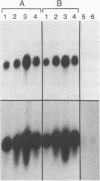
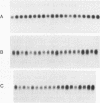
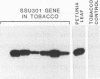
Abstract
The petunia rbcS gene SSU301 was introduced into tobacco using Agrobacterium tumefaciens-mediated transformation. The time at which rbcS expression was maximal after transfer of the tobacco plants to the greenhouse was determined. The expression level of the SSU301 gene varied up to 9 fold between individual tobacco plants which had been standardized physiologically as much as possible. The presence of adjacent pUC plasmid sequences did not affect the expression of the SSU301 gene. In an attempt to reduce the between-transformant variability in expression, the SSU301 gene was introduced into tobacco surrounded by 10kb of 5' and 13 kb of 3' DNA sequences which normally flank SSU301 in petunia. The longer flanking regions did not reduce the between-transformant variability of SSU301 gene expression.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachy R. N., Chen Z. L., Horsch R. B., Rogers S. G., Hoffmann N. J., Fraley R. T. Accumulation and assembly of soybean beta-conglycinin in seeds of transformed petunia plants. EMBO J. 1985 Dec 1;4(12):3047–3053. doi: 10.1002/j.1460-2075.1985.tb04044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Elzen P., Tamaki S., Dunsmuir P., Bedbrook J. Differential expression of the eight genes of the petunia ribulose bisphosphate carboxylase small subunit multi-gene family. EMBO J. 1985 Dec 1;4(12):3055–3061. doi: 10.1002/j.1460-2075.1985.tb04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Favreau M., Dunsmuir P., Bedbrook J. Confirmation of the relative expression levels of the Petunia (Mitchell) rbcS genes. Nucleic Acids Res. 1987 Jun 11;15(11):4655–4668. doi: 10.1093/nar/15.11.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., van den Elzen P., Tamaki S., Dunsmuir P., Bedbrook J. Linkage and homology analysis divides the eight genes for the small subunit of petunia ribulose 1,5-bisphosphate carboxylase into three gene families. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4964–4968. doi: 10.1073/pnas.82.15.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaese P., De Greve H., Decraemer H., Schell J., Van Montagu M. Rapid mapping of transposon insertion and deletion mutations in the large Ti-plasmids of Agrobacterium tumefaciens. Nucleic Acids Res. 1979 Dec 11;7(7):1837–1849. doi: 10.1093/nar/7.7.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmuir P., Smith S., Bedbrook J. A number of different nuclear genes for the small subunit of RuBPCase are transcribed in petunia. Nucleic Acids Res. 1983 Jun 25;11(12):4177–4183. doi: 10.1093/nar/11.12.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Jones J. D., Dunsmuir P., Bedbrook J. High level expression of introduced chimaeric genes in regenerated transformed plants. EMBO J. 1985 Oct;4(10):2411–2418. doi: 10.1002/j.1460-2075.1985.tb03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F., Morelli G., Fraley R. T., Rogers S. G., Chua N. H. Photoregulated expression of a pea rbcS gene in leaves of transgenic plants. EMBO J. 1985 Dec 1;4(12):3063–3068. doi: 10.1002/j.1460-2075.1985.tb04046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell J. T., Nagy F., Chua N. H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. 1985 Feb 28-Mar 6Nature. 313(6005):810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townes T. M., Lingrel J. B., Chen H. Y., Brinster R. L., Palmiter R. D. Erythroid-specific expression of human beta-globin genes in transgenic mice. EMBO J. 1985 Jul;4(7):1715–1723. doi: 10.1002/j.1460-2075.1985.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]