INTRODUCTION
An individual’s normal gait speed is a straightforward performance measure with powerful predictive capacity. Numerous studies show that a decline in gait speed is highly associated with greater mortality [1,2], greater functional disability [3], poorer quality of life [4], diminished cognition [5,6], increased health care spending [3,7], and thus, increased loss of independence. Critically, there are defined interventions to increase gait speed in at-risk community dwelling individuals [8,9]. Improved gait speed may lower the risk of the above health-related outcomes [10].
In the above studies, gait speed typically is measured in clinic, with individuals timed while walking a short, predetermined distance (e.g. 4–16 m). This approach is straightforward, and can be done with little clinical disruption or subject inconvenience. However, in older individuals, gait speed (and other aspects of physical performance) decline over long time periods [11], and thus require repeated assessments. Physical activity is also subject to ultradian, circadian, and seasonal changes that cannot be assessed during brief clinical visits [12], yet may be vital to interpret activity status. For example, activity measured over one week in a group of healthy Canadian seniors showed that while most subjects achieved an age-appropriate target for total daily activity count, only a minority of subjects had activity bouts that were ten minutes or longer, as recommended by the Canadian Physical Activity Guide [13]. Finally, these sorts of measures are too imprecise to provide effective feedback for subjects engaged in interventions designed to improve gait speed and physical activity. These limitations justify new approaches to continuously measure gait speed in a noninvasive, inexpensive, robust, and easily adopted manner.
A promising way to accomplish the above task is to repurpose widely used personal electronics to measure gait speed. This “ubiquitous computing” approach has been successfully used to create “smart stretchers” [14], heart rate monitors [15], personal diabetes management systems [16], and a variety of related healthcare devices from inexpensive, widely available technologies. Cellular telephones are particularly appealing devices for ubiquitous healthcare computing. Inexpensive smartphones are routinely equipped with a three-dimensional accelerometer. With appropriate software, these phones can function as actimeters. In fact, many software companies offer downloadable pedometer applications that use this functionality. None of these applications or devices, however, has been validated against any standard measure of gait or activity.
Here, we provide the first steps toward validation of cell-phone accelerometer measures of gait speed. The purpose of this study was to determine if physical activity counts as measured by cell phones correlate with treadmill gait speeds. We also determined where to place the cell phone (ankle, hip, wrist, or chest) to obtain the most accurate measures of treadmill gait speed. Determining whether cell phones can accurately predict treadmill gait speed is a waypoint on the path toward cell phone prediction of gait speed in naturalistic conditions, and has the advantage that gait speed for this study is under investigator control.
METHODS
Subjects
This study’s protocol was approved by the UNMC Institutional Review Board (IRB). All participants provided informed consent. We recruited a total of 55 young, middle-aged, and older individuals. This convenience sample included UNMC students and employees, who constituted most of the younger and a sizeable minority of middle-aged subjects. Older adults (or their spouses or children) who received primary care from the UNMC Home Instead Center for Successful Aging constituted most of the older and a majority of the middle-aged subjects. We also recruited and enrolled interested members of the Omaha/Council Bluffs metropolitan area community (through UNMC-approved social media). Overall, we recruited 17 young (20–35 years old, 8 male, 9 female), 19 middle-aged (36–65 years old, 8 male, 11 female), and 19 older (65+ years old, 5 male, 14 female) subjects. Table 1 lists major subject demographic criteria. To be included in the study, subjects had to be ≥19 years of age and community-dwelling. Exclusion criteria included any self-reported abnormalities of gait, uncontrolled medical or psychiatric illness, and inability to walk on a treadmill. Subjects were also required to have the ability to provide informed consent. One subject (male from older group) was removed from analysis because he entered an assisted living facility shortly after completion of treadmill testing. All of the other enrolled subjects retained sufficient functional capacity to remain independent in the community.
TABLE 1.
Study demographic characteristics
| Cohort | ||||
|---|---|---|---|---|
| Characteristic | Measurement Type | Young | Middle-aged | Aged |
| Sample size | n | 17 | 19 | 18 |
| Age (yrs) | mean ± SEM median, IRQ [1st qrt, 3rd qrt] |
27 ± 1.0 27, [25, 30] |
48.9 ± 1.9 49, [41, 55] |
74.9 ± 1.5 75, [70, 80] |
| Female Gender | n (%) | 9 (53) | 11 (58) | 14 (70)♦ |
| Weight (kg) | mean ± SEM median, IRQ [1st qrt, 3rd qrt] |
73.9 ± 3.1 72.6, [65.8, 79.4] |
80.7 ± 4.3 77.6, [63.5, 90.7] |
68.3 ± 3.8 67.6, [57.1, 72.1] |
| Tested at HICSA | n (%) | 5 (30)♦ | 11 (58) | 17 (95)♦ |
SEM = standard error of the mean.
IRQ = Inter-Quartile Range, i.e. the middle 50% of the data, b/n the 1st and 3rd quartiles
HICSA = UNMC Home Instead Center for Successful Aging.
= null hypothesis that samples came from population with 50% male, 50% female gender (row 3), or 50% UNMC Center for Cardiac Rehabilitation, 50% Home Instead Center for Successful Aging (row 5) rejected (p<0.05) by binomial distribution.
Data acquisition
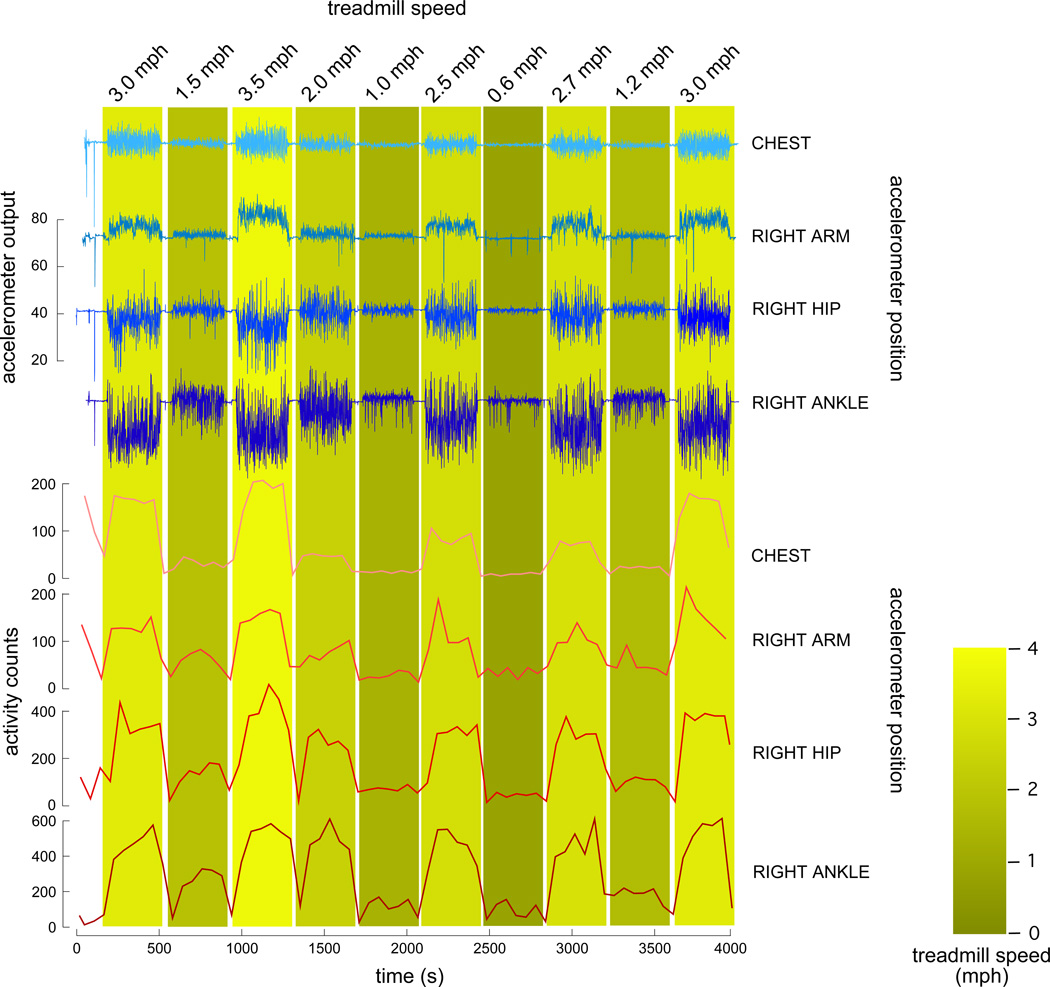
Activity was measured using Nokia N79 cell phones. These phones have an intrinsic 3D accelerometer (sourced from STMicroelectronics, LIS302DL, by Nokia) with a dynamic range of ± 2 g. The accelerometer was sampled using Python for S60 version 1.9.7 (sensor framework) running on the Symbian V3FP2 operating system. This software sampled the accelerometer at approximately 12 Hz; 1–2 epochs of data loss (always <6 s, usually <2 s) occurred every 500–600 minutes of data collection. This data loss represents an operating system interrupt process. Time series from the multiple phones used for data collection were synchronized to one another through the GSM network signal. We chose physical activity (PA) counts [17] as our outcome measure. PA counts provide a graded measure of accelerometer magnitude over a broad variety of movements. The algorithm to determine PA counts [18] is straightforward and well-accepted (which is currently not the case for more complex accelerometer measures, such as footfalls or gait speed). Briefly, raw accelerometer data was conditioned by calculating the acceleration magnitude (x, y, and z axes), bandpass filtering the signal using a 48 component finite impulse response filter (Nyquist frequency of 0.5 Hz, bandpass cutoffs at 0.1 Hz and 0.4 Hz, respectively), normalizing the filtered time series by resampling at 1 Hz, and then integrating over one minute bins [per 18] to obtain PA counts. We used a classical windowed linear phase impulse response filter (implemented by the fir1 MATLAB function). Figure 1 provides an example of both raw accelerometer traces and the resulting PA counts for a representative subject.
Figure 1. Activity counts from cell-phone accelerometers provide an accurate measure of treadmill gait speed regardless of where the sensor is worn.
The top 4 traces depict raw data from a representative trial (43 y/o man) showing acceleration magnitude versus time for sensors worn at the chest, right arm, right hip, and right ankle (1st through 4th traces from top, respectively). For all traces the baseline is centered at 64 (midscale between sensor output of 0 for −2g, and 128 for +2g), the amount of deflection from this baseline is per the common scale provided left of these traces. The bottom 4 traces show activity counts versus time for the sensors worn at the chest, right arm, right hip, and right ankle, respectively. Counts were calculated over one minute nonoverlapping bins. Treadmill speed is given at the top of each epoch bar.
Protocol
Subjects were allowed to change into clothing comfortable for walking and (if desired) jogging. Subjects were weighed while fully dressed. For some subjects, we used the weight from their most recent clinical encounter. Cell phones were set for data acquisition, and placed next to the left ankle (held by an elastic sweatband or sock), right ankle, left hip (placed in the pocket), right hip, left hand (held by an elastic sweatband or manually gripped), right hand, and chest (placed in a pendant). We chose our cell phones from a pool of 10 identical phones, and assigned specific phones to different sites (ankle, hip, etc.) in a randomized manner. A video camera recorded footfalls at 30 frames/s. The subject climbed onto the treadmill and straddled the belt. We used treadmills located at either the UNMC Center for Cardiac Rehabilitation (Quinton MedTrack CR-60, Bothel WA) or the Home Instead Center for Successful Aging (SciFit AC5000, Tulsa OK). The subject made three short jumps in place to synchronize accelerometer time series to video. The subject then alternated one minute periods of inactivity (straddling the treadmill belt) with five minute periods of walking at different, defined speeds. Subjects were asked to walk or jog at speeds that they would normally achieve during their day-to-day routines. Figure 1 shows results from a typical trial. Subjects were clearly instructed not to attempt treadmill speeds that were in any way strenuous. For most subjects, we evaluated 5 minutes of treadmill locomotion at a minimum speed of 0.3 to 1 km/hr (0.2–0.6 mi/hr), a maximum speed per their tolerance, and multiple intermediate speeds. For most subjects, we replicated the starting speed (usually between 1.6 and 5.6 km/hr; 1.0 and 3.5 mi/hr) at the end of the trial. When subjects wanted to be tested at higher speeds, we alternated trials of a higher speed (e.g. 9.7 km/hr; 6.0 mi/hr) with trials at a lower speed. No individuals were tested at faster than 11.3 km/hr (7.0 mi/hr). Usually, older individuals could be tested to speeds of about 4 km/hr (2.5 mi/hr); in these cases, we evaluated treadmill gait at finer speed increments. No adverse events occurred during testing, and all subjects tolerated the protocol well.
Data quality control and analysis
Some of our older subjects held the treadmill grip bars, rather than let their arms swing freely. Wrist data from these individuals was removed from analysis. Custom MATLAB (Mathworks, Natick, MA) software was created to ensure that all time series were continuous; we segmented and separately filtered each segment for any trials showing more than 5 sec of data loss. For all subjects, activity count time series were inspected to identify the bouts of walking occurring between the 1 minute rest bouts. These 5 values (one for each minute spent walking at that speed) are repeated measures for the specific subject at the given treadmill speed.
Statistical analysis
Our primary goal for this validation study was to determine (1) if cell phone derived PA counts correlated with treadmill gait speed, (2) optimum location (if any) for placing the sensors, (3) if our technology (e.g. specific cell phone used) or subjects (e.g. age, weight) introduced important confounding factors, and (4) if the performance of cell phone accelerometers was linear over our anticipated operating ranges. We created a linear regression model of PA counts versus study independent variables for each of the seven locations where we placed study cell phones. For example, the (dependent) variable of PA counts was statistically modeled on the (predictor) variables treadmill speed while adjusting for the covariates weight, age, gender, location, cell phone ID, and operator. Predictor variable treadmill speed was limited to no more than 6.4 km/hr (4.0 mi/hr), because the linearity assumption was not met for higher speeds. Calculated models were then inverted to determine treadmill speed as a function of PA counts.
The statistical model used was a linear regression mixed model (aka repeated measures ANOVA) because the data were longitudinal repeated measures. Linear regression mixed models also avoid biasing results by accounting for the within-subject correlation [19]. Table 2A presents the models determined for all cell phone locations. The Bayesian Information Criterion (BIC) was used to select the model that best fit the data (smaller BIC indicates better model). For cases where we created distinct models for gender, BIC was calculated from a combined dataset to ensure that all BICs were derived from datasets with approximately the same n. Since the data were non-normal, we transformed them before analyses by the van der Waerden inverse rank transformation,
| (1) |
where Y is the Van der Waerden transformed cell phone activity counts, Φ(z) is the cumulative distribution function of the standard normal distribution N(0, 1), Φ−1(p) is its inverse function (a.k.a. the probit function, e.g. Φ−1 (0.975) = 1.96), r is the rank of the respective value of the cell phone counts, n is the size of the sample of cell phone counts used to build the statistical model, and r/(n+1) is the percentile of the cell count value. For example, if a person’s chest cell phone PA count is 80, that would correspond to the 60th percentile (Table 2B), which yields Φ−1 (.60) = 0.25335 using, e.g., function normsinv() in MS Excel or norminv() in MATLAB, among others. The level of significance α (for individual predictor variables and the overall models) was always kept at 0.05. The statistical package SAS 9.2 (SAS Inst, Cary, NC) was used for this analysis.
Table 2.
A. Regression models and their corresponding inversions for van der Waerden-transformed cell phone counts (Y) as a function of significant independent factors. Refer to Table 2B to convert raw counts to percentiles for calculation of Y per equation (1). For all identified models, p<0.0001. The contribution of each individual predictor to the overall estimate of treadmill gait speed can be appreciated by examining the predictor coefficients. For all models, activity counts are clearly the dominant factor influencing the estimate of gait speed. B. Conversion of raw cell count value to percentile p, for subsequent use in the probit function Φ−1(p).
| A | |||
|---|---|---|---|
| Site | Sex | Formula | BIC |
| Chest | M/F | Speed = 1.24*Y − 0.0039*Age + 2.42 | 2221.8 |
| Left Wrist | M/F | Speed = 1.29*Y + 0.02*Age + 2.02 | 3505.7 |
| Left Hip | M | Speed = 1.30*Y + 2.00 | 2031.6 |
| F | Speed = 1.30*Y + 4.54 − 0.0075*Age − 0.0261*Weight | 2031.6 | |
| Left Ankle | M/F | Speed = 1.35*Y + 2.26 | 2679.3 |
| Right Wrist | M/F | Speed = 1.70*Y + 1.79 + 0.0095*Age | 3481.8 |
| Right Hip | M | Speed = 1.32*Y + 1.95 | 1968.3 |
| F | Speed = 1.27*Y + 3.76 − 0.0053*Age | 1968.3 | |
| Right Ankle | M/F | Speed = 1.31*Y + 2.24 | 2635.4 |
| B | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentile | |||||||||||
| Site | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
| Chest | 13 | 24 | 33 | 42 | 55 | 69 | 80 | 94 | 111 | 139 | 876 |
| Left Wrist | 11 | 38 | 54 | 66 | 78 | 90 | 104 | 118 | 142 | 192 | 763 |
| Left Hip | 12 | 54 | 77 | 100 | 131 | 177 | 242 | 320 | 389 | 466 | 1054 |
| Left Leg | 18 | 75 | 116 | 170 | 255 | 365 | 455 | 521 | 582 | 672 | 1230 |
| Right Wrist | 13 | 38 | 55 | 68 | 82 | 95 | 110 | 130 | 157 | 209 | 716 |
| Right Hip | 12 | 51 | 76 | 99 | 131 | 177 | 245 | 324 | 393 | 484 | 1054 |
| Right Leg | 17 | 79 | 117 | 163 | 236 | 359 | 464 | 540 | 614 | 709 | 1144 |
To validate the calculated models (for the hip) against observed treadmill gait speeds, we generated 10 “jackknifed” data sets 20% the original data set size by resampling with replacement (MATLAB randsample). Observed treadmill gait speed was then plotted against predicted treadmill gait speed. Regression with 95% confidence intervals on observations was determined (Supplemental Figure 1 shows representative analysis for one jackknifed data set). Of note, the regression line slope was not significantly different from unity. This analysis suggested that changes in hip cell phone activity counts had equal sensitivity to detect changes in treadmill gait speed when compared to changes in treadmill gait speed evoked by the investigator manually programming in a new treadmill speed.
RESULTS
Dataset characteristics
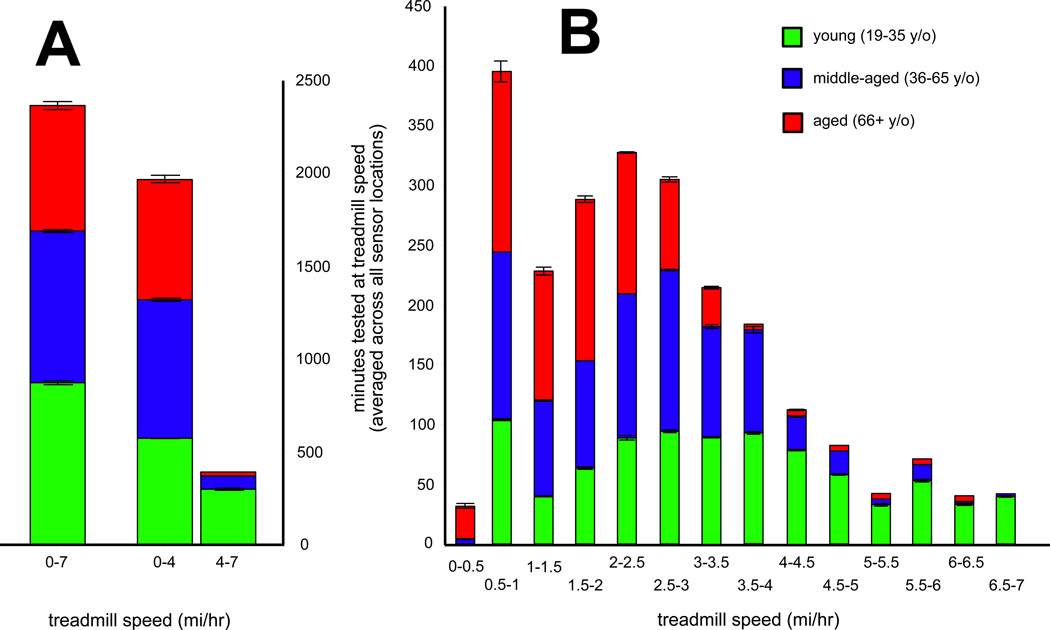
Since study participants all had significantly differing capacities for physical activity, it is not surprising that different age cohorts contributed different amounts of data over tested treadmill speeds. Figure 2 shows the cumulative walking time over three different speed ranges, and the percentage that each group contributed to that total. Young subjects clearly contributed a majority of physical activity data (75% of dataset) for gait speeds >4 mi/hr. Older individuals contributed slightly more physical activity data for gait speeds <2 mi/hr (in the interest of time, younger subjects were not exhaustively evaluated at the slower treadmill speeds). Gait speeds of 2.35 km/hr (1.46 mi/hr) and 4 km/hr (2.5 mi/hr) (dashed lines) correspond to well-demonstrated clinical cutoffs of 0.6 m/s (1 ft/s) and 1.0 m/s (3.3 ft/s). Middle-aged and older individuals contribute a majority of the activity count values for speeds immediately surrounding these critical values.
Figure 2. Relative contributions of each age cohort to the study data.
At each tested speed, the average (over all tested sensors) total duration of locomotion is shown. A. Contributions to all treadmill speeds (leftmost bar) and to walking versus jogging/running speeds (right most pair). B. Contributions to treadmill speeds by 0.5 mph increment. The 0.5–1.0 bin, for example, covers all speeds above 0.5 and up to 1.0 mph. Green shading indicates young (19–35 y/o) cohort, blue shading indicates middle-aged (36–65 y/o) cohort, and red shading indicates aged (65+ y/o) cohort. Error bars are ± 1 standard error of the mean for the given treadmill speed range and given cohort.
Treadmill gait speeds estimated from cell phone accelerometer are robust to individual cell phone placement
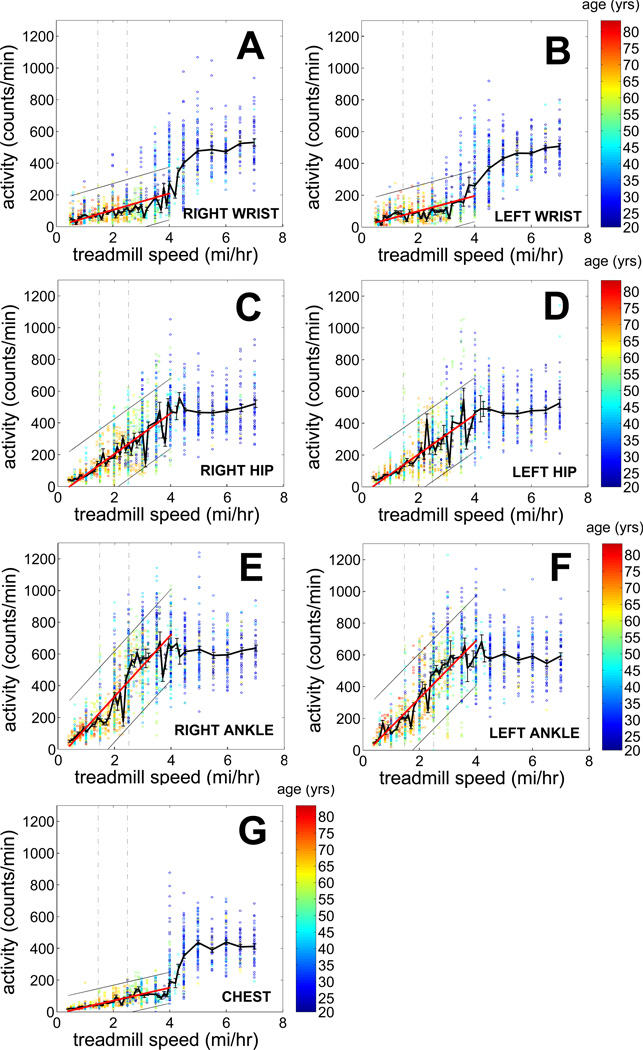
Figure 3 shows the relationship between activity counts and treadmill gait speed for phones placed on the left and right wrist (panel A, B), left and right hip (panel C, D), left and right ankle (panel E, F), and chest (panel G). Table 2A provides the results of the maximum likelihood regression models of treadmill speed as a function of study independent variables, and their respective inversions to obtain a formula predicting treadmill gait speed from cell phone activity counts. In this table, Y denotes the van der Waerden inverse rank transformed raw cell phone activity count, whereas Speed denotes the variable treadmill speed. If the same model is valid for both sexes, then sex is shown as M/F; otherwise, a separate formula is provided for each sex. Since our major development focus at this time is to develop appropriate technology to assess and treat older, frailer individuals, we focus further analyses on treadmill speeds of 6.4 km/hr (4 mi/hr) or less.
Figure 3. Activity count versus treadmill speed relationships for all sensor locations.
For all figures, the solid red line shows the linear regression between treadmill speed and activity counts (fit for all data between 0.0 and 6.4 km/hr (0–4 mph) gait speeds); the thin surrounding black lines are 95% confidence boundaries on this regression. The thick black line connects mean activity count values for each of the evaluated treadmill speeds; bars surrounding this point are ± 1 standard error of the mean. Individual observations of activity counts are shown as open colored circles. Subject age is color coded as circle color; refer to colorbar at right side for key. The dashed lines at gait speeds of 2.35 km/hr (1.46 mph) and 4 km/hr (2.5 mph) highlight system performance at two critical functional thresholds. These relationships come from cell phones placed at the right wrist (A), left wrist (B), right hip (C), left hip (D), right ankle (E), left ankle (F), and neck (G).
Regardless of where we placed the cell phone, all predictor models are highly significant (p<0.0001), and relatively equivalent in terms of performance. By BIC, cell phones placed on the wrists had models with the poorest performance predicting treadmill speed, while cell phones worn in the hip pocket had models with the best performance. Cell phones worn at the chest and ankles had models with slightly poorer performance compared with cell phones worn at the hip.
We also note that neither the testing location, individual who performed the testing, or the specific cell phone used to collect data were found significant by this analysis. Furthermore, when examining the models of Table 2A, we find that activity counts are by far the most significant predictive factor for treadmill speed. While some of the models find age (chest, left and right wrists, left and right hips in females), weight (left hip in females) or gender (left and right hip) to be significant predictors, the weight of these factors is far less than that of activity counts.
The above models can be used to estimate treadmill speed from cell phone activity counts as follows. When a one-minute-bin activity count is generated, that count is ranked relative to our dataset of the respective counts (on which we have built the particular model). Next, the so-obtained rank is substituted into the formula for the van der Waerden inverse rank transformation. Finally, the obtained value is substituted in the formula for treadmill speed, as suggested by the respective model.
DISCUSSION
This study is the first to validate cell phone accelerometers as tools for accurate, easy, and inexpensive treadmill gait speed measures across a broad population of community dwelling adults. These gait speed measures are precise even if the subjects wear the cell phone casually (loose in pocket, in a neck pendant, etc.). Much of our validation dataset evaluated treadmill gait speeds around the critical range of 2.4 to 4 km/hr (1.5 to 2.5 mph). Prior studies have demonstrated that individuals unable to walk faster than 2.35 km/hr (1.46 mi/hr) are at a higher risk for a variety of adverse health-related outcomes; this risk is no longer present in persons able to walk faster than 4 km/hr (2.5 mi/hr, [20,7]). These findings further justify our ubiquitous computing approach using cell phones to measure individual functional status [21].
Gait speed is a critical performance measure to assess an individual’s functional status. A recent review of high-quality studies determined that slow gait speed is a significant risk factor for disability, cognitive impairment, loss of independence, falls, and mortality [22]. Tools that allow non-invasive and pervasive gait speed measures offer promise to screen large populations for early functional status loss [23]. Efficacy of interventions designed to preserve functional status may also be determined with high face validity by measuring improvements in gait speed [24]. The ubiquitous computing approach we describe would be an inexpensive and appropriate manner to implement these large-scale gait-based interventions.
While the concept of using cellular telephones to measure activity in community-dwelling adults is new, the underlying semiconductor-based accelerometer technology is well-established and has been validated in a number of contexts. Activity quantification from early, unidimensional semiconductor accelerometers had good agreement with both trained observers and earlier generation mechanical accelerometers [25]. Physical activity measurements with accelerometers also had less bias and more validity than survey-based instruments [26]. Further validation studies demonstrated that accelerometers could readily differentiate activity levels across different groups of older adults, including persons residing in a Veteran’s Administration nursing home, persons receiving in-home nonaerobic therapy, and persons attending an aerobic exercise program at a community center [27]. Similarly, activity counts strongly correlated with pedometer counts (and weakly correlated with the Zutphen Physical Activity Questionnaire) in older adults [28]. These and many more studies imply that current accelerometer technologies have strong face validity for measuring physical activity in many differently-abled groups.
We recognize that the process of validating cell phones to measure activity in independent, community-dwelling adults is at its earliest stages. There remain limitations to our current analysis. We suspect that walking in regions with significant built infrastructure (e.g., sidewalks, paved roads, shopping centers, homes, and other structures with relatively smooth, flat walking surfaces) or extensive grading (e.g., golf courses) may yield arm, hip, and limb acceleration profiles similar to those obtained from an exercise treadmill (since the walking path under these conditions has been significantly smoothed and leveled). Studies are ongoing to test this hypothesis. Our cell phone-based approach must also be validated against standard survey and performance battery based instruments that assess gait. Furthermore, many outdoor activities, such as hiking and gardening, occur over rougher terrain. Sporting activities (tennis, softball, basketball, etc.) require irregular bursts of movement interspersed with side-to-side movements. Under these conditions, our current cell phone derived measures of gait speed will be less accurate. Inclusion of additional independent measures of gait speed, including those derived from high temporal resolution GPS coordinates, hold additional promise for refining our model to provide gait speed predictions for individuals, as well as groups. Finally, even the healthiest individuals spend a relatively small percentage of time each day in active ambulation. Automated classification of nonlocomotor behaviors (e.g., per [29–32]) has the potential to provide a more nuanced representation of individual physical activity over long time periods, and may provide better tools to evaluate features of energy balance currently difficult to measure, such as non-exercise activity thermogenesis [33,34].
In summary, we demonstrate that cell phones provide a validated, inexpensive, accurate, noninvasive, and highly robust way to continuously measure treadmill gait speed. This finding justifies future efforts to validate cell phones measurements of naturalistic gait speed in ambulatory, community dwelling, independent adults.
Highlights.
Trial primary outcome was to determine if measurements from cell phone accelerometers correlated with treadmill walking speed.
Trial secondary outcome was to determine if the location where the subject kept the cell phone (ankle, hip, wrist, etc.) affected the results.
Enrollment: 17 young (age 19–35), 19 middle-aged (36–65), 17 aged (66+) community dwelling, independent adults with no functional gait deficits.
Physical activity counts derived from cell phone accelerometer strongly correlated with gait speed.
The hip was the best location to place the cell phone; however, all positions yielded suitable models.
Supplementary Material
Acknowledgments
Sponsors: This work was funded by a grant to SJB from the Vada Kinman Oldfield Foundation, The Dr. and Mrs. Robert A. Steven Endowment for Research in Geriatrics, and startup support from the University of Nebraska Medical Center. EHG supported by K08 MH071671. Sponsors did not have any role in design, methods, subject recruitment, data collection, analysis or preparation of the manuscript.
Other: The authors thank Dr. Scott Shurmur, UNMC Division of Cardiology, Department of Medicine, the staff of UNMC cardiac rehabilitation, and the staff of the UNMC Home Instead Center for Successful Aging for use of their treadmills. MATLAB code and analysis protocol for accelerometer data available upon request from SJB or AKS. We greatly thank all the research subjects who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Initial concepts and framework developed by SJB, AKS, MCB, and EHG. Software development by AKS, RHC, and SJB. Subject testing by DRH, JW, SJB, CAH, and RHC. Data analysis by GH, AKS, and SJB. Manuscript preparation by SJB, AKS, GH, MCB, EHG, and JFP.
REFERENCES
- 1.Blain H, Carriere I, Sourial N, Berard C, Favier F, et al. Balance and walking speed predict subsequent 8-year mortality independently of current and intermediate events in well-functioning women aged 75 years and older. J Nutr Health Aging. 2010;14:595–600. doi: 10.1007/s12603-010-0111-0. [DOI] [PubMed] [Google Scholar]
- 2.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, et al. Gait speed and survival in older adults. Jama. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cesari M, Kritchevsky SB, Newman AB, Simonsick EM, Harris TB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. J Am Geriatr Soc. 2009;57:251–259. doi: 10.1111/j.1532-5415.2008.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helbostad JL, Sletvold O, Moe-Nilssen R. Home training with and without additional group training in physically frail old people living at home: effect on health-related quality of life and ambulation. Clin rehabil. 2004;18:498–508. doi: 10.1191/0269215504cr761oa. [DOI] [PubMed] [Google Scholar]
- 5.Duff K, Mold JW, Roberts MM. Walking speed and global cognition: results from the OKLAHOMA Study. Neuropsychol dev cogn. 2008;15:31–39. doi: 10.1080/13825580701531904. [DOI] [PubMed] [Google Scholar]
- 6.Inzitari M, Newman AB, Yaffe K, Boudreau R, de Rekeneire N, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. 2007;29:156–162. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 8.Lord SR, Lloyd DG, Nirui M, Raymond J, Williams P, Stewart RA. The effect of exercise on gait patterns in older women: a randomized controlled trial. J Gerontol. 1996;51:M64–M70. doi: 10.1093/gerona/51a.2.m64. [DOI] [PubMed] [Google Scholar]
- 9.VanSwearingen JM, Perera S, Brach JS, Cham R, Rosano C, Studenski SA. A randomized trial of two forms of therapeutic activity to improve walking: effect on the energy cost of walking. J Gerontol. 2009;64:1190–1198. doi: 10.1093/gerona/glp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong CH, Wong SF, Yusoff AM, Karunananthan S, Bergman H. The effect of later-life health promotion on functional performance and body composition. Aging Clin Exp Res. 2008;20:454–460. doi: 10.1007/BF03325152. [DOI] [PubMed] [Google Scholar]
- 11.Ko SU, Hausdorff JM, Ferrucci L. Age-associated differences in the gait pattern changes of older adults during fast-speed and fatigue conditions: results from the Baltimore longitudinal study of ageing. Age Ageing. 2010;39:688–694. doi: 10.1093/ageing/afq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis MG, Fox KR, Hillsdon M, Sharp DJ, Coulson JC, Thompson JL. Objectively Measured Physical Activity in a Diverse Sample of Older Urban UK Adults. Med Sci Sport Exer. 2011;43:647–654. doi: 10.1249/MSS.0b013e3181f36196. [DOI] [PubMed] [Google Scholar]
- 13.Copeland JL, Esliger DW. Accelerometer assessment of physical activity in active, healthy older adults. J Aging Phys Activ. 2009;17:17–30. doi: 10.1123/japa.17.1.17. [DOI] [PubMed] [Google Scholar]
- 14.Ohashi K, Kurihara Y, Watanabe K, Tanaka H. Safe patient transfer system with monitoring of location and vital signs. J Med Dent Sci. 2008;55:33–41. [PubMed] [Google Scholar]
- 15.Zakrzewski M, Kolinummi A, Vanhala J. Contactless and unobtrusive measurement of heart rate in home environment. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2060–2063. doi: 10.1109/IEMBS.2006.260714. [DOI] [PubMed] [Google Scholar]
- 16.Park KS, Kim NJ, Hong JH, Park MS, Cha EJ, Lee TS. Personal diabetes management system based on ubiquitous computing technology. Stud Health Technol Inform. 2006;122:967–968. [PubMed] [Google Scholar]
- 17.Chen KY, Bassett DR., Jr The technology of accelerometry-based activity monitors: current and future. Med Sci Sport Exer. 2005;37:S490–S500. doi: 10.1249/01.mss.0000185571.49104.82. [DOI] [PubMed] [Google Scholar]
- 18.Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sport Exer. 2005;37:S531–S543. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- 19.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer-Verlag; 2000. [Google Scholar]
- 20.Kwon S, Perera S, Pahor M, Katula JA, King AC, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009;13:538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schenk AK, Witbrodt BC, Hoarty CA, Carlson RH, Jr, Goulding EH, et al. Cellular telephones measure activity and lifespace in community-dwelling adults: proof of principle. J Am Geriatr Soc. 2011;59:345–352. doi: 10.1111/j.1532-5415.2010.03267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 23.Studenski S. Bradypedia: is gait speed ready for clinical use? J Nutr Health Aging. 2009;13:878–880. doi: 10.1007/s12603-009-0245-0. [DOI] [PubMed] [Google Scholar]
- 24.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55:1727–1734. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 25.Klesges RC, Klesges LM, Swenson AM, Pheley AM. A validation of two motion sensors in the prediction of child and adult physical activity levels. Am J Epidemiol. 1985;122:400–410. doi: 10.1093/oxfordjournals.aje.a114121. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Kochersberger G, McConnell E, Kuchibhatla MN, Pieper C. The reliability, validity, and stability of a measure of physical activity in the elderly. Arch Phys Med Rehabil. 1996;77:793–795. doi: 10.1016/s0003-9993(96)90258-0. [DOI] [PubMed] [Google Scholar]
- 28.Harris TJ, Owen CG, Victor CR, Adams R, Ekelund U, Cook DG. A comparison of questionnaire, accelerometer, and pedometer: measures in older people. Med Sci Sports Exerc. 2009;41:1392–1402. doi: 10.1249/MSS.0b013e31819b3533. [DOI] [PubMed] [Google Scholar]
- 29.Hong Y-J, Kim I-J, Ahn SC, Kim H-G. Mobile health monitoring system based on activity recognition using accelerometer. Simul Model Pract Th. 2010;18:446–455. [Google Scholar]
- 30.Kwapisz JR, Weiss GM, Moore SA. Activity recognition using cell phone accelerometers. ACM SIGKDD Explorations. 2010;12:74–82. [Google Scholar]
- 31.Li M, Rozgica V, Thatte G, Lee S, Emken A, et al. Multimodal physical activity recognition by fusing temporal and cepstral information. IEEE Trans Neural Syst Rehabil Eng. 2010;18:369–380. doi: 10.1109/TNSRE.2010.2053217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pruitt LA, Glynn NW, King AC, Guralnik JM, Aiken EK, et al. Use of accelerometry to measure physical activity in older adults at risk for mobility disability. J Aging Phys Activ. 2008;16:416–434. doi: 10.1123/japa.16.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levine JA, Vander Weg MW, Hill JO, Klesges RC. Non-exercise activity thermogenesis: the crouching tiger hidden dragon of societal weight gain. Arterioscler Thromb Vasc Biol. 2006;26:729–736. doi: 10.1161/01.ATV.0000205848.83210.73. [DOI] [PubMed] [Google Scholar]
- 34.Tremblay MS, Esliger DW, Tremblay A, Colley R. Incidental movement, lifestyle-embedded activity and sleep: new frontiers in physical activity assessment. Can J Public Health. 2007;98(Suppl 2):S208–S217. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.