The biomedical research community has been quick to apply the most modern methods in molecular and cellular biology to the study of human pathological specimens. This approach has fostered the molecular understanding of human disease, while at the same time it has provided unique insight into the normal functions of individual molecules. In settings where disease phenotypes have been less informative, the development of targeted gene disruptions in mice has provided an important alternative approach, which avoids the ambiguities resulting from use of chemical inhibitors. Critical to all of these studies is the existence of precise physiological methods for studying the functions of tissues and organs, but this technology has unfortunately been neglected in recent years because of a misperception that classical physiology is not fashionable.
The paper by Schnermann and colleagues (1) in this issue of the Proceedings is an excellent example of how the application of classical physiological measurements to tissues from transgenic mice successfully answered an important biological question: how is water reabsorbed by renal proximal tubules? The human kidney plays an important role in waste removal by filtering approximately 200 liters of plasma per day from which essential solutes are reabsorbed along with most of the water. Renal proximal tubules and descending thin limbs of Henle’s loop are the sites where approximately 80% of this fluid is reabsorbed. The vectorial distribution of salt and sugar transporters at the apical membranes (facing the urinary lumen) or basolateral membranes (facing the interstitium) together create a small-standing osmotic gradient across the tubular epithelium. Thus, the interstitium is slightly hyperosmolar with respect to the urinary lumen, providing the driving force for water reabsorption that is essential for the countercurrent mechanism by which urine is concentrated to osmolalities far above the plasma. It has long been debated whether water is reabsorbed through the renal proximal tubular epithelial cells (transcellular pathway) or through the spaces between cells (paracellular pathway).
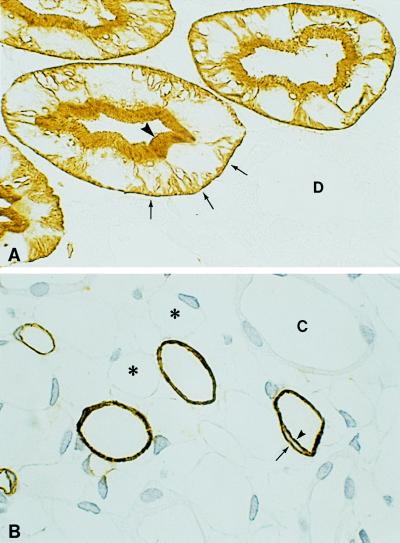
Fortunately, classical renal physiologists were ready when AQP1 knockout mice became available (1), and they demonstrated that this water channel protein is critical to the transcellular absorption of water by renal proximal tubules. These studies make beautiful sense because the earliest observations that AQP1 resides in apical and basolateral membranes of renal proximal tubules and descending thin limbs (2) provided the essential clue that AQP1 functions as a water transporter (3). Moreover, the abundance of AQP1 at these sites is so striking (ref. 4 and Fig. 1) that calculations predicted AQP1 would fully explain the water permeability of the proximal nephron (5). Surprisingly, the rare humans lacking the Colton blood group antigens were found to bear disrupting mutations in the AQP1 gene (6); however, none exhibited obvious signs of kidney dysfunction. This discrepancy now warrants reanalysis because the studies of kidneys from AQP1 knockout mice were found to have a marked solute concentration defect, and the studies also predicted that compensatory mechanisms will diminish the phenotype in the unstressed animals. Thus, careful water deprivation studies may be needed to uncover renal defects caused by AQP1 deficiency in humans.
Figure 1.
Thin cryosections (1 μm) of rat kidney immunolabeled with anti-AQP1 and counterstained with peroxidase. (A) Cortex contains proximal tubules with abundant immunolabeling of the apical brush border (arrowhead) and basolateral plasma membranes with infoldings (arrows). Other nephron segments including distal-convoluted tubules (D) failed to label. (B) Inner medulla contains descending thin limbs that exhibit extensive immunolabeling of both apical (arrowhead) and basolateral (arrow) plasma membranes domains. Vascular structures, ascending thin limbs (∗), and collecting ducts (C) failed to label. ×650. Figure provided by Søren Nielsen, University of Aarhus, Denmark.
Interest in this problem by classical physiologists was not simply a coincidence, for investigators in the National Institutes of Health Laboratory of Kidney and Electrolyte Metabolism (LKEM), the institutional home of two authors of ref. 1, have focused their attention on renal transport processes ever since their doors were opened 50 years ago this spring. Previous studies at the LKEM contributed many fundamental discoveries in renal physiology, including development of the isolated perfused tubule by Maurice Burg and his colleagues (7), a technique essential to the analysis of AQP1 knockout mice (1). Several scientific groups are now directing their attention to the aquaporins, a large family of water transport molecules whose members each have unique tissue distributions in kidney (8, 9). Mutations in AQP2 have been recognized to cause some forms of nephrogenic diabetes insipidus (10). AQP2 also is secondarily involved in numerous defects of water metabolism including lithium toxicity, postobstructive polyuria, congestive heart failure, and pregnancy (11). Recognition that at least six different aquaporins are expressed in kidney indicate that the full repertoire of renal physiological methods may be needed to probe the significance of aquaporins, as well the other transport molecules, which are expressed in this complex organ.
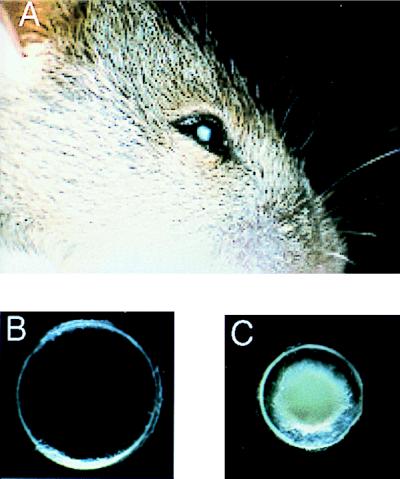
Although nephrologists have led the way in transport physiology, aquaporins are expressed in numerous other tissues, and the array of clinical defects involving aquaporins is likely to be exceedingly diverse. Thus classical physiological analyses of other tissues including lung (12), hepatobiliary tract (13), salivary gland (14), and eye (15) may provide insight into other normal and pathological roles of this family of proteins. Mutations in the gene encoding the lens protein AQP0, also known as major intrinsic protein (16), were found to underlie the “CAT mouse” phenotype (congenital cataracts, Fig. 2). This suggests that mutations in the AQP0 gene may cause human cataracts or that secondary defects in the protein may contribute to presbyopia. The recent development of a targeted gene disruption of AQP4 in mice revealed a minor renal phenotype (17); however, the abundance of this protein in brain predicts a physiological role in water metabolism within the central nervous system (18).
Figure 2.
CAT mouse features microphthalmia and congenital cataracts resulting from a mutation in the gene encoding lens AQP0 (major intrinsic protein of lens). (A) Head of a 3-week-old CAT mouse, revealing the unusually small eye. (B) Lens from a normal 21-day-old mouse. (C) Lens from a 21-day-old CAT mouse is small and contains a dense opacity. Figure provided by Alan Shiels, Washington University, St. Louis, MO.
As expected for a fundamental process such as water transport, members of the aquaporin family have been identified in virtually all nonmammalian species including invertebrates, microorganisms, and plants (19). A mutation in the Drosophila homolog of AQP4 has been linked previously to the defect known as big brain (20). AqpZ in Escherichia coli has been shown to confer a distinct growth advantage under hypo-osmolar conditions (21), an example in which bacterial physiology may explain the need to repeatedly clean our bathroom toilet bowls. Numerous genes encoding members of the aquaporin family are being identified in plants where these proteins are being found to participate in many physiological and pathological processes (22). Development of a transgenic Arabidopsis thaliana expressing antisense specific for aquaporin PIP1b demonstrated the importance of this protein in root water uptake (23). The antisense plants are able to maintain normal xylem pressure by sending out an increased number of rootlets (Fig. 3). Multiple physiological processes of plants such as seed dessication, transpiration, and inhibition of self-pollination are being reported to involve aquaporins (24).
Figure 3.
Morphology of transgenic Arabidopsis thaliana. Plant expressing antisense RNA to aquaporin PIP1b (Left) and control plant (Right). Except for a fivefold amplification of root arborization, the morphology of the antisense plant is equivalent to the control. Modified with permission (23). Figure provided by Ralf Kaldenhoff, University of Würzburg, Germany.
The complexity of the aquaporin family of water transport molecules will certainly be expanded by the genome-sequencing projects. As with the AQP1 null mice (1), simple defects in cellular plumbing are the molecular basis of human nephrogenic diabetes insipidus, CAT mice, and thirsty plants (25). It is worth remembering that rigorous elucidation of these abnormal functions will require the sophisticated methods of classical physiology.
Footnotes
The companion to this commentary is published on pages 9660–9664.
References
- 1.Schnermann J, Chou C-L, Ma T, Traynor T, Knepper M A, Verkman A S. Proc Natl Acad Sci USA. 1998;95:9660–9664. doi: 10.1073/pnas.95.16.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denker B M, Smith B L, Kuhajda F P, Agre P. J Biol Chem. 1988;263:15634–15642. [PubMed] [Google Scholar]
- 3.Preston G M, Carroll T P, Guggino W B, Agre P. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen S, Smith B L, Christensen E I, Knepper M A, Agre P. J Cell Biol. 1993;120:371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maeda Y, Smith B L, Agre P, Knepper M A. J Clin Invest. 1995;95:422–428. doi: 10.1172/JCI117672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preston G M, Smith B L, Zeidel M L, Moulds J J, Agre P. Science. 1994;265:1585–1587. doi: 10.1126/science.7521540. [DOI] [PubMed] [Google Scholar]
- 7.Burg M, Grantham J, Abramow M, Orloff J. Am J Physiol. 1966;210:1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- 8.Knepper M A, Wade J B, Terris J, Ecelbarger C A, Marples D, Mandon B, Chou C L, Kishore B K, Nielsen S. Kidney Int. 1996;49:1712–1717. doi: 10.1038/ki.1996.253. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki S, Ishibashi K, Marumo F. Annu Rev Physiol. 1998;60:199–220. doi: 10.1146/annurev.physiol.60.1.199. [DOI] [PubMed] [Google Scholar]
- 10.Deen P M, Verdijk M A, Knoers N V, Wieringa B, Monnens L A, van Os C H, Van Oost B A. Science. 1994;264:92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen, S., Frøkiær, J. & Knepper, M. A. (1998) Curr. Opin. Nephrol. Hypertens., in press. [DOI] [PubMed]
- 12.Matthay M A, Folkesson H G, Verkman A S. Am J Physiol. 1996;270:L487–L503. doi: 10.1152/ajplung.1996.270.4.L487. [DOI] [PubMed] [Google Scholar]
- 13.Marinelli R A, LaRusso N F. Semin Liver Dis. 1996;16:221–229. doi: 10.1055/s-2007-1007234. [DOI] [PubMed] [Google Scholar]
- 14.Turner R T. Ann N Y Acad Sci. 1993;694:24–35. doi: 10.1111/j.1749-6632.1993.tb18339.x. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Dept. of Health and Human Services, National Eye Institute. Vision Research, A National Plan: 1999–2003. A Report of the National Advisory Eye Council. Bethesda: Natl. Inst. of Health; 1998. , No. 98–4120, pp. 41–93. [Google Scholar]
- 16.Shiels A, Bassnett S. Nat Genet. 1996;12:212–215. doi: 10.1038/ng0296-212. [DOI] [PubMed] [Google Scholar]
- 17.Chou C L, Ma T, Yang B, Knepper M A, Verkman A S. Am J Physiol. 1998;274:C549–C554. doi: 10.1152/ajpcell.1998.274.2.C549. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen S, Nagelhus E A, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen O P. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J H, Saier M H., Jr J Membr Biol. 1996;153:171–180. doi: 10.1007/s002329900120. [DOI] [PubMed] [Google Scholar]
- 20.Rao Y, Jan L Y, Jan Y N. Nature (London) 1990;345:163–167. doi: 10.1038/345163a0. [DOI] [PubMed] [Google Scholar]
- 21.Calamita G, Kempf B, Bonhivers M, Bishai W R, Bremer E, Agre P. Proc Natl Acad Sci USA. 1998;95:3627–3631. doi: 10.1073/pnas.95.7.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maurel C. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:399–429. doi: 10.1146/annurev.arplant.48.1.399. [DOI] [PubMed] [Google Scholar]
- 23.Kaldenhoff R, Grote K, Zhu J-J, Zimmermann U. Plant J. 1998;14:121–128. doi: 10.1046/j.1365-313x.1998.00111.x. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda S, Nasrallah J B, Dixit R, Preiss S, Nasrallah M E. Science. 1997;276:1564–1566. doi: 10.1126/science.276.5318.1564. [DOI] [PubMed] [Google Scholar]
- 25.Agre P, Bonhivers M, Borgnia M J. J Biol Chem. 1998;273:14659–14662. doi: 10.1074/jbc.273.24.14659. [DOI] [PubMed] [Google Scholar]