Abstract
Lead toxicity is a significant problem in the U.S. with elevated blood lead levels being highest among very young children and older adults > 50 years old. Bone is the major reservoir of body lead, accounting for 75% in children and 90% in adults. Very little is known about the effect of lead on bone mineral properties in adults. We investigated the effect of lead on the femora from adult, 6 month old female C57/BL6 mice who were administered lead in the drinking water (250 ppm, blood lead 33 μg/dl) for 4 months. Bone mineral properties were examined using Fourier Transform Infrared Microscopy (FTIRM), quantitative microcomputed tomography (microCT) and whole bone mechanical testing. Lead significantly decreased the bone mineral density in the cortical and proximal cancellous bone and increased the marrow area in the cortical bone with microCT. Whole bone three-point bending showed a trend of decreased maximum and failure moments in the lead treated bones compared to controls. Lead significantly decreased the mineral/matrix ratio, collagen maturity and crystallinity in the trabecular bone as measured by FTIRM. In the cortical bone lead significantly decreased collagen maturity and bone crystal size by FTIRM. In contrast to cell culture studies, lead significantly increased serum osteocalcin levels. Lead also significantly increased the bone formation and resorption markers suggesting increased bone turnover. These data show lead increases bone turnover resulting in weaker cortical bone in adult female mice and suggest that lead may exacerbate bone loss and osteoporosis in the elderly.
Keywords: lead, bone, mice, Fourier Transform Infrared Imaging, microcomputed tomography, biomarkers
INTRODUCTION
Lead toxicity remains a significant public health problem in the United States with elevated blood lead levels (> 0.5 μM, 10 μg/dL) being reported in very young children (aged 1–5 yrs) and adults > 50 years old [1]. In addition, negative effects of lead have recently been reported at levels lower than 10 μg/dL leading to a proposal to reduce the definition of elevated blood lead levels from 10 μg/dL to 5 μg/dL [2]. This criterion would significantly increase the number of people with reportable elevations in blood lead.
Bone is the major reservoir of body lead accounting for 75% of total body lead in affected children and over 90% in adults [3,4]. Elevated bone lead values have been found in lead-intoxicated children [5] and in adults who have either been occupationally exposed or have had past exposure to higher environmental levels [6,7]. Bone is an important endogenous source capable of releasing stored lead to other soft tissues and organs in normal and pathological states. Low blood lead levels can cause a significant accumulation of Pb2+ in bone over time. Bone lead is a marker of past exposure with a half-life of approximately 20 years [8] and increases with age [9]. This places the elderly, who have past exposure to higher environmental levels of lead, at risk since they have an increased amount of bone lead compared to other age groups [10].
Lead has detrimental effects on bone. In-vitro lead reduced the production of osteocalcin and inhibited alkaline phosphatase activity in osteoblasts [11,12]. Lead suppressed type II and type X collagen expression in chondrocytes and altered growth factors and second messenger signaling responses during chondrocyte maturation [13,14]. In osteoclasts lead stimulated bone resorption [15].
In-vivo, controlled studies specifically investigating the effect of lead alone have primarily focused on bone from younger animals. This focus was due to the reported associations of decreased stature and chest circumference in children less than 7 years of age with chronic low level lead exposure [16,17]. The detrimental effects of lead on young animal bones included inhibition of matrix production [18], increased bone resorption [18,19], disruption of mineralization and chondrocyte organization (20) and inhibition of axial bone development [19]. Also, lead exposure was found to delay fracture healing by inhibiting endochondral ossification in 12 week old mice [21].
Yet, a very recent epidemiological study showed significant associations between older women with higher blood lead levels and an increased risk of nonspine fractures and higher risk of falls [22]. A study providing strong evidence in support of the hypothesis that lead plays a contributing role in osteoporosis [22]. Previous studies on the effect of lead on bone in adult animals which have attained peak bone density have been limited. An early study showed lead exposure of adult male rats on a reduced calcium diet resulted in reduced bone density and impaired mineralization [23]. However, a subsequent study, by the same group, showed no detrimental effect of lead when adult rats were fed a calcium replete diet [24]. Likewise, a second study found no effect of lead on bone mineral density in adult mice [25]. However, neither of the studies in a calcium replete state, reported elevated blood lead levels (≥ 10 μg/dL) in the animals. Controlled animal studies investigating the effect of elevated lead alone on adult bone mineral properties are needed to provide mechanistic information for the reported associations between elevated blood lead and increased risk of fractures in older women [22].
The purpose of this study was to investigate the sole effect of elevated lead on bone (cortical and cancellous) mineral properties and bone strength in femora from female adult mice fed a normal diet as contrasted with age matched controls. Biomarkers for bone formation and resorption were measured in serum. Fourier Transform Infrared Imaging (FTIRI) was used to measure detailed mineral properties such as mineral/matrix ratio, collagen maturity and bone crystal size and perfection. Bone mineral density and geometry were investigated using quantitative microcomputed tomography (microCT) and whole bone strength was measured in three point bending.
MATERIALS AND METHODS
Experimental animals
Two month old female C57/BL6 mice were purchased (Jackson Laboratories, Bar Harbor, ME). Groups of 10 animals each were given 250 ppm lead acetate (treatment) or sodium acetate (control) in the drinking water and fed a normal diet for a period of 4 months. The mice were 6 months of age when sacrificed and mature as the C57/BL6 mouse has been shown to attain peak bone density at 4 months of age [26]. Blood lead was measured using atomic absorption spectroscopy of blood samples collected via periorbital blood collection with appropriate anesthetization. Serum was obtained from a portion of the blood samples for bone biomarker measurements. At euthanasia, the right and left femora from each animal were dissected and cleaned of soft tissue. One femur was used for FTIRM and the second femur was used for microCT, three point bending, bone lead and mineral bound osteocalcin measurements. Bone lead was measured in marrow depleted femora dried to constant weight at 110 °C and then ashed for 24 hours at 650 °C. Ash weights were recorded, and the ash was dissolved in concentrated HCl for lead and calcium measurements using atomic absorption spectroscopy.
Bone Biomarker Measurements
The bone formation markers osteocalcin and P1NP, and the bone resorption marker CTX were assayed in serum from control and lead exposed mice. Osteocalcin was measured by an in-house equilibrium radioimmunoassay that has been published previously [27]. Mineral bound osteocalcin was extracted from bone and then also measured with the in-house equilibrium radioimmunoassay. Serum P1NP [28] and CTX (RatLaps) [29] were assayed by rodent specific kits (Immunodiagnostic Systems Inc, Fountain Hills, AZ).
FTIRM
Femurs from each animal were briefly washed in ice-cold water, and the bone marrow was flushed with saline using a 25-gauge 5/8-inch needle (Becton-Dickinson, Franklin Lakes, NJ). Bones were split longitudinally to facilitate penetration of the embedding media, polymethylmethacrylate (PMMA), and longitudinal undecalcified sections of the femur were cut (HM360 microtome, Microm, Waldorf, Germany) at 2 μM thickness and mounted on infrared windows. Femur sections were examined by FTIRI to acquire spectral images using the Perkin Elmer Spotlight Imaging system (Perkin Elmer Instruments, Shelton, CT, USA). The Fourier-Transform infrared microscope is equipped with a mercury-cadmium-telluride detector operating under nitrogen purge, and automated x-y stage drive. Spectra were collected from areas of 400 × 400 μm2, at a resolution of 4 cm−1, and a spatial resolution of ~ 7 μm. Background spectra were collected under identical conditions from clear glass from the same BaF2 windows. IR data were collected for three areas per anatomical bone site for cortical and trabecular tissue. After acquisition, spectra were baseline corrected and the spectral contribution of PMMA embedding media was subtracted using ISYS Chemical Imaging Software (Malvern, Worcestershire, UK). Four spectroscopic parameters were calculated: mineral-to-matrix ratio, carbonate-to-phosphate ratio, crystallinity, and collagen cross-link ratio. The mineral to (collagen) matrix ratio is the integrated area ratio of the ν1 ν3 PO4 band (900–1200 cm−1)/amide I band (1590–1712 cm−1) and the carbonate to mineral ratio is the integrated area ratio of the carbonate band (840–890 cm−1)/ν1 ν3 PO 4 band (900–1200 cm−1). The collagen maturity parameter is the ratio of nonreducible (pyridinoline) to reducible (dihydroxylysinonorleucine – DHLNL) collagen cross-links which is expressed as the intensity ratio of 1660 cm−1/1690 cm−1 [30]. The mineral crystallinity parameter corresponds to the crystallite size and perfection as determined by x-ray diffraction and is calculated from the intensity ratios of subbands at 1030 cm−1 (stoichiometric apatite) and 1020 cm−1 (nonstoichiometric apatite) [31]. Areas in the image devoid of mineral were set equal to zero and masked to be excluded from the calculations. The results for each parameter were expressed as a histogram describing the pixel distribution, mean value and standard deviation of the distribution and associated color coded images were generated at the same time by ISYS. For cortical regional analysis, point spectra were extracted from the periosteal edge, the central cortex and the endosteal edge. The spectra were baseline corrected, PMMA subtracted and analyzed for the parameters previously described.
For the FTIRM measurements a power analysis indicated 6 samples would be sufficient to detect a difference at a significance level of at least p < 0.05. We collected 10 bones for each treatment to ensure there was a large enough sample size. The measured parameters in Figures 3 and 4 have at least 7 samples and for some parameters we added an extra sample or 2 where standard deviations with n=7 were large.
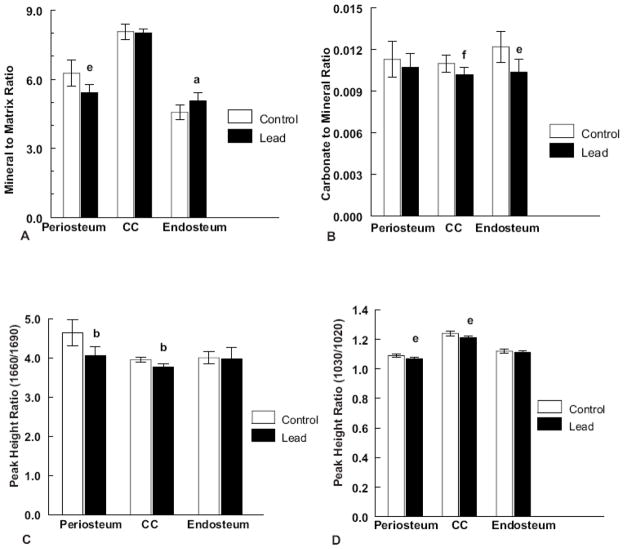
Figure 3.
Regional graphs of (A) Mineral to matrix ratio, (N = 8 control and 8 lead treated). (B) Carbonate to mineral ratio, (N = 8 control and 8 lead treated). (C) Collagen maturity (1660/1690 intensity ratio) (N = 9 control and 8 lead treated) and (D) Crystallinity (1030/1020 intensity ratio) for the periosteum, central cortex and endosteal regions of cortical bone (N = 8 control and 8 lead treated) in control and lead exposed cortical bone samples. Significance is indicated by letter ((a) p < 0.02, (b) p < 0.001, (e) p < 0.01, (f) p < 0.05) as compared to the respective controls. Values are reported as mean ± SD.
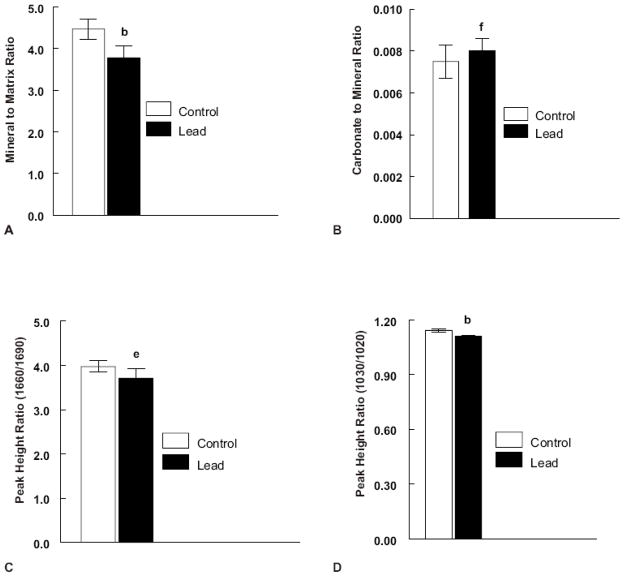
Figure 4.
Plots of (A) Mineral to matrix ratio (N = 8 control and 8 lead treated), (B) Carbonate to mineral ratio (N = 8 control and 7 lead treated), (C) Collagen maturity (1660/1690 intensity ratio) (N = 8 control and 7 lead treated) and (D) Crystallinity (1030/1020 intensity ratio) (N = 8 control and 7 lead treated) for control and lead exposed cancellous bone FTIRI images. Significance is indicated by letter ((b) p < 0.001, (e) p < 0.01, (f) p < 0.05) as compared to the respective controls. Values are reported as mean ± SD.
Microcomputed Tomography (MicroCT)
High-resolution microcomputed tomography (microCT) was used to provide 3-D information on bone geometry. Femoral 3-D morphology was determined from whole femurs by microCT (MS-8 In Vitro μCT scanner; GE Healthcare, London, Ontario, Canada). Femora, cleaned of soft tissue, were scanned in saline (0.9% sodium chloride; Baxter Healthcare, Deerfield, IL, USA). To minimize the noise, a large number of views (400), increased frame averaging (7 frames/view), and increased shutter exposure time (3000 msec) were used, resulting in 4-hr scans at 12 μm isotropic voxel resolution. A multi-level phantom was included to calibrate the attenuation levels (Hounsfield units) to mineral density (mg/cc). A modified Parker algorithm was used during reconstruction. Cortical and cancellous volumes of interest (VOI) were extracted and segmented using individual thresholds determined from the attenuation histogram of the VOI (Microview Software v. 2.2, GE Healthcare). The threshold value was also visually confirmed to correlate to the grey scale image at the cortical bone after binarization of the image and exclusion of lower values. Threshold analysis of the trabecular bone was accomplished by segmentation at different values and visual inspection of the masked bone volume. At the diaphysis, a 4.0 mm high segment was analyzed centered at the midsection. For the proximal metaphyses, 0.84 mm high proximal cancellous volumes were segmented and analyzed. Bone mineral density was calculated for cortical and proximal trabecular tissue and bone marrow area was calculated from the scans of the cortical diaphysis.
Whole Bone Mechanical Testing
The whole bone stiffness and strength were measured by loading the femora to failure in three-point bending. Femoral bending tests measure the load-bearing capacity of the diaphysis, a cortical site, by inducing compressive and tensile stresses in the cortex. In the mouse, the small volumes of cancellous bone present at the ends of long bones and in the vertebrae confounds measurement of cancellous properties. Each femur was positioned with the posterior surface on the supports with a span of 7.1 mm. The load was applied to the anterior surface at a constant rate of 0.1mm/sec using a servohydraulic testing system (MiniBionix 858, MTS Systems, Eden Prairie, MN and MLP 25 load cell, Transducer Techniques, Temecullah, CA). Load and displacement data were sampled at 20 Hz. Maximum and failure moments, bending stiffness and displacement at maximum and failure loads were determined.
Statistical Methods
Data are expressed as mean ± standard deviation for each treatment group. Statistical significance was determined using the Student-t-test where a value of p ≤ 0.05 was accepted as statistically significant. A trend is defined as a p value within the range 0.10>p>0.05.
RESULTS
Lead exposure and whole-blood and bone Pb2+ and Ca2+ measurements
We established a reproducible protocol to administer lead to mice in the drinking water at levels that increased lead values in blood that were environmentally relevant and were also detectable in the bone mineral after 4 months. At euthanasia the blood lead was 33 ± 8 μg/dL in lead exposed mice versus 0.15 ± 0.1 μg/dL for controls given an equivalent concentration of sodium acetate. These blood lead levels reflect environmentally relevant exposures (10 – 40 μg/dL) for lead toxicity. The concentration of lead in dry bone ash from treated animals was 164 ± 30 μg Pb/gm dry bone. The bone calcium levels for controls, 333.4 ± 25 mg Ca/g bone ash, were not significantly different from lead treated femora, 328.2 ± 20 mg Ca/g bone ash (no significant difference was found in percent ash for control (63.9 ± 3.0 %) and lead treated bone (65.0 ± 1.7 %)). In addition, the mice exhibited no physical or behavioral changes with lead exposure and the body weights of the two groups were equivalent at necropsy.
MicroCT and Biomechanical Measurements
Micro-computed tomography scans were collected on control and lead treated mice femora. Lead treatment significantly decreased the bone mineral density in cortical (p < 0.02) and proximal trabecular (p < 0.001) bone tissue (Figure 1). In the cortex, lead also significantly increased the medullary area (p < 0.001, Figure 1A). The decreased bone mineral density and increased marrow area with lead exposure is shown in a selected image in Figure 1 with numerical values showing significant differences given in Table I.
Figure 1.
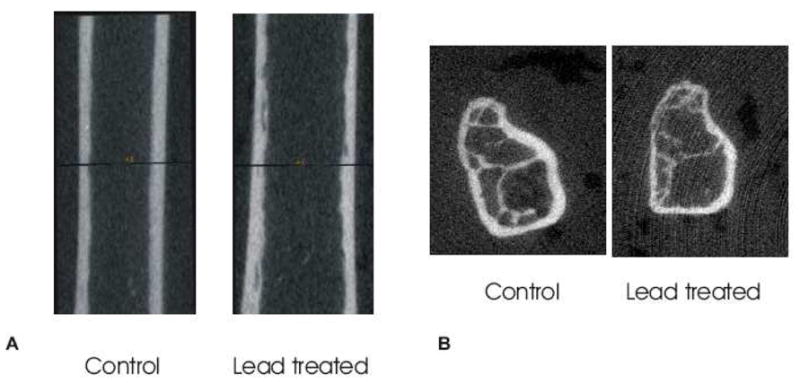
Representative three dimensional μCT images of control and lead treated cortical diaphysis (A) and control and lead treated proximal trabecular bone (B). Lead exposed cortical and trabecular bone showed a reduced bone mineral density (A,B). Lead was also shown to increase the bone marrow area in cortical bone (A).
Table I.
Bone Mineral Properties for Control and Lead Treated Mice
| Sample | Control | Lead |
|---|---|---|
| Cortical Bone | ||
| Bone Mineral Density (mg/cc) | 1023.2 ± 13 | 1000.9 ± 21a |
| Bone Marrow Area (mm2) | 0.903 ± 0.05 | 0.975 ± 0.05b |
| N | 9 | 10 |
| Biomechanical Properties | ||
| Maximum Moment | 73.8 ± 5.4 | 64.7 ± 13.5c |
| Failure Moment | 58.0 ± 17.7 | 44.4 ± 14.1d |
| N | 10 | 10 |
| Cancellous Bone | ||
| Bone Mineral Density (mg/cc) | 590 ± 44 | 517 ± 42b |
| N | 9 | 8 |
-Significance is indicated by letter
p < 0.02,
p < 0.001,
p = 0.066,
p = 0.08 as compared to respective controls.
Values are reported as mean ± SD.
Lead treatment produced a trend toward reduced bone strength (Table 1). The lead treated cortices were not significantly different from the controls but showed a trend toward a decreased Maximum moment (p=0.066) and a decreased Failure moment (p=0.08). To determine whether this difference in strength was due to a difference in size or in material properties, the maximum moment (Mmax) and bending stiffness (EI) were normalized by the moment of inertia corresponding to plane of bending testing (Imin). Lead significantly reduced (p = 0.02) the Mmax/Imin ratio (710.06 ± 83.94 vs. 595.19 ± 105.7 for control and lead treated respectively) and produced a trend toward a decreased EI/Imin ratio (8247.46 ± 1599.9 vs 7188.67 ± 817.1 for control and lead treated respectively), (p = 0.08) suggesting that shape changes were insufficient to explain treatment differences.
Fourier Transform Infrared Imaging
FTIRI images were collected on cortical bone sections to determine whether lead induced differences in detailed bone mineral properties. A representative color-coded image of control and lead treated cortical mineral-to-matrix ratios is shown in Figure 2A. Figure 3A shows regional differences in the mineral to matrix ratios between the control and lead treated cortex in the periosteum, central cortex and endosteum. Lead significantly (p < 0.01) decreased the mineral to matrix ratio in the periosteum, and significantly increased (p < 0.02) the mineral to matrix ratio in the endosteum. Lead treatment significantly decreased (p < 0.05) the carbonate to mineral ratio in the central cortex and endosteum (p < 0.01) (Figure 3B). Carbonate to phosphate in the periosteum was not affected by lead treatment.
Figure 2.
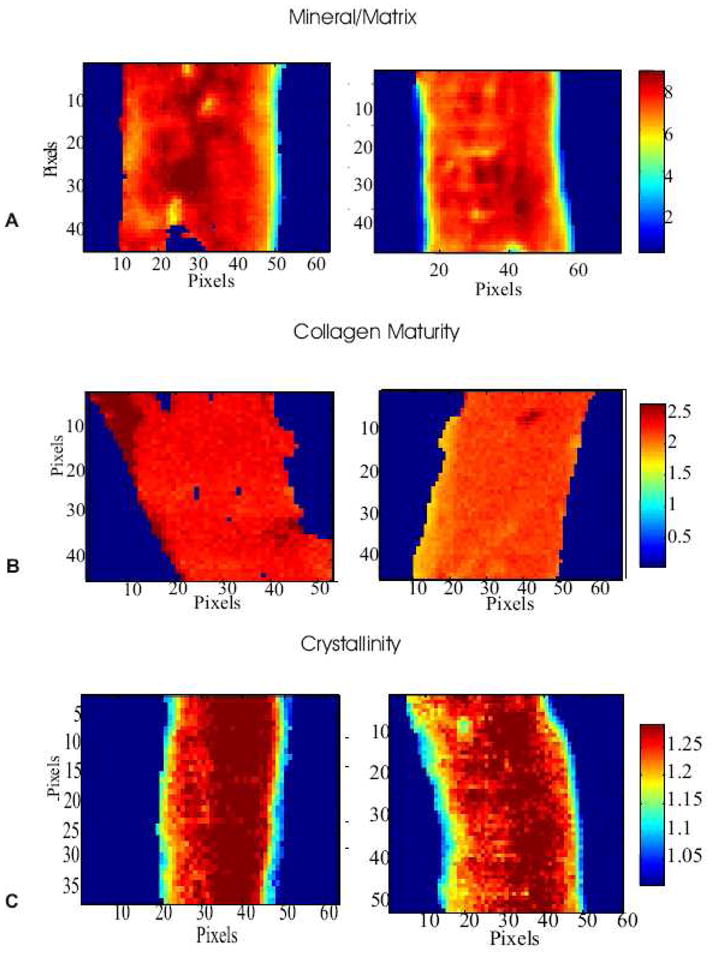
Typical FTIRIs of various spectral parameters in control and leaded treated cortices. Representative images of (A) mineral to matrix ratio, (B) collagen maturity (peak height ratio 1660/1690) and (C) crystallinity (peak height ratio 1030/1020).
Collagen maturity was assessed by the peak height ratio of the mature nonreducible crosslink, pyridinoline, to that of the immature reducible crosslink DHLNL (1660/1690). Lead significantly decreased the collagen maturity in the periosteum (p < 0.001) and in the central cortex (p < 0.001) (Figure 2B and Figure 3C). No significant difference was observed in the endosteum.
Crystallinity is a parameter, which is correlated to the size and perfection of the bone crystals. Representative color-coded images of control and lead treated crystallinity images are shown in Figure 2C. Figure 3D shows the regional differences in crystallinity between control and lead treated cortices in the periosteum, central cortex and endosteum. Lead significantly decreased the crystallinity in the periosteum (p < 0.01) and central cortex (p < 0.01). No significant difference was found in the endosteum.
Mineral properties were also assessed for cancellous bone. Since the periosteal and endosteal edges of the trabecular bone were not clearly distinguishable the reported values reflect those of the entire trabecular bone image area. Figure 4A shows differences in the mineral and matrix properties of the cancellous bone in control and lead treated samples. Lead was found to significantly reduce (p < 0.001) the mineral to matrix ratio in the trabecular bone. Lead was found to significantly increase (p < 0.05) the carbonate to mineral ratio in trabecular bone. Lead also significantly decreased (p < 0.01) the collagen crosslink parameter (Figure 4C) and significantly decreased (p < 0.001) the crystallinity in the trabecular bone (Figure 4D).
Serum Biomarkers and Matrix Osteocalcin
Lead significantly (p < 0.001) increased the serum concentrations of both osteocalcin and P1NP indicating an increase in matrix production and new bone formation (Figure 5). Lead significantly (p < 0.001) increased the concentration of CTX in the mice serum compared to untreated controls (Figure 5) indicating lead increased bone resorption as well. An increase in bone formation and resorption markers indicates that lead increases bone turnover. Lead exposure did not alter the bone content of osteocalcin (1.37 ± 0.097 and 1.34 ± 0.067 μg osteocalcin/mg bone powder for lead exposed and control bones, respectively).
Figure 5.
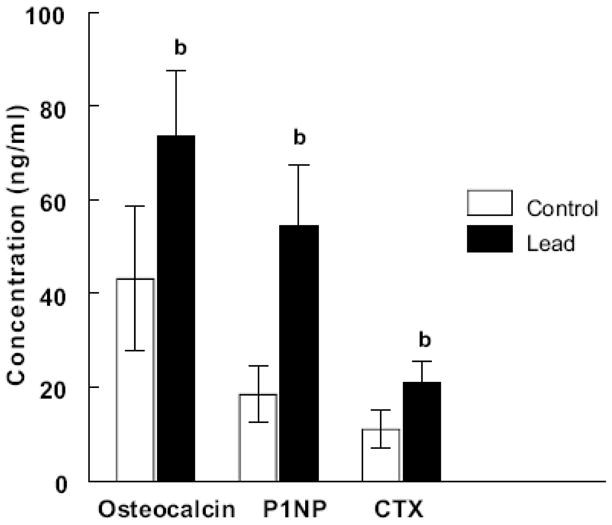
Plots of osteocalcin (N = 10 control and 10 lead exposed), P1NP (N = 10 control and 10 lead exposed) and CTX (N = 9 control and 7 lead exposed) concentrations in serum from control and lead exposed samples.
DISCUSSION
The aim of this study was to provide mechanistic data to explain an epidemiological study [22] showing an association between elevated blood lead levels and increased risk of fractures in elderly women. This study is the first to report the effect of lead exposure on detailed bone mineral properties and whole bone mechanical behavior in adult bone of female mice. Lead decreased bone mineral density, collagen maturity and mineral crystallinity, altered mineral/matrix ratios and increased bone marrow area and bone turnover, resulting in a trend toward weaker bones
Controlled animal studies investigating the effect of lead alone on bone mineral in older animals are few in number and the findings are limited. A previous study found no effect of lead exposure on bone mineral density in older male mice [25], which was probably due to the very low levels of lead given to the mice (less than 1 ppm) as compared to the lead levels given in our study and those previously reported [21,23] (100 – 580 ppm). The blood lead was not measured in that study so assessing whether the mice had even attained elevated blood lead levels seen in the population is difficult. A study by Gruber et al demonstrated osteopenia in lead exposed rats on a reduced calcium diet [23]. However, these results may not be due solely to lead since a reduced calcium diet can also independently reduce bone mineral density. A following study by Gruber [24] reported no detrimental effect of lead on bone histomorphometric properties in rats suggesting that an adequate calcium diet alleviates the detrimental effect of lead on bone. However, the blood lead in that study (1.7 μg/dL) was significantly below the toxic limit (10 μg/dL). Indeed, correlations between increased blood lead levels and increased risk of fractures were observed with older women with blood lead levels (> 8μg/dL) [22]. The results in our paper demonstrate that toxic levels of lead have detrimental effects on bone in older female mice in the presence of an adequate calcium diet.
In our study, lead significantly reduced the cortical and proximal bone mineral density in female adult femora using micro-computed tomography. Lead is known to displace calcium in hydroxyapatite mineral [32] and since lead has a higher atomic number and higher attenuation coefficient than calcium, higher bone mineral densities might be expected with lead exposed bone. In contrast, we observed a significant decrease in bone mineral density with lead, suggesting that the observed decreased mineral density measured by micro-CT may be underestimated.
The increase in bone remodeling with lead, as shown by the increase in the biomarkers for bone formation (P1NP) and bone resorption (CTX), provides an explanation for the observations presented here. The increase in P1NP biomarker with lead indicates increased collagen matrix production, explaining the decreased mineral/matrix ratio observed at the periosteum with lead exposure, the site of active bone formation. The decreased peak height ratio (1660/1690) indicates a decrease in the ratio of mature to immature collagen crosslinks with lead. The immature divalent crosslink DHLNL is converted into the mature crosslink pyridinoline over time. Therefore, the decreased collagen maturity can be attributed to increased bone turnover whereby the collagen crosslinks do not mature before they are resorbed. The decreased carbonate to phosphate ratio is also related to the increased bone turnover since carbonate impurities in the mineral would be resorbed as well. The crystallinity parameter, related to crystal size and perfection, was significantly decreased with lead exposure in the periosteum and central cortex, which is also consistent with an increase in bone formation in this region with lead. Increased bone formation is associated with the production of smaller bone crystals. In the endosteum, however, the crystallinity was not different with lead treatment. This finding is also consistent with increased bone resorption as smaller crystals are resorbed first, resulting in a population containing an increased proportion of larger crystals on the endosteal edge. Increased resorption may also explain the increased marrow area observed in lead exposed bone.
Similar patterns and slightly larger effects were seen in the cancellous bone. A possible explanation for this differential effect is the higher turnover of cancellous bone than cortical bone. Also, lead has been shown to concentrate more in the cancellous bone in the beginning of the exposure and after a period of time moves to the cortical bone for long term storage [33].
Lead exposure produced a trend toward a decreased maximum moment and a decreased failure moment. In the postyield region, permanent damage may begin to accumulate within the material through microcracks within the matrix and disruption of the collagen fibrils [34]. The failure moment is the point at which the bone breaks. The reduced moments demonstrate lead exposure diminishes the ability of the matrix to withstand applied forces and weakens the bone such that fractures would occur more readily in lead exposed subjects. Bone strength is determined by cortical geometry and material properties. Lead exposure significantly reduced the Mmax/Imin ratio and produced a trend toward decreased EI/Imin ratio, indicating that material differences were present as the reduced strength of bone following lead exposure was not explained by differences in the size of the lead treated verses control bones.
It is known that osteoporosis in the elderly results in compression fractures of the spine. The whole bone test of mouse long bones test cortical bone and the small volumes of tissue at cancellous sites limit equivalent tests of cancellous bone. Bending creates both compressive and tensile stresses in the sample, so bending failure reflects both types of loading. Our compositional results showed similar effects at cortical and cancellous sites, suggesting that load-bearing would be altered in both tissue types.
The lead induced alterations in mineral, collagen and bone turnover parameters reported in this study explain the observed trend toward a reduction in bone strength. Bone strength and stiffness have been shown to increase with increasing mineral density and crystallinity (stoichiometric perfection and size of bone crystals) [35,36]. Both parameters correlate with elastic and post-yield mechanical properties of bone tissue [35,36]. Larger crystals may provide additional tensile strength and stability to the collagen matrix [36]. Thus, smaller bone crystals produced during lead exposure also play a role in the reduction in bone strength. Collagen contributes to the toughness of bone. The mature collagen crosslink, pyridinoline, was shown to be involved in maintaining the mechanical integrity of the collagen network after denaturation [37]. Both the mature (pyridinoline) [38] and immature (DHLNL) [39] collagen crosslinks have been reported to correlate with bone strength. Bone turnover rate can also influence bone strength [40]. An increased bone formation produces smaller bone crystals and increased bone resorption prevents the maturation of mineral and collagen crosslinks, Also, accelerated bone turnover has been shown to increase fragility due to osteoid accumulation and decreased secondary mineralization [40] resulting in reduced bone strength [41].
The mechanism of lead’s detrimental effect on bone most likely involves multiple processes. Lead may affect both the osteoblast and the osteoclast. In organ culture lead stimulated radiolabeled calcium release [15] and enhanced the production of osteoclast-like multinucleated cells in bone marrow culture by a mechanism involving increases in PGE2, cAMP and calcium ions [42]. In addition, we have previously shown that lead increased intracellular calcium in osteoblasts [43]. Hormones such as vitamin D3, IGF and PTH play a major role in bone remodeling and all three are capable of increasing intracellular calcium by different mechanisms. RANKL activation of RANK also induces a rise in intracellular calcium in osteoclastogenesis. Increases in intracellular calcium, via activation of calmodulin, can play a role in regulating the differentiation and proliferation of osteoblasts [44] and the differentiation and function of osteoclasts [45]. During bone resorption lead released from bone mineral, due to current or past exposure, may interfere with intracellular calcium signaling and play a role in the detrimental effects reported here.
Increased blood lead levels were recently associated with an increased risk of fractures in older women [22]. Furthermore, it has been suggested that significantly high blood lead levels in postmenopausal women may be involved in osteoporosis [46]. The results presented in this paper provide a mechanism to explain these associations. We show detrimental effects due solely to lead on bone mineral density, bone mineral and matrix composition, and bone turnover markers, that result in a trend toward a reduced whole bone strength in femurs from adult female mice. The data in this paper demonstrate why lead exposure, if present in the elderly could result in a higher risk of fractures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pirkle JL, Kaufmann RB, Brody DJ, Hickman T, Gunter EW, Paschal DC. Exposure of the U.S. population to lead, 1991–1994. Env Health Perspect. 1998;106:745–750. doi: 10.1289/ehp.98106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canfield RL, Henderson CR, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microgram per deciliter. N Engl J Med. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry PSI. Concentrations of lead in the tissues of children. Br J Ind Med. 1981;38:61–71. doi: 10.1136/oem.38.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry PSI. A comparison of concentrations of lead in human tissues. Br J Ind Med. 1975;32:119–139. doi: 10.1136/oem.32.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen JF, Markowitz ME, Bijur PE, Jenks ST, Wielopolski L, Kalef-Ezra JA, Slatkin DN. L-line x-ray fluorescence of cortical bone lead compared with the CaNa2EDTA test in lead-toxic children: Public health implications. Proc Natl Acad Sci. 1989;86:685–689. doi: 10.1073/pnas.86.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleeker ML, McNeil FE, Lindgren KN, Masten VL, Ford DP. Relationship between bone lead and other indices of lead exposure in smelter workers. Toxicology Letters. 1995;77:241–248. doi: 10.1016/0378-4274(95)03303-3. [DOI] [PubMed] [Google Scholar]
- 7.Kim R, Landrigan C, Mossmann P, Sparrow D, Hu H. Age and secular trends in bone lead levels in middle-aged and elderly men: three year longitudinal follow-up in the normative aging study. Am J Epid. 1997;146:586–591. doi: 10.1093/oxfordjournals.aje.a009318. [DOI] [PubMed] [Google Scholar]
- 8.Skerfving S, Nilsson U. Assessment of accumulated body burden of metals. Toxicol Lett. 1992;64/65:17–24. doi: 10.1016/0378-4274(92)90168-j. [DOI] [PubMed] [Google Scholar]
- 9.Gamblin C, Gordon CL, Meir DCF, Chettle DR, Webber CE. In-vivo measurements of bone lead content in residents of Southern Ontario. Appl Radiat Isot. 1994;45:1035–1038. doi: 10.1016/0969-8043(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 10.Vig EK, Hu H. Lead toxicity in older adults. J Am Geriatr Soc. 2000;48:1501–1506. [PubMed] [Google Scholar]
- 11.Long GJ, Rosen JF, Pounds JG. Lead impairs the production of osteocalcin by rat osteosarcoma (ROS 17/2.8) cells. Toxicology and Applied Pharmacology. 1990;106:270–277. doi: 10.1016/0041-008x(90)90246-q. [DOI] [PubMed] [Google Scholar]
- 12.Angle CR, Thomas DJ, Swanson SA. Lead inhibits the basal and stimulated responses of rat osteoblast-like cell line ROS 17/2.8 to 1α,25-Dihydroxyvitamin D3 and IGF-I. Toxicology and Applied Pharmacology. 1990;103:281–287. doi: 10.1016/0041-008x(90)90230-r. [DOI] [PubMed] [Google Scholar]
- 13.Hicks DG, O’Keefe RJ, Reynolds KJ, Cory-Schlecta DA, Puzas JE, Judkins A, Rosier RN. Effect of Lead on Growth Plate Chondrocyte Phenotype. Toxicology and Applied Pharmocology. 1996;140:164–172. doi: 10.1006/taap.1996.0209. [DOI] [PubMed] [Google Scholar]
- 14.Zuscik MJ, Pateder DB, Puzas JE, Schwarz EM, Rosier RN, O’Keefe RJ. Lead alters parathyroid hormone-related peptide and transforming growth factor-β1 effects and AP-1 and NF-κB signaling in chondrocytes. Journal of Orthopaedic Research. 2002;20:811–818. doi: 10.1016/S0736-0266(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 15.Miyahara T, Koniyama H, Miyanishi A, Takata M, Nagai M, Kozuka H, Hayashi T, Yamamoto M, Ito Y, Odake H, Koizumi F. Stimulative effects of lead on bone resorption in organ culture. Toxicology. 1995;97:191–197. doi: 10.1016/0300-483x(94)02948-t. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz J, Angle C, Pitcher H. Relationship between childhood blood lead levels and stature. Pediatrics. 1986;77:281–288. [PubMed] [Google Scholar]
- 17.Shukla R, Bornschein RL, Dietrich KN, Buncher CR, Berger O, Hammond PB, Succop PA. Effects of fetal and infant lead exposure on growth in stature. Pediatrics. 1989;84:604–612. [PubMed] [Google Scholar]
- 18.Hass GM, Brown DVL, Eisenstein R, Hemmens A. Relations between lead poisoning in rabbit and man. The American Journal of Pathology XLV. 1964;N.5:691–727. [PMC free article] [PubMed] [Google Scholar]
- 19.Escribano A, Revilla M, Hernández ER, Seco C, González-Riola J, Villa LF, Rico H. Effect of lead on bone development and bone mass: A morphometric, densitometric, and histomorphometric study in growing rats. Calcif Tissue Int. 1997;60:202–203. doi: 10.1007/s002239900214. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton JD, O’Flaherty EJ. Effects of lead exposure on skeletal development in rats. Fundamental and Applied Toxicology. 1994;22:594–604. doi: 10.1006/faat.1994.1066. [DOI] [PubMed] [Google Scholar]
- 21.Carmouche JJ, Puzas JE, Zhang X, Tiyapatanaputi P, Cory-Slechta DA, Gelein R, Zuscik M, Rosier RN, Boyce BF, O’Keefe RJ, Schwarz EM. Lead exposure inhibits fracture healing and is associated with increased chondrogenesis, delay in cartilage mineralization, and a decrease in osteoprogenitor frequency. Environ Health Perspectives. 2005;113:749–755. doi: 10.1289/ehp.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalil N, Cauley JA, Wilson JW, Talbott EO, Morrow L, Hochberg MC, Hillier TA, Muldoon SB, Cummings SR. Relationship of blood lead levels to incident nonspine fractures and falls in older women: the study of osteoporotic fractures. J Bone Miner Res. 2008;23:1417–1425. doi: 10.1359/JBMR.080404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruber HE, Gonick HC, Khalil-Manesh F, Sanchez TV, Motsinger S, Meyer M, Sharp CF. Osteopenia induced by long-term, low- and high-level exposure of the adult rat to lead. Miner Electrolyte Metab. 1997;23:65–73. [PubMed] [Google Scholar]
- 24.Gruber HE, Ding Y, Stasky AA, Meyer M, Pandian MR, Pandian D, Vaziri ND, Grigsby J, Gonick HC. Adequate dietary calcium mitigates osteopenia induced by chronic lead exposure in adult rats. Miner Electrolyte Metab. 1999;25:143–146. doi: 10.1159/000057438. [DOI] [PubMed] [Google Scholar]
- 25.Massie HR, Aiello VR. Lead accumulation in the bones of aging male mice. Gerontology. 1992;38:13–17. doi: 10.1159/000213302. [DOI] [PubMed] [Google Scholar]
- 26.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 27.Gundberg CM, Hauschka PV, Lian JB, Gallop PM. Osteocalcin: Isolation, Characterization, and Detection. Methods in Enzymology. 1984;107:516–544. doi: 10.1016/0076-6879(84)07036-1. [DOI] [PubMed] [Google Scholar]
- 28.Hale LV, Sells Galvin RJ, Risteli J, Ma YL, Harvey AK, Yang X, Cain RL, Zeng Q, Frolik CA, Sato M, Schmid AL, Geiser AG. PINP: A serum biomarker of bone formation in the rat. Bone. 2007;40:1103–1109. doi: 10.1016/j.bone.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 29.Garnero P, Ferreras M, Karsdal MA, Nicamhlaoibh R, Risteli J, Borel O, Qvist P, Delmas PD, Foged NT, Delaisse JM. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J Bone Miner Res. 2003;18:859–867. doi: 10.1359/jbmr.2003.18.5.859. [DOI] [PubMed] [Google Scholar]
- 30.Paschalis EP, Verdelis K, Mendelsohn R, Boskey A, Yamauchi M. Spectroscopic determination of collagen cross-links in bone. J Bone Miner Res. 2001;16:1821–1828. doi: 10.1359/jbmr.2001.16.10.1821. [DOI] [PubMed] [Google Scholar]
- 31.Mendelsohn R, Paschalis EP, Boskey AL. Infrared spectroscopy, microscopy, and microscopic imaging of mineralizing tissues. Spectra-structure correlations from human iliac crest biopsies. J Biomed Opt. 1999;4:14–21. doi: 10.1117/1.429916. [DOI] [PubMed] [Google Scholar]
- 32.Miyake M, Ishigaki K, Suzuki T. Structure refinements of Pb2+ ion-exchanged apatites by x-ray powder pattern-fitting. J Sol State Chem. 1986;61:230–235. [Google Scholar]
- 33.Christoffersson JO, Schutz A, Skerfving S, Ahlgren L, Mattsson S. A model describing the kinetics of lead in occupationally exposed workers. In: Ellis KT, Yasumura S, Morgan WD, editors. In Vivo Body Composition Studies. Institute of Physical Sciences in Medicine; London, England: 1987. pp. 334–347. [Google Scholar]
- 34.Rubin C, Rubin J. Biomechanics and Mechanobiology of Bone. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 6. American Society for Bone and Mineral Research; Washington DC, USA: pp. 36–41. [Google Scholar]
- 35.Currey JD. The effect of porosity and mineral content on the Young’s modulus of elasticity of compact bone. J Biomech. 1988;21(2):131–139. doi: 10.1016/0021-9290(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 36.Yerramshetty JS, Akkus O. The associations between mineral crystallinity and the mechanical properties of human cortical bone. Bone. 2008;42:476–482. doi: 10.1016/j.bone.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Li X, Bank RA, Agrawal CM. Effects of collagen unwinding and cleavage on the mechanical integrity of the collagen network in bone. Calif Tissue Int. 2002;71:186–192. doi: 10.1007/s00223-001-1082-2. [DOI] [PubMed] [Google Scholar]
- 38.Oxlund H, Berckman M, Ortoft G, Anderson TT. Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone. 1995;17:365S–371S. doi: 10.1016/8756-3282(95)00328-b. [DOI] [PubMed] [Google Scholar]
- 39.Knott L, Whitehead CC, Fleming RH, Bailey AJ. Biochemical changes in the collagenous matrix of osteoporotic avian bone. Biochem. 1995;310:1045–1051. doi: 10.1042/bj3101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinstein RS. Perspective: True strength. J Bone Miner Res. 2000;15:621. doi: 10.1359/jbmr.2000.15.4.621. [DOI] [PubMed] [Google Scholar]
- 41.Lloyd SA, Yuan YY, Kostenuik PJ, Ominsky MS, Lau AG, Morony S, Stolina M, Asuncion FJ, Bateman TA. Soluble RANKL induces high bone turnover and decreases bone volume, density, and strength in mice. Calcif Tissue Int. 2008;82:361–372. doi: 10.1007/s00223-008-9133-6. [DOI] [PubMed] [Google Scholar]
- 42.Miyahara T, Komiyama H, Miyanishi A, Matsumoto M, Xue-Ya W, Takata M, Takata S, Nagai M, Kozuka H, Yokoyama K, Kanamoto Y. Effects of lead on osteoclast-like cell formation in mouse bone marrow cell cultures. Calcif Tissue Int. 1994;54:165–169. doi: 10.1007/BF00296069. [DOI] [PubMed] [Google Scholar]
- 43.Schanne FAX, Dowd TL, Gupta RK, Rosen JF. Lead increases free Ca2+ concentration in cultured osteoblastic bone cells: simultaneous detection of intracellular free Pb2+ by 19F NMR. Proc Natl Acad Sci USA. 1989;86:5133–5135. doi: 10.1073/pnas.86.13.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zayzafoon M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J Cell Biochem. 2006;97:56–70. doi: 10.1002/jcb.20675. [DOI] [PubMed] [Google Scholar]
- 45.Seales EC, Micoli KJ, McDonald JM. Calmodulin is a critical regulator of osteoclastic differentiation, function, and survival. J Cell Biochem. 2006;97:45–55. doi: 10.1002/jcb.20659. [DOI] [PubMed] [Google Scholar]
- 46.Silbergeld EK, Schwartz J, Mahaffey K. Lead and osteoporosis: mobilization of lead from bone in postmenopausal women. Environ Res. 1988;47:79–94. doi: 10.1016/s0013-9351(88)80023-9. [DOI] [PubMed] [Google Scholar]