed] [Google Scholar]
- Tian Q. & Wang J. Role of serine/threonine protein phosphatase in Alzheimer's disease. Neurosignals 11, 262–9 (2002). [DOI] [PubMed] [Google Scholar]
- Vogelsberg-Ragaglia V., Schuck T., Trojanowski J. Q. & Lee V. M. PP2A mRNA expression is quantitatively decreased in Alzheimer's disease hippocampus. Exp Neurol 168, 402–12 (2001). [DOI] [PubMed] [Google Scholar]
- Gong C. X. et al. Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J Neurochem 65, 732–8 (1995). [DOI] [PubMed] [Google Scholar]
- Hooper C., Killick R. & Lovestone S. The GSK3 hypothesis of Alzheimer's disease. J Neurochem 104, 1433–9 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni R. B., Otth C., Concha II. & Munoz J. P. The protein kinase Cdk5. Structural aspects, roles in neurogenesis and involvement in Alzheimer's pathology. Eur J Biochem 268, 1518–27. (2001). [DOI] [PubMed] [Google Scholar]
- Whittington R. A., Papon M.-A., Chouinard F. & Planel E. Hypothermia and Alzheimer's disease neuropathogenic pathways. Curr Alzheimer Res 7, 717–25 (2010). [DOI] [PubMed] [Google Scholar]
- Davies S. P., Reddy H., Caivano M. & Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351, 95–105 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdane M. et al. Mitotic-like tau phosphorylation by p25-Cdk5 kinase complex. J Biol Chem 278, 34026–34 (2003). [DOI] [PubMed] [Google Scholar]
- Han P. et al. Suppression of cyclin-dependent kinase 5 activation by amyloid precursor protein: a novel excitoprotective mechanism involving modulation of tau phosphorylation. J Neurosci 25, 11542–52 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt R., Hundelt M. & Shahani N. Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochim Biophys Acta 1739, 331–354 (2005). [DOI] [PubMed] [Google Scholar]
- Arendt T., Holzer M., Brückner M. K., Janke C. & Gärtner U. The use of okadaic acid in vivo and the induction of molecular changes typical for Alzheimer's disease. Neuroscience 85, 1337–40 (1998). [DOI] [PubMed] [Google Scholar]
- Mailliot C., Bussière T., Caillet-Boudin M. L., Delacourte A. & Buée L. Alzheimer-specific epitope of AT100 in transfected cell lines with tau: toward an efficient cell model of tau abnormal phosphorylation. Neurosci Lett 255, 13–6 (1998). [DOI] [PubMed] [Google Scholar]
- Nuydens R. et al. Okadaic acid-induced apoptosis in neuronal cells: evidence for an abortive mitotic attempt. J Neurochem 70, 1124–33 (1998). [DOI] [PubMed] [Google Scholar]
- Bretteville A. & Planel E. Tau aggregates: toxic, inert, or protective species? J Alzheimers Dis 14, 431–436 (2008). [DOI] [PubMed] [Google Scholar]
- Spires-Jones T. L., Kopeikina K. J., Koffie R. M., de Calignon A. & Hyman B. T. Are tangles as toxic as they look? J Mol Neurosci 45, 438–44 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planel E., Yasutake K., Fujita S. C. & Ishiguro K. Inhibition of protein phosphatase 2A overrides tau protein kinase I/glycogen synthase kinase 3 beta and cyclin-dependent kinase 5 inhibition and results in tau hyperphosphorylation in the hippocampus of starved mouse. J Biol Chem 276, 34298–306 (2001). [DOI] [PubMed] [Google Scholar]
- Henry M., Grob M. & Mouginot D. Endogenous angiotensin II facilitates GABAergic neurotransmission afferent to the Na+-responsive neurons of the rat median preoptic nucleus. Am J Physiol Regul Integr Comp Physiol 297, R783–92 (2009). [DOI] [PubMed] [Google Scholar]
- Hamdane M. et al. p25/Cdk5-mediated retinoblastoma phosphorylation is an early event in neuronal cell death. 118, 1291Journal of cell science–8 (2005). [DOI] [PubMed] [Google Scholar]
- Jämsä A., Hasslund K., Cowburn R. F., Bäckström A. & Vasänge M. The retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cell line as a model for Alzheimer's disease-like tau phosphorylation. Biochem Biophys Res Commun 319, 993–1000 (2004). [DOI] [PubMed] [Google Scholar]
- Weaver C. L., Espinoza M., Kress Y. & Davies P. Conformational change as one of the earliest alterations of tau in Alzheimer's disease. Neurobiol Aging 21, 719–27 (2000). [DOI] [PubMed] [Google Scholar]
- Jicha G. A. et al. A conformation- and phosphorylation-dependent antibody recognizing the paired helical filaments of Alzheimer's disease. J Neurochem 69, 2087–95 (1997). [DOI] [PubMed] [Google Scholar]
- Otvos L. et al. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res 39, 669–73 (1994). [DOI] [PubMed] [Google Scholar]
- Whittington R. A. et al. Propofol directly increases tau phosphorylation. PLoS One 6, e16648 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A. & Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol 101, 1371–1378 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: LiCl treatment dose establishment in SH-SY5Y 3R tau cells. SH-SY5Y 3R tau cells were subjected to indicated LiCl dose in hypothermic conditions for 2 hours