Abstract
Cystic fibrosis (CF) is the most common lethal autosomal recessive disease in the Caucasian population. It is due to mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. To date, over 1910 mutations have been identified in the CFTR gene. Among these mutations, the CF-causing missense mutation G551D-CFTR (approx. 5% of cases) encodes for a CFTR chloride channel with normal expression on the cell surface. Nevertheless, it is associated with severe disease due to its altered channel activation. The aim of the present study was to identify specific interacting proteins of G551D-CFTR. Co-immunoprecipitated proteins with G551D-CFTR were resolved by 2D-gel electrophoresis (2-DE). Mass Spectrometry revealed that calumenin was present in the protein complex linked to G551D-CFTR. Despite its basal expression was not modified in G551D-CFTR expressing cells when compared to Wt-CFTR expressing cells, it was more abundant in the G551D-CFTR complex detected by immunoprecipitation. The calumenin-CFTR interaction was also shown by Surface Plasmon Resonance and further confirmed by computational analysis of the predicted calumenin’s partners. Because in our cellular model calumenin was found in the endoplasmic reticulum (ER) by immunofluorescence experiments, we suggest that calumenin is likely involved in the mutated CFTR’s maturation. In conclusion, we showed for the first time that calumenin binds to CFTR and that it is increased in the G551D-CFTR complex. We suggest that it may be involved in the physiopathology of G551D-CFTR and that G551D-CFTR may follow a specific maturation and trafficking pathway. We also hypothesize that UPR may be triggered independently of the retention of G551D-CFTR in the ER because Grp78/Bip expression is increased in the cells. Finally, we propose here that Calumenin is a new CFTR chaperone.
Introduction
Cystic fibrosis (CF) is the most common lethal autosomal recessive disease in the Caucasian population. It is due to mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene [1]–[3]. CFTR is an ATP-binding cassette transporter functions as a chloride (Cl-) channel [4]–[6] and comprises two hydrophobic core regions, two nucleotide-binding domains (NBDs) with ATP-binding activity [7] and a regulatory domain (R domain). CFTR channel opening requires phosphorylation by cAMP-dependent protein kinases (PKA) [8] and hydrolyzable MgATP [9], [10]. Both genetic and interacting proteins seem to be involved in the CFTR regulation. Indeed, CFTR regulation is complex and involves dimerization of the protein [11], [12] and interdomain interactions [13]. Syntaxin 1A, EBP50, E3KARP, the µ subunit of the endocytic clathrin adaptor complex, cysteine string proteins and annexin A5 are CFTR-binding proteins [14]–[19], but the extent to which CFTR channels are regulated by protein-protein interactions remains largely unknown.
To date, over 1910 mutations (www.genet.sickkids.on.ca/cftr/) have been identified in the CFTR gene and a classification of mutations by which different mechanisms induce CF has been proposed [20]. Among these mutations, the CF-causing missense mutation G551D-CFTR (Gly to Asp at position 551) exhibits normal expression at the cell surface but it is associated with severe disease [21], [22]. Indeed, it lacks channel activation mediated by ATP [23], [24]. G551D-CFTR is not a common mutation in the CF patients (approx. 5% of cases) but the clinical phenotype is considered very severe. Therefore, efforts have been taken to overcome the G551D-CFTR defect. Biochemical studies on purified and reconstituted G551D-CFTR showed the potentiation of the ATPase activity by VRT-532 [25]. Nevertheless, VRT-532 did not affect the ATPase activity of the Wt (wild-type) CFTR. This supported the idea that this compound corrects the specific molecular defect of this mutant by a direct or indirect binding, stabilizing an intramolecular interaction. This potentiator seems to have a mutant specificity which may be due to CFTR interacting proteins [26].
As it is suggested that G551D-CFTR has different binding partners when compared to Wt-CFTR, the aim of the present study was to identify specific interacting proteins of G551D-CFTR. The proteins linked to G551D-CFTR were resolved by 2D-gel electrophoresis (2-DE). Among the detected spots, one spot exhibited a high intensity and was subjected to Mass Spectrometry (MS). MS revealed that the corresponding protein was calumenin. We found that its basal expression was not modified in G551D-CFTR expressing cells when compared to Wt-CFTR expressing cells. Using co-immunoprecipitation, we found that calumenin was bound to the G551D-CFTR protein. The co-immunoprecipitation experiment also indicated that calumenin was also bound to Wt-CFTR. Nevertheless, the amount of bound calumenin was higher in the G551D-CFTR complex than in the Wt-CFTR one. The calumenin - CFTR interaction was further confirmed by computational interaction prediction and by Surface Plasmon Resonance (SPR). In order to know whether calumenin was localized in the Endoplasmic Reticulum (ER) in our cell model and whether the interaction takes place in the ER in which calumenin is known to be present, immunofluorescence was performed. We found that the calumenin - CFTR interaction is indeed only present in the ER of the cells. Because calumenin expression is modulated in cells expressing the most frequent CFTR mutant (F508del) together with Grp78/Bip which is a hallmark of the Unfolded Protein Response (UPR) and because we found an increased level of calumenin linked to G551D-CFTR, we assessed the Grp78 expression in the cells. We found that Grp78 expression was increased in G551D-CFTR expressing cells, suggesting that calumenin is likely involved in the maturation of CFTR within the ER, that G551D-CFTR may follow a specific maturation and trafficking pathway and that UPR may be triggered in CF independently of the retention of G551D-CFTR in the ER. Finally, we propose that Calumenin is a new CFTR’s chaperone.
Results and Discussion
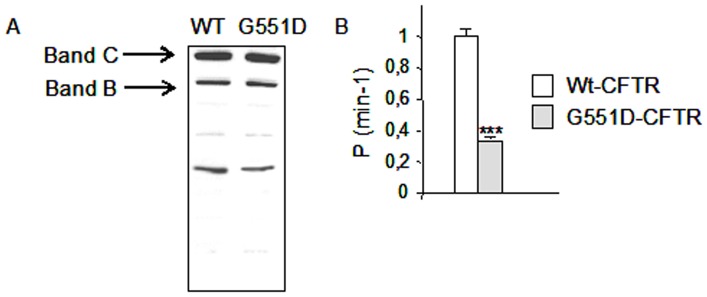
Wt-CFTR and G551D-CFTR were expressed in Hela cells which do not endogenously express CFTR and their function was measured by [27], [28]. The expression of the CFTR protein was assessed by western blotting. As shown in Fig. 1A the mature fully glycosylated CFTR and core-glycosylated CFTR were expressed in both cell types. Therefore, we found that in our cell model the mutant protein can fold and traffic normally to the plasma membrane. This was in accordance with previous results showing that G551D-CFTR exhibits no trafficking defects [23], [29]. We investigated the cAMP-dependent halide flux through Wt-CFTR and G551D-CFTR (Fig. 1B) in the stably transfected cells. We found that it was deeply decreased in G551D-CFTR cells, as previously described [9], [29]. Therefore, we showed that viral tranfection of the cells led to the expression of Wt-CFTR and G551D-CFTR and that their expression and function were in accordance with previous results, indicating that the cell model could be used for further experiments.
Figure 1. Validation of the cell model.
A, Wt-CFTR and G551D-CFTR expression in Hela cells. Example of western blot showing the mature fully glycosylated CFTR and core-glycosylated CFTR labeled as Band C (170 kDa) and Band B, respectively. B, Study of the cyclic AMP-induced I− efflux from Wt-CFTR (n = 3) and G551D-CFTR (n = 3) using SPQ experiments. The halide permeability (p) is the rate of SPQ dequenching. The graphs show a deeply decreased activity in G551D-CFTR expressing cells. Data are the means ± S.E.M., ***: p<0.001.
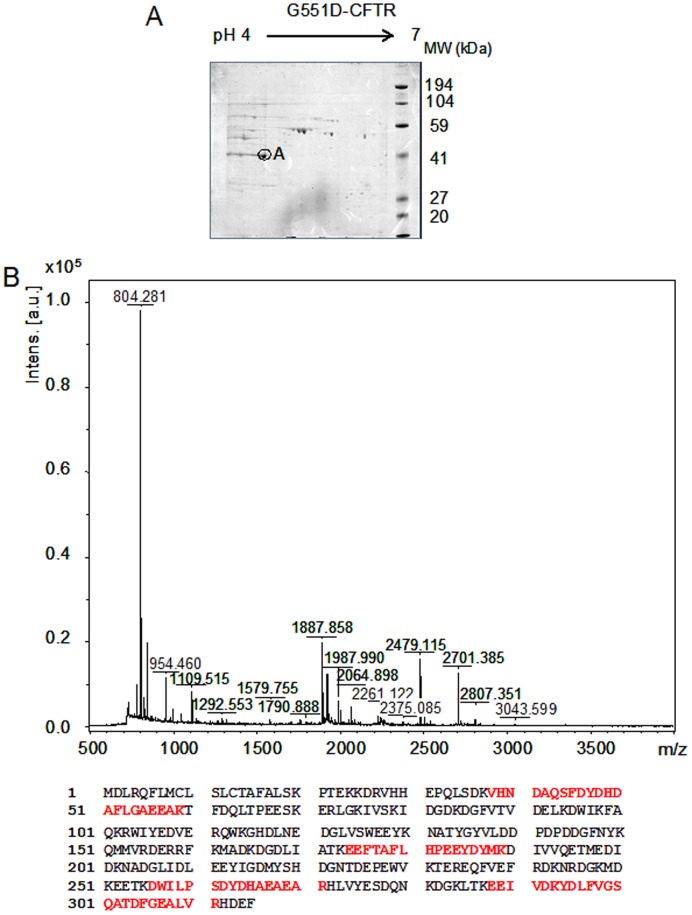
A reference 2-DE (pH 4–7) of 6 gels performed with proteins complex linked to G551D-CFTR obtained by immunoprecipitation is presented in Fig. 2A. The image data analysis detected 165 protein spots in the complex. The spot showing the higher expression was further analyzed (A, on Fig. 2A). It was excised from the gel, trypsined and analyzed by MS. The results indicate that the spot corresponded to calumenin (table 1). The MS/MS results leading to calumenin identification are summarized in table 2. The MS spectrum and the coverage of the calumenin sequence are presented in Fig. 2B.
Figure 2. Calumenin belongs to the G551D-CFTR’s complex.
A, Example of a reference 2-DE (pH 4–7) of 6 gels performed with immunoprecipitated G551D-CFTR. The image analysis detected 165 protein spots. The analyzed spot is noted as A. B, MS spectra obtained for the A spot and protein coverage (24%) shown in bold red on the calumenin amino acid sequence (gi/2809324).
Table 1. Identification of spots A by Mass Spectrometry.
| Spot | RefSeqAC | Protein Name | Gene | Sequence Coverage | Queries Matched | Molecular weight/Isoelectric point | Observed in (cell type) |
| A | NM001219 | Calumenin | CALU (Homo sapiens) | 67 | 66 | 37084/4.47 | G551D-CFTR |
Table 2. MS/MS results for Calumenin identification.
| Tree hierarchy | Meas. M/z | Calc. MH+ | Meas. Mr | Calc. Mr | Int. | Dev.(ppm) | Score | Mascot Score | Range |
| MSMS 25 | 1887,858 | 1887,851 | 1886,851 | 1886,843 | 18320,6 | 3,746 | 25 | 81 | 256–271 |
| MSMS 38 | 2064,898 | 2064,89 | 2063,891 | 2063,882 | 3936,262 | 4,134 | 7 | 7 | 174–189 |
| MSMS 50 | 2479,115 | 2479,08 | 2478,108 | 2478,072 | 11680,34 | 14,325 | 89 | 109 | 38–59 |
| MSMS 55 | 2701,385 | 2701,336 | 2700,378 | 2700,328 | 8222,202 | 18,254 | 2059 | 152 | 288–311 |
Search Parameter: Charge = 1+, Trypsin, Mascot 2.3.01.241, NCBInr NCBInr_20120204.fasta; Modifications: Global: Carboxymethyl (C), Optional: Oxidation (M). Individual ions scores >51 indicate identity or extensive homology (p<0.05). Protein scores are derived from ions scores as a non-probabilistic basis for ranking protein hits.
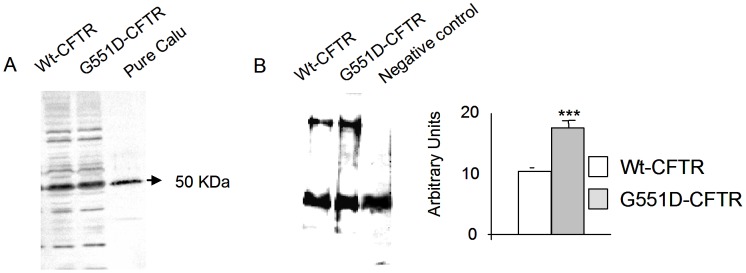
Calumenin is a multiple EF-hand Ca2+-binding protein located in the ER. It belongs to the CREC family of Ca2+-binding proteins. It is also found in the secretory pathway and can be secreted to the extracellular space. It interacts with different ligands and it inhibits several proteins in the ER membrane such as the ryanodine receptor (RyR2) and the Ca2+-transporting ATPase (SERCA2). Some other functions concern participation in the secretory process, ER lumenal chaperone activity and signal transduction. It is also involved in a large variety of disease processes (for review [30]) and it was shown to be involved in the Ca2+ homeostasis of the ER, due to a direct interaction with SERCA2 and RyR2. Indeed, an inhibition of SERCA2-mediated Ca2+ uptake into SR in cardiomyocyte due to an interaction with calumenin was observed [31]. To assess the expression of calumenin in G551D-CFTR expressing cells, western blots (n = 5) were performed to compare its basal level with that of wt-CFTR expressing cells. As shown in Fig. 3A, no difference was observed between Wt- or G551D-CFTR expressing cells suggesting that it is not involved in a difference of the Ca2+ homeostasis between Wt- and G551D-CFTR cells. Whereas, calumenin was detected in the CFTR’s ER-Associated Folding Proteome and seems to be modulated in Fdel508-CFTR expressing cells, its direct interaction with CFTR has never clearly been established [32], [33]. Therefore, we assessed its interaction with CFTR by coimmunoprecipitation. As shown in Fig. 3B, we found that calumenin was bound to CFTR and that it was more abundant in the G551D-CFTR complex than in the Wt-CFTR complex. The reverse coimmunoprecipitation indicated that the amount of bound G551D-CFTR onto calumenin was higher than the amount of bound Wt-CFTR (not shown). Because we found that calumenin binds to the mature G551D-CFTR form (170 kDa) and because human calumenin is found in the ER as well as in the Golgi complex [34], our results suggest that it is likely involved in the CFTR maturation.
Figure 3. More calumenin is bound onto G551D-CFTR than onto Wt-CFTR.
A, Example of detection of calumenin in Wt-CFTR and G551D expressing cells, assessed by western blotting using whole cell lysates. No difference was observed between the two cell lines. Pure Calumenin was loaded as a molecular weight control because of the presence of non-specific bands on the membrane. B, Example of coimmunoprecipitation showing the calumenin-CFTR interaction in total proteins (left). Histogram represents the quantitation indicating that more calumenin is bound onto G551D-CFTR than onto Wt-CFTR (right).
Grp78/Bip is a major sensor for the Unfolded Protein Response (UPR) triggering in the ER. It is modulated as well as calumenin in F508del-CFTR expressing cells and we previously showed that it is involved in the observed UPR in CF cells [33], [35]. Therefore, we assessed Grp78 expression in G551D-CFTR expressing cells, using Fdel508-CFTR cells as a positive control. As shown in Fig. 4A, we observed an increased Grp78 expression in G551D-CFTR cells when compared to Wt-CFTR expressing cells. The quantitation indicated that its expression was not significantly different to that observed in Fdel508-CFTR cells, suggesting the UPR triggering (Fig. 4B). Because it was previously shown that G551D-CFTR is not retained in the ER [36] this result suggests that UPR, or at least an ER stress, may be triggered in G551D-CFTR expressing cells due to another mechanism involving calumenin.
Figure 4. UPR is likely triggered in G551D-CFTR expressing cells.
A, Example of detection of Grp78 in Wt-CFTR, Fdel508-CFTR and G551D-CFTR expressing cells, assessed by western blotting using whole cell lysates. A Fdel508-CFTR expressing cell lysate was used as a positive control. B, Histogram represents the quantitation indicating that the amount of Grp78 is increased in Fdel508-CFTR and G551D-CFTR expressing cells whereas no difference is observed between Fdel508-CFTR and G551D-CFTR expressing cells (right).
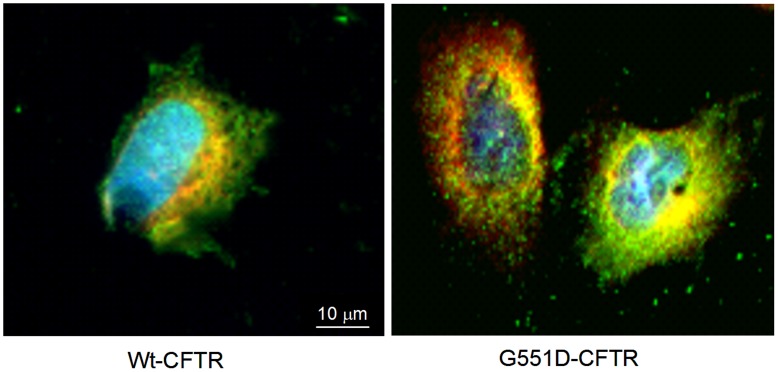
Because the calumenin - CFTR interaction in the ER of cells had never been shown before and because some extra bands were observed when calumenin was detected in the cells using western blottings, we had to further provide evidence showing this interaction. For this purpose, Surface Plasmon Resonance was used [37]. The sensorgrams obtained when pure calumenin was linked on a sensorchip and pure CFTR was injected (0.15 to 1.5 nM), showing an interaction followed by a dissociation phase which was not zero when the injection was stopped indicated that the interaction occured (Fig. 5A). The obtained value for the dissociation constant was 3.8×10−12 M showing a strong interaction. The opposite experiment in which purified CFTR was linked on the sensorchip was also performed (not shown). The SPR experiments confirming the direct interaction permitted to rule out the possibility of an unspecific binding during co-immunoprecipitation experiments. The calumenin - CFTR interaction was also evaluated with the online Search Tool for the Retrieval of Interacting Genes/Protein (STRING 9.0, http://string-db.org) to predict both direct and indirect interactions [38]. As shown in Fig. 5B CFTR was found to be a calumenin’s network. Among the predicted calumenin’s partners which are listed in Table 3, some are depicted and characterized by biochemical experiments such as RyR and ATP2A2 [30], [31]. In order to show that in our cell model calumenin is located within the ER, as described in other cell types, immunofluorescence was performed. The nuclei of the cells as well as the ER and calumenin were labelled in wt- and G551D-CFTR expressing cells (Fig. 6). The results indicated that in our cell model calumenin is indeed localized in the ER and that therefore the described interaction between Calumenin and wt- or G551D-CFTR takes place within the ER.
Figure 5. Evidence of the direct calumenin-CFTR interaction.
A, Example of sensorgrams obtained when pure Calumenin was linked on the sensorchip and pure CFTR was injected (0.15 to 1.5 nM), showing an interaction followed by a dissociation phase which was not zero when the injection was stopped. The obtained value for the dissociation constant was 3.8e−12 M showing a strong interaction. SPR was performed to rule out a possible non specific binding in co-immunoprecipitation experiment. B, Network of the predicted interactions for Calumenin obtained by STRING. Green connecting lines indicates that the interaction in mentioned in PubMed abstracts with no or insignificant experimental data. Pink connecting lines indicates that the interaction has been demonstrated with available biochemical data. A pink line was observed for the Calumenin - CFTR interaction [32]. Partners are depicted in Table 3.
Table 3. Predicted Functional Partners for Calumenin.
| Name | Ensembl project number(http://www.ensembl.org) | Role | Interactionscore (STRING) |
| AURKA | ENSP00000216911, Homo sapiens | May play a role in cell cycle regulation during anaphase and/or telophase,in relation to the function of the centrosome/spindle pole region duringchromosome segregation. May be involved in microtubule formationand/or stabilization. | 0.974 |
| APCS | ENSP00000255040, Homo sapiens | Amyloid P component, serum. Can interact with DNA and histones and may scavenge nuclear material released from damaged circulating cells. May also function as a calcium-dependent lectin | 0.929 |
| ATP2A2 | ENSP00000324892, Homo sapiens | ATPase, Ca2+ transporting, cardiac muscle, slow twitch 2. This magnesium-dependent enzyme catalyzes the hydrolysis of ATP coupled with thetranslocation of calcium from the cytosol to the sarcoplasmic reticulumlumen. Isoform SERCA2A is involved in the regulation of thecontraction/relaxation cycle | 0.860 |
| RYR2 | ENSP00000355533, Homo sapiens | ryanodine receptor 2 (cardiac); Communication between transverse-tubulesand sarcoplasmic reticulum. Contraction of cardiac muscle is triggered byrelease of calcium ions from SR following depolarization of T- tubules(By similarity) | 0.853 |
| RYR1 | ENSP00000352608, Homo sapiens | ryanodine receptor 1 (skeletal); Communication between transverse-tubulesand sarcoplasmic reticulum. Contraction of skeletal muscle is triggered byrelease of calcium ions from SR following depolarization of T-tubules | 0.815 |
| CFTR | ENSP00000003084, Homo sapiens | cystic fibrosis transmembrane conductance regulator (ATP-binding cassettesub-family C, member 7); Involved in the transport of chloride ions. Mayregulate bicarbonate secretion and salvage in epithelial cells by regulatingthe SLC4A7 transporter | 0.801 |
| VKORC1 | ENSP00000378426, Homo sapiens | vitamin K epoxide reductase complex, subunit 1; Involved in vitamin K metabolism. Catalytic subunit of the vitamin K epoxide reductase (VKOR)complex which reduces inactive vitamin K 2,3-epoxide to active vitamin K | 0.778 |
| IL8 | ENSP00000306512, Homo sapiens | interleukin 8; IL-8 is a chemotactic factor that attracts neutrophils, basophils,and T-cells, but not monocytes. It is also involved in neutrophil activation. It isreleased from several cell types in response to an inflammatory stimulus.IL-8(6–77) has a 5–10-fold higher activity on neutrophil activation,IL-8(5–77) has increased activity on neutrophil activation and IL-8(7–77)has a higher affinity to receptors CXCR1 and CXCR2 as comparedto IL-8(1–77), respectively | 0.764 |
| TJP1 | ENSP00000281537, Homo sapiens | tight junction protein 1 (zona occludens 1); The N-terminal may be involvedin transducing a signal required for tight junction assembly, while theC-terminal may have specific properties of tight junctions. The alphadomain might be involved in stabilizing junctions | 0.750 |
| KCNN4 | ENSP00000262888, Homo sapiens | potassium intermediate/small conductance calcium-activated channel,subfamily N, member 4; Forms a voltage-independent potassium channelthat is activated by intracellular calcium. Activation is followed bymembrane hyperpolarization which promotes calcium influx. The channel isblocked by clotrimazole and charybdotoxin but is insensitive to apamin | 0.748 |
Figure 6. The calumenin – CFTR interaction takes place in the ER.
Immunofluorescent localization of Calumenin within Wt- (left) and G551D-CFTR (right) expressing cells. The nuclei were labelled in blue by DAPI. The ER was visualized using PDI labelling (red) and Calumenin was seen in green. Overlapping of PDI and Calumenin is yellow showing the described interaction between Calu and CFTR takes place within the ER.
Conclusion
Our results suggest that the G551D-CFTR’s maturation/trafficking is different from the one of Wt-CFTR because it likely involves more calumenin. Furthermore, we suggest that UPR is likely triggered in G551D-CFTR expressing cells. Nevertheless, the UPR pathway may be different than the one observed in Fdel508-CFTR cells because the G551D-CFTR protein is not retained in the ER. In conclusion, we show here a direct calumenin - wt-CFTR and calumenin - G551D-CFTR interaction. Because we found the interaction within the ER and because the amount of bound calumenin is likely higher with the mutated CFTR, we propose that the G551D-CFTR protein may be directed to a specific trafficking pathway. The exact role of calumenin upon UPR in G551D-CFTR expressing cells is now under investigation in our laboratory as well as its possible role as a chaperone for the most frequent mutation found in CF (F508del) which is retained in the ER. Furthermore, according to the Bip/Grp78 expression, we propose that UPR may be triggered in G551D-CFTR expressing cells, independently of any retention of the mutated CFTR in the ER.
Materials and Methods
Plasmid Construction
The pLenti6/V5-CFTR mammalian expression lentiviral construct, driven by the cytomegalovirus promoter was constructed using ViraPower™ Lentiviral Directional TOPO® Expression Kit (Invitrogen). To clone the cDNA encoding the human wild type CFTR (Wt-CFTR, Transgene SA, pTG5985, France, access Genbank no. M28668) into the pLenti vector, a forward primer sequence with 4 nucleotides CACC- at the 5′ end, based on the TOPO-cloning technology, was designed according to manufacturer’s instruction. The cDNAs encoding G551D-CFTR and F508del-CFTR were generated by site-directed mutagenesis (QuickChange II XL Site-Directed Mutagenesis Kit, Stratagene).
Viral Particle Production and Cell Transfection
6x106 293FT cells (provided with the ViraPower™ Lentiviral Directional TOPO® Expression Kit, InVitrogen) were plated 24 hours prior to the addition of a complex comprising Wt-CFTR, G551D-CFTR or F508del-CFTR plasmid and Lipofectamine™ 2000 (Invitrogen). The HIV type 1 (HIV-1) gag-pol construct pLP1, rev construct pLP2 and the Vesicular Stomatitis Virus G glycoprotein (VSV-G) construct pLP/VSVG were delivered together with the pLenti expression vector into the cells at a ratio of 3∶3:3∶3 µg, respectively. Cells were washed 24 hours post-transfection and incubated with fresh media. Supernatants containing viral particles were harvested 72 hours post-transfection and titrated on HeLa cells. The remaining virus was stored at −80°C before use. The transfection of HeLa cells was performed in the presence of Polybrene (6 µg/ml, Chemicon). To transfect 2×105 cells, 1 mL supernatants containing lentivirus was added (3×103 TU/ml). Cells were incubated with fresh media 24 hours after transfection and were subsequently treated by Blasticitin for antibiotic selection 48 hours after transfection.
Cell Culture
293FT cells and HeLa cells (from ATCC, N°CRM-CCL-2, USA) were cultured in Dulbecco’s Modified Eagle’s medium supplemented with 10% fetal bovine serum. HeLa cells stably expressing either Wt-CFTR, G551D-CFTR and F508del-CFTR were selected using Blasticitin (2 µg/ml) for at least 10 days.
Protein Preparation and Immunoblot Analysis
Wt-CFTR, G551D-CFTR and F508del-CFTR expressing cells were collected and homogenized using a glass homogenizer in 2 mL of RIPA buffer. Lyzates were ultra-centrifuged (32500 rpm, 30 min., 4°C). The pellets were resuspended in 500 µL of RIPA. The Wt-CFTR, G551D-CFTR and F508del-CFTR expression were assessed by western blottings. Proteins (50 µg) were loaded on a Bis/Tris precasted polyacrylamide gel (10%, Invitrogen) and transferred onto a PVDF membrane. CFTR proteins were probed using a monoclonal anti-CFTR antibody (24-1, R&D System) and a horseradish peroxidase (HRP) conjugated secondary antibody (Amersham). Other used antibodies were polyclonal anti-calumenin antibody (D-19, Santa Cruz Biotechnology) and polyclonal anti-Grp78 antibody (H-129, Santa Cruz Biotechnology). Horseradish peroxidase (HRP) conjugated secondary antibodies were from Amersham. Blots were developed by enhanced chemiluminescence kit (ECL Plus, Amersham) and analyzed using Chemi-Smart 5100 ECL imaging system (Vilber Lourmat). Intensity of each band was quantified using densitometric analysis of the signals (GelDoc 2000, Biorad). Each value was normalized by the total amount of the loaded proteins per lane which was estimated by staining of the membranes by coomassie blue. Normalization was also performed by the actin signal obtained on the same membranes. The results did not vary with the normalization type.
Methoxy-N-(3-sulfopropyl)quinolinium (SPQ) Fluorescence Assay
Stably transfected cells expressing either Wt-CFTR or G551D-CFTR were used in SPQ experiments as previously described, using forskolin as CFTR activator because it induces cAMP synthesis which regulates the CFTR chloride channel function [19], [27]. Cells were loaded with intracellular SPQ dye by incubation in Ca2+ -free hypotonic (50% dilution) medium containing 10 mM SPQ, 15 min, at 37°C. Coverslips were mounted on the stage of an inverted microscope equipped for fluorescence and illuminated at 360 nm. The emitted light was collected at 456+/−33 nm by a high-resolution image intensifier coupled to a video camera connected to a digital image processing board controlled by FLUO software (Imstar, France). Cells were maintained at 37°C and continuously superfused with an extracellular solution containing in mM:145 NaCl, 4 KCl, 1 MgCl2, 1 CaCl2, 5 HEPES and 5 glucose, pH 7.4. A microperfusion system allowed local application and rapid change of the different experimental mediums. Iodide (I−) and nitrate (NO3−) containing media were identical to the extracellular solution except that I− and NO3− replaced Cl− as the dominant extracellular anions. All extracellular media also contained 10 µM bumetanide to inhibit the Cl−/Na+/K+ co-transporter. Single-cell fluorescence intensity was measured from digital image processing and displayed against time. Fluorescence intensity was standardized according to the equation F = (F−Fo)/Fo×100, where F is the relative fluorescence and Fo, the fluorescence intensity measured in presence of I-. The halides membrane permeability (p) was determined as the rate of SPQ dequenching upon perfusion with nitrates. At least three successive data points were collected immediately after the NO3− containing medium application, and then fitted using a linear regression analysis. The slope of the straight line reflecting the membrane permeability to halides (noted p in min-1) was used as an index of CFTR activity [19], [27].
Immunoprecipitation
Stably transfected HeLa cells expressing G551D-CFTR were washed in cold PBS, scraped into PBS, pelleted and resuspended in the RIPA buffer (25 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 1% Na deoxycholate, 0.1% SDS, 10 mM iodoacetamide, 100 µM PMSF, pH 7.5) in the presence of fresh protease inhibitor cocktail (P8340, Sigma-Aldrich) at a final concentration of 4.5 µl/ml. Anti-CFTR monoclonal antibody M3A7 (Millipore) or anti-calumenin antibody was incubated with magnetic beads (Dyna beats Protein G, Invitrogen) at a final concentration of 0.2 mg per ml of wet beads at 4°C for 1 hour with rocking. 500 µg of whole cell lysate were applied to the beads-antibody complex. Beads were then incubated at 4°C overnight and washed 3 times in cold PBS. Immunoprecipitated proteins were subsequently eluted with 30 µl of SDS-PAGE loading buffer at 95°C for 10 min. Control experiments were performed without any antibody linked to the beads.
Immunofluorescence
Cells were fixed in 4% PFA for 10 min. After fixation and three 5-min washes with PBS-T (PBS 1X plus Tween 0.1%), cells were permeabilized with 0.5% Triton X-100 in PBS for 4 min. After 5-min washes with PBS-T, nonspecific binding was prevented by blocking with 2% BSA in PBS 1X f (30 min, room temperature (RT)). Primary antibody against Calu (H-40, Santa Cruz; 1∶100 in BSA 2%) was applied for 2 h at RT. After three 5-min washes with PBS-T, cells were incubated for 1 h 30 at RT with Alexa fluor 488 goat anti-rabbit IgG (Molecular probes) secondary antibody diluted 1∶1000 in BSA 2%. Then, after three 5-min washes with PBS-T, a second labelling against PDI (Protein Disulfide Isomerase, Invitrogen) was performed. Primary antibody against PDI (1∶1000 in 2% BSA) was applied (12 h, 4°C). After three 5-min washes with PBS-T, cells were incubated for 1 h 30 at RT with Goat anti mouse texas red (Molecular probes) secondary antibody diluted (1∶1000 in BSA 2%). Nuclei were labelled with DAPI (Sigma; 1∶1000 in PBS 1X). Slides were mounted with Fluorsave (Calbiochem) and examined under a Zeiss AxioStar-Plus microscope. Images were collected with Zeiss 100× objectives.
Two-dimensional Gel Electrophoresis (2-DE)
To perform isoelectric focusing, proteins (100 micrograms) were diluted in rehydration buffer (6 M urea, 2 M thiourea, 4% CHAPS, 0.02 M DTT, 0.5% IPG Buffer) to obtain a 125 µL final volume. Samples were loaded onto 7 cm linear pH 4–7 strips (Immoboline DryStrip, GE Healthcare). An Ettan IPGphor Isoelectric Focusing system (GE Healthcare) was used for the first dimension. The second dimension (10% NuPage Bis/Tris precast acrylamide gel) was performed on XCell SureLock® Mini-Cell system (Invitrogen). Coomassie Blue staining was used for image analysis and MS experiments.
Image Acquisition and 2-D Gel Analysis
Coomassie Blue 2-DE gels were scanned and digitized using the GS-800 Calibrated Densitometer (Bio-Rad). Gel alignment, spot detection and quantification were performed using the PDQuest software (Sensitivity: 31.70, Size scale: 3, Min peak: 160, Bio-Rad). Data are the means of six gels from six different experiments.
Mass Spectrometry
Maldi-TOF/TOF analysis of protein spot was performed. The target protein spot was excised from the stained gels, destained and denatured. The gel-piece was then incubated in digestion solution containing 20 µg/mL proteomics grade trypsin for 10–12 h at 37°C. The products of tryptic digestion were deposited on PAC (Prespotted AnchorChip) from Bruker Daltonics. The tryptic peptides were analysed using an autoflex III MALDI-TOF TOF equipped with a smartbeam laser and controlled by the FlexControl 3.0 and FlexAnalysis 3.0 software package. The mass spectrometer was operated with positive polarity in reflectron mode and spectra acquired in the range of m/z 500–4000. A timed ion gate was used for precursor ion selection and the fragments generated were further accelerated with 21 kV in the LIFT cell, and detected following passage through the reflectron. The used software was BioTools 3.2 (Bruker Daltonics). MS spectrum was performed in data-depended mode in which up to 4 precursor ions above an intensity threshold of 7 counts/s (cps) were selected for MS analysis from each survey “scan”. The tandem mass spectrometry data was converted into a PKL file using Masslynx v 4.0 software (Micromass, Waters, USA) and then was imported into a local Mascot 3.2 search engine. The search parameters in MatrixScience Mascot were as follows: the enzyme was trypsin; the taxonomy was selected as Homo sapiens; the mass tolerance was ±0.3 Da; the MS/MS tolerance was ±0.5 Da; the missed cleavage sites were allowed up to 1; the fixed modifications in NCBI nr were selected as carboxymethyl (cysteine); the variable modification was selected as oxidation (methylation) or none; the data format was selected as micromass PKL format; and the instrument was selected as MALDI-TOF/TOF. Individual ions scores >51 indicate identity or extensive homology (P<0.05). MS tolerance was 70 ppm.
Surface Plasmon Resonance
Pure recombinant human calumenin was obtained from Prospec (Israel) and purified full length CFTR was a generous gift from Pr Robert Ford (University of Manchester, UK). CM5 Sensor chip, amine coupling kit (N-hydroxysuccinimide, (NHS), N-ethyl-N-(3-diethylaminopropyl) carbodiimide hydrochloride (EDC), ethanolamine (1 M, pH 8.5) and HBS-EP buffer (10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.005% surfactant P20 at pH 7.4) were obtained from GE Healthcare Bio-Sciences AB. The experiments were performed at the PurIProb core facility (Inserm, UMR1078, Brest). Real-time detection of the Calu - Wt-CFTR were performed using a Biacore system (Biacore 3000; GE Healthcare) and its Control Software version 3.2. All injections were performed at 25°C in HBS-EP 1× running buffer (GE Healthcare). Biacore 3000 was set at 25°C for all steps during the analytical process, and experimental data were collected at a medium rate. Biacore sensorgrams were analyzed using the BIAevaluation software. For each sample the obtained RU value was the value on the active flow cell (FC) minus the value of the reference FC, 20 seconds after the beginning of the dissociation phase. Binding of pure calumenin was performed according to Biacore’s recommendations on the CM5 sensor chips using an Amine Coupling Kit (GE Healthcare) to achieve about 2000 RU on FC2 and FC4, respectively. The surfaces were then blocked with 1 M ethanolamine hydrochloride (pH 8.5). The reference channels (FC1) were activated with equal volumes of NHS and EDC and immediately saturated with ethanolamine. Binding experiments were carried out at a flow rate of 5 µl/min using HBS-EP buffer pH 7.4 as running buffer, in triplicate, at 25°C. In between injections, the surface was regenerated by a 2 microliters pulse of 50 mM NaOH, which was found to be a suitable condition for removing the bound analyte on the immobilized antibody with a very low impairment of the ligands. The results presented here are from FC2-FC1. The reverse experiment in which CFTR was bound was also performed (not shown). Bovine Serum Albumin was used as a negative control. Affinity constants were calculated using the Biacore 3000 Control Software’s wizard (GE Healthcare).
STRING Query of Protein Interaction Network
The calumenin - CFTR interaction which was identified experimentally was evaluated with the online Search Tool for the Retrieval of Interacting Genes/Protein (STRING 9.0, http://string-db.org) to predict both direct and indirect interactions [38]. The confident scoring of the interaction was based on known and predicted protein interactions collected from direct (physical) and indirect (functional) associations. The score was set at 0,4 which represents a medium confidence. The database quantitatively integrated interaction data from four sources - genomic context, high-throughput experiments, co-expression and previous knowledge from research publications. The results are presented with the input protein (calumenin) in the context of a graphical network of interaction partners. Associations in STRING are provided with a probabilistic confidence score which represents an estimate of how likely a given association describes a functional linkage between two proteins. The score is computed and indicates higher confidence when more than one type of information supports the association (direct physical interactions, genetic interaction, text mining, and experiments).
Statistics
Results are expressed as means ± SEM for n cells. To compare data, Student t tests were performed. P<0.01 was considered statistically significant.
Acknowledgments
We thank the association Gaëtan Salaün, the “Etablissement Français du Sang”, the SFR Scinbios (Brest) and the French foundation “Vaincre la Mucoviscidose”. We also thank Simon Delagneau for his technical aid.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the association Gaëtan Salaün, the “Etablissement Français du Sang” and the French CF foundation “Vaincre la Mucoviscidose”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kerem BS, Rommens JM, Buchana JA, Markiewicz D, Cox TK, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 2.Riordan JR, Rommens JM, Kerem BS, Alon N, Rozmahel R, et al. Identification of the cystic fibrosis gene: clonic and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 3.Rommens JM, Lannuzzi MC, Kerem BS, Drumm ML, Melmer G, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 4.Welsh MJ, Tsui LC, Boat TF, Beaudet AL. In CR Scriver, AL Beaudet, WS Sly, Valle D (1995) The Metabolic and Molecular Bases of Inherited Disease (7th edition., McGraw-Hill, New York), 3799–3876. 1995.
- 5.Drumm ML, Pope HA, Cliff WH, Rommens JM, Marvin SA, et al. Correction of the cystic fibrosis defect in vitro by retrovirus-madiated gene transfer. Cell. 1990;62:1227–1233. doi: 10.1016/0092-8674(90)90398-x. [DOI] [PubMed] [Google Scholar]
- 6.Rich DP, Anderson MP, Gregory RJ, Cheng SH, Paul S, et al. Expression of the cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990;347:358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- 7.Szellas T, Nagel G. Aparent affinity of CFTR for ATP is increased by continous kinase activity. FEBS Letters. 2003;535:141–146. doi: 10.1016/s0014-5793(02)03892-9. [DOI] [PubMed] [Google Scholar]
- 8.Cheng SH, Rich DP, Marshall J, Gregory RJ, Welsh MJ, et al. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell. 1991;66:1027–1036. doi: 10.1016/0092-8674(91)90446-6. [DOI] [PubMed] [Google Scholar]
- 9.Anderson MP, Welsh MJ. Regulation by ATP and ADP of CFTR chloride channels that contain mutant nucleotide-binding domains. Science. 1992;257:1701–1704. doi: 10.1126/science.1382316. [DOI] [PubMed] [Google Scholar]
- 10.Quinton PM, Reddy MM. Control of CFTR chloride conductance by ATP levels through non-hydrolytic binding. Nature. 1992;360:79–81. doi: 10.1038/360079a0. [DOI] [PubMed] [Google Scholar]
- 11.Howell LD, Borchardt R, Kole J, Kaz AM, Randak C, et al. Protein kinase A regulates ATP hydrolysis and dimerization by a CFTR domain. Biochem. J. 2004;378:151–159. doi: 10.1042/BJ20021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramjeesingh M, Ugwu F, Li C, Dhani S, Huan LJ, et al. Stable dimeric assembly of the second membrane-spanning domain of CFTR reconstitutes a chloride-selective pore. Biochem. J. 2003;375:633–641. doi: 10.1042/BJ20030774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naren AP, Cormet-Boyaka E, Fu J, Villain M, Blalock JE, et al. CFTR chloride channel regulation by an interdomain interaction. Science. 1999;286:544–548. doi: 10.1126/science.286.5439.544. [DOI] [PubMed] [Google Scholar]
- 14.Naren AP, Kirk KL. CFTR Chloride Channels: Binding Partners and Regulatory Networks. News Physiol. Sci. 2000;15:57–61. doi: 10.1152/physiologyonline.2000.15.2.57. [DOI] [PubMed] [Google Scholar]
- 15.Naren AP, Nelson DJ, Xie W, Jovov B, Pevsner J, et al. Regulation of CFTR chloride channels by syntaxin and Munc 18 isoforms. Nature. 1997;390:302–305. doi: 10.1038/36882. [DOI] [PubMed] [Google Scholar]
- 16.Naren AP, Quick MW, Collawn JF, Nelson DJ, Kirk KL. Syntaxin 1A inhibits CFTR chloride channels by means of domain-specific protein-protein interactions. Proc. Natl. Acad. Sci. USA. 1998;95:10972–10977. doi: 10.1073/pnas.95.18.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun F, Hug MJ, Lewarchik CM, Yun CHC, Bradbury NA, et al. E3KARP mediates the association of ezrin and protein kinase A with the cystic fibrosis transmembrane conductance regulator in airway cells. J. Biol. Chem. 2000;275:29539–29546. doi: 10.1074/jbc.M004961200. [DOI] [PubMed] [Google Scholar]
- 18.Trouvé P, Le Drévo MA, Kerbiriou M, Fichou Y, Gillet D, et al. Annexin V is directly involved in cystic fibrosis transmembrane conductance regulator’s chloride channel function. Biochim. Biophys. Acta. 2007;1772(10):1121–33. doi: 10.1016/j.bbadis.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Le Drévo MA, Benz N, Kerbiriou M, Gioux M, Pennec JP, et al. Annexin A5 increases the cell surface expression and the chloride channel function of the ΔF508-Cystic Fibrosis Transmembrane Regulator. Biochim. Biophys. Acta. 1782 605–614. 2008. [DOI] [PubMed]
- 20.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993;73:1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- 21.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N. Engl. J. Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 22.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 23.Bompadre SG, Sohma Y, Li M, Hwang TC. G551D and G1349D, two CF-associated mutations in the signature sequences of CFTR, exhibit distinct gating defects. J. Gen. Physiol. 2007;129:285–298. doi: 10.1085/jgp.200609667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bompadre SG, Li M, Hwang TC. Mechanism of G551D-CFTR potentiation by a high affinity ATP analog. J. Biol. Chem. 2008;283:5364–5369. doi: 10.1074/jbc.M709417200. [DOI] [PubMed] [Google Scholar]
- 25.Pasyk S, Li C, Ramjeesingh M, Bear CE. Direct interaction of a small-molecule modulator with G551D-CFTR, a cystic fibrosis-causing mutation associated with severe disease. Biochem. J. 2009;418:185–190. doi: 10.1042/BJ20081424. [DOI] [PubMed] [Google Scholar]
- 26.Caputo A, Hinzpeter A, Caci E, Pedemonte N, Arous N, et al. Mutation-specific potency and efficacy of cystic fibrosis transmembrane conductance regulator chloride channel potentiators. J Pharmacol Exp Ther. 2009;330(3):783–791. doi: 10.1124/jpet.109.154146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leblais V, Demolombe S, Vallette G, Langin D, Baro I, et al. β3-adrenoreceptor control the CFTR conductance through a cAMP/PKA- independent pathway. J.Biol.Chem. 1999;274:6107–6113. doi: 10.1074/jbc.274.10.6107. [DOI] [PubMed] [Google Scholar]
- 28.Anderson MP, Rich DP, Gregory RJ, Smith AE, Welsh MJ. Generation of cAMP-activated chloride currents by expression of CFTR. Science. 1991;251(4994):679–82. doi: 10.1126/science.1704151. [DOI] [PubMed] [Google Scholar]
- 29.Gregory RJ, Rich DP, Cheng SH, Souza DW, Paul S, et al. Maturation and function of cystic fibrosis transmembrane conductance regulator variants bearing mutations in putative nucleotide-binding domains 1 and 2. Mol Cell Biol. 1991;11(8):3886–93. doi: 10.1128/mcb.11.8.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honoré B. The rapidly expanding CREC protein family: members, localization, function, and role in disease. Bioessays. 2009;31(3):262–77. doi: 10.1002/bies.200800186. [DOI] [PubMed] [Google Scholar]
- 31.Sahoo SK, Kim do H. Calumenin interacts with SERCA2 in rat cardiac sarcoplasmic reticulum. Mol Cells. 2008;26(3):n265–9. [PubMed] [Google Scholar]
- 32.Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, et al. Hsp90 Cochaperone Aha1 Downregulation Rescues Misfolding of CFTR in Cystic Fibrosis. Cell. 2006;127(4):503–815. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 33.Gomes-Alves P, Couto F, Pesquita C, Coelho AV, Penque D. Rescue of F508del-CFTR by RXR motif inactivation triggers proteome modulation associated with the unfolded protein response. Biochim Biophys Acta. 2010;1804(4):856–65. doi: 10.1016/j.bbapap.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Vorum H, Hager H, Christensen BM, Nielsen S, Honoré B. Human calumenin localizes to the secretory pathway and is secreted to the medium. Exp Cell Res. 1999;248(2):473–81. doi: 10.1006/excr.1999.4431. [DOI] [PubMed] [Google Scholar]
- 35.Kerbiriou M, Le Drévo MA, Férec C, Trouvé P. Coupling cystic fibrosis to endoplasmic reticulum stress: differential role of Grp78 and ATF6. Biochimica et Biophysica Acta. 2007;1772:1236–49. doi: 10.1016/j.bbadis.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Devor DC, Engelhardt JF, Ernst SA, Strong TV, et al. Molecular basis of defective anion transport in L cells expressing recombinant forms of CFTR. Human Molecular Genetics 2. 1993;8:1253–1261. doi: 10.1093/hmg/2.8.1253. [DOI] [PubMed] [Google Scholar]
- 37.Trouvé P, Kerbiriou M, Le Hir S, Benz N, Férec C. Surface Plasmon Resonance shows a gender difference in circulating annexin A5 in Human. Talanta 15. 2012;93:219–223. doi: 10.1016/j.talanta.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Szklarcyk D, Franchescini A, Kuhn M, Simonovic M, Roth A, et al. The STRING Database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011;39:D561–8. doi: 10.1093/nar/gkq973. [DOI] [PMC free article] [PubMed] [Google Scholar]