Abstract
The marine copepod Tigriopus californicus lives in intertidal rock pools along the Pacific coast, where it exhibits strong, temporally stable population genetic structure. Previous allozyme surveys have found high frequency private alleles among neighboring subpopulations, indicating that there is limited genetic exchange between populations. Here we evaluate the factors responsible for the diversification and maintenance of alleles at the phosphoglucose isomerase (Pgi) locus by evaluating patterns of nucleotide variation underlying previously identified allozyme polymorphism. Copepods were sampled from eleven sites throughout California and Baja California, revealing deep genetic structure among populations as well as genetic variability within populations. Evidence of recombination is limited to the sample from Pescadero and there is no support for linkage disequilibrium across the Pgi locus. Neutrality tests and codon-based models of substitution suggest the action of natural selection due to elevated non-synonymous substitutions at a small number of sites in Pgi. Two sites are identified as the charge-changing residues underlying allozyme polymorphisms in T. californicus. A reanalysis of allozyme variation at several focal populations, spanning a period of 26 years and over 200 generations, shows that Pgi alleles are maintained without notable frequency changes. Our data suggest that diversifying selection accounted for the origin of Pgi allozymes, while McDonald-Kreitman tests and the temporal stability of private allozyme alleles suggests that balancing selection may be involved in the maintenance of amino acid polymorphisms within populations.
Introduction
Although there is considerable interest in quantifying adaptive variation among naturally occurring populations [1] and using this information in the management of populations [2], prospecting for adaptive variation in unknown genomes often requires considerable resources [3] or long-term experimental studies [4]. However, the advent of genomic resources and new integrative approaches has greatly expanded our ability to identify adaptive genetic variation in non-model organisms [5] and motivates a reexamination of previously identified polymorphism at functional allozyme loci. In particular, phosphoglucose isomerase (Pgi, E.C. 5.3.1.9, also known as glucose-6-phosphate isomerase) frequently exhibits polymorphism in natural populations and has been linked with natural selection in several taxa [6]–[10]. This has led Wheat [11] to argue that Pgi could be a useful candidate gene for studying adaptive genetic variation in a variety of arthropods.
The marine copepod Tigriopus californicus is known to have genetic polymorphism at the Pgi locus, with strong population divergence over a very small spatial scale [12]. For example, a single rocky outcrop at Pescadero, California, maintained a fast-migrating allele (Pgi F) at approximately 50% in samples taken between 1978 and 1996 [13]. This allele was either absent or found at extremely low frequency at the neighboring outcrop (designated Site 10) 500 m to the south [14]. Site 10 similarly maintained a slow-migrating allele (Pgi S) that has been recorded only intermittently at very low frequency at the Pescadero site [12]. While Pgi variability in each population is not exceptional, the fact that variation is maintained when local populations are subject to fluctuations in population density due to their ephemeral tidepool habitat [15] suggests that Pgi polymorphism might be maintained by selection.
T. californicus populations are among the most sharply structured of any marine organism [12], [16]. Phylogeographic studies indicate long-term divergence among populations at a small geographical scale [17], which is further emphasized by uncorrected mitochondrial DNA (mtDNA) interpopulation divergences frequently exceeding 15% [16]. Fine scale allozyme surveys have also suggested historical isolation of local subpopulations, with high frequency private alleles found in many populations [12]–[14], [18]. At migration-drift equilibrium, the average frequency of neutral private alleles is inversely related to the extent of gene flow among neighboring populations [19]. And in most cases, private alleles at several allozyme loci in T. californicus are restricted to narrow (i.e., <25 km) stretches of coastline and in exceptional cases are primarily restricted to individual rocky outcrops. The geographic distribution of alleles is also stable through time [13], suggesting that individual rocky outcrops throughout California are effectively closed to immigration and emigration over periods spanning hundreds of generations. While the deep genetic subdivision suggests populations of T. californicus are independent evolutionary lineages, laboratory tests have shown that they remain reproductively compatible [18], lacking either assortative mating or offspring inviability at the F1 stage in interpopulation crosses.
Here we characterize Pgi allozyme polymorphism in T. californicus at the DNA sequence level. We investigate whether (1) natural selection has contributed to the origin of genetic polymorphism among divergent lineages, and (2) what processes are maintaining genetic variation at the Pgi locus. We report DNA polymorphism data for 43 chromosomes sampled from populations throughout the southern range of the species and conduct additional allozyme surveys at populations that have been the subject of previous genetic studies. Using neutrality tests and a codon-based model of substitution, we find evidence of positive selection acting on multiple charge-changing amino acid sites. Our allozyme surveys demonstrate that allele frequencies of Pgi in T. californicus populations show stable demographic trends through a 26-year time span. In contrast to the expectation of alleles under positive selection, this temporal analysis of Pgi allele frequencies demonstrates stability of charge-changing polymorphisms over the course of approximately 200 generations of the Tigriopus lifecycle. Based on results from McDonald-Kreitman neutrality tests, we discuss how Pgi polymorphism is likely maintained by balancing selection in multiple independent populations of T. californicus.
Methods
Sample Collection
T. californicus inhabits tidepools along the supralittoral fringe of rocky intertidal habitats on the Pacific coast of North America. Animals were collected from locations throughout California and Baja California, Mexico between May, 2001 and December, 2004 and transported live to Scripps Institution of Oceanography, La Jolla, California. Copepods from Playa Altamira, Mexico, have been shown to be partially reproductively incompatible with T. californicus from California and we include them here as an out-group. With the exception of samples from two new sites, Mussel Rock (37° 20′ 55″ N/122° 24′ 08″W) and Pomponio Beach (37° 17′ 48″ N/122° 24′ 25″ W), collection sites are the same as those reported previously [12]–[14], [18], [20]. Although allozyme studies of Punta Morro and Playa Altamira, Mexico, were completed soon after collection, DNA analyses were based on animals maintained in the laboratory for a period of up to three years. All other samples were either prepared for molecular analysis immediately or maintained at 20° C with a 12∶12 light-dark cycle for a maximum of five days. The latter samples were carefully checked to ensure that no mortality had taken place prior to preparation for molecular analysis.
Cloning of Phosphoglucose-isomerase
Pgi was amplified from cDNA using degenerate primers designed from an alignment of sequences from Drosophila melanogaster (Accession number: NP_523663.1), Gryllus veletis (AAG15513.1), Danio rerio (AAH44450.1), and Homo sapiens (AAP72966.1). Primers were designed to match seven or more amino acids which consisted of low degeneracy sites that were completely conserved among the four species in the alignment, at six different regions of the Pgi alignment. Total RNA was extracted with TRI Reagent (Sigma) from copepods collected from the Ocean Beach Pier collecting site, San Diego, CA. First strand cDNA synthesis was primed with an oligo(dT) primer with an extension at the 5′ end to facilitate subsequent 3′RACE. Touchdown PCR was performed with cycling conditions consisting of a 30 s 95° denaturation step, an initial annealing step of 30 s at 56° C and an extension step of 2 m at 72° C. Every 2 cycles for the first 12 cycles, the annealing temperature was stepped down by 1°C. The final 23 cycles were conducted with an annealing temperature of 50°C. A second round of PCR utilizing the same PCR primers was performed with a 50°C annealing temperature for 35 cycles with 1.0 µl of the original unpurified PCR product as template. Following two rounds of PCR, two degenerate primer pairs (5′CCIYTNATGGTNACNGARGC3’/5′TCCATRTCNCCYTGYTGRAARTA3’ and 5′TAYTTYCARCARGGNGAYATGGA3’/5′ARYTCIACNCCCCAYTGRTC3’) each yielded a single PCR product of the size expected from the original Pgi alignment. These products were gel purified, cloned using the pCR-4-TOPO vector (TOPO TA cloning Kit, Invitrogen), and sequenced on a MegaBace sequencer (GE Health Sciences). The section of the gene between the two original products was subsequently amplified from cDNA with T. californicus-specific primers. The 5′ and 3′ ends of the gene were obtained by RLM-RACE (Generacer Kit, Invitrogen).
Allozyme Electrophoresis, PCR, and DNA Sequencing
A subset of nine samples from California and northern Baja California were examined for allozyme variation. Animals were homogenized in 10 µl of chilled buffer (0.1M Tris-Borate-EDTA, pH 8.9, with 0.12 g/ml sucrose, 10 µg/ml bromphenol blue added for loading and tracking). Following Burton [21], five µl of the homogenate was loaded directly on an acrylamide gel for allozyme electrophoresis and gels were stained for Pgi (NADP was replaced with NAD for use with a recombinant glucose-6-phosphate dehydrogenase coupling enzyme from Leuconostoc mesenteroides, E.C. 1.1.1.49, Sigma).
Some of the individuals used for allozyme electrophoresis were also included in the sequencing analysis. A 5 µl aliquot from the allozyme homogenate was prepared for PCR immediately following loading of allozyme gels. Samples were treated with five µl of Proteinase K (0.2 mg/ml) and incubated at 65°C for one hour and 85°C for 15 minutes. Samples from Pescadero (n = 13), San Diego (n = 14), and Santa Cruz (n = 5) were selected randomly (i.e., independent of allozyme genotype) for sequencing to allow for estimation of population genetic parameters. In contrast, of the five alleles sequenced from Site 10, two Pgi M/Pgi S heterozygotes were selected specifically to characterize the Pgi S/Pgi.89 allele [14]. However, we were unable to unambiguously characterize the amino acid replacement responsible for this allozyme allele. Single Pgi S/Pgi S and Pgi M/Pgi M homozygotes from Laguna Beach were selected for sequencing based upon allozyme genotype. Single sequences were obtained from additional populations from Carpinteria, Abalone Cove, Punta Morro, and Playa Altamira populations.
All Pgi sequences were obtained by amplifying the entire structural gene of approximately 2.5 kb from genomic DNA with primers located in the 5′ and 3′ untranslated regions (UTR). PCR products were then cloned to determine the gametic phase of polymorphisms. Full length Pgi was amplified from Carpinteria and Playa Altamira samples with primers TcPGI-5′-34F and TcPGI-STOP+21R. All other samples were amplified with primers TcPGI-5′UTR-F and TcPGI-3′UTR-R (Table S1). Initially, at least one homozygote for Pgi F and Pgi M (i.e., Pgi 1.05 and Pgi 1.00 in previous nomenclature) allozyme alleles from San Diego and Pescadero populations were amplified and sequenced directly for characterization of charge-changing residues [14]. Subsequent PCR products were gel extracted, concentrated by ethanol precipitation, and cloned using either the pCR-4-TOPO or TOPO XL vectors (TOPO TA cloning kit, Invitrogen). Inserts were amplified with a set of four partially overlapping pairs of primers (Table S1) and both strands of the PCR products were sequenced on a Megabace capillary sequencer. Sequences are deposited at NCBI’s GenBank with accession numbers: JX089404-JX089454.
Analysis of Population Structure
Sequences were edited with Sequencer version 4.5 (Genecodes, Ann Arbor, Mich.) and aligned by pairwise alignment, followed by minor adjustments to the alignment made by eye. MrBayes 3.1.2, an unrooted Bayesian method [22], was used to reconstruct the genealogical history of Pgi alleles. To determine which DNA substitution model would serve as an appropriate prior for gene-tree estimation, we used Akaike Information Criteria (AIC) in the program MrModeltest v.2 [23] and selected the general time reversible (GTR) model with gamma-distributed rate variation. In each of two independent MrBayes runs, four chains were sampled every 1000 steps over a total of 30 million steps. Runs were checked for convergence using Tracer [24], 10,000 samples were discarded as burnin, and a majority-rule consensus tree was estimated from the two runs.
We also estimated population structure (FST) and migration rates (Nm) between regional populations, and calculated interpopulation genetic distances based on the Jukes and Cantor substitution model [25] in MEGA version 2.1 [26]. Introns and a small fragment of the 3′UTR were excluded due to alignment ambiguities among some populations and distances were calculated as net between-population means (i.e., corrected for within population distances).
Site Frequency Tests of Recombination, Linkage Disequilibrium and Neutrality
We first tested for evidence of recombination and linkage disequilibrium using DnaSP v.5.0 [27]. The per gene recombination parameter R [28], equivalent to 4Nr, and the minimum number of recombination events Rm [29] were estimated for the entire dataset, as well as geographic subsets of the data. Linkage disequilibrium (LD) between pairs of polymorphic sites was estimated using the following summary statistics: average LD measured by ZnS, average LD between adjacent sites measured by Za, the difference between Za-ZnS measured by ZZ, LD among segregating sites measured by B, and LD among unique data partitions measured by Q [30]–[32]. Additionally, the Genetic Algorithm Recombination Detection (GARD) in Datamonkey [33] and the SiScan, MaxChi, Chimaera, and 3seq methods calculated in RDP2 [34] were used to identify recombinant alleles.
Estimation of population genetic parameters and tests of neutrality were conducted with DnaSP. We tested the assumption of neutrality at segregating sites in Pgi using several summary statistics, including Tajima’s D [35], Fu and Li’s DFL and F [36], the Hudson-Kreitman-Aguadé (HKA) statistic, and the McDonald-Kreitman (MK) statistic. A negative Tajima’s D test statistic signifies an excess of low frequency polymorphisms, as a result of positive selection or recent population expansion [35]. Under Fu and Li’s tests, negative values indicate an excess of mutation in external branches, which can arise when an advantageous allele becomes fixed or purifying selection removes deleterious alleles [36]. The HKA test examines polymorphism within species and divergence between species at two different genetic regions, testing the idea that divergence between species will be increased for a gene under positive selection. We assessed variation in Pgi in reference to nucleotide variation in the Rieske iron-sulfur protein, RISP [17]. The MK test examines the synonymous and non-synonymous variation within and between species, comparing the ratio of fixed substitutions to the ratio of polymorphisms. This test would support positive selection if there is an excess of non-synonymous fixed differences, and conversely supports balancing selection if there is an excess of non-synonymous polymorphism. The DFL, F, and MK statistics were run using the Playa Altamira sample as an out-group.
Test for Positive Selection Based on Codon Models
Most neutrality tests examine the ratio of non-synonymous to synonymous substitutions (ω) averaged over the entire coding region of a gene, requiring values of ω>1 for evidence of positive selection. Because selection is frequently directed at a few amino acid sites of a gene, standard neutrality tests are very stringent and often fail to detect positive selection when it occurs [37]. Yang and Nielsen [37] developed an alternative approach to detecting positive selection, using a maximum-likelihood framework to examine patterns of substitution at individual codon sites. The method is implemented in a series of nested models, where more parameter-rich models allow ω to vary across sites and include terms for positive selection. The likelihood of the complex models are evaluated against the simpler model using a likelihood ratio test and the chi-square distribution to assess statistical significance (with degrees of freedom equal to the number of free parameters). Because this method assumes a fixed phylogeny, it is sensitive to any recombinant alleles present in the dataset [38]. We removed recombinant alleles identified in the RDP2 analysis, re-estimated the Bayesian phylogeny with Playa Altamira as an out-group, and tested for evidence of positive selection at Pgi. Six substitution models were evaluated, including model M0 with ω fixed at all sites, model M1a where sites are nearly-neutral in two site classes (ω = 1 or ω<1), the positive selection model M2a adding a third class (ω>1, ω = 1 or ω<1), the discrete distribution model M3 allowing ω to vary unconstrained among three discrete classes, the model M7 where ω is drawn from a beta distribution (for 0<ω<1), and the M8 model adding a second site class to the beta distribution model (for 0<ω<1, or ω>1) [39]–[41]. Nested models were compared to test for heterogeneous ω-ratios among sites (M3 vs. M0) and to test for positive selection using two model parameterizations (M2a vs. M1a and M8 vs M7). The naïve empirical Bayes (NEB) method and Bayes empirical Bayes (BEB) method were used to test the significance of ω-ratios at each codon position identified as positively selected under models M2a and M8.
We also implemented two similar tests that examine codon substitutions on a per site basis, the random effects likelihood (REL) model and the internal fixed effects likelihood (iFEL) model [42], [43]. The added feature of these methods is to provide additional testing for codon sites subject to purifying selection (ω<<1). The REL model is an extension of the Yang and Nielsen [37] method, allowing for synonymous rate variation and using empirical Bayes factors for model testing (we use a BF>40 as a cut-off for significance testing). The iFEL test implements a site by site likelihood ratio test to detect population-level adaptation by testing for selection along internal branches (we use a p<0.05 for significance testing). Both tests were implemented in the Datamonkey software [33].
Temporal Analysis of Allozyme Allele Frequencies
We obtained a single-locus estimate of the effective population size change in the San Diego, Pescadero, and Site 10 populations based upon temporal changes in Pgi allele frequencies. The temporal samples included in the analysis were collected at different intervals between April 1978 and December 2004. We used the maximum likelihood-based approach of Beaumont [44] to estimate effective population size in a coalescent model from multiple temporal samples of allele counts in a single population. The method utilizes MCMC importance sampling to 1) estimate the harmonic mean effective population size and 2) the joint ancestral and contemporary effective population size based on allele frequency variation, assuming a model of exponential growth or decline over the temporal interval. Our focus is on the joint ancestral and contemporary effective population size, which provides an indication of demographic trends in allozyme allele classes over time. Analyses were run for each population using the default parameters (maximum iterations 100, thinning interval 10, and 0.5 proposal distribution). The number of generations between each temporal sample is required as input for the analysis. In the laboratory, the developmental time (from egg production in generation N to egg production in generation N+1) of T. californicus varies with temperature, averaging 25 days at 20°C and 32 days at 15°C, while is typically four months at those temperatures [45]. Natural populations of T. californicus reproduce continuously throughout the year with no periods of dormancy (or resting eggs) in its life cycle. Given the extensive variation in temperature experienced by natural populations, it is difficult to accurately assess generation times in the field; we conducted analyses assuming a conservative generation time of two months. We also examined allele frequency changes across years using goodness of fit G-tests implemented in the R software [46].
Results
Gene Structure and Organization
The full length cDNA contained an open reading frame of 558 codons with a putative methionine start codon and a stop codon near the 3′ end of the transcript. Comparison of the translated cDNA from the San Diego population to Pgi sequences from Homo sapiens, Drosophila melanogaster, and Anopheles gambiae revealed identical amino acids at roughly 70% of the sites. Genomic DNA sequences indicated the presence of ten exons and nine small introns between 64 and 181 base-pair (bp) long in the San Diego population. Small indel polymorphisms ranged in size from one bp to approximately 20 bp and occurred in all nine introns in interpopulation comparisons when the Playa Altamira sequence was included in the alignment. One indel restricted to this population resulted from a compound (GT)5(CT)4 short tandem repeat located in the second intron. The final alignment of 2,668 bp included the entire structural gene, with 10 exons, 9 introns, and 12 bp of the 3′UTR.
Replacement Polymorphism and Charge-changing Residues
The Pgi M/Pgi F polymorphism at Pescadero was traced to an Arg/Gln replacement polymorphism at codon position 77 in exon 3 (Table S2). In Pescadero, medium-migrating (Pgi M) alleles contained an Arg at this site and fast-migrating (Pgi F) alleles contained a Gln at this position. In contrast, the Pgi M and Pgi F allele classes in San Diego was traced to Gly and Asp residues, respectively, at codon position 66 in exon 2 (Table S3). In Punta Morro, Baja California, a Pgi F allele also contained an Asp at codon position 66 and no additional charge-changing replacements relative to the San Diego Pgi F allele consistent with a single mutational origin for the fast-migrating alleles in these populations. Unfortunately, we were unable to obtain a Pgi M allele from this location [18]. However, the widespread Pgi M electromorph found in southern California does not appear to be due to convergence on charge in different populations. Finally, the Pgi M/Pgi S polymorphism at Laguna Beach resulted from two charge-changing replacements (Asp/Asn at position 318, Lys/Glu at codon position 463).
Gene Tree and Population Structure
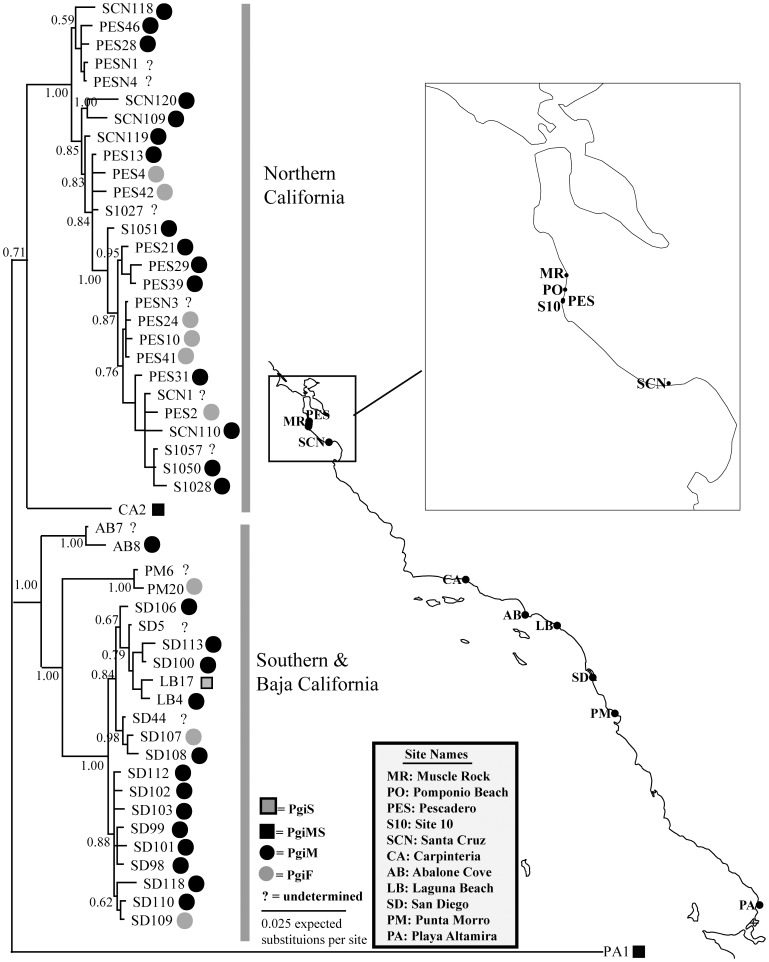
A Bayesian gene tree analysis showed that related alleles were distributed across multiple sample sites ( Figure 1 ), but a high degree of genetic structure was evident between regional populations in northern California and southern California. These populations formed reciprocally monophyletic clades (with 1.0 posterior probability branch support) separated by an average sequence divergence of 3.75% per site. The northern and southern clades did not share alleles and the alleles with charge-changing amino acids in each lineage either evolved multiple times or recombined over time. Additionally, the gene tree indicated that the partially reproductively isolated population from Playa Altamira, Baja California, Mexico was deeply divergent (∼12% average sequence divergence per site) from both the northern and southern California clades. Interpopulation distances (corrected for within population variation) at Pgi (Table S4) were comparable to other T. californicus nuclear genes [16], [17]. Similar to previous studies, there was evidence of multiple regionally distributed allopatric lineages with interpopulation genetic distances ranging as high as 1.5–2.5%.
Figure 1. Bayesian majority-rule consensus phylogeny of Pgi.
Sequences are labeled according to sample site, individual number, and allozyme class (slow, medium-slow, medium, fast denoted by the colored shapes).
Recombination, Linkage Disequilibrium, and Neutrality Tests
Recombination was evident between segregating sites in Pgi as estimated from the recombination rate per gene (R, Table 1 ) and the minimum number of recombination events (Rm) in samples from Santa Cruz (SCN) and Pescadero (PES). The GARD analysis suggested that there were two break points at positions 642 and 1714 (ΔAICC 107.13), both located within coding regions. Specifically, samples PES31, PES29, PES21, PES24, PES10, PES41, and PES39 showed evidence of recombination in the RDP2 analysis. Statistical tests for linkage disequilibrium (ZnS, Za, ZZ, B, and Q) were not significant.
Table 1. Site frequency tests of recombination, linkage disequilibrium and neutrality at Pgi.
| N | R | Rm | ZnS | Za | ZZ | B | Q | θπ | θS | D | DNS | DS | DFL | F | HKA | ||
| All Samples | 43 | 0.40 | 48 | 0.19 | 0.23 | 0.04 | 0.04 | 0.07 | 0.03 | 0.050 | −1.74 | −2.79* | −1.52 | −3.46* | −3.05* | ||
| Northern Clade | 24 | 5.30 | 14 | 0.16 | 0.50 | 0.34 | 0.16 | 0.29 | 0.01 | 0.02 | −1.67 | −2.50* | −0.82 | −3.24* | −3.27* | ||
| PESN | 13 | 41.80 | 5 | 0.01 | 0.01 | −0.51 | −2.05* | 0.60 | −1.41 | −1.39 | 3.455 | ||||||
| SCN | 5 | 475.00 | 3 | 1.531 | |||||||||||||
| Southern Clade | 18 | 0.001 | 9 | 0.30 | 0.34 | 0.04 | 0.23 | 0.27 | 0.01 | 0.02 | −1.89* | −2.32* | −1.63 | −2.17 | −2.55* | ||
| SD | 14 | 3.467 | |||||||||||||||
N sample size; R recombination rate per gene; Rm min. no. recombination events; ZnS average LD; Za average LD between adjacent sites; ZZ = Za- ZnS; B LD among segregating sites; Q LD among unique partitions; D Tajima’s D for all sites, non-synonymous sites (DNS) and synonymous sites (DS); DFL Fu and Li’s D; F Fu and Li’s F; HKA neutrality test of Pgi compared to RISP gene.
Neutrality tests based on site frequency spectra, including Tajima’s D, Fu and Li’s D and F, were used to test for selection in all samples and in separate analyses of both the northern and southern clades ( Table 2 ), but only a subset of these tests were statistically significant. Tajima’s D was negative and significant at non-synonymous coding sites at the species-level and within populations, suggesting positive selection and/or demographic change at Pgi. However, Tajima’s D was non-significant at synonymous sites. HKA tests in local populations (San Diego, Pescadero, and Santa Cruz) did not provide evidence of selection. In comparisons of the northern and southern clade to the Playa Altamira out-group, McDonald-Kreitman tests showed significant excess of replacement polymorphisms, indicating balancing selection could be maintaining non-synonymous variation within each T. californicus clade.
Table 2. Site frequency tests of interpopulation genetic structure, gene flow and neutrality at Pgi.
| Fst | Nm | Sfixed | Spolymorphic | NSfixed | NSpolymorphic | MK p-value | |
| Northern Clade vs. Playa Altamira | n/a | n/a | 86 | 39 | 26 | 47 | 0.000*** |
| Southern Clade vs. Playa Altamira | n/a | n/a | 83 | 61 | 23 | 37 | 0.014* |
| Northern vs. Southern Clade | 0.68 | 0.12 | 1 | 95 | 0 | 82 | 1 |
| Pescadero vs. Site 10 | 0.043 | 5.6 | 0 | 17 | 0 | 30 | n/a |
| Pescadero vs. Santa Cruz | 0.122 | 1.8 | 0 | 24 | 0 | 35 | n/a |
| San Diego vs. Long Beach | 0.386 | 0.4 | 2 | 19 | 0 | 25 | 0.203 |
FST among-population genetic differentiation; Nm gene flow rate between populations; S number of segregating sites; NS number of non-synonymous sites; MK McDonald-Kreitman neutrality test.
Codon-based Test for Selection
We tested for variation in ω across codon positions in Pgi to determine if certain amino acid sites had elevated levels of substitution (ω>1) indicating positive selection ( Table 3 ). Likelihood-ratio tests indicated that a model with discrete rate categories of ω (Model 3) fits the data significantly better (p<0.001) than a single rate (Model 0). Two separate model comparisons were made to look for evidence of positive selection. Based on likelihood-ratio tests, both selection models were a better fit to the data, either in comparison to a nearly neutral model (Model 2a vs. 1a, p<0.05) or to a model with a Beta distribution (Model 8 vs. Model 7, p<0.01). A naïve empirical Bayes (NEB) analysis indicated that positive selection is evident only at a small fraction of sites, including charge-changing amino acid shifts in the San Diego population (Asp-Gly) and the Pescadero population (Arg-Gln). All amino acid sites with elevated ω fell outside the active sites and dimer interfaces of the predicted Pgi protein model. The REL analysis provided additional support for positive selection at two of the same codon sites (66 and 301) with strong Bayes factor scores (>40). In addition, six codon sites were identified as under purifying selection (10, 162, 167, 182, 386, and 510) with very strong Bayes factor scores (>50). The iFEL analysis detected positive selection at three codon sites (66, 77, and 301 at p<0.05), notably the two charge-changing sites, and provided support for purifying selection at seven sites (10, 167, 182, 268, 388, 405, and 510 at p<0.05), four of which are shared with the REL analysis.
Table 3. Comparison of codon substitution models using likelihood-ratio tests and amino acid sites showing elevated non-synonymous substitution ratios (ω).
| Amino acid sites under selection | |||||||||
| PAML Model | DF | LnL | Nested ModelComparison | Likelihood RatioTest statistic | CodonPosition | Amino AcidChange | NEB Probω >1 | BEB Probω >1 | ω ± SE |
| M0(one-ratio) | 1 | −4107.2882 | 3 vs. 0 | 32.310*** | 45 | Ser-Gln | 0.676 | 0.643 | 1.241±0.594 |
| M1a (nearly-neutral) | 2 | −4094.4102 | 2 vs. 1 | 6.554* | 66 | Asp-Gly | 1.000** | 0.933 | 1.551±0.385 |
| M2a (selection) | 4 | −4091.1332 | 8 vs. 7 | 13.581** | 77 | Arg-Gln | 0.987* | 0.849 | 1.474±0.463 |
| M3 (discrete) | 5 | −4091.1332 | 301 | Cys-Gly-Ser-Ala | 0.997** | 0.890 | 1.513±0.423 | ||
| M7 (β) | 2 | −4097.9314 | 404 | Ala-Val-Met | 0.837 | 0.713 | 1.331±0.529 | ||
| M8 (β+ω) | 4 | −4091.1408 | 472 | Thr-Glu | 0.795 | 0.709 | 1.321±0.549 | ||
DF degrees of freedom; LnL log-likelihood; NEB naïve empirical Bayes test; BEB Bayes empirical Bayes test; SE standard error.
Significant tests are indicated with an asterisk.
Allozyme Allele Frequencies Over Time
Pgi allele frequencies remained relatively stable at Pescadero, San Diego and Site 10 over a period of 26 years and G-tests across sampling periods were not significantly different ( Table 4 ). During this time, Pgi genotypes rarely deviated from Hardy-Weinberg expectations, with the two exceptions occurring at Pescadero in June 1978 (p<0.05; G-test, G = 10.85, df = 3) and July 2004 (p<0.05; G-test, G = 5.67, df = 1) showing a deficit of heterozygotes. The Pescadero site maintained the widespread Pgi M and a private Pgi F allele, but the Pgi F allele was extremely rare at the immediately adjacent outcrop (Site 10) 500 m to the south. Instead, a private slow-migrating, Pgi S, allele occurred at Site 10 and was not found at Pescadero. North of Pescadero, the closest suitable habitat to the north is at Pomponio Beach and Mussel Rock (4.2 km and 9.9 km from Pescadero, respectively) and these sites were nearly monomorphic for the Pgi M allele. An additional private allele (medium-slow) was found segregating at low frequency at these two locations, but the Pgi F allele was not observed. Carpinteria and Santa Cruz samples were monomorphic, while Pgi variation at Laguna Beach included an additional private allele and allele frequencies were comparable to a previous sample.
Table 4. Pgi allozyme allele and genotype frequencies in Tigriopus californicus.
| Allozyme allele frequencies | Allozyme genotype frequencies | ||||||||||||||||
| Site | Datea | N | F | M | MS | S | VS | F/F | M/F | M/M | M/MS | MS/MS | MS/S | F/S | M/S | S/S | p |
| Mussel Rock | 12/28/04 | 38 | - | .987 | .013 | - | – | – | – | .974 | – | 0.26 | – | – | – | – | n.s. |
| Pomponio | 12/28/04 | 36 | – | .972 | .028 | – | – | – | – | .944 | – | 0.56 | – | – | – | – | n.s. |
| Pescadero | 4/78 | 50 | .52 | .48 | – | – | – | .32 | .40 | .28 | – | – | – | – | – | – | n.s. |
| 6/78 | 84 | .375 | .613 | – | .012 | – | .143 | .464 | .381 | – | – | – | – | – | .012 | .013 | |
| 8/78 | 104 | .5 | .5 | – | – | – | .26 | .48 | .26 | – | – | – | – | – | – | n.s. | |
| 8/79 | 130 | .485 | .515 | – | – | – | .223 | .523 | .254 | – | – | – | – | – | – | n.s. | |
| 12/2/81 | 30 | .483 | .517 | – | – | – | – | – | – | – | – | – | – | – | – | n.a. | |
| 8/27/82 | 83 | .515 | .466 | – | .015 | – | – | – | – | – | – | – | – | – | – | n.a. | |
| 5/10/83 | 97 | .459 | .536 | – | .005 | – | .268 | .381 | .340 | – | – | – | – | .010 | – | n.s. | |
| 8/95 | 66 | .38 | .62 | – | – | – | .121 | .515 | .364 | – | – | – | – | – | – | n.s. | |
| 7/96 | 70 | .51 | .49 | – | .014 | – | .286 | .414 | .271 | – | – | – | .029 | – | – | n.s. | |
| 7/15/04 | 46 | .511 | .489 | – | – | – | .348 | .326 | .326 | – | – | – | – | – | – | .017 | |
| 12/28/04 | 40 | .488 | .513 | – | – | – | .25 | .475 | .275 | – | – | – | – | – | – | n.s. | |
| Site 10 | 8/79 | 70 | – | .864 | – | .136 | – | – | – | .729 | – | – | – | – | .271 | – | n.s. |
| 8/95 | 66 | .04 | .84 | – | .12 | – | – | .076 | .682 | – | – | – | – | .242 | – | n.s. | |
| 7/15/04 | 48 | – | .969 | – | .031 | – | – | – | .938 | – | – | – | – | .063 | – | n.s. | |
| Santa Cruz | 11/92 | >50 | – | 1.0 | – | – | – | – | – | 1.0 | – | – | – | – | – | – | n.s. |
| 2004 | 20 | – | 1.0 | – | – | – | – | – | 1.0 | – | – | – | – | – | – | n.s | |
| Carpinteria | 2004 | 48 | – | – | 1.0 | – | – | – | – | – | – | 1.0 | – | – | – | – | n.s. |
| Laguna Beach | 10/92 | 69 | .014 | 0.78 | – | 0.21 | – | – | .014 | .609 | – | – | – | .014 | .319 | .043 | n.a. |
| 2004 | 43 | .058 | .709 | – | .221 | 0.12 | – | – | – | – | – | – | – | – | – | n.a. | |
| San Diego | 10/87 | >35 | .11 | .89 | – | – | – | – | – | – | – | – | – | – | – | – | n.a. |
| 10/92 | >50 | .22 | .78 | – | – | – | .046 | .230 | .724 | – | – | – | – | – | – | n.a. | |
| 9/11/04 | 47 | .202 | .798 | – | – | – | .043 | .319 | .638 | – | – | – | – | – | – | n.s. | |
| 9/19/04 | 24 | .146 | .854 | – | – | – | .042 | .208 | .750 | – | – | – | – | – | – | n.s. | |
| Punta Morro | 12/92 | >50 | .31 | .69 | – | – | – | – | – | – | – | – | – | – | – | – | n.a. |
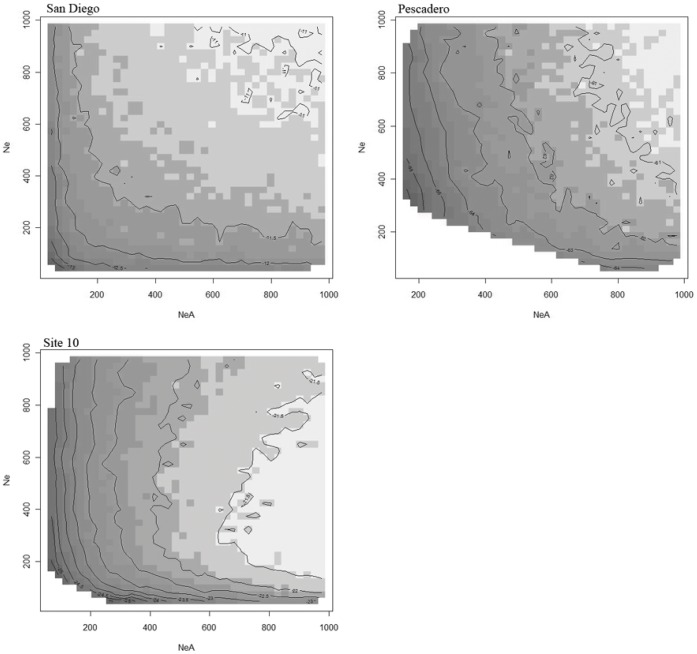
We used temporal samples of allele frequencies to estimate the joint log-likelihood surface for ancestral (N eA) and contemporary population size (N e) and found no evidence of a shift in population size in either the San Diego or Pescadero populations ( Figure 2 ). The stability of allele frequency estimates through time also suggests that selection did not increase or decrease the frequency of different electromorphs. In contrast, the Site 10 population showed a reduction in contemporary population size relative to ancestral population size, marked by a decline in the slow allele.
Figure 2. Log-likelihood surface plot of ancestral population size (N e A) versus contemporary population size (N e).
Estimates are based upon temporal changes in allozyme allele frequencies at Pgi in San Diego, Pescadero, and Site 10. The highest log-likelihood values are indicated by the white shaded contours.
Discussion
Origins of Variability at the Phosphoglucose Isomerase Locus in Tigriopus Californicus
Based on previous observations of sharp population structure [12], [14], [18], stability of allele frequencies through time [13], and evidence of adaptive variation in other arthropods [11], we set out to examine whether allozyme polymorphism observed at Pgi in Tigriopus californicus provided evidence of natural selection. Our analysis of sequence variation focused on identifying charge-changing amino acid polymorphisms and estimating variability in previously studied populations from Baja California and California. Due to the strong phylogeographic structure and evidence of independent lineages in Tigriopus [17], tests based on phylogenetic as well as population-genetic methods are appropriate for evaluating evidence of natural selection. Results from these multiple statistical tests provided evidence of selection operating on amino acid polymorphisms in Pgi.
Site frequency tests of neutrality, including Tajima’s D, Fu and Li’s D and F, indicate that nucleotide variation has arisen by positive or purifying selection [35]. Statistically significant and negative values were found at both the species level and at the population level. In Tigriopus, there is little evidence from other genes to support population expansion in our focal populations [17], [47] and we note that these D values were negative despite underlying population structure. When D was calculated exclusively at non-synonymous and synonymous sites in T. californicus, only tests at non-synonymous sites were significant. While this suggested a stronger role for purifying selection, tests at synonymous sites were also negative and the lack of significance might result from reduced power due to a smaller number of sites.
Site-specific tests provided stronger evidence for positive selection acting in Pgi. The codon model of Yang and Nielsen [37] provided statistical support for elevated levels of non-synonymous replacements across Pgi codons at a small number of sites. Additional tests using REL [42] and iFEL [43] also supported positive selection at the same codon positions. We conducted these tests after removing recombinant alleles from the dataset, to avoid any bias in the phylogeny introduced by recombination [38]. Two of the selected sites were the same charge-changing amino acid polymorphisms responsible for the independently evolved Pgi F and Pgi M alleles in the northern and southern T. californicus clades. Linkage appeared to have decayed around these amino acid replacements, which was unexpected for a very recently derived allele because a new mutation will initially be in complete linkage disequilibrium with all polymorphisms on the chromosome on which it arises. Therefore, the decay of linkage suggested that positive selection acting on these regions is historical rather than recent.
Maintenance of Divergent Pgi Alleles
Populations of T. californicus remain polymorphic for charge-changing amino acid polymorphisms and have maintained these alleles at stable frequencies for at least 100–200 generations. The population inhabiting a rocky outcrop at Pescadero, California has maintained the private Pgi F allele at stable frequency for a period now spanning at least 26 years. Although the local abundance of T. californicus frequently fluctuates over several orders of magnitude [45], the temporal changes in allele frequencies in our data are minor and could be consistent with that of a neutral allele in a population of moderate size, where genetic drift is a weak force. Although our analysis suggests that Pgi charge-changing amino acid polymorphisms originated as a result of positive diversifying selection, it is clear that there must be some evolutionary process acting to maintain polymorphism in local populations.
The maintenance of genetic variation in a variable environment can be advantageous to natural populations as a form of bet-hedging, insofar as the demands of natural selection require raw materials for rapid genetic change. However, the stochastic process of genetic drift, exacerbated in small or fluctuating populations, as well as selection (purifying and positive), make it difficult to sustain high levels of genetic variation. In addition to the generative role of mutation, three mechanisms act to maintain variation in natural populations. First, gene flow among structured populations can substantially increase the effective population size at neutral loci [48]. Second, recombination can act to generate variation by creating novel combinations of alleles [49]. Third, selective forces, in the form of balancing, frequency dependent or fluctuating selection [50], can actively maintain alleles in natural populations. Because these mechanisms could clearly act in concert to maintain genetic variation at a particular locus, we discuss the relative importance of each mechanism at the Pgi locus in T. californicus.
The role of gene flow in maintaining variation in T. californicus seems quite limited. Populations at adjacent rock-pools can be distinguished by high frequency private alleles, suggesting low rates of gene exchange [14], [19]. In a survey of populations inhabiting narrow (i.e., <25 km) stretches of coastline in central California, Burton and Feldman [14] reported high-frequency private alleles at all five allozyme loci examined (Got 1.07 at Monterey (30%), Pgm 1.07 at Capitola (25%), Pgi F at Pescadero (48%), Gpt 1.06 at Santa Cruz (25%), Got 1.03 at Bodega Bay (50%), and Est.97 at Moss Beach (30%)). Numerous low frequency polymorphisms are also restricted in distribution. As a result estimated rates of interpopulation gene flow are very low [12], [17] in T. californicus.
Recombination can act to generate allelic variation and has been suggested to play an important supporting role in maintaining adaptive polymorphisms in Pgi in Colias butterflies [51]. In our dataset, recombinant alleles were detected in the northern California Pescadero population. At a neutral locus, recombination has to be quite high (r ≥10µ) and the population size quite large (4Nµ ≥2) for recombination to have a significant effect on standing levels of genetic variation [52]. We currently lack independent estimates of either the recombination rate or the effective population size of local populations of T. californicus; however, we would expect to see evidence of recombination in the San Diego and Santa Cruz populations if it was acting as a general mechanism in generating Pgi polymorphism throughout the range of T. californicus.
Balancing selection remains the most probable explanation for the maintenance of variation in T. californicus. McDonald-Kreitman tests of both the northern and southern clades with Playa Altamira as an out-group indicate balancing selection due to a statistically significant excess of replacement polymorphisms. The preservation of polymorphism at the charge-changing sites, when nearby sites are under negative selection, is also consistent with balancing selection. Several statistical tests provide evidence of purifying selection acting on other coding regions of Pgi, including Tajima’s D and the REL and iFEL codon-based tests.
Balancing selection has also been implicated in studies of Pgi in two intertidal amphipods, two butterflies and a cricket [6], [8]–[10]. The amphipod species in the genus Gammarus are notable for their similarity to T. californicus, in terms of life history, habitat preference and multigenerational maintenance of Pgi allozyme variation. Patarnello and Battaglia [10] have further shown that clear fitness tradeoffs exist for particular Pgi genotypes and temperature conditions. Similar efforts to characterize the fitness of different genotypes in an ecological context will be needed to provide stronger evidence on whether balancing selection is acting to maintain variation at Pgi in T. californicus.
Supporting Information
Oligonucleotide primers used for amplification and sequencing of Tigriopus californicus phosphoglucose isomerase ( Pgi ).
(PDF)
Non-singleton polymorphisms from three sites in northern California. The polymorphism responsible for the Pgi F allele is indicated in bold. The boxed region indicates the haplotype block that is conserved in all Pgi F alleles. The electrophoretic allele class, Pgi F or Pgi M, of each sequence is indicated by an M or an F next to the sample ID. Sample abbreviation are PES = Pescadero, S10 = Site 10, and SCN = Santa Cruz. Singleton sites have been omitted from the alignment. Numbers at the top of the table are the positions in the global alignment of all nine populations in this study.
(PDF)
Non-singleton polymorphisms from San Diego (SD) and Laguna Beach (LB). The polymorphism responsible for the Pgi F allele is indicated in bold. The electrophoretic allele class, Pgi F, Pgi M or Pgi S, of each sequence is indicated by an F, M, or S next to the sample ID. Singleton sites have been omitted from the alignment. Numbers at the top of the table are the positions in the global alignment of all populations.
(PDF)
Inter-population distances at Pgi based upon coding regions corrected by the method of Jukes and Cantor [25] . Distances are net between-population means (i.e., corrected for within population distances).
(PDF)
Acknowledgments
We would like to thank the following people who were instrumental in various aspects of this work. We thank C. Ellison for scaling a cliff in the rain to obtain a sample, E. Anderson for providing assistance with effective population size estimation, L. Dingding for help with allozyme gel electrophoresis, and R. Byrne for general laboratory assistance. We also thank C. Wheat, an anonymous reviewer and the editor for comments that greatly improved the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors gratefully acknowledge financial support from National Science Foundation grants to R.S.B. (DEB 0521176 and DEB 0717178). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McKay JK, Latta RG. Adaptive population divergence: markers, QTL and traits. Trends Ecol Evol. 2002;17:285–291. [Google Scholar]
- 2.Fraser D, Bernatchez L. Adaptive evolutionary conservation: towards a unified concept for defining conservation units. Mol Ecol. 2001;10:2741–2752. [PubMed] [Google Scholar]
- 3.Morin PA, Luikart G, Wayne RK the SNP workshop group. SNPs in ecology, evolution and conservation. Trends Ecol Evol. 2004;19:208–216. [Google Scholar]
- 4.Ellegren H, Sheldon BC. Genetic basis of fitness differences in natural populations. Nature. 2008;452:169–175. doi: 10.1038/nature06737. [DOI] [PubMed] [Google Scholar]
- 5.Storz JF, Wheat CW. Integrating evolutionary and functional approaches to infer adaptation at specific loci. Evolution. 2010;64:2489–2509. doi: 10.1111/j.1558-5646.2010.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheat C, Haag C, Marden J, Hanski I, Frilander M. Nucleotide polymorphism at a gene (Pgi) under balancing selection in a butterfly metapopulation. Mol Biol Evol. 2010;27:267–281. doi: 10.1093/molbev/msp227. [DOI] [PubMed] [Google Scholar]
- 7.Dahlhoff EP, Rank NE. Functional and physiological consequences of genetic variation at phosphoglucose isomerase: Heat shock protein expression is related to enzyme genotype in a montane beetle. Proc Natl Acad Sci USA. 2000;97:10056–10061. doi: 10.1073/pnas.160277697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz LA, Harrison RG. Balancing selection on electrophoretic variation of phosphoglucose isomerase in two species of field cricket: Gryllus veletis and G. pennsylvanicus. Genetics. 1997;147:609–621. doi: 10.1093/genetics/147.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watt WB. Adaptation at specific loci. II. Demographic and biochemical elements in the maintenance of the Colias PGI polymorphism. Genetics. 1983;103:691–724. doi: 10.1093/genetics/103.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patarnello T, Battaglia B. Glucosephosphate isomerase and fitness: effects of temperature on genotype dependent mortality and enzyme activity in two species of the genus Gammarus (Crustacea: Amphipoda). Evolution. 1992;46:1568–1573. doi: 10.1111/j.1558-5646.1992.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 11.Wheat C. Phosphoglucose isomerase (Pgi) performance and fitness effects among arthropods and its potential role as an adaptive marker in conservation genetics. Conserv Genet. 2010;11:387–397. [Google Scholar]
- 12.Burton RS, Feldman MW, Curtsinger JW. Population genetics of Tigropus californicus (Copepoda: Harpacticoida): I. Population structure along the Central California coast. Mar Ecol Prog Ser. 1979;1:29–39. [Google Scholar]
- 13.Burton RS. Genetic evidence for long term persistence of marine invertebrate populations in an ephemeral environment. Evolution. 1997;51:993–998. doi: 10.1111/j.1558-5646.1997.tb03681.x. [DOI] [PubMed] [Google Scholar]
- 14.Burton RS, Feldman MW. Population genetics of Tigriopus californicus. II. Differentiation among neighboring populations. Evolution. 1981;35:1192–1205. doi: 10.1111/j.1558-5646.1981.tb04989.x. [DOI] [PubMed] [Google Scholar]
- 15.Dybdahl MF. Extinction, recolonization, and the genetic structure of tidepool copepod populations. Evol Ecol. 1994;8:113–124. [Google Scholar]
- 16.Burton RS, Lee B-N. Nuclear and mitochondrial gene genealogies and allozyme polymorphism across a major phylogeographic break in the copepod Tigriopus californicus. Proc Natl Acad Sci USA. 1994;91:5197–5201. doi: 10.1073/pnas.91.11.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willett CS, Ladner JT. Investigations of fine-scale phylogeography in Tigriopus californicus reveal historical patterns of population divergence. BMC Evol Biol. 2009;9:139. doi: 10.1186/1471-2148-9-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganz HH, Burton RS. Genetic differentiation and reproductive incompatibility among Baja California populations of the copepod Tigriopus californicus. Mar Biol. 1995;123:821–827. [Google Scholar]
- 19.Slatkin M. Rare alleles as indicators of gene flow. Evolution. 1985;39:53–65. doi: 10.1111/j.1558-5646.1985.tb04079.x. [DOI] [PubMed] [Google Scholar]
- 20.Burton RS, Swisher SG. Population structure of the intertidal copepod Tigriopus californicus as revealed by field manipulation of allele frequencies. Oecologia. 1984;65:108–111. doi: 10.1007/BF00384471. [DOI] [PubMed] [Google Scholar]
- 21.Burton RS. Hybrid breakdown in developmental time in the copepod Tigriopus californicus. Evolution. 1990;44:1814–1822. doi: 10.1111/j.1558-5646.1990.tb05252.x. [DOI] [PubMed] [Google Scholar]
- 22.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 23.Nylander JAA. MrModeltest v2. Evolutionary Biology Centre, Uppsala University, Uppsala: Program distributed by the author. 2004.
- 24.Rambaut A, Drummond AJ. TRACER: MCMC Trace Analysis Tool Version v1.5.0. University of Oxford, Oxford. 2009.
- 25.Jukes TH, Cantor CR. Munro HN, editor. Evolution of protein molecules. 1969. pp. 21–132. editor. Mammalian Protein Metabolism. New York: Academic Press.
- 26.Kumar S, Tamura K, Jakobsen IB, Nei M. MEGA2: Molecular Evolutionary Genetics Analysis Software. Bioinformatics. 2001;17:1244–1245. doi: 10.1093/bioinformatics/17.12.1244. [DOI] [PubMed] [Google Scholar]
- 27.Librado P, Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 28.Hudson RR. Estimating the recombination parameter of a finite population-model without selection. Genet Res. 1987;50:245–250. doi: 10.1017/s0016672300023776. [DOI] [PubMed] [Google Scholar]
- 29.Hudson RR, Kaplan NL. Statistical properties of the number of recombination events in the history of a sample of DNA sequences. Genetics. 1985;111:147–164. doi: 10.1093/genetics/111.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wall JD. Recombination and the power of statistical tests of neutrality. Genet Res. 1999;74:65–69. [Google Scholar]
- 31.Kelly JK. A test of neutrality based on interlocus associations. Genetics. 1997;146:1197–1206. doi: 10.1093/genetics/146.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rozas J, Gullaud M, Blandin G, Aguadé M. DNA variation at the rp49 gene region of Drosophila simulans: Evolutionary inferences from an unusual haplotype structure. Genetics. 2001;158:1147–1155. doi: 10.1093/genetics/158.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delport W, Poon AF, Frost SDW, Kosakovsky Pond SL. Datamonkey 2010: a suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics. 2010;26:2455–2457. doi: 10.1093/bioinformatics/btq429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin DP, Williamson C, Posada D. RDP2: recombination detection and analysis from sequence alignments. Bioinformatics. 2005;21:260–262. doi: 10.1093/bioinformatics/bth490. [DOI] [PubMed] [Google Scholar]
- 35.Tajima F. Statistical-method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu YX, Li WH. Statistical tests of neutrality of mutations. Genetics. 1993;133:693–709. doi: 10.1093/genetics/133.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Z, Nielsen R. Codon-substitution models for detecting molecular adaptation at individual sites along specific lineages. Mol Biol Evol. 2002;19:908–917. doi: 10.1093/oxfordjournals.molbev.a004148. [DOI] [PubMed] [Google Scholar]
- 38.Anisimova M, Nielsen R, Yang Z. Effect of recombination on the accuracy of the likelihood method for detecting positive selection at amino acid sites. Genetics. 2003;164:1229–1236. doi: 10.1093/genetics/164.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong WSW, Yang Z, Goldman N, Nielsen R. Accuracy and power of statistical methods for detecting adaptive evolution in protein coding sequences and for identifying positively selected sites. Genetics. 2004;168:1041–1051. doi: 10.1534/genetics.104.031153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Z, Nielsen R, Goldman N, Pedersen A-MK. Codon substitution models for heterogeneous selection pressure at amino acid sites. Genetics. 2000;155:431–449. doi: 10.1093/genetics/155.1.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Z, Nielsen R. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol Biol Evol. 2000;17:32–43. doi: 10.1093/oxfordjournals.molbev.a026236. [DOI] [PubMed] [Google Scholar]
- 42.Kosakovsky Pond SL, Frost SDW. Not so different after all: A comparison of methods for detecting amino acid sites under selection. Mol Biol Evol. 2005;22:1208–1222. doi: 10.1093/molbev/msi105. [DOI] [PubMed] [Google Scholar]
- 43.Kosakovsky Pond SL, Frost SDW, Grossman Z, Gravenor MB, Richman DD, et al. Adaptation to different human populations by HIV-1 revealed by codon-based analyses. PLoS Comput Biol. 2006;2:e62. doi: 10.1371/journal.pcbi.0020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beaumont MA. Estimation of population growth or decline in genetically monitored populations. Genetics. 2003;164:1139–1160. doi: 10.1093/genetics/164.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vittor BA. Effects of the environment on fitness-related life history characteristics in Tigriopus californicus: Univ. of Oregon, Eugene. 1971.
- 46.R Development Core Team. R version 2.13.2. The R Foundation for Statistical Computing. 2011.
- 47.Edmands S. Phylogeography of the intertidal copepod Tigriopus californicus reveals substantially reduced population differentiation at northern latitudes. Mol Ecol. 2001;10:1743–1750. doi: 10.1046/j.0962-1083.2001.01306.x. [DOI] [PubMed] [Google Scholar]
- 48.Wakeley J. The coalescent in an island model of population subdivision with variation among demes. Theor Popul Biol. 2001;59:133–144. doi: 10.1006/tpbi.2000.1495. [DOI] [PubMed] [Google Scholar]
- 49.Morgan K, Strobeck C. Is intragenic recombination a factor in the maintenance of genetic variation in natural populations? Nature. 1979;277:383–384. doi: 10.1038/277383a0. [DOI] [PubMed] [Google Scholar]
- 50.Gillespie JH. A general model to account for enzyme variation in natural populations. V. The SAS-CFF model. Theor Popul Biol. 1978;14:1–45. doi: 10.1016/0040-5809(78)90002-3. [DOI] [PubMed] [Google Scholar]
- 51.Wang B, DePasse JM, Watt WB. Evolutionary genomics of Colias phosphoglucose isomerase (PGI) introns. J Mol Evol. 2012. [DOI] [PubMed]
- 52.Hudson RR. Properties of a neutral allele model with intragenic recombination. Theor Popul Biol. 1983;23:183–201. doi: 10.1016/0040-5809(83)90013-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Oligonucleotide primers used for amplification and sequencing of Tigriopus californicus phosphoglucose isomerase ( Pgi ).
(PDF)
Non-singleton polymorphisms from three sites in northern California. The polymorphism responsible for the Pgi F allele is indicated in bold. The boxed region indicates the haplotype block that is conserved in all Pgi F alleles. The electrophoretic allele class, Pgi F or Pgi M, of each sequence is indicated by an M or an F next to the sample ID. Sample abbreviation are PES = Pescadero, S10 = Site 10, and SCN = Santa Cruz. Singleton sites have been omitted from the alignment. Numbers at the top of the table are the positions in the global alignment of all nine populations in this study.
(PDF)
Non-singleton polymorphisms from San Diego (SD) and Laguna Beach (LB). The polymorphism responsible for the Pgi F allele is indicated in bold. The electrophoretic allele class, Pgi F, Pgi M or Pgi S, of each sequence is indicated by an F, M, or S next to the sample ID. Singleton sites have been omitted from the alignment. Numbers at the top of the table are the positions in the global alignment of all populations.
(PDF)
Inter-population distances at Pgi based upon coding regions corrected by the method of Jukes and Cantor [25] . Distances are net between-population means (i.e., corrected for within population distances).
(PDF)