Abstract
In the mature mammalian auditory system, inner hair cells are responsible for converting sound-evoked vibrations into graded electrical responses, resulting in release of neurotransmitter and neuronal transmission via the VIIIth cranial nerve to auditory centres in the central nervous system. Before the cochlea can reliably respond to sound, inner hair cells are not merely immature quiescent pre-hearing cells, but instead are capable of generating ‘spontaneous’ calcium-based action potentials. The resulting calcium signal promotes transmitter release that drives action potential firing in developing spiral ganglion neurones. These early signalling events that occur before sound-evoked activity are thought to be important in guiding and refining the initial phases of development of the auditory circuits. This review will summarise our current knowledge of the mechanisms that underlie spontaneous action potentials in developing inner hair cells and how these events are triggered and regulated.
Keywords: action potentials, calcium, inner hair cells, cochlea, development, auditory, spiral ganglion neuron
Introduction
Our exquisite ability to hear and discriminate sounds relies upon precise organisation of auditory pathways. The mature auditory system is a highly ordered structure and the neural connections are intricately mapped throughout the auditory brain regions. A major challenge in auditory research has been to gain an understanding of how these complex but highly ordered neuronal circuits are generated during development.
Although the development of hearing is a multi-step process, for the purpose of this review, the ‘onset of hearing’ will be considered to be when auditory neurones reliably respond to normal intensities of airborne sound; in rats, this is around postnatal day (P)10–12. It is generally accepted that much of the complex circuitry that underpins our ability to hear is established before the cochlea can reliably respond to sound and is therefore independent of sound evoked activity. After this, neuronal connections undergo a period of experience-driven refinement, generated by sound-evoked electrical activity in the auditory system, that leads to the mature, adult neural connections (for reviews, see Friauf and Lohmann (1999), Rubel and Fritzsch (2002), Kandler et al. (2009), and Blankenship and Feller (2010)). However, an interesting question arises: how is such an intricate auditory map generated before the onset of sound evoked activity? Initially, this early mapping was thought to be a result of molecular cues present in the very early embryo, effectively creating a system that was predetermined. However, this view has been radically modified as it has become increasingly evident that many other systems, such as the retina, also require experience-independent firing to promote, for example, neuronal survival, and synaptic remodelling (reviewed in Moody (1998), Friauf and Lohmann (1999), and Blankenship and Feller (2010)). Many authors have drawn comparisons between the correlated bursting activities seen in the pre-hearing auditory system to the light independent waves of activity that underlie the development of the retina. Such comparisons have led to the suggestion that spontaneous activity in auditory neurones, before the onset of hearing, is likely to play a role in the development and refinement of auditory maps. However, despite this, only relatively recently is direct evidence of a role for sound-independent activity emerging. For example, Leao et al. (2006a, b) made recordings from neurones in the medial nucleus of the trapezoid body (MNTB) and demonstrated that their neuronal membrane properties were tonotopically arranged with gradients in ion channel function. In hearing-impaired mice that lacked auditory nerve activity before (and after) the onset of hearing, these normal tonotopic gradients were absent at the onset of hearing (Leao et al. 2006a, b). These studies provide convincing evidence that auditory nerve activity before the onset of hearing is required for normal tonotopic development of neurones in the MNTB. The contribution of pre- and post-hearing activity to the development and refinement of auditory brain maps is likely to be an area of great interest and debate and the reader is referred to several reviews (Moody 1998; Friauf and Lohmann 1999; Rubel and Fritzsch 2002; Walmsley et al. 2006; Huberman et al. 2008; Kandler et al. 2009; Blankenship and Feller 2010).
Although it is well established that spontaneous activity occurs in the developing cochlea, until relatively recently, how such activity was generated was not fully understood. During the last two decades, it has been clearly established that developing inner hair cells (IHCs) are not merely quiescent cells awaiting the onset of hearing, but are generating electrical activity in the form of calcium-based action potentials (Kros et al. 1998). These action potentials have much slower kinetics than sodium-based action potentials and can generate sufficient calcium influx to trigger transmitter release even in cells with relatively immature synapses (Beutner and Moser 2001; Marcotti et al. 2003b, 2004). The release of neurotransmitter activates afferent boutons and drives bursts of firing activity in spiral ganglion neurones (Glowatzki and Fuchs 2002; Tritsch et al. 2010). As I will discuss in this review, it is now proposed that the early electrical activity in IHCs, together with the genetically determined molecular cues, are the mechanism by which the auditory system can establish the detailed circuitry present before the cochlea can reliably respond to sound.
Although we have considerable information on action potentials in IHCs, details of whether their generation is entirely spontaneous or externally evoked still remains an area of debate. In addition, how they might influence the connectivity and refinement of the early maps has yet to be established. This review will outline our current knowledge and understanding of the mechanisms that generate and regulate these early sound-independent signalling events and their transmission to auditory nerve fibres.
Action potential firing in immature IHCs
Mice, rats and other altricial animals have provided excellent models for studying the development of hearing because they are born deaf and develop hearing during the first two postnatal weeks, with the cochlea able to reliably respond to sound at around postnatal day (P)10–12. During this developmental period, the IHCs do not respond to sound but instead are capable of firing spontaneous and evoked calcium action potentials that disappear close to P10–12 as a large, fast potassium current develops and changes the cell from an excitable cell, capable of firing action potentials, to one that responds with graded receptor potentials as it does in the mature system (Kros et al. 1998; Beutner and Moser 2001; Marcotti et al. 2003b). It is worth noting that outer hair cells are also able to fire similar action potentials, but their function is currently unknown (Oliver et al. 1997; Beurg et al. 2008).
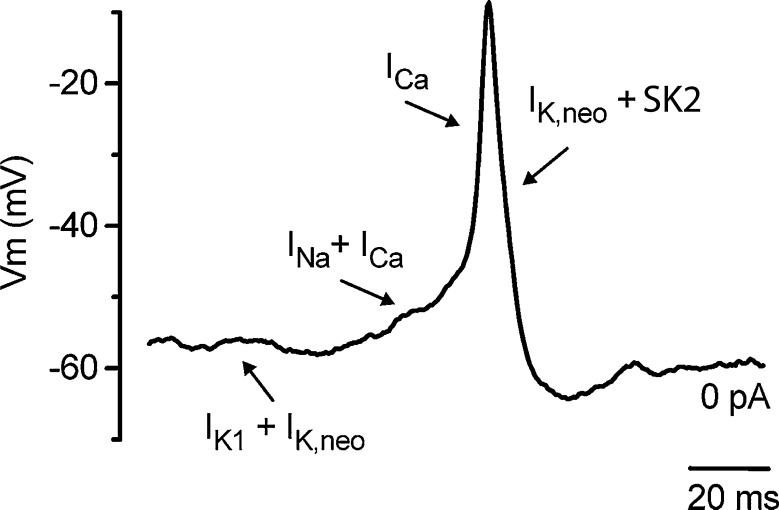
Unlike neurones, the IHC action potentials are generated by calcium influx as removing external calcium or blocking calcium channels completely prevents firing. About 70 % of immature IHCs also have a fast activating and inactivating sodium current that disappears in the second postnatal week between P10–12. Blocking the sodium current with TTX has no effect on the amplitude of the action potential, although it does slow the firing rate (Marcotti et al. 2003b). The currents involved in action potential firing are summarised in Figure 1, where the slow depolarisation preceding the action potential is initiated by sodium and calcium currents that set the firing rate, but the upstroke of the action potential is wholly dependent on calcium influx. Spontaneous firing first occurs in apical IHCs as early as embryonic (E) day 18.5, (where E19–20 is the day of birth) when they are broad and only depolarise the cell by about 20 mV. However, as development proceeds, both the calcium current and action potential amplitude increase steadily until age P7 (Beutner and Moser 2001; Marcotti et al. 2003b). At this point in development, intracellular calcium signals generated by step depolarisation of IHCs under voltage clamp are broadly distributed around the base of the cell and not confined to discrete hotspots (Kennedy and Meech 2002) as they are in the mature system (Frank et al. 2009). This suggests that the large calcium current present during this first postnatal week may be generated by the combined activity of calcium channels aggregated at synaptic zones and extra-synaptic calcium channels. The latter may be required to generate sufficient inward current to maintain spontaneous spiking. The calcium current that generates spiking is an L-type (long lasting) current carried by the Cav1.3 subunit (Platzer et al. 2000; Brandt et al. 2003). This current activates at relatively negative potentials and studies in IHCs have reported varying degrees of calcium-dependent inactivation, from almost no inactivation to approximately 40 % (Kennedy 2002; Tarabova et al. 2007; Grant and Fuchs 2008; Johnson and Marcotti 2008).
FIG. 1.
Membrane currents involved in spiking in developing IHCs. A spontaneous action potential recorded from a 3-day-old mouse showing the different phases of the action potential and how these are generated by the underlying basolateral currents (arrows). Initially, both sodium and calcium currents generate a slow depolarisation before the upstroke of the action potential which is only dependent on the calcium current. Repolarisation results from the reduction in calcium current and activation of the delayed rectifier current present in neonatal IHCs (IK,neo) and SK2 potassium currents. Reproduced with permission from Marcotti et al. (2004).
Action potential repolarisation results from a combination of calcium channel inactivation, voltage activation of the delayed rectifier current and activation of small conductance calcium activated potassium (SK2) currents. In immature IHCs, the SK2 current first appears between P0 and P2 depending on cochlea location (Marcotti et al. 2004; Roux et al. 2011); and by P2–3, it contributes to the robust repolarisation of the action potential by reducing the half width and generating the after-hyperpolarisation. Inhibition of the SK2 current by the bee venom apamin, an SK2-specific blocker, leads to a loss of the after-hyperpolarisation and a gradual failure of firing, initially causing broad or prolonged action potential plateaus but eventually leading to a maintained depolarisation (Marcotti et al. 2003a, b, 2004; Johnson et al. 2007). The delayed rectifier current present in neonatal IHCs (IK,neo) also aids repolarisation but has not as yet been identified at the molecular level (Fig. 1). Thus, the generation and kinetics of the action potentials are dependent on the interactions between the calcium channel, calcium signal, and the SK2 and delayed rectifier potassium currents as shown in Figure 1. Of critical importance is the calcium current that generates both the upstroke of the action potential and the intracellular calcium signal that activates the SK2 current responsible for the robust repolarisation and maintenance of action potential firing. Recordings of calcium currents from immature IHCs (<P12) made at body temperature are three times larger than those made at room temperature (Grant and Fuchs 2008), although recordings from older animals (P13–16) show less temperature sensitivity (Nouvian 2007). In immature IHCs, such an effect of temperature is likely to have a significant impact on all components of the action potential as both the size of the upstroke and intracellular calcium signal are critically dependent on calcium influx. Additionally, calcium buffer concentration, binding kinetics and mobility will help to determine the rise and spread of the intracellular calcium signal (Roberts 1994) important in SK2 activation. If endogenous buffering levels are perturbed during whole cell patching, this is likely to have an impact on action potential kinetics and may affect regularity of firing as calcium buffers are known to vary with age and cochlear location (Hackney et al. 2003, 2005). It is perhaps not surprising therefore that different experimental conditions can have a significant impact on action potential firing (Johnson et al. 2011).
Action potential firing generates transmitter release
Clearly, if IHC action potential firing is to generate signals within the auditory pathways, then not only should the action potentials generate neurotransmitter release but the IHCs must receive afferent innervation capable of detecting and responding to this release. Afferent nerve fibres invade the sensory epithelium even before terminal differentiation of hair cells occurs. As soon as IHCs differentiate, they are contacted by afferent nerve fibres, but whether these contacts are functional so early has not been clearly demonstrated. At birth, IHCs already have the specialised dense structures surrounded by transmitter release vesicle known as ribbon structures. However, during the first postnatal weeks, the ribbons generally have an immature round form and may be present in clusters, with or without adjacent nerve fibres. During the second postnatal week, the ribbon structures begin to take their more mature plate-like form (Sobkowicz et al. 1982).
Two different approaches have been used to study synaptic activity of IHCs. Firstly, direct measurements of changes in IHC capacitance, a measure presumed to reflect vesicle fusion with the cell membrane (Neher and Marty 1982), provide measurements from all of the ribbon synapses in any given cell (Moser and Beutner 2000; Johnson et al. 2005). A second approach of recording activity from afferent boutons directly attached to an IHC gives information on activity at a single synapse (Glowatzki and Fuchs 2002). Such approaches have demonstrated that immature IHCs are capable of transmitter release, albeit with a reduced sensitivity to calcium, compared to the hearing system, from as early as E17.5 (Johnson et al. 2005); although at this early stage, it is not known whether the afferent terminals are functional. Capacitance measurements demonstrate that a single action potential generates sufficient calcium influx to trigger neurotransmitter release (Beutner and Moser 2001; Johnson et al. 2005). Furthermore, recordings from afferent boutons attached to P7–11 IHCs show that the glutamate release initiates excitatory post-synaptic currents (EPSCs) in the afferent boutons. In a series of pioneering experiments, Goutman and Glowatzki (2007) recorded simultaneously from pre-hearing rat IHCs and a single afferent bouton attached to the IHC. They demonstrated that depolarisation of the IHC caused a corresponding large response in the afferent bouton. During long depolarisation, the initial peak was followed by a mix of single EPSCs with a wide range of amplitudes and multiphasic EPSCs, reflecting both highly co-ordinated and less coordinated release from the IHC (Goutman and Glowatzki 2007; Grant et al. 2010). These experiments demonstrate that immature IHCs are capable of transmitter release and that the afferent boutons express the appropriate receptors that allow them to respond to the transmitter release.
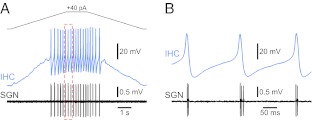
More recently, Tritsch et al. (2010) made recordings from spiral ganglion neurones (SGNs) and demonstrated that their activity occurred in bursts that repeated every 2–3 s. Within these bursts, the activity was not random but occurred as mini-bursts that repeated every 100–300 ms. The firing patterns recorded were not only consistent over time but also between SGNs, suggesting that the cells were being driven by a pacemaker. Recordings from IHCs showed similar patterns of activity with periodic depolarisations generating burst of action potentials with similar inter-spike intervals to those seen in SGN mini-bursts. Unequivocal evidence of the role for IHCs in driving the activity in SGNs came from simultaneous recordings from IHCs and their corresponding postsynaptic SGN (Fig. 2). It can be seen that initially the IHCs and SGN are silent before current-evoked depolarisation in the IHC generates a period of spiking. As seen in Figure 2, each IHC action potential generated a small burst of two to three action potentials in the SGN (Tritsch et al. 2010).
FIG. 2.
IHC action potentials drive spontaneous firing in SGNs in the pre-hearing ear. A Simultaneous recordings from an IHC (whole cell, blue trace) and corresponding postsynaptic SGN (extracellular recording, black trace) in a pre-hearing rat. IHC resting potential −80 mV, stimulated with a 40 pA current injection to initiate firing. The dashed red box is shown in more detail (B) showing that each IHC action potential initiates several action potentials in the SGN. Adapted by permission from Macmillan Publishers Ltd: Nature Neuroscience (Tritsch et al. 2010), copyright (2010).
Together, these data indicate that after birth, IHCs release neurotransmitter onto functional afferent nerve terminals and generate patterns of activity in SGNs (Tritsch et al. 2010). It is possible that such activity may occur even before birth as IHCs are capable of transmitter release at that stage (Johnson et al. 2005). Given that, some interesting questions arise: How are the action potentials generated? And what controls whether the firing is continuous or in bursts? There are two main possibilities: One is that the action potentials are entirely self-generated and are wholly reliant on the balance and kinetics of the ion channels and calcium signals present in hair cells, but that the pattern of firing can also be regulated by external mechanisms. The other is that an external stimulus generates depolarisation that initiates firing and controls the pattern of firing. Both of these possibilities are considered in the following sections.
The role of efferents in the control of IHC action potential firing
Early in development, IHCs are transiently innervated by medial olivocochlear efferent fibres (Simmons et al. 1996) that disappear after the onset of hearing (Simmons 2002). These efferent fibres first make contact with IHCs as early as E16 and increase in density until the end of the first postnatal week (Sobkowicz and Emmerling 1989). By P1, these synapses appear fully functional as ACh-activated currents can be recorded from IHCs (Roux et al. 2011). Opposite the efferent fibres, the IHC membrane expresses ACh receptors (AChRs) that are also transiently expressed with a time frame that matches the nerve innervation. The AChR α9 subunit can be detected as early as E18 with α10 being detected a few days later at E21, and both subunits show clear basal to apical gradients in expression (Simmons and Morley 1998, 2011; Elgoyhen et al. 2001; Morley and Simmons 2002) suggesting that the influence of efferent innervation could vary with cochlear location. The two subunits form an unusual nicotinic AChR composed of two α9 and three α10 subunits, a combination of subunits that renders the receptor highly permeable to calcium (Elgoyhen et al. 1994, 2001; Glowatzki and Fuchs 2000;Sgard et al. 2002; Weisstaub et al. 2002; Ballestero et al. 2005; Plazas et al. 2005). Similar to the case in outer hair cells, upon activation the resulting intracellular calcium signal activates nearby SK2 currents that hyperpolarise the cell briefly (Fuchs and Murrow 1992; Doi and Ohmori 1993; Yuhas and Fuchs 1999; Glowatzki and Fuchs 2000; Oliver et al. 2000). IHCs respond to exogenously applied ACh as early as P1 but the maximum response is seen between P7–9 after which it begins to decline again (Katz et al. 2004; Roux et al. 2011). Towards the onset of hearing, direct efferent innervation of IHCs is lost and these changes are accompanied by a loss of transcription of the gene encoding the α10 (Chrna10) but not the α9 (Chrna9) AChR subunit (Elgoyhen et al. 1994, 2001; Simmons and Morley 1998, 2011; Morley and Simmons 2002). In fact, Chrna9 continues to be transcribed into adult stages (Elgoyhen et al. 1994) although the reason for this remains unclear. However, the lack of cholinergic responses in these mature cells is not solely due to the loss of α10 as even when α10 cDNA is constitutively expressed under the control of the mouse Pou4f3 gene promoter, a hair cell transcription factor (Erkman et al. 1996), the response to ACh is still lost with the same developmental time frame as seen in wild-type animals. In addition, the lack of chrna10 seems to cause no developmental defects in the development of IHC currents or on the action potential activity (Gomez-Casati et al. 2009). The pattern of nerve innervation, expression of subunits of the AChR and responsiveness of the cells to exogenously applied ACh all peak at the end of the first postnatal week, a time that coincides with peak spontaneous action potential activity in the developing cochlea.
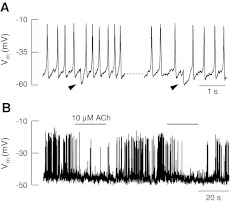
The first demonstration of the effects of efferent innervation on action potential firing came in a series of experiments by Glowatzki and Fuchs (2000). They recorded action potential firing in developing rat IHCs and showed the occurrence of small hyperpolarising events (marked by the arrows in Fig. 3A) that delayed the subsequent action potential, thereby lengthening the inter-spike interval. These events could be blocked by the application of strychnine to block the AChR and similar hyperpolarisations could be generated by brief external application of ACh (Fig. 3B). These experiments demonstrated that IHC action potential activity could be modulated by the efferent system. Goutman et al. (2005) then went on to use electrical field stimulation to drive the efferent fibres at different frequencies to mimic activity of the intact system. These experiments showed that stimulation of the efferent system at 2 kHz could delay action potential firing and higher frequencies of 5 kHz could prevent firing altogether. More recently, experiments by Johnson et al. (2011) have demonstrated that more than a simple on/off inhibition of firing, the efferent system may exert a more subtle modulatory effect on the firing patterns of developing IHCs. They showed that inhibition of the AChR by strychnine causes the IHC membrane potential to depolarise slightly leading to an increase in firing rate in basal hair cells and interestingly changed the apical hair cell firing from a burst-like pattern to more sustained firing (Johnson et al. 2011). These data suggest that the efferent system could be responsible for modulating IHC action potential firing into bursts and this bursting activity could drive the corresponding pattern of activity found in auditory nerve in vivo (Jones et al. 2007). Indeed as each efferent fibre can contact several IHCs, it is likely that activation of the efferent system may produce coordinated firing in groups of hair cells and therefore in groups of auditory nerves as previously suggested (Jones et al. 2007). Interestingly, because there are gradients in the expression of both of the AChR subunits (Simmons and Morley 1998, 2002; Elgoyhen et al. 2001; Simmons and Morley 2011) the influence of efferent innervation could vary with cochlear location and could partially account for the patterned activity observed in apical and basal hair cells (Johnson et al. 2011).
FIG. 3.
Efferent activity modulates IHC action potential firing. A Whole cell recording from a pre-hearing rat IHC showing action potential firing in response to current injection. Arrows indicate spontaneous hyperpolarisations that briefly pause action potential firing. B Brief extracellular application of acetylcholine temporarily inhibits spontaneous firing in IHCs; the effect is fully reversible. Adapted from Glowatzki and Fuchs (2000). Reprinted with permission from AAAS.
The role of ATP in the control of IHC action potential firing
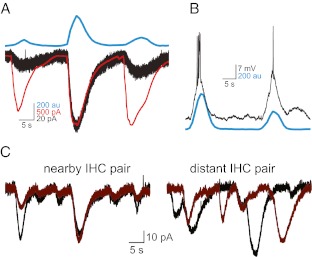
In a series of elegant experiments, Tritsch et al. (2007) recorded ATP-dependent activity in Kölliker’s organ. This activity generated large inward currents in a population of support cells near the organ of Corti, cells that start to disappear between P13 and P19, after the cochlea can reliably respond to sound (Tritsch et al. 2007; Tritsch and Bergles 2010). In their recording conditions, IHCs were not firing spontaneously, but instead required ATP release to generate depolarisation and initiate bursts of firing, similar to those reported in SGNs (Jones et al. 2007). The authors also observed optical changes in Kölliker’s organ that resulted from light scattering produced when support cells crenellated, an effect that could be mimicked by application of ATP. Simultaneous recordings from inner support cells and IHCs along with optical changes within Kölliker’s organ showed that all three responded to ATP in a coincident manner demonstrating that ATP release can depolarise IHCs (Fig. 4A) leading to bursts of action potential firing. Furthermore, using spontaneous optical changes as an indicator of the timing of ATP release, the authors demonstrate that ATP also causes bursts of firing in spiral ganglion neurones (Fig. 4B). Dual recordings from IHCs showed that ATP could synchronise the activity of closely located hair cells but not those located further away (Fig. 4C) suggesting that coordination by ATP could form the basis of tonotopic signalling along the length of the cochlea. Tritsch et al. (2007) conclude that support cells generate bursts of electrical activity in spiral ganglion neurones through ATP-dependent activation of IHCs, coordinated in groups of neighbouring cells.
FIG. 4.
ATP release from Kölliker’s organ activates IHCs. A Simultaneous recordings of IHC currents (black trace) and a nearby support cell (red trace) and optical changes (blue trace) imaged in the adjacent Kölliker’s organ. B Depolarisation and action potential firing in a P9 IHC (black trace) that was coincident with optical changes in Kölliker’s organ (blue trace). C Inward currents recorded from pairs of IHC located either in close proximity to each other or more distant showing synchronisation of activity in IHC that are located close together. Adapted by permission from Macmillan Publishers Ltd: Nature (Tritsch et al. 2007) copyright (2007).
Position dependence of the patterns of action potential firing
Very recently, evidence has emerged that the pattern of action potential firing during development has a clear basal to apical gradient (Johnson et al. 2011). The authors recorded spontaneous firing in mice, rats and gerbils using the cell-attached patch technique which does not disrupt the intracellular environment by introducing exogenous calcium buffers. As IHC action potentials depend on calcium for both the upstroke and activation of SK2 currents, and since there is evidence that calcium buffering differs along the length of the cochlea both in the mature and immature systems (Hackney et al. 2005), this technique has clear advantages. Importantly, Johnson et al. (2011) also carried out their experiments at 35–37 °C, as temperature is known to affect the size of the calcium current in immature IHCs (Grant and Fuchs 2008). Using this approach, they demonstrated spontaneous action potential firing that had a more burst-like pattern in apical IHCs compared to basal IHCs, a difference that remained throughout the first postnatal week, suggesting that the basal pattern of firing was not just a manifestation of delayed development along the length of the cochlea (Johnson et al. 2011). The spontaneous bursting activity in apical hair cells was abolished by strychnine, an AChR antagonist, suggesting that the bursting behaviour seen in these experiments was due to the efferent system effectively ‘dampening’ fast firing into a burst-like pattern. The inhibitory effects of the efferent system seen in these experiments is in agreement with earlier experiments where inter-spike interval was increased or firing inhibited altogether by activation of the efferent system (Fig. 3; Glowatzki and Fuchs 2000; Goutman et al. 2005).
Johnson et al. (2011) also recorded the effects of exogenously applied ATP to mimic the activity of Köllikers organ, but using experimental conditions (higher extracellular potassium) that would tend to favour spontaneous firing. In the experiments of Tritsch et al. (2007) and Tritsch and Bergles (2010), the IHCs were essentially quiescent in the absence of ATP with a resting potential of −72 mV. This is presumably due to the choice of lower extracellular potassium concentrations that would favour a negative resting potential and reduce the likelihood of spontaneous firing. They clearly observed that ATP is able to robustly depolarise the IHCs and initiate bursts of firing. In broad agreement, Johnson et al. (2011) demonstrated that micromolar concentrations of ATP could depolarise IHCs and cause increases in firing rate. However, they also demonstrated that much lower concentrations of ATP were able to hyperpolarise the hair cells and thus reduce firing rate, an effect that would be impossible to detect if the IHC was already silent. Taken together, these data suggest that if IHCs are spontaneously firing in vivo then the release of ATP from Kölliker’s organ could have a more subtle modulatory effect by increasing or decreasing firing in a dose-dependent manner. As yet, the question of whether the IHCs are firing spontaneously or are normally silent and require an external stimulus to initiate firing is not fully understood. However, it seems that when IHCs fire they do so in a burst-like manner, and that the pattern of bursting is arranged in a longitudinal gradient along the cochlea.
Conclusions
Over the past 10–15 years, enormous progress has been made into understanding the early developmental signalling events occurring within the cochlea. A picture of action potential firing within developing hair cells driving the activity of spiral ganglion neurones in an experience-independent manner has now clearly emerged. Despite this, there are still very important questions that remain unanswered. It is now well established that IHCs can generate bursts of action potential activity, but two mechanisms have been proposed to generate coordinated bursting in developing IHCs that may be regulated in completely different ways. Understanding how release of ATP from Kolliker’s organ and the modulation of firing by the efferent system interact to produce patterned firing is likely to prove of critical importance. In order to achieve this, we must determine the activity of the efferent system in vivo and how ATP release from support cells is generated and regulated. The recent discovery of longitudinal gradients in action potential firing hints at a mechanism that, in addition to molecular cues, helps develop a tonotopic gradient in the neural circuitry. Although establishing that coordinated bursts of firing in spiral ganglion neurones originate from the IHCs in the organ of Corti represents a huge advance in our understanding of auditory development, it leaves open the question of how this early coordinated activity mediates refinement of neural connections during the development of hearing. Furthermore, determining how genetic defects may alter or prevent these early signalling events and understanding how these changes may lead to hearing loss still represent considerable challenges.
Acknowledgments
I would like to thank Dr Robert Meech, Dr Dawn Davies and Dr Krasimara Tsaneva-Atanasova for their helpful discussions and comments on the manuscript. Work in the author’s lab was supported by a project grant from the Wellcome Trust (072809).
References
- Ballestero JA, Plazas PV, Kracun S, Gomez-Casati ME, Taranda J, Rothlin CV, Katz E, Millar NS, Elgoyhen AB. Effects of quinine, quinidine, and chloroquine on alpha9alpha10 nicotinic cholinergic receptors. Mol Pharmacol. 2005;68(3):822–829. doi: 10.1124/mol.105.014431. [DOI] [PubMed] [Google Scholar]
- Beurg M, Safieddine S, Roux I, Bouleau Y, Petit C, Dulon D. Calcium- and otoferlin-dependent exocytosis by immature outer hair cells. J Neurosci. 2008;28(8):1798–1803. doi: 10.1523/JNEUROSCI.4653-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Moser T. The presynaptic function of mouse cochlear inner hair cells during development of hearing. J Neurosci. 2001;21(13):4593–4599. doi: 10.1523/JNEUROSCI.21-13-04593.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11(1):18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Striessnig J, Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci. 2003;23(34):10832–10840. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Ohmori H. Acetylcholine increases intracellular Ca2+ concentration and hyperpolarizes the guinea-pig outer hair cell. Hear Res. 1993;67(1–2):179–188. doi: 10.1016/0378-5955(93)90245-V. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79(4):705–715. doi: 10.1016/0092-8674(94)90555-X. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci USA. 2001;98(6):3501–3506. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O’Connell SM, Keithley EM, Rapaport DH, Ryan AF, Rosenfeld MG. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381(6583):603–606. doi: 10.1038/381603a0. [DOI] [PubMed] [Google Scholar]
- Frank T, Khimich D, Neef A, Moser T. Mechanisms contributing to synaptic Ca2+ signals and their heterogeneity in hair cells. Proc Natl Acad Sci USA. 2009;106(11):4483–4488. doi: 10.1073/pnas.0813213106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E, Lohmann C. Development of auditory brainstem circuitry. Activity-dependent and activity-independent processes. Cell Tissue Res. 1999;297(2):187–195. doi: 10.1007/s004410051346. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Murrow BW. Cholinergic inhibition of short (outer) hair cells of the chick’s cochlea. J Neurosci. 1992;12(3):800–809. doi: 10.1523/JNEUROSCI.12-03-00800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science. 2000;288(5475):2366–2368. doi: 10.1126/science.288.5475.2366. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5(2):147–154. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Gomez-Casati ME, Wedemeyer C, Taranda J, Lipovsek M, Dalamon V, Elgoyhen AB, Katz E. Electrical properties and functional expression of ionic channels in cochlear inner hair cells of mice lacking the alpha10 nicotinic cholinergic receptor subunit. J Assoc Res Otolaryngol. 2009;10(2):221–232. doi: 10.1007/s10162-009-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutman JD, Glowatzki E. Time course and calcium dependence of transmitter release at a single ribbon synapse. Proc Natl Acad Sci USA. 2007;104(41):16341–16346. doi: 10.1073/pnas.0705756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutman JD, Fuchs PA, Glowatzki E. Facilitating efferent inhibition of inner hair cells in the cochlea of the neonatal rat. J Physiol. 2005;566(Pt 1):49–59. doi: 10.1113/jphysiol.2005.087460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant L, Fuchs P. Calcium- and calmodulin-dependent inactivation of calcium channels in inner hair cells of the rat cochlea. J Neurophysiol. 2008;99(5):2183–2193. doi: 10.1152/jn.01174.2007. [DOI] [PubMed] [Google Scholar]
- Grant L, Yi E, Glowatzki E. Two modes of release shape the postsynaptic response at the inner hair cell ribbon synapse. J Neurosci. 2010;30(12):4210–4220. doi: 10.1523/JNEUROSCI.4439-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney CM, Mahendrasingam S, Jones EM, Fettiplace R. The distribution of calcium buffering proteins in the turtle cochlea. J Neurosci. 2003;23(11):4577–4589. doi: 10.1523/JNEUROSCI.23-11-04577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney CM, Mahendrasingam S, Penn A, Fettiplace R. The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J Neurosci. 2005;25(34):7867–7875. doi: 10.1523/JNEUROSCI.1196-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Marcotti W. Biophysical properties of CaV1.3 calcium channels in gerbil inner hair cells. J Physiol. 2008;586(4):1029–1042. doi: 10.1113/jphysiol.2007.145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Marcotti W, Kros CJ. Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells. J Physiol. 2005;563(Pt 1):177–191. doi: 10.1113/jphysiol.2004.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Adelman JP, Marcotti W. Genetic deletion of SK2 channels in mouse inner hair cells prevents the developmental linearization in the Ca2+ dependence of exocytosis. J Physiol. 2007;583(Pt 2):631–646. doi: 10.1113/jphysiol.2007.136630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Eckrich T, Kuhn S, Zampini V, Franz C, Ranatunga KM, Roberts TP, Masetto S, Knipper M, Kros CJ, Marcotti W. Position-dependent patterning of spontaneous action potentials in immature cochlear inner hair cells. Nat Neurosci. 2011;14(6):711–717. doi: 10.1038/nn.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Leake PA, Snyder RL, Stakhovskaya O, Bonham B. Spontaneous discharge patterns in cochlear spiral ganglion cells before the onset of hearing in cats. J Neurophysiol. 2007;98(4):1898–1908. doi: 10.1152/jn.00472.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci. 2009;12(6):711–717. doi: 10.1038/nn.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E, Elgoyhen AB, Gomez-Casati ME, Knipper M, Vetter DE, Fuchs PA, Glowatzki E. Developmental regulation of nicotinic synapses on cochlear inner hair cells. J Neurosci. 2004;24(36):7814–7820. doi: 10.1523/JNEUROSCI.2102-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy HJ. Intracellular calcium regulation in inner hair cells from neonatal mice. Cell Calcium. 2002;31(3):127–136. doi: 10.1054/ceca.2001.0267. [DOI] [PubMed] [Google Scholar]
- Kennedy HJ, Meech RW. Fast Ca2+ signals at mouse inner hair cell synapse: a role for Ca2+-induced Ca2+ release. J Physiol. 2002;539(Pt 1):15–23. doi: 10.1113/jphysiol.2001.013171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros CJ, Ruppersberg JP, Rusch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394(6690):281–284. doi: 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- Leao RN, Naves MM, Leao KE, Walmsley B. Altered sodium currents in auditory neurons of congenitally deaf mice. Eur J Neurosci. 2006;24(4):1137–1146. doi: 10.1111/j.1460-9568.2006.04982.x. [DOI] [PubMed] [Google Scholar]
- Leao RN, Sun H, Svahn K, Berntson A, Youssoufian M, Paolini AG, Fyffe RE, Walmsley B. Topographic organization in the auditory brainstem of juvenile mice is disrupted in congenital deafness. J Physiol. 2006;571(Pt 3):563–578. doi: 10.1113/jphysiol.2005.098780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol. 2003;548(Pt 2):383–400. doi: 10.1113/jphysiol.2002.034801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Rusch A, Kros CJ. Sodium and calcium currents shape action potentials in immature mouse inner hair cells. J Physiol. 2003;552(Pt 3):743–761. doi: 10.1113/jphysiol.2003.043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Kros CJ. A transiently expressed SK current sustains and modulates action potential activity in immature mouse inner hair cells. J Physiol. 2004;560(Pt 3):691–708. doi: 10.1113/jphysiol.2004.072868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody WJ. Control of spontaneous activity during development. J Neurobiol. 1998;37(1):97–109. doi: 10.1002/(SICI)1097-4695(199810)37:1<97::AID-NEU8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Morley BJ, Simmons DD. Developmental mRNA expression of the alpha10 nicotinic acetylcholine receptor subunit in the rat cochlea. Brain Res Dev Brain Res. 2002;139(1):87–96. doi: 10.1016/S0165-3806(02)00514-X. [DOI] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci USA. 2000;97(2):883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci USA. 1982;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouvian R (2007) Temperature enhances exocytosis efficiency at the mouse inner hair cell ribbon synapse. J Physiol 584:535–542 [DOI] [PMC free article] [PubMed]
- Oliver D, Plinkert P, Zenner HP, Ruppersberg JP. Sodium current expression during postnatal development of rat outer hair cells. Pflugers Arch. 1997;434(6):772–778. doi: 10.1007/s004240050464. [DOI] [PubMed] [Google Scholar]
- Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B. Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron. 2000;26(3):595–601. doi: 10.1016/S0896-6273(00)81197-6. [DOI] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102(1):89–97. doi: 10.1016/S0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Plazas PV, Katz E, Gomez-Casati ME, Bouzat C, Elgoyhen AB. Stoichiometry of the alpha9alpha10 nicotinic cholinergic receptor. J Neurosci. 2005;25(47):10905–10912. doi: 10.1523/JNEUROSCI.3805-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WM. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J Neurosci. 1994;14(5 Pt 2):3246–3262. doi: 10.1523/JNEUROSCI.14-05-03246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux I, Wersinger E, McIntosh JM, Fuchs PA, Glowatzki E. Onset of cholinergic efferent synaptic function in sensory hair cells of the rat cochlea. J Neurosci. 2011;31(42):15092–15101. doi: 10.1523/JNEUROSCI.2743-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Sgard F, Charpantier E, Bertrand S, Walker N, Caput D, Graham D, Bertrand D, Besnard F. A novel human nicotinic receptor subunit, alpha10, that confers functionality to the alpha9-subunit. Mol Pharmacol. 2002;61(1):150–159. doi: 10.1124/mol.61.1.150. [DOI] [PubMed] [Google Scholar]
- Simmons DD. Development of the inner ear efferent system across vertebrate species. J Neurobiol. 2002;53(2):228–250. doi: 10.1002/neu.10130. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Morley BJ. Differential expression of the alpha 9 nicotinic acetylcholine receptor subunit in neonatal and adult cochlear hair cells. Brain Res Mol Brain Res. 1998;56(1–2):287–292. doi: 10.1016/S0169-328X(98)00056-4. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Morley BJ. Spatial and temporal expression patterns of nicotinic acetylcholine alpha 9 and alpha 10 subunits in the embryonic and early postnatal inner ear. Neuroscience. 2011;194:326–336. doi: 10.1016/j.neuroscience.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DD, Moulding HD, Zee D. Olivocochlear innervation of inner and outer hair cells during postnatal maturation: an immunocytochemical study. Brain Res Dev Brain Res. 1996;95(2):213–226. doi: 10.1016/0165-3806(96)00084-3. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Emmerling MR. Development of acetylcholinesterase-positive neuronal pathways in the cochlea of the mouse. J Neurocytol. 1989;18(2):209–224. doi: 10.1007/BF01206663. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Rose JE, Scott GE, Slapnick SM. Ribbon synapses in the developing intact and cultured organ of Corti in the mouse. J Neurosci. 1982;2(7):942–957. doi: 10.1523/JNEUROSCI.02-07-00942.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabova B, Lacinova L, Engel J. Effects of phenylalkylamines and benzothiazepines on Ca(v)1.3-mediated Ca2+ currents in neonatal mouse inner hair cells. Eur J Pharmacol. 2007;573(1–3):39–48. doi: 10.1016/j.ejphar.2007.06.050. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Bergles DE. Developmental regulation of spontaneous activity in the mammalian cochlea. J Neurosci. 2010;30(4):1539–1550. doi: 10.1523/JNEUROSCI.3875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450(7166):50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Rodriguez-Contreras A, Crins TT, Wang HC, Borst JG, Bergles DE. Calcium action potentials in hair cells pattern auditory neuron activity before hearing onset. Nat Neurosci. 2010;13(9):1050–1052. doi: 10.1038/nn.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B, Berntson A, Leao RN, Fyffe RE. Activity-dependent regulation of synaptic strength and neuronal excitability in central auditory pathways. J Physiol. 2006;572(Pt 2):313–321. doi: 10.1113/jphysiol.2006.104851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisstaub N, Vetter DE, Elgoyhen AB, Katz E. The alpha9alpha10 nicotinic acetylcholine receptor is permeable to and is modulated by divalent cations. Hear Res. 2002;167(1–2):122–135. doi: 10.1016/S0378-5955(02)00380-5. [DOI] [PubMed] [Google Scholar]
- Yuhas WA, Fuchs PA. Apamin-sensitive, small-conductance, calcium-activated potassium channels mediate cholinergic inhibition of chick auditory hair cells. J Comp Physiol A. 1999;185(5):455–462. doi: 10.1007/s003590050406. [DOI] [PubMed] [Google Scholar]