Abstract
Objective
Assess risks and benefits of conjugated equine estrogen (CEE) and medroxyprogesterone (MPA) in postmenopausal Chinese women.
Methods
A retrospective cohort study was undertaken using the Taiwan National Health Insurance Research Database, a population-based healthcare claims dataset. Eligible women aged 50–79 were classified as exposed to CEE 0.625mg/day, MPA 5.0mg/day (E+P, n=4712) or CEE 0.625mg/day alone (E-only, n=1208) and age-matched to unexposed women (n=10,125). Follow up was complete on 96% of participants. The primary outcomes were coronary heart disease (CHD) and invasive breast cancer. The global index summarized risks of primary outcomes, stroke, pulmonary embolism, colon and endometrial cancers, hip fractures and death. Time-to-event analyses were performed.
Results
Median durations of exposure in the E+P and E-only groups were 6.9 months and 9 months, respectively. Median follow-up was 110 months. Hazard ratios (95%CI) for E+P exposure were: myocardial infarction (MI), 0.78 (0.51–1.19); CHD death, 1.21 (0.53–2.70); breast cancer, 1.48 (1.20–1.83); global index, 0.79 (0.72–0.87). Hazard ratios for E-only exposure were: MI, 0.76 (0.35–1.68); CHD death, 0.57 (0.11–2.80); breast cancer 1.44 (0.99–2.10); global index, 1.09 (0.92–1.28). Per 10,000 person-years, there were 12 excess breast cancer cases with E+P exposure; there were 39 fewer global index events with E+P exposure. Adjusting for age, statin and aspirin use, hypercholesterolemia, diabetes and hypertension did not significantly change estimates.
Conclusions
In postmenopausal Chinese women, CEE with or without MPA was not associated with increased rates of coronary heart disease, but was CEE with MPA may be associated with a higher breast cancer rate. E+P exposure conferred lower global index event rates.
Keywords: Asian, Menopause, Hormone Therapy, Hormone Replacement
Introduction
Menopausal hormone therapy (MHT) impacts many aspects of postmenopausal women’s health, including coronary heart disease, cancer risk, thromboembolic risk and bone loss. The Women’s Health Initiative (WHI) was a large randomized controlled trial (RCT) that examined risks and benefits of conjugated equine estrogen (CEE) and medroxyprogesterone (MPA) in healthy, predominantly white women. WHI trial and follow up data do not support an overall benefit in use of MHT in this population 1, 2, but the generalizability of these data across racial and ethnic groups has not been demonstrated.
The risk-benefit ratio of MHT may differ by race and ethnicity. U.S. population-based data demonstrate significantly lower underlying cancer and heart disease risks in Asian Americans, compared to whites. Of all racial and ethnic groups in the U.S., Asian Americans have the lowest incidence of and mortality from all cancers combined 3. Heart disease risk is also lower in Asian Americans; 2004 age-adjusted death rates from heart disease were 216/100,000 for whites compared to 118/100,000 for Asian Americans 4.
Following these observations, we hypothesized that the risks and benefits of MHT in Asian women may be different from that of white women. Specifically, we sought to determine if CEE and MPA exposure or CEE-only exposure in postmenopausal Chinese women increases the risks of cardiovascular disease and breast cancer using data from the Taiwan National Health Insurance Research Database (NHIRD), a population-based health claims database. . By examining the impact of these MHT regimens on cardiovascular disease, cancer, fractures and death, we tested if CEE and MPA exposure or CEE-only exposure yields a favorable risk-benefit ratio.
Methods
Study design
The retrospective cohort study was designed to incorporate a similar study time frame, inclusion and exclusion criteria, and treatment protocol as the WHI RCT (Table 1) 5. The study was approved by the institutional review boards of the University of Pennsylvania and University of California, San Diego.
Table 1.
Comparison of WHI and NHIRD study designs
| Study protocol | WHI RCT | NHIRD | |
|---|---|---|---|
| Study enrollment | 1993–1998 | 1997–2000 | |
| Study dates | E+P | 1993–2002* | 1997–2007 |
| E only | 1993–2003* | ||
| Participant age at enrollment | 50–79 | 50–79 | |
| Menopausal status | No vaginal bleeding for 12 months | Assumed menopausal based on age ≥ 50 | |
| Controls | RCT 1:1 randomization | Age-matched 1:2 | |
| Exclusion criteria** | Medical condition associated with predicted survival < 3 years*** | ||
| Prior breast cancer | |||
| Prior other prior cancers within 10 years | |||
| Endometrial hyperplasia | |||
| Alcoholism, drug dependency | |||
| Dementia, mental illness | |||
| Acute MI, CVA, TIA within 6 months | |||
| Severe hypertension | |||
| Chronic hepatitis or cirrhosis | |||
| Prior PE or DVT | |||
| Exposure | E+P | CEE 0.625 MPA 2.5 mg | CEE 0.625, MPA 5 mg |
| E-only | CEE 0.625 mg | Same | |
| Duration of exposure median (range) | E+P | cumulative drop out 42% | 0.57 years (0.15–9.8) |
| E-only | cumulative drop-out 54% | 0.76 years (0.15–10.3) | |
| Duration of follow up mean (range) | E+P | 5.2 years (3.5–8.5) | 9.5 (0.1–10.6) |
| E-only | 6.8 years (5.7–10.7) | 9.4 (0.1–10.6) | |
Post-intervention phase 2002–2005
WHI exclusionary criteria not available in the NHIRD: abnormal pelvic exam, hysterectomy
AIDS, COPD, CHF, ESRD
Data source
National Health Insurance (NHI) of Taiwan provides comprehensive healthcare coverage for 99% of Taiwan’s 22 million people, 98% of whom are Han Chinese, since 1995 6. Since January of 1997, NHI claims data including all outpatient care, inpatient care and prescription drug use have been captured. The International Classification of Diseases (ICD), 9th Revision, Clinical Modification codes were used to classify medical diagnoses. From these data, the NHIRD research cohort was created. This research cohort is comprised of 1 million participants randomly sampled from the entire population. For these 1 million individuals, claims data from January 1, 1997 through December 31, 2007 have been incorporated into the de-identified research dataset used in this study.
The NHI formulary was searched for all estrogen- or progesterone-containing drugs. After identifying compounds used for MHT, all MHT prescriptions between June 1, 1997 and December 31, 2007 that were filled by potential eligible participants were extracted to assign exposure status.
Study population
Women were potentially eligible for the study if they reached age 50 and were younger than age 80 during the enrollment interval (June 1, 1997 to May 31, 2000). This interval was selected to coincide with the onset of NHIRD data capture and to close prior to the reporting of WHI data. Women older than age 50 were considered to be menopausal 7.
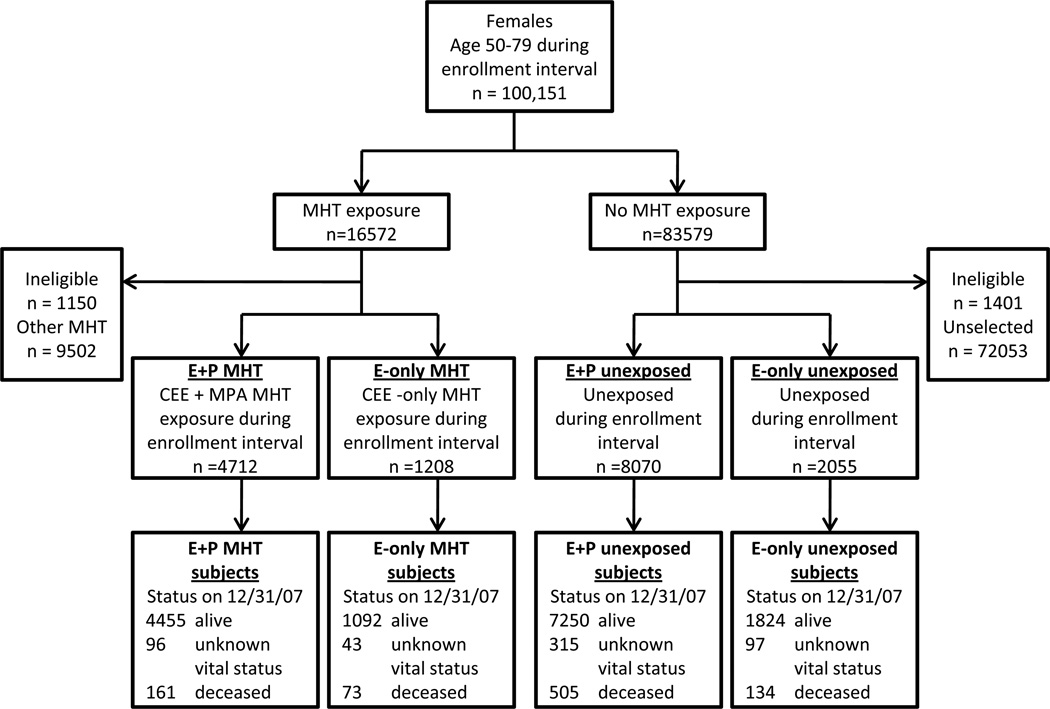
Participants were selected based on the following algorithm (Figure 1). The same inclusion and exclusion criteria except for exposure status were applied to exposed and unexposed participants to minimize selection bias. Potential eligible participants who filled at least two monthly prescriptions within three continuous months during the enrollment interval were categorized as exposed to MHT. For each MHT-exposed participant, the first date when the MHT prescription was filled was deemed her study enrollment date. Two MHT exposure groups were selected based on prescription data. Those who filled prescriptions for daily CEE (0.625 mg daily) and MPA (5 mg daily) were considered exposed to E+P MHT; participants who filled prescriptions for only CEE (0.625 mg daily) and no progestins were considered exposed to E-only MHT. For E-only MHT participants, hysterectomy status could not be reliably ascertained in this dataset, as surgical procedures prior to 1997 were not captured. Women who filled other types of MHT regimens, including other doses of CEE and MPA, other estrogens and progesterones, other delivery routes (transdermal, vaginal, intramuscular), during the recruitment interval were excluded from this analysis (57%, n=9,502). Unexposed participants were randomly selected from the remainder of the cohort. Matched by date of birth within five years, two age-matched unexposed participants were randomly selected for each exposed participant and designated the same enrollment date. A total of 72,053 unexposed women were not included due to not being randomly selected.
FIG. 1.
Study flow diagram. CEE, conjugated equine estrogens; MHT, menopausal hormone therapy; E, estrogen; P, progestin; MPA, medroxyprogesterone acetate.
Baseline characteristics were captured by ICD-9 and NHI formulary codes for hypertension, hypercholesterolemia, angina, myocardial infarction (MI), deep venous thrombosis (DVT), pulmonary embolism (PE), stroke, bony fracture, smoking, obesity, statin use, and aspirin use that occurred before her enrollment date (Supplemental Table 1 and 2, See Tables, Supplemental Digital Content, http://links.lww.com/MENO/A18). Women were excluded if they incurred an exclusionary diagnosis in the 6 months prior to the enrollment date (Supplemental Tables 1 and 2, See Tables, Supplemental Digital Content, http://links.lww.com/MENO/A18). Six months was selected based on the extent to which historical NHIRD data were available for a significant portion of participants.
Outcomes
For each study participant, outcome events that occurred after the enrollment date were identified by ICD-9 codes. The primary outcomes were coronary heart disease (myocardial infarction [MI] and coronary heart disease [CHD] death) and invasive breast cancer. CHD deaths were defined as death occurring within 28 days of hospitalizations when MI was diagnosed. The global index was a composite outcome summarizing the earliest occurrence of breast cancer, stroke, PE, endometrial cancer, colorectal cancer, hip fracture or death 1. Home deaths were not captured in the NHIRD. As the Taiwan national death index is not de-identified, it was not possible to link the NHIRD participants with the public death records.
Statistical analysis
The primary analysis used time-to-event methods. Participants were classified as exposed (E+P MHT or E-only MHT) or unexposed (E+P MHT unexposed or E-only MHT unexposed). Akin to the intention to treat (ITT) analysis in an RCT, time to outcomes was calculated regardless of length of MHT exposure, drop-out of MHT or drop-in to MHT5. In this analysis, the follow-up period of each participant was defined from the participant’s enrollment date until the date of the respective outcome diagnosis, death, loss of NHI coverage, or December 31, 2007, whichever was the earliest. After determining that the assumptions of proportional hazards were met, Cox proportional hazard ratios (HR) and 95% confidence intervals (CI) were estimated for each primary outcome as well as the global index.
Pre-specified secondary analyses were performed. First, the adjusted association between hormone exposure and each outcome was determined using multivariable Cox models while controlling for confounding. Covariates (Table 2) that are clinically known confounders or that changed the crude HR by more than 10% were included in multivariable models8.
Table 2.
Baseline characteristics of study participants (n= 16445) by exposure group
| E+P MHT (n=4712) |
E+P unexposed (n=8070) |
E-only MHT (n=1208) |
E-only unexposed (n=2055) |
|
|---|---|---|---|---|
| Age at study entry, mean±SD (95% CI), years | 58.2±6.3 (50.0–79.8) | 58.9±6.2 (50.0–79.7) | 59.2±6.9 (50.0–79.6) | 59.7±6.7 (50.0–79.8) |
| Age group at study entry | ||||
| 50–59 (%) | 3219 (68.3) | 4978 (61.7) | 736 (60.9) | 1156 (56.3) |
| 60–69 (%) | 1202 (25.5) | 2545 (31.5) | 362 (30.0) | 707 (34.4) |
| 70–79 (%) | 291 (6.2) | 547 (6.8) | 110 (9.1) | 192 (9.3) |
| Smoking | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Obesity | 2 (0.04) | 2 (0.03) | 1 (0.08) | 1 (0.01) |
| Hypertension (%) | 503 (10.6) | 529 (6.6) | 157 (13.0) | 143 (7.0) |
| Hypercholesterolemia (%) | 194 (4.1) | 126 (1.6) | 52 (4.3) | 41 (2.0) |
| Statin use (%) | 329 (6.9) | 294 (3.6) | 101 (8.4) | 96 (4.7) |
| Aspirin use (%) | 262 (5.5) | 313 (3.9) | 90 (7.4) | 79 (3.8) |
| Treated for diabetes (%) | 373 (7.9) | 662 (8.2) | 137 (11.3) | 178 (8.7) |
| History of fracture ≥age 55 (%) | 42 (0.89) | 59 (0.73) | 12 (0.99) | 21 (1.0) |
| Exposure in days median (95% CI) | 211 (56–3569) | 0 (0–1974) | 276 (56–3760) | 0 (0–1966) |
Second, we analyzed the effect of E+P or E-only MHT on participants who were 55 and younger at study entry, because the effect of hormones on outcomes may be modified by age or years since menopause9. In this analysis, the same procedures as in the primary analysis were undertaken for the 30% of the study population that met these age criteria.
Finally, an as-treated analysis was performed to consider duration of hormone use and drop-out and drop-ins. Unexposed participants were considered drop-ins when they fulfilled the inclusion criteria for exposure to MHT: filling two monthly prescriptions of CEE 0.625 mg within 3 months. For the drop-ins, the date of the first of two prescriptions was considered their stop point. Exposed participants who had a gap of at least 6 months of no filled hormone prescriptions were considered drop-outs. Six months after the last prescription was considered the stop point. Unexposed participants who were matched to the drop-outs were also assigned the same stop point if they had not experienced an outcome, death or loss of NHI coverage by the stop point date. The as-treated analysis encompassed all participants, but censored event histories after stop points in this subset of individuals.
Sample size was determined by the number of eligible women in the enrollment interval (1997–2000). A priori power calculations assumed an annualized incidence of breast cancer to be 0.21% in unexposed women (30% lower than reported in the WHI population). Based on 5000 E+P MHT users and 10,000 E+P unexposed participants, the study was powered to detect a relative risk of breast cancer of 2.5 or more with 80% power and alpha error of 0.05. Assuming an annualized incidence of 0.15% for CHD in unexposed women (50% lower than reported in the WHI population), the study was powered to detect a relative risk of 2.9 or more.
Results
16,045 study participants were in the final dataset: 4,712 participants who were exposed to E+P MHT and 1,208 participants who were exposed to E-only MHT (Figure 1). For E+P MHT exposed participants, there were 8,070 E+P MHT unexposed controls; for E-only MHT exposed participants, there were 2,055 E-only unexposed controls. During the study period, the number of participants lost to follow up was small (n=551, 3.4%).
Baseline characteristics of study participants are summarized in Table 2. At study entry, mean ages (SD) were 58.2 (6.3) for E+P MHT participants and 58.9 (6.2) for E+P unexposed participants. Mean ages (SD) were 59.2 (6.9) for E-only MHT participants and 59.7 (6.7) for E-only unexposed participants. Baseline prevalence of risk factors for cardiovascular disease was low. Compared to unexposed participants, MHT participants were more likely to have a history of hypertension, hypercholesterolemia, statin use and aspirin use. Participants exposed to E-only MHT were also more likely to be treated for diabetes than E-only unexposed participants. Obesity and smoking were rarely identified by ICD-9 codes. Overall, the median durations of exposure (range) in the E+P and E-only groups were 6.9 months (2–117) and 9 months (2–124), respectively. Median follow-up was 9.2 years and similar among all groups.
Primary ITT analysis
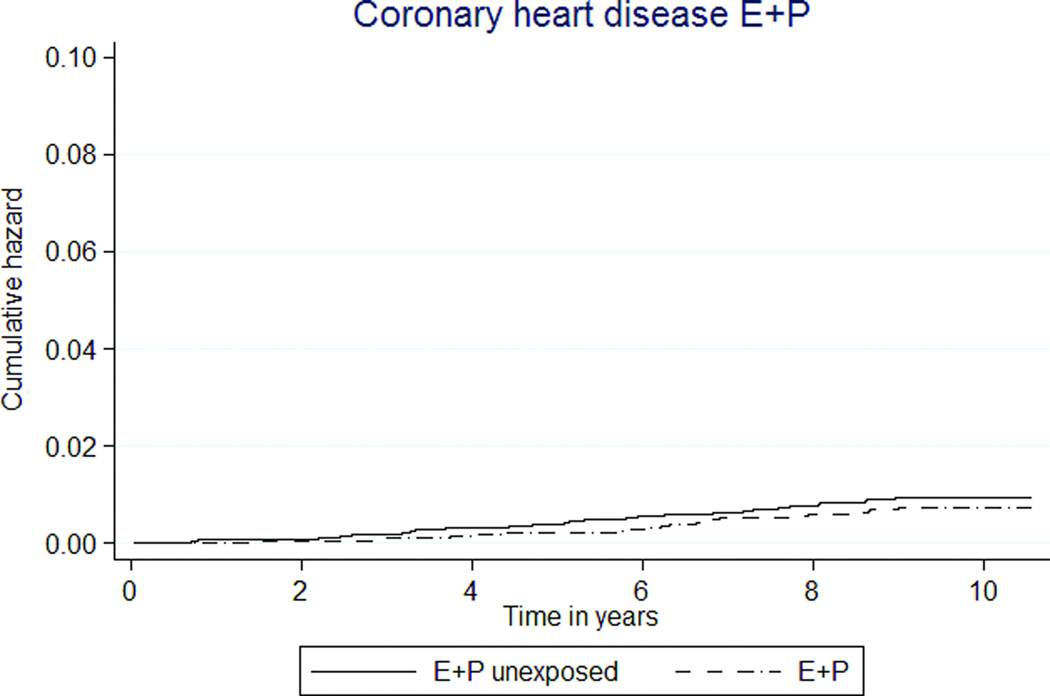
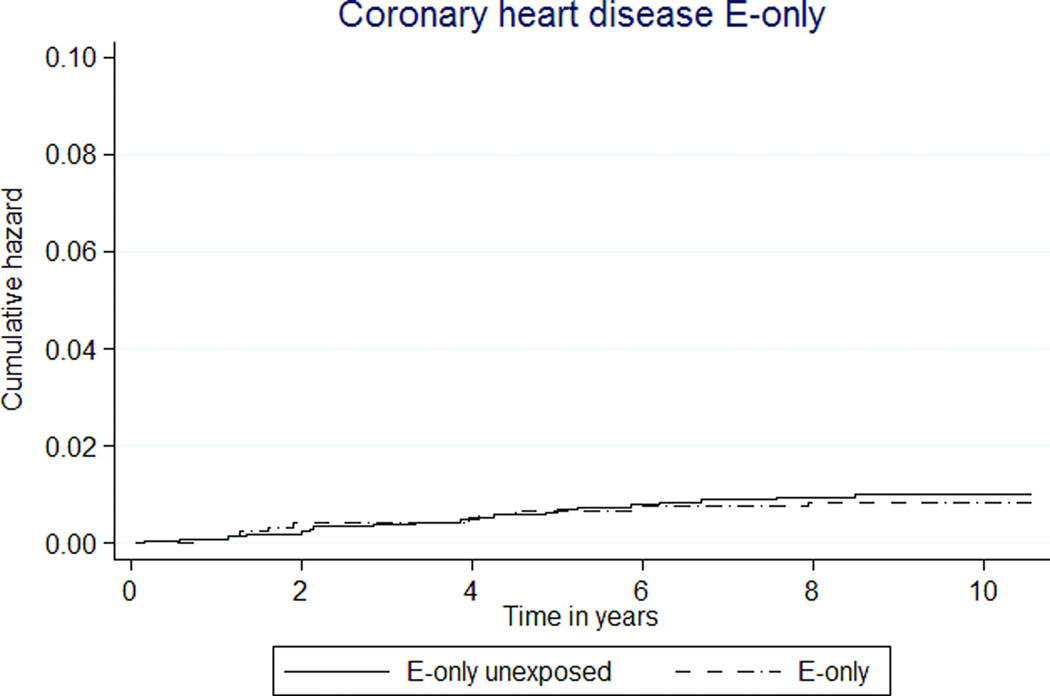
The overall rates of cardiovascular disease outcomes were low (Tables 3, 4; Figures 2a, 2b). The combined rates of experiencing an acute MI or a CHD death were not statistically significantly different between women exposed to MHT and unexposed women. The rate was 7 per 10,000 person-years in the E+P MHT group, compared to 10 per 10,000 person-years in the unexposed group (HR 0.76 [95% CI 0.49–1.15]). For E-only MHT, the combined rate was 9 per 10,000 person-years in the exposed and 10 per 10,000 person-years in the unexposed (HR 0.85 [95% CI 0.39–1.81]).
Table 3.
Comparison of outcomes between E+P MHT and unexposed participants
| Outcome | No. of participants (annualized %) | Unadjusted HR (95% CI) |
Adjusted HR (95% CI)* |
|
|---|---|---|---|---|
| E+P MHT (n=4,712) |
E+P unexposed (n=8,070) |
|||
| Follow-up time in months mean (range) | 111.8 (5.6–126.9) | 110.2 (0.4–127.0) | NA | NA |
| Acute MI | 32 (0.07) | 69 (0.09) | 0.78 (0.51–1.19) | 0.76 (0.50–1.16) |
| CHD Death | 10 (0.02) | 14 (0.02) | 1.21 (0.53–2.70) | 1.16 (0.51–2.64) |
| Breast cancer | 160 (0.36) | 183 (0.24) | 1.48 (1.20–1.83) | 1.44 (1.16–1.79) |
| Stroke | 184 (0.41) | 493 (0.65) | 0.62 (0.53–0.74) | 0.62 (0.53–0.74) |
| CABG/PTCA | 58 (0.13) | 50 (0.06) | 1.95 (1.34–2.85) | 1.82 (1.25–2.68) |
| PE | 9 (0.02) | 16 (0.02) | 0.95 (0.42–2.14) | 0.80 (0.35–1.85) |
| DVT | 18 (0.04) | 35 (0.05) | 0.87 (0.49–1.53) | 0.90 (0.51–1.60) |
| Colon cancer | 23 (0.05) | 67 (0.09) | 0.58 (0.36–0.93) | 0.62 (0.39–1.00) |
| Endometrial | 38 (0.09) | 30 (0.04) | 2.15 (1.33–3.47) | 2.22 (1.37–3.61) |
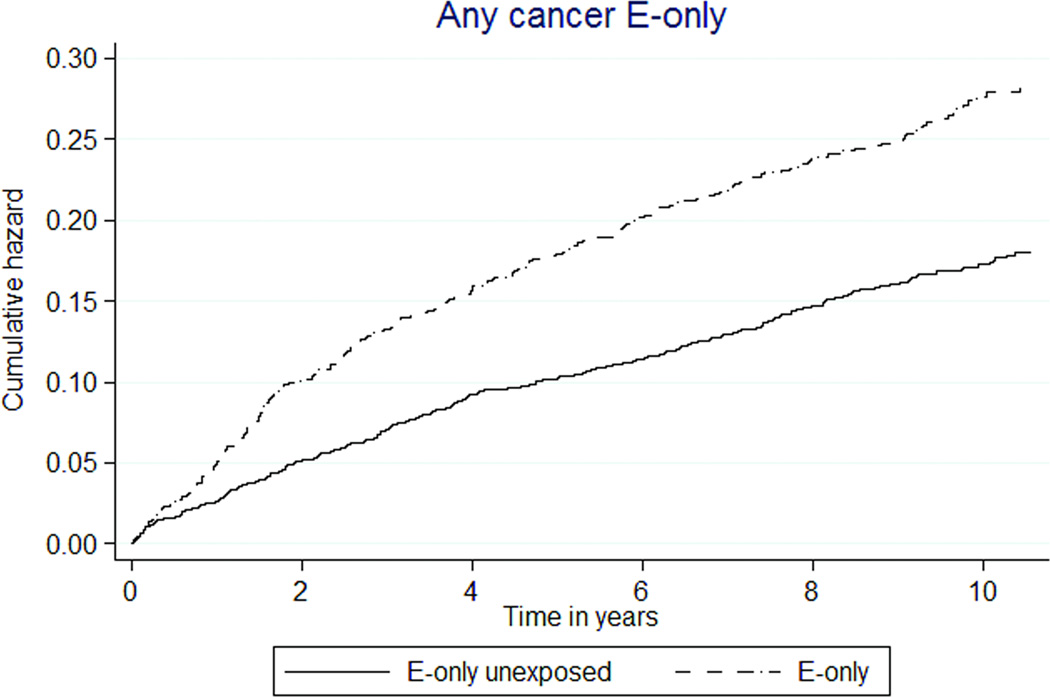
| Any cancer | 806 (1.81) | 1112 (1.46) | 1.26 (1.15–1.38) | 1.29 (1.18–1.42) |
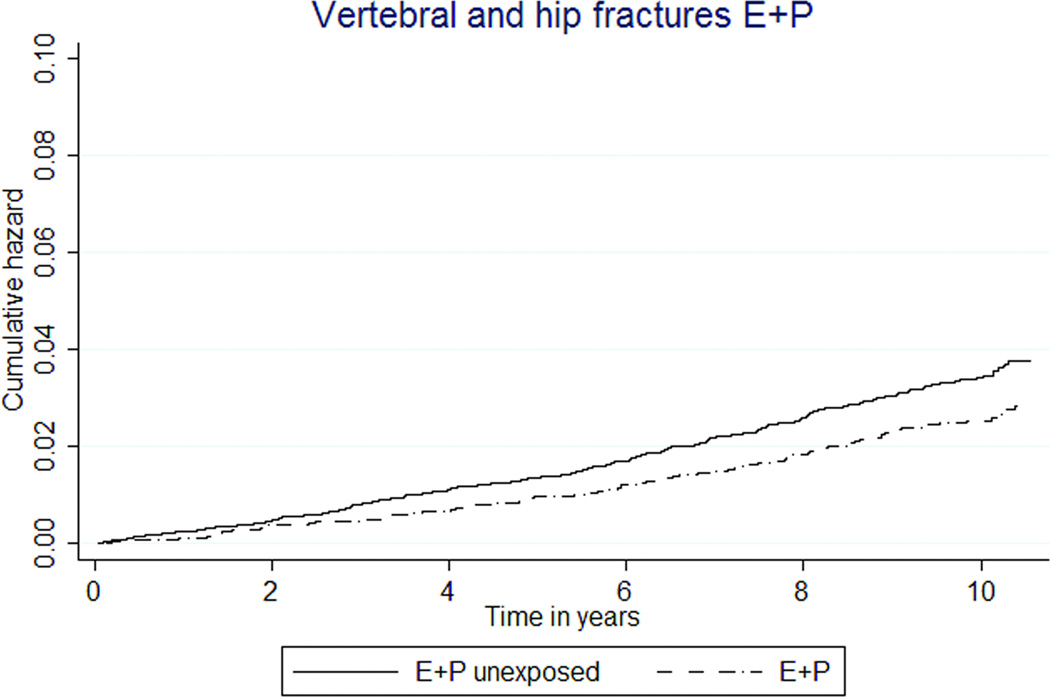
| Vertebral and hip fractures | 111 (0.26) | 251 (0.34) | 0.74 (0.59–0.93) | 0.80 (0.64–1.0) |
| Any fracture | 304 (0.71) | 604 (0.84) | 0.82 (0.72–0.95) | 0.89 (0.78–1.03) |
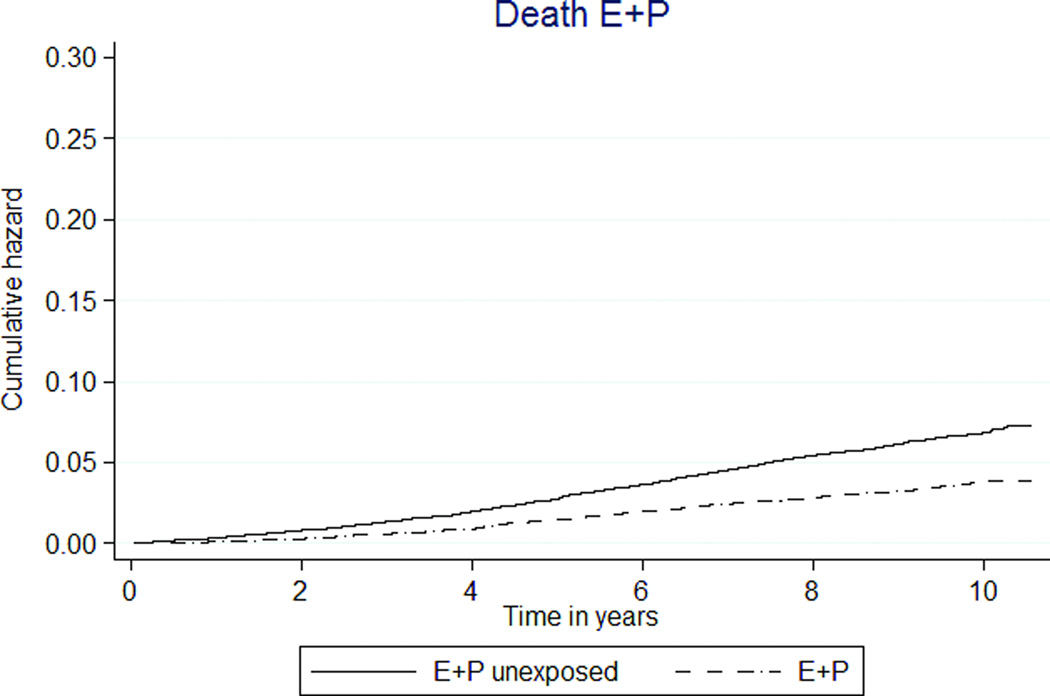
| Death | 161 (0.36) | 505 (0.66) | 0.54 (0.45–0.64) | 0.56 (0.47–0.67) |
| Global index | 626 (1.50) | 1324 (1.89) | 0.79 (0.72–0.87) | 0.81 (0.74–0.90) |
Adjusted for age, statin use, aspirin use, hypercholesterolemia, diabetes medication use, hypertension
Table 4.
Comparison of outcomes between E-only MHT and unexposed participants
| Outcome | No. of participants (annualized %) | Unadjusted HR (95% CI) |
Adjusted HR (95% CI)* |
|
|---|---|---|---|---|
| E-only MHT (n=1,208) |
E-only unexposed (n=2,055) |
|||
| Follow-up time in months mean (range) | 111.6 (6.5–126.9) | 111.3 (0.75–126.9) | NA | NA |
| Acute MI | 9 (0.08) | 20 (0.10) | 0.76 (0.35–1.68) | 0.69 (0.31–1.52) |
| CHD Death | 2 (0.02) | 6 (0.03) | 0.57 (0.11–2.80) | 0.45 (0.09–2.30) |
| Breast cancer | 50 (0.43) | 59 (0.31) | 1.44 (0.99–2.10) | 1.39 (0.95–2.03) |
| Stroke | 81 (0.69) | 135 (0.71) | 1.02 (0.77–1.35) | 0.97 (0.73–1.28) |
| CABG/PTCA | 12 (0.10) | 16 (0.08) | 1.35 (0.63–2.89) | 1.11 (0.52–2.40) |
| PE | 3 (0.03) | 2 (0.01) | 2.53 (0.42–15.2) | 2.75 (0.45–16.8) |
| DVT | 16 (0.14) | 7 (0.04) | 3.89 (1.60–9.46) | 3.63 (1.48–8.89) |
| Colon cancer | 7 (0.06) | 12 (0.06) | 0.99 (0.39–2.51) | 0.99 (0.38–2.57) |
| Endometrial cancer | 10 (0.09) | 12 (0.06) | 1.41 (0.61–3.28) | 1.31 (0.56–3.09) |
| Any cancer | 277 (2.37) | 313 (1.65) | 1.59 (1.35–1.87) | 1.63 (1.39–1.93) |
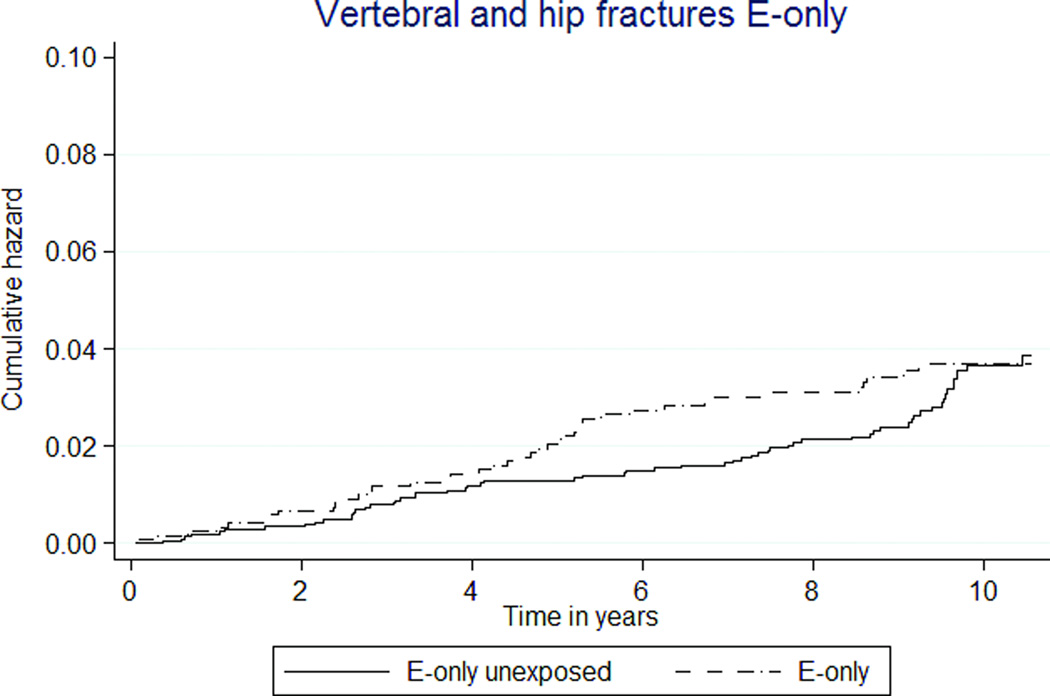
| Vertebral and hip fractures | 41 (0.37) | 61 (0.32) | 1.14 (0.77–1.70) | 1.14 (0.75–1.70) |
| Any fracture | 91 (0.83) | 178 (0.97) | 0.86 (0.67–1.11) | 0.87 (0.67–1.12) |
| Death | 73 (0.62) | 134 (0.70) | 0.92 (0.69–1.23) | 0.89 (0.67–1.19) |
| Global Index | 225 (2.20) | 357 (2.0) | 1.09 (0.92–1.28) | 1.08 (0.92–1.29) |
Adjusted for age, statin use, aspirin use, hypercholesterolemia, diabetes medication use, hypertension
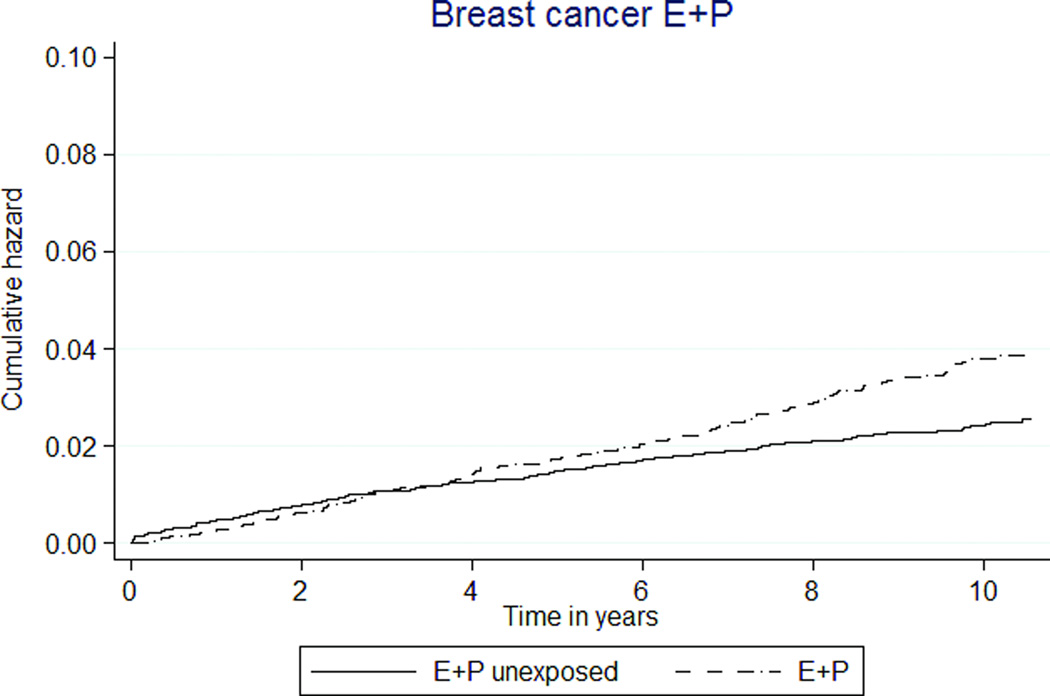
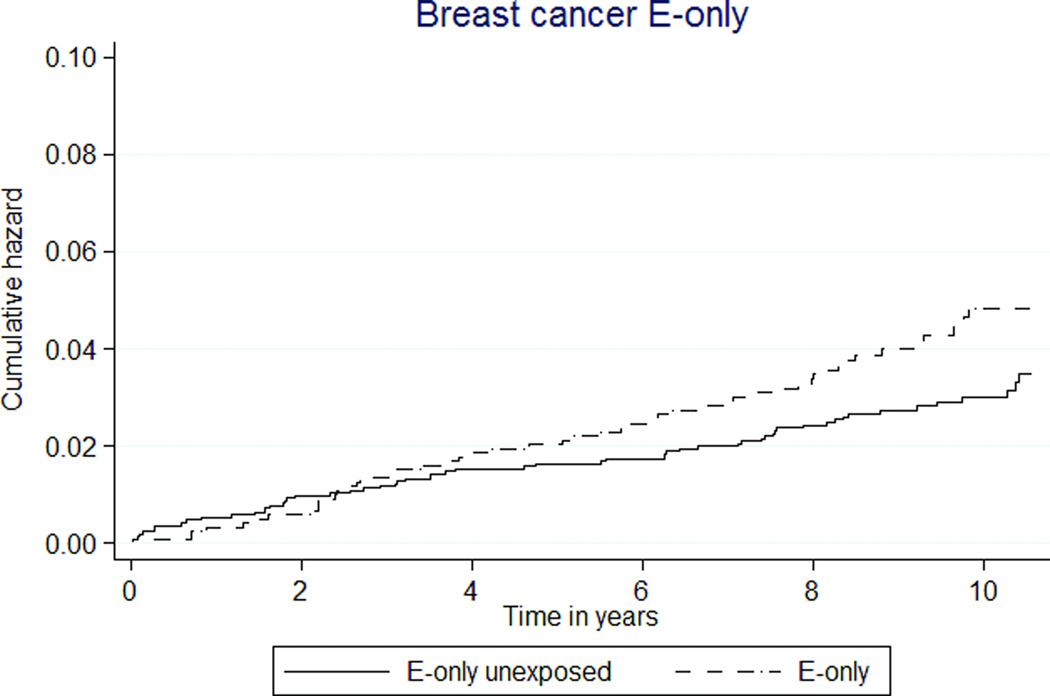
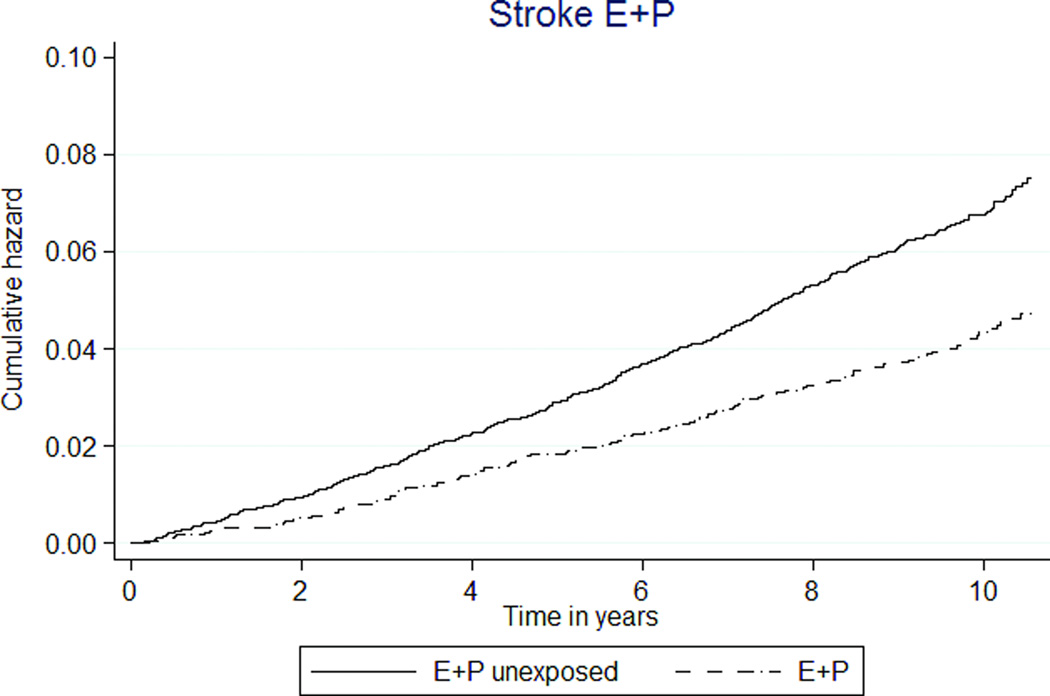
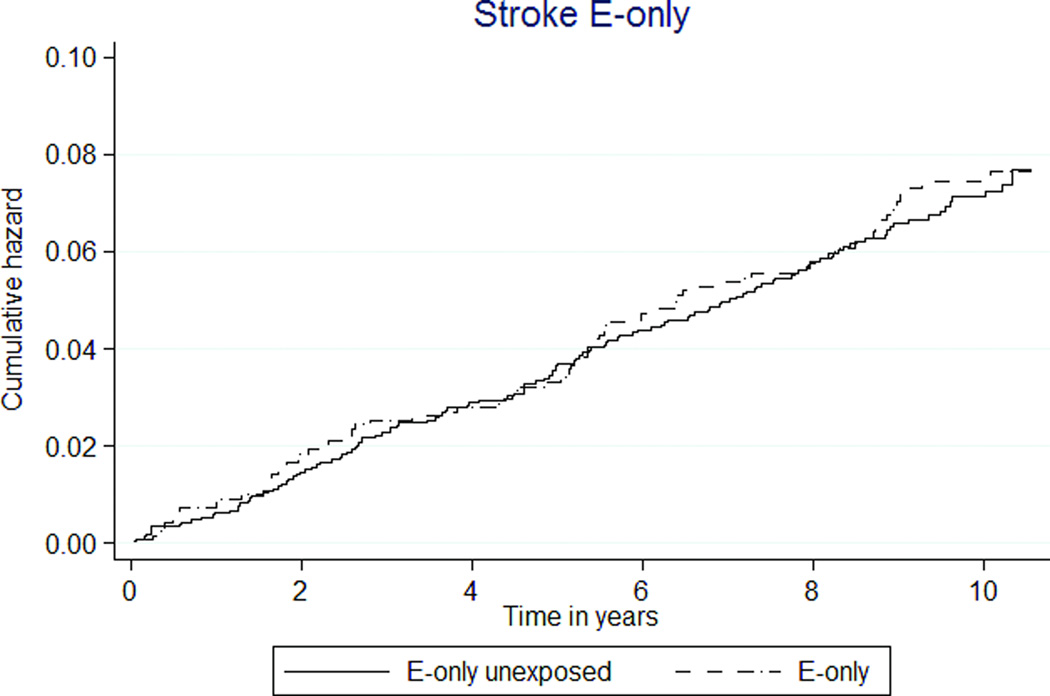
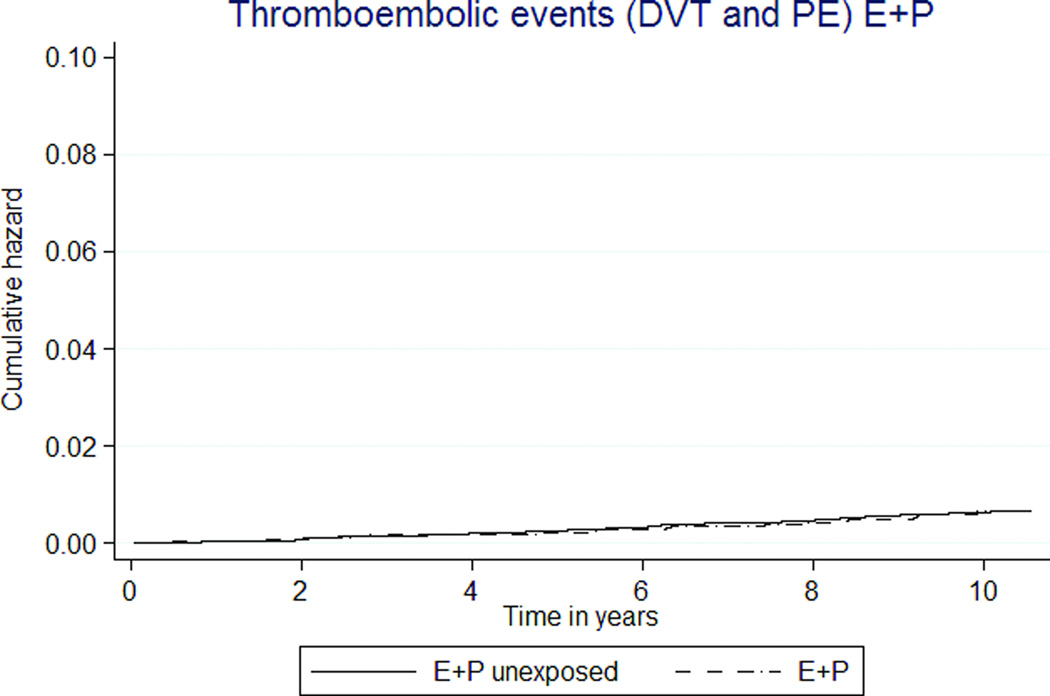
FIG. 2.
Kaplan-Meier estimates of cumulative hazards for clinical outcomes by MHT exposure status. MHT, menopausal hormone therapy; DVT, deep venous thrombosis; PE, pulmonary embolism; E, estrogen; P, progestin.
E+P MHT use was associated with a 50% higher rate of breast cancer (36 versus 24 per 10,000 person-years, HR 1.48 [95% CI 1.20–1.83]). E-only MHT also appeared to be associated with a higher rate, but this did not reach statistical significance (HR 1.44 [95% CI 0.99–2.10], Tables 3, 4; Figures 2c, d).
Among global index events, E+P exposure was associated with 39% lower stroke rates. In E+P MHT participants, the rate was 41 per 10,000 person-years, compared to 65 in unexposed participants (HR 0.62 [95% CI 0.53–0.74]) (Table 3, Figure 2e). The rates of stroke were similar between E-only MHT and E-only unexposed participants (69 versus 71 per 10,000 person-years, HR 1.02 [95% CI 0.77–1.35]) (Table 4, Figure 2f).
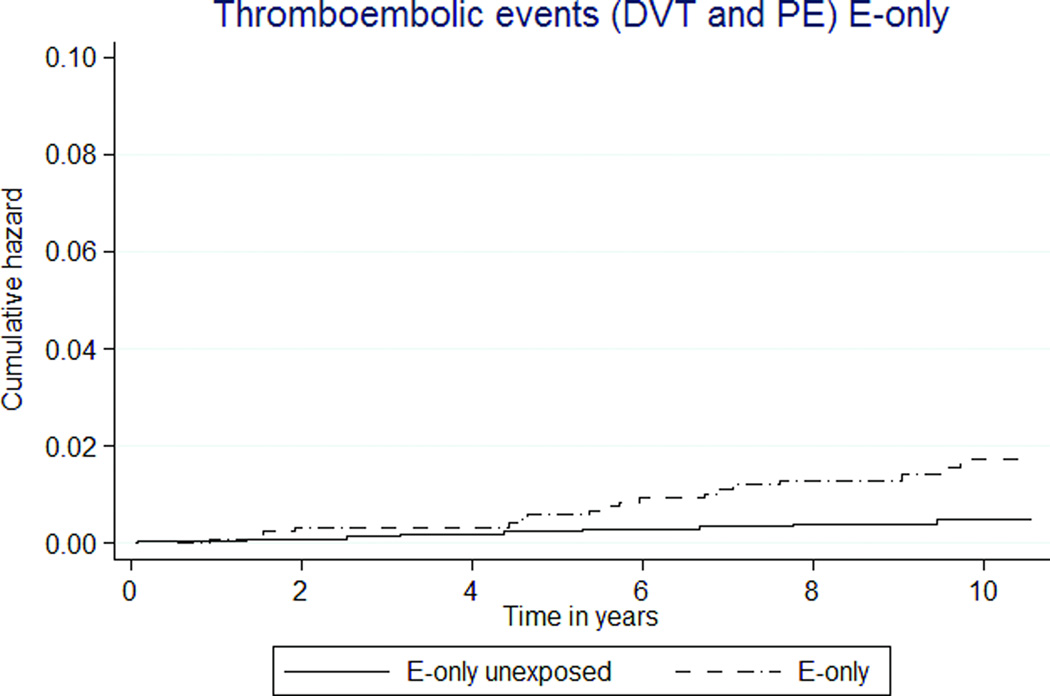
Rates for pulmonary embolism (PE) and deep venous thrombosis (DVT) were low (Tables 3, 4; Figures 2g, h). MHT exposure was not associated with combined thromboembolic rates. However, the rate of DVT was almost three-fold higher in the E-only MHT group than E-only unexposed group, HR 3.89 (1.60–9.46).
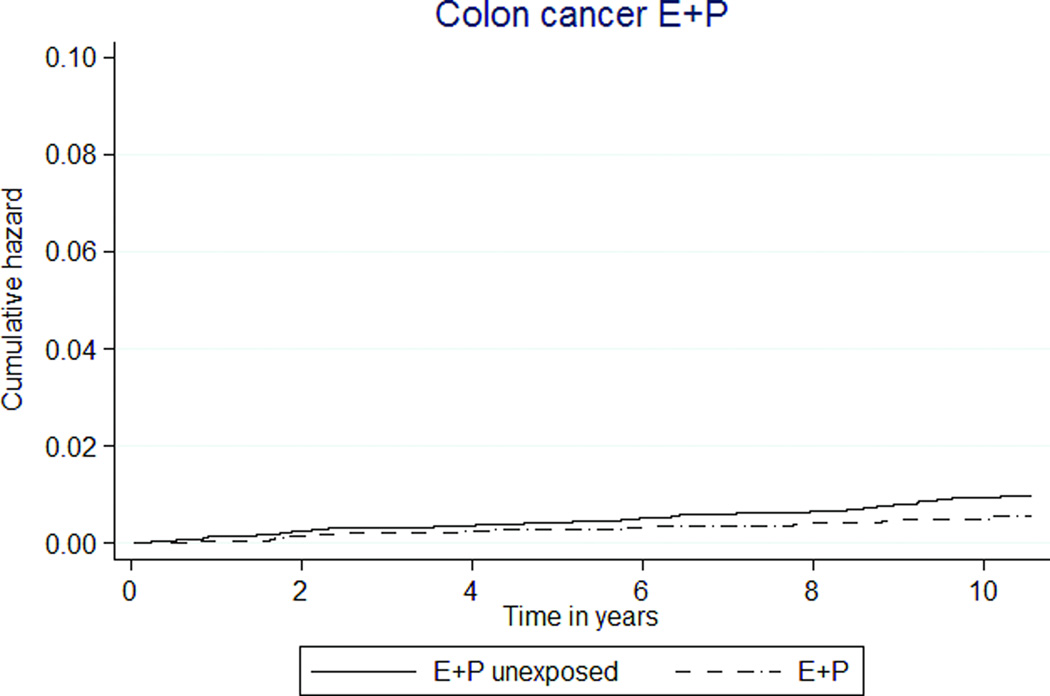
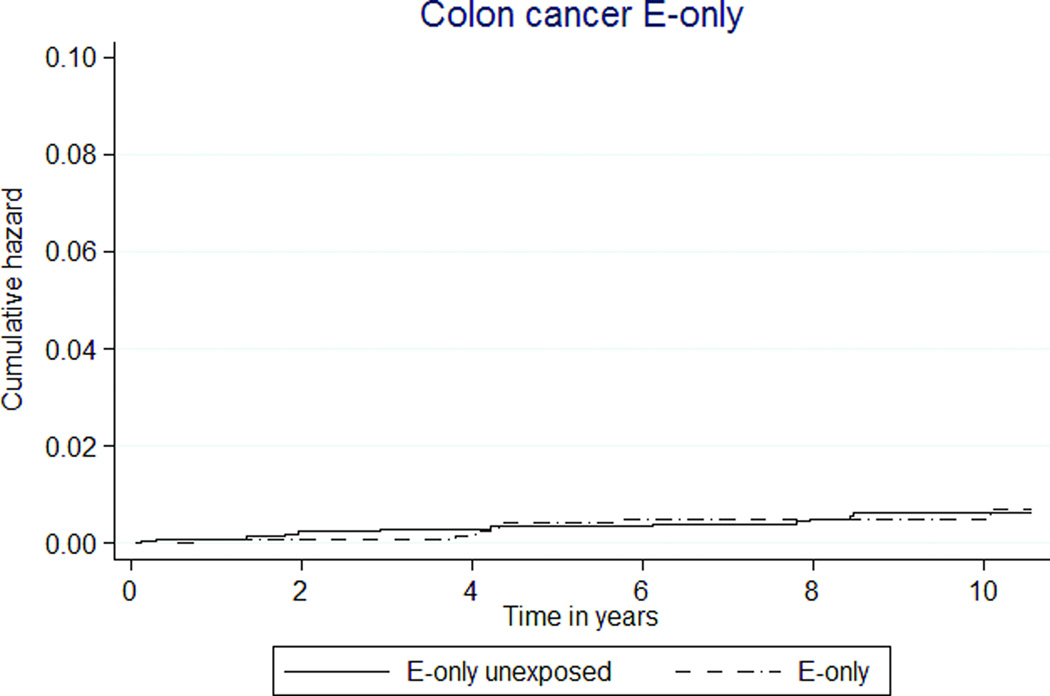
E+P exposure was associated with lower rates of colorectal cancer (5 versus 9 per 10,000 person-years, HR 0.58 [95% CI 0.36–0.93]) (Table 3, Figure 2i). E-only MHT was not associated with colorectal cancer, HR 0.99 (95% CI 0.39–2.51) (Table 4, Figure 2j).
A higher rate of endometrial cancer was observed with E+P use, but not with E-only use (Tables 3, 4). E+P MHT participants had a 2-fold increased risk (9 versus 4 per 10,000 person-years, HR 2.15 [1.33–3.47]). For E-only MHT participants, the HR was 1.41 (95% CI 0.61–3.28).
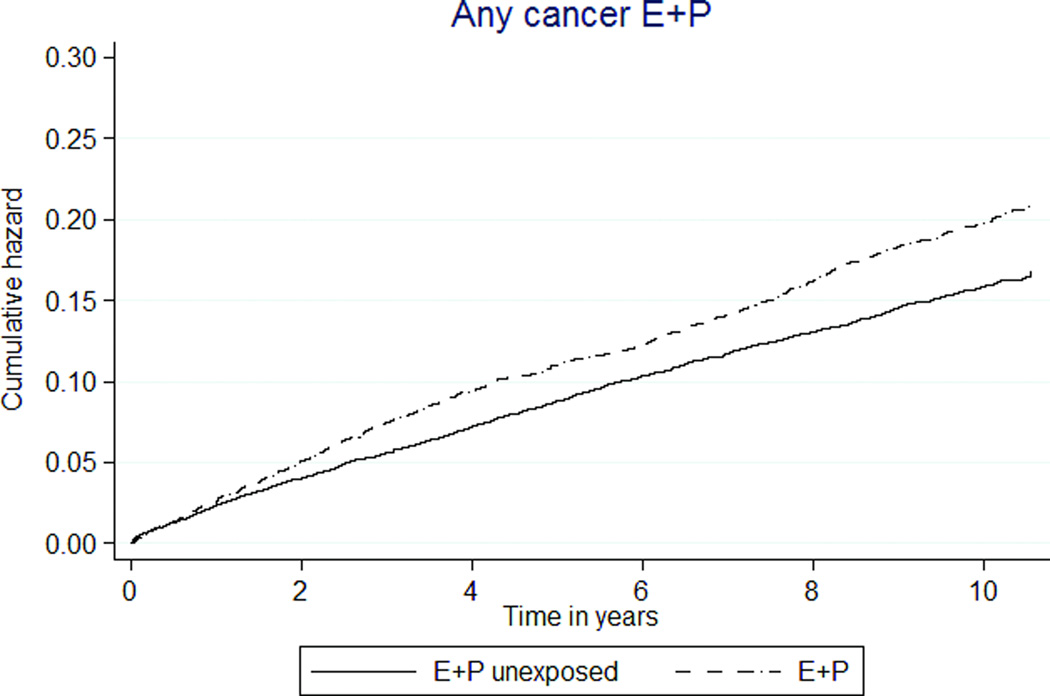
Total cancer incidence was 24% higher in E+P MHT participants than E+P unexposed (181 versus 146 per 10,000 person-years, HR1.26 [95% CI 1.15–1.38]). As well, cancer incidence was 44% higher in E-only MHT participants than unexposed participants (237 versus 165 per 10,000 person-years, HR 1.59 [1.35–1.87], Figures 2k, 2l).
Lower rates of vertebral and hip fractures were associated with E+P MHT use (26 vs. 34 per 10,000 person-years, HR 0.72 [95% CI 0.58–0.90]), but fracture rates not different by E-only exposure (37 vs. 32 per 10,000 person-years, HR 1.14 [95% CI 0.77–1.70], Figures 2m, 2n).
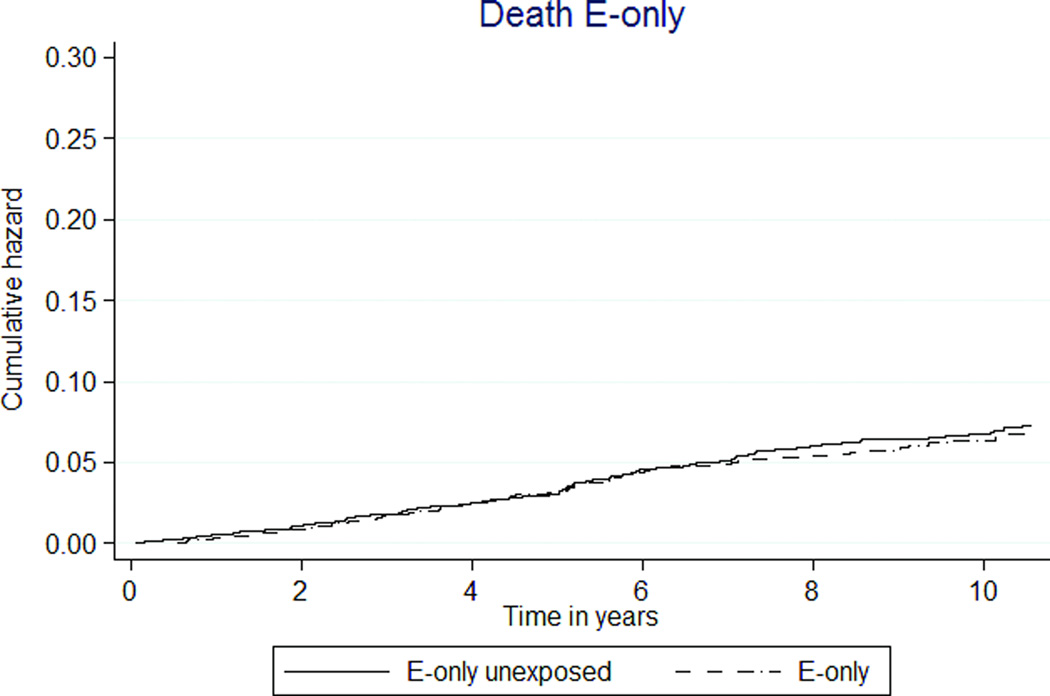
Mortality was significantly lower in E+P participants than unexposed participants (36 versus 66 per 10,000 person-years, HR 0.54 [95% CI 0.45–0.64], Figure 3a). The E-only MHT group had a similar rate of death as the unexposed group (62 versus 70 per 10,000 person-years, HR 0.92 [95% CI 0.69–1.23], Figure 3b).
FIG. 3.
Kaplan-Meier estimates of cumulative hazards of global index and death by MHT exposure status. MHT, menopausal hormone therapy; E, estrogen; P, progestin.
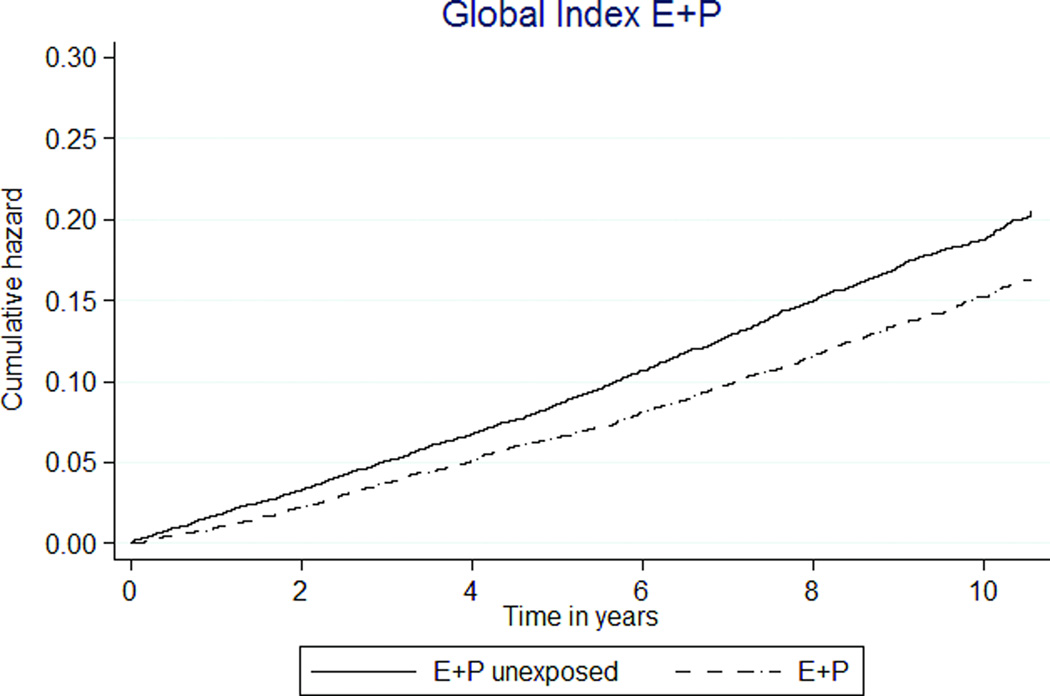
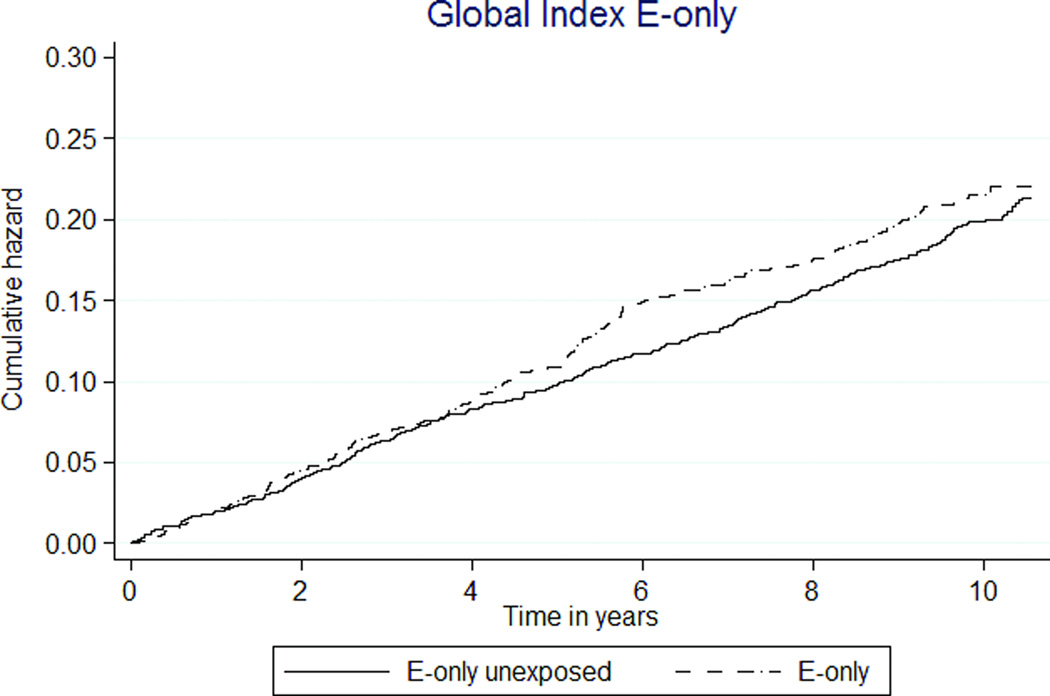
The global index composite outcome showed E+P MHT associated with decreased event rates (150 vs. 189 per 10,000 person-years, HR 0.79 [95% CI 0.72–0.87], Table 3, Figure 3c). In the E-only analysis, the global index was not associated with E-only MHT exposure (HR 1.09 [0.92–1.28], Table 4, Figure 3d).
Adjusted analysis
Multivariable Cox models for each outcome were generated to adjust for age, hypercholesterolemia, hypertension, statin use, aspirin use and anti-diabetes drug use. Given low incidence of obesity (0.04%) and smoking (0%) diagnoses, they were not included in adjusted models. The direction and magnitude of hazard ratios in the adjusted models were similar to the primary analysis (Tables 3, 4).
Analysis of age younger than 55
Participants who were 55 years old and younger at study entry represented 30.5% of the entire cohort. Similar to the overall cohort, E+P MHT and E-only MHT participants were more likely to have hypertension and hypercholesterolemia compared to their unexposed controls. The median duration of E+P MHT exposure was 7.9 months (range 2–93), and the median duration of E-only MHT exposure was 12.3 months (range 2–121). In this younger group, the number of events was lower.
Tables 5 and 6 summarize the rates of outcomes and hazard ratios in this sub-population. Overall, hazard ratios appear similar to the entire study cohort. However, E+P MHT was associated with a significantly lower rate of MI events (HR 0.26 [95% CI 0.07–0.90]), an observation that remained significant after adjusting for confounders. The reduction in stroke, reduction in death, increase in breast cancer and reduction in global index events with E+P MHT exposure remained in this analysis. In the E-only analysis, breast cancer and any cancer rates remained higher in the exposed than in the unexposed group.
Table 5.
Comparison of outcomes between Unexposed and E+P MHT participants who were 55 years and younger at study entry
| Outcome | No. of participants (annualized %) | Unadjusted HR (95% CI) |
Adjusted HR (95% CI)* |
|
|---|---|---|---|---|
| E+P MHT (n=1,774) |
E+P unexposed (n=2,171) |
|||
| Follow-up time, mo mean (range) | 113.5 (7.2–126.9) | 111.9 (1.0–126.9) | NA | NA |
| Acute MI | 3 (0.02) | 14 (0.07) | 0.26 (0.07–0.90) | 0.23 (0.06–0.80) |
| CHD Death | 0 (0) | 4 (0.02) | - | - |
| Breast cancer | 66 (0.40) | 55 (0.28) | 1.46 (1.02–2.09) | 1.49 (1.04–2.13) |
| Stroke | 29 (0.17) | 73 (0.37) | 0.47 (0.31–0.73) | 0.43 (0.28–0.67) |
| CABG/PTCA | 13 (0.08) | 13 (0.06) | 1.19 (0.55–2.58) | 0.95 (0.42–2.14) |
| PE | 2 (0.01) | 2 (0.01) | 1.21 (0.17–8.59) | 1.14 (0.16–8.37) |
| DVT | 5 (0.03) | 2 (0.01) | 3.0 (0.58–15.49) | 2.66 (0.50–14.1) |
| Colon cancer | 5 (0.03) | 10 (0.05) | 0.60 (0.20–1.76) | 0.56 (0.19–1.68) |
| Endometrial | 9 (0.05) | 6 (0.03) | 1.82 (0.65–5.11) | 1.56 (0.55–4.49) |
| Any cancer | 251 (1.6) | 239 (1.2) | 1.30 (1.09–1.55) | 1.30 (1.09–1.56) |
| Vertebral and hip fractures | 17 (0.10) | 25 (0.12) | 0.82 (0.44–1.52) | 0.81 (0.44–1.52) |
| Any fracture | 66 (0.39) | 96 (0.48) | 0.82 (0.60–1.12) | 0.81 (0.59–1.10) |
| Death | 26 (0.15) | 69 (0.34) | 0.45 (0.29–0.71) | 0.44 (0.28–0.70) |
| Global index | 147 (0.90) | 217 (1.1) | 0.82 (0.66–1.00) | 0.79 (0.64–0.98) |
Adjusted for age, statin use, aspirin use, hypercholesterolemia, diabetes medication use, hypertension
Table 6.
Comparison of outcomes between Unexposed and E-only MHT participants who were 55 years and younger at study entry
| Outcome | No. of participants (annualized %) | Unadjusted HR (95% CI) |
Adjusted HR (95% CI)* |
|
|---|---|---|---|---|
| E-only MHT (n=434) |
E-only unexposed (n=515) |
|||
| Follow-up time, mean (range), mo | 114.9 (7.9–126.9) | 113.5 (0.75–126.9) | NA | NA |
| Acute MI | 0 (0) | 2 (0.04) | - | - |
| CHD Death | 0 (0) | 0 (0) | - | - |
| Breast cancer | 21 (0.51) | 13 (0.0.26) | 1.91 (0.96–3.82) | 1.85 (0.92–3.73) |
| Stroke | 17 (0.41) | 18 (0.37) | 1.11 (0.57–2.16) | 0.99 (0.50–1.95) |
| CABG/PTCA | 1 (0.02) | 4 (0.08) | 0.29 (0.03–2.64) | 0.18 (0.02–1.89) |
| PE | 2 (0.05) | 0 (0) | - | - |
| DVT | 6 (0.14) | 0 (0) | - | - |
| Colon cancer | 3 (0.07) | 2 (0.04) | 1.78 (0.30–10.6) | 1.81 (0.29–11.2) |
| Endometrial cancer | 2 (0.04) | 3 (0.06) | 0.78 (0.13–4.69) | 0.71 (0.11–4.54) |
| Any cancer | 86 (2.33) | 52 (1.12) | 2.06 (1.46–2.91) | 2.11 (1.49–3.00) |
| Vertebral and hip fractures | 3 (0.07) | 6 (0.12) | 0.58 (0.14–2.32) | 0.58 (0.14–2.38) |
| Any fracture | 15 (0.37) | 26 (0.54) | 0.67 (0.36–1.27) | 0.62 (0.32–1.19) |
| Death | 12 (0.29) | 15 (0.31) | 0.94 (0.44–2.01) | 0.97 (0.45–2.10) |
| Global Index | 53 (1.3) | 53 (1.1) | 1.19 (0.81–1.74) | 1.12 (0.77–1.66) |
Adjusted for age, statin use, aspirin use, hypercholesterolemia, diabetes medication use, hypertension
As treated analysis
Tables 7 and 8 reflect estimates from the as-treated analysis. Eight percent of unexposed participants (n=808) became drop-ins during the study. Overall, the duration of follow up was shortened with censoring at drop-out or drop-in, rendering fewer events. No acute MI or CHD death events occurred in E+P MHT participants, while 9 events per 10,000 person-years were observed in unexposed participants. In the E+P analysis, breast cancer rates were not significantly increased (HR 0.96 [95% CI 0.63–1.46]) and colon cancer rates were not significantly decreased (HR 0.50 [95% CI 0.21–1.16]). In the E-only analysis, breast cancer rates were not increased in MHT users, HR 0.79 (95% CI 0.40–1.58). But the global index demonstrated significantly higher risk of outcomes associated with E-only MHT use (HR 1.24 [95% CI 1.05–1.47]).
Table 7.
As-treated comparison of outcomes between E+P MHT and unexposed subjects
| Outcome | No. of subjects (annualized %) | Unadjusted HR (95% CI) |
Adjusted HR (95% CI)* |
|
|---|---|---|---|---|
| E+P MHT (n=4,712) |
E+P unexposed (n=7,867) |
|||
| Follow-up time in months Mean (range) | 27.8 (5.6–126.9) | 27.2 (2.6–126.9) | NA | NA |
| Acute MI | 0 (0) | 13 (0.07) | - | - |
| CHD Death | 0 (0) | 3 (0.02) | - | - |
| CABG/PTCA | 3 (0.03) | 3 (0.02) | 1.56 (0.31–7.73) | 1.35 (0.26–6.65) |
| Breast cancer | 34 (0.31) | 58 (0.33) | 0.96 (0.63–1.46) | 0.96 (0.63–1.48) |
| Stroke | 30 (0.27) | 95 (0.54) | 0.51 (0.34–0.77) | 0.50 (0.33–0.76) |
| PE | 3 (0.02) | 2 (0.01) | 2.34 (0.39–14.0) | 2.52 (0.42–15.4) |
| DVT | 3 (0.03) | 8 (0.04) | 0.61 (0.16–2.29) | 0.61 (0.16–2.33) |
| Colon cancer | 7 (0.06) | 23 (0.13) | 0.50 (0.21–1.16) | 0.48 (0.21–1.13) |
| Endometrial | 19 (0.17) | 9 (0.05) | 3.45 (1.56–7.63) | 3.74 (1.68–8.33) |
| Any cancer | 265 (2.5) | 327 (1.9) | 1.33 (1.13–1.56) | 1.36 (1.15–1.60) |
| Vertebral and hip fractures | 73 (0.67) | 121 (0.69) | 0.97 (0.73–1.30) | 1.06 (0.79–1.43) |
| Any fracture | 213 (2.0) | 386 (2.3) | 0.89 (0.75–1.05) | 0.92 (0.78–1.09) |
| Death | 88 (0.79) | 316 (1.7) | 0.46 (0.36–0.58) | 0.50 (0.39–0.63) |
| Global index | 555 (5.36) | 1000 (5.87) | 0.90 (0.81–0.99) | 0.94 (0.85–1.04) |
Adjusted for age, statin use, aspirin use, hypercholesterolemia, diabetes medication use, hypertension
Table 8.
As-treated comparison of outcomes between E-only MHT and unexposed subjects
| Outcome | No. of subjects (annualized %) | Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
|
|---|---|---|---|---|
| E-only MHT (n=1,208) |
E-only unexposed (n=1,997) |
|||
| Follow-up time in months mean (range) | 33.6 (6.5–127.0) | 32.7 (4.6–126.9) | NA | NA |
| Acute MI | 2 (0.06) | 5 (0.09) | 0.65 (0.13–3.33) | 0.57 (0.11–2.99) |
| CHD Death | 0 (0) | 2 (0.04) | - | - |
| CABG/PTCA | 0 (0) | 2 (0.02) | - | - |
| Breast cancer | 12 (0.36) | 24 (0.45) | 0.79 (0.40–1.58) | 0.82 (0.41–1.67) |
| Stroke | 22 (0.65) | 30 (0.56) | 1.16 (0.67–2.01) | 1.08 (0.62–1.90) |
| PE | 1 (0.03) | 1 (0.02) | 1.54 (0.10–24.73) | 1.93 (0.12–31.3) |
| DVT | 3 (0.09) | 1 (0.02) | 4.76 (0.50–45.83) | 3.99 (0.39–40.0) |
| Colon cancer | 2 (0.06) | 5 (0.09) | 0.64 (0.12–3.28) | 0.56 (0.11–3.0) |
| Endometrial cancer | 6 (0.18) | 4 (0.07) | 2.41 (0.68–8.56) | 2.20 (0.61–7.91) |
| Any cancer | 132 (4.3) | 112 (2.2) | 2.0 (1.55–2.57) | 2.01 (1.56–2.59) |
| Vertebral and hip fractures | 31 (0.93) | 48 (0.91) | 1.02 (0.65–1.60) | 1.07 (0.68–1.70) |
| Any fracture | 73 (2.2) | 128 (2.5) | 0.89 (0.67–1.19) | 0.90 (0.68–1.21) |
| Death | 47 (1.3) | 96 (1.8) | 0.77 (0.54–1.09) | 0.85 (0.59–1.22) |
| Global Index | 232 (7.8) | 315 (6.3) | 1.24 (1.05–1.47) | 1.27 (1.07–1.51) |
Adjusted for age, statin use, aspirin use, hypercholesterolemia, diabetes medication use, hypertension
Hazard ratios for other outcomes remained similar to the ITT analysis. Lower stroke (HR 0.51 [95% CI 0.34–0.77]) and death rates (HR 0.46 [95% CI 0.36–0.58]) remained significantly associated with E+P MHT. Higher endometrial cancer rate was observed in the E+P exposure group (HR 3.45 [95% CI 1.56–7.63]).
Discussion
In postmenopausal Chinese women, the risk of coronary heart disease was low and was not associated with exposure to menopausal hormone therapy using conjugated equine estrogen, with or without medroxyprogesterone. A higher rate of breast cancer may be associated with CEE and MPA exposure. The global index demonstrated a significantly decreased event rate associated with CEE and MPA exposure, but not with CEE-only exposure.
Absolute rates of acute myocardial infarction and CHD death were low but similar among exposed and unexposed women, despite both hormone exposure groups having more risk factors for cardiovascular outcomes at baseline. The low rates may reflect the younger age of study participants as well as the underlying Asian population. Among women younger than 55, combination MHT exposure was associated with lower rates of MI and CHD death. In contrast to the WHI and Heart and Estrogen/Progestin Replacement Study 10, no increased risk of CHD outcomes was observed at initiation. While the findings are novel and contribute to limited published data on CHD and MHT in this population, it should be noted that the low event rates limited the study power and ability to demonstrate statistical certainty. In addition, some known confounders such as diet, body size and physical activity could not be adequately assessed in this dataset.
Increased breast cancer risk was associated with CEE with MPA use. The absolute increases were 12 more breast cancers per 10,000 person-years for E+P MHT exposure. Similar to WHI data, the rates in unexposed and E+P MHT groups were similar in the first 4 years but diverged thereafter. However, this effect disappeared in the as-treated analysis, which censored event history 6 months after drug discontinuation. The as-treated result may reflect low power to detect events once follow up was limited or long latency between HT exposure and breast cancer that are not captured by restricting follow up time, The data comparing the E-only MHT group to the unexposed group did not demonstrate a protective effect. This is consistent with prior cohort data including a comprehensive meta-analysis 11 and the WHI observational arm 12. A constraint of this dataset in studying breast cancer risks was the inability to control for some risk factors such as parity, age at first birth, body size, family history and age at menopause.
Lower stroke risk was consistently associated with combination MHT, despite higher baseline risks of hypertension and hypercholesterolemia in these women. Most prior data suggest increased risk of strokes regardless of age 10,13–15. To date, there are no other data on risk of stroke with menopausal hormone therapy exposure in postmenopausal Asian women. Moreover, a recent study supports the validity of the stroke diagnosis in the NHIRD dataset 16. While our results should be interpreted with caution and may be due to uncontrolled confounding, the findings are consistent with the hypothesis of differential risks and benefits of MHT by population and warrants further future investigation.
Colorectal cancer rates were lower in E+P participants compared to E+P unexposed participants. Our data are consistent with both the WHI and observational studies of E+P. Endometrial cancer risk was higher among MHT exposed women, with or without MPA. In the CEE-only group, this risk likely resulted from unopposed estrogen exposure in women with a uterus. It is unclear why the combined MHT group also had a significantly higher risk of uterine cancer given evidence of filled progesterone prescriptions, but it is a limitation of administrative databases that compliance cannot be measured. Nonetheless, these data highlight the relationship between estrogen alone and uterine cancer to providers and patients.
Thromboembolic disease risks were very low and likely reflect shorter MHT exposure. An increased risk of DVT and PE was expected with any estrogen exposure, but was observed only in the E-only group as compared to the unexposed. Through the entire follow up, the rate of DVT and PE for the E+P group never deviated from that of controls.
Fracture risks in the population were similar to self-reported data in postmenopausal Chinese women 17. Hazard ratios in both MHT treatment groups support the protective effect of estrogen exposure against fractures, but only the combination MHT group had significantly lower fracture rates. The lack of significant association with E-only MHT may be a consequence of limited power.
Death rates were similar in the E-only MHT and unexposed groups, but significantly lower in the E+P MHT group compared to E+P unexposed group. The decreased death rate cannot be explained by known baseline co-morbidities or the sum of all major outcomes. Because deaths captured in the NHIRD do not account for deaths that occur outside of the hospital, it is possible that the rate of home deaths differed by MHT exposure groups. In the WHI CEE and MPA or CEE only trials, all-cause mortality were similar between exposed and unexposed women, but a reanalysis of the data demonstrated a reduced relative risk (0.7 [0.51–0.96]) among younger women from 50–59 9. Our population of younger Asian women supports this observation.
MHT remains a controversial treatment for which there are very limited data in Asian women. This study is the largest to date in this population and offers two important advantages. It is population-based, as national health insurance covers 99% of the population, and generalizable. Second, the dataset captures drug utilization and outcomes comprehensively because of the broad scope of medications and services covered by NHI. In comparison, a multi-center RCT of 1100 Asian women evaluated efficacy of three doses of combination conjugated equine estrogen/ medroxyprogesterone (0.625/2.5, 0.45/1.5, 0.3/1.5) for relief of vasomotor and vaginal atrophy symptoms, but the study is limited by sample size and short follow-up 18. Finally, the study was able to examine a number of clinically important outcomes in postmenopausal Asian women.
There are several additional considerations in interpreting the data from this observational study. Because of the limited sample size, there may be effects of MHT that we were underpowered to detect, and the confidence intervals of many of the effect estimates are relatively wide. Furthermore, compared to the WHI, the duration of hormone exposure in this study was short and the NHIRD population was younger. Shorter hormone exposure may reflect limited adherence or the practice pattern in this population. While these data are generalizable to the large number of postmenopausal Taiwanese women, the difference in duration of exposure limits comparisons of the two studies.
Because randomization did not occur, selection bias and uncontrolled confounding are limitations of the study design. Unexpectedly, women in this general population who received hormone therapy had more co-morbidities at baseline, which may bias estimates on outcomes such as CHD toward the null. This may be attributed to the timing of the NHIRD study entry, when MHT was commonly prescribed for secondary prevention of cardiovascular disease. In adjusted analyses, we accounted for known confounding and report that hazard ratios did not substantially change. However, as discussed, there are other confounders that could not be accounted for in this dataset. One example is exposure to dietary phytoestrogens. Soy products are common in the diet of this population, but food intake is not a part of the data acquired by the NHIRD. There are neither reported data on the intake of isoflavones in postmenopausal Taiwanese women nor if this intake varies with use of MHT. Therefore, we were unable to control for this factor.
Third, using a de-identified claims database relies on ICD-9 diagnostic and procedure codes. The two major limitations to this approach are that clinic and hospital coding may over-or under-count the number of true diagnoses and contribute to information bias. Also, because the dataset is de-identified, we were unable to perform traditional validation steps such as verifying diagnoses through medical chart review of a subset of patients or have diagnoses undergo adjudication. This limitation was approached by additional analyses restricting outcome diagnoses to those with a corroborating clinical procedure or prescription (data not shown). Hazards did not significantly change in direction or magnitude. Finally, exclusion criteria were limited to diagnoses in the 6 months before study entry as the beginning of data collection for the NHIRD preceded the enrollment period by a short interval. However, because medications required monthly physician visits and prescriptions, it is less likely that significant exclusionary diagnoses were missed.
Conclusion
In postmenopausal Chinese women, risks of cardiovascular disease outcomes were low. In the setting of limited exposure to CEE 0.625 mg with and without MPA 5mg, the rates of MI and CHD death did not differ with MHT exposure. E+P MHT may be associated with an increased rate breast cancer. Overall, women who were exposed to combination MHT had lower rates of global index events. While many outcomes were similar to those reported by the WHI, there were notable differences in some absolute risks and outcome rates that warrant further investigation in this population.
Supplementary Material
Acknowledgments
Funding: ASRM/Ortho Research Grant in Reproductive Medicine (HIS), HD-058799 (HIS), ACS MRSG-08-110-01-CCE (HIS), K24HD060687 (KTB). Sponsors had no role in the design, analysis or presentation of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: No authors have non-financial interests that may be relevant to the submitted work.
Contributor Information
H. Irene Su, University of California, San Diego, Department of Reproductive Medicine, La Jolla, CA, USA.
Yu-Chun Chen, National Yang-Ming University, Taipei, Taiwan.
Wei-Ting Hwang, University of Pennsylvania, Center for Clinical Epidemiology and Biostatistics, Philadelphia, PA, USA.
Ziyue Liu, University of Pennsylvania, Center for Clinical Epidemiology and Biostatistics, Philadelphia, PA, USA.
Tung-Ping Su, National Yang-Ming University, Taipei, Taiwan.
Tzeng-Ji Chen, National Yang-Ming University, Taipei, Taiwan.
Kurt T. Barnhart, University of Pennsylvania, Center for Clinical Epidemiology and Biostatistics, Philadelphia, PA, USA.
Yu-Xiao Yang, University of Pennsylvania, Center for Clinical Epidemiology and Biostatistics, Philadelphia, PA, USA.
References
- 1.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002 Jul 17;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008 Mar 5;299(9):1036–1045. doi: 10.1001/jama.299.9.1036. [DOI] [PubMed] [Google Scholar]
- 3.Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer: 1975–2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. 2006 Oct 15;107(8):1711–1742. doi: 10.1002/cncr.22193. [DOI] [PubMed] [Google Scholar]
- 4.Statistics NCfH. Hyattsville MD: Health, United States: 2006 with Chartbook on Trends in the Health of Americans; 2006. [PubMed] [Google Scholar]
- 5.Tannen RL, Weiner MG, Xie D, Barnhart K. A simulation using data from a primary care practice database closely replicated the women's health initiative trial. J Clin Epidemiol. 2007 Jul;60(7):686–695. doi: 10.1016/j.jclinepi.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Cheng SH, Chiang TL. The effect of universal health insurance on health care utilization in Taiwan. Results from a natural experiment. JAMA. 1997 Jul 9;278(2):89–93. doi: 10.1001/jama.278.2.89. [DOI] [PubMed] [Google Scholar]
- 7.Morabia A, Costanza MC. International variability in ages at menarche, first livebirth, and menopause. World Health Organization Collaborative Study of Neoplasia and Steroid Contraceptives. Am J Epidemiol. 1998 Dec 15;148(12):1195–1205. doi: 10.1093/oxfordjournals.aje.a009609. [DOI] [PubMed] [Google Scholar]
- 8.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989 Jan;129(1):125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 9.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007 Apr 4;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 10.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998 Aug 19;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 11.Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000 Jan 26;283(4):485–491. doi: 10.1001/jama.283.4.485. [DOI] [PubMed] [Google Scholar]
- 12.Prentice RL, Chlebowski RT, Stefanick ML, et al. Conjugated equine estrogens and breast cancer risk in the Women's Health Initiative clinical trial and observational study. Am J Epidemiol. 2008 Jun 15;167(12):1407–1415. doi: 10.1093/aje/kwn090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. JAMA. 2003 May 28;289(20):2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 14.Grodstein F, Manson JE, Stampfer MJ, Rexrode K. Postmenopausal hormone therapy and stroke: role of time since menopause and age at initiation of hormone therapy. Arch Intern Med. 2008 Apr 28;168(8):861–866. doi: 10.1001/archinte.168.8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prentice RL, Manson JE, Langer RD, et al. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol. 2009 Jul 1;170(1):12–23. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the national health insurance research database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. Dec 29; doi: 10.1002/pds.2087. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Shu XO, Li H, et al. Prospective cohort study of soy food consumption and risk of bone fracture among postmenopausal women. Arch Intern Med. 2005 Sep 12;165(16):1890–1895. doi: 10.1001/archinte.165.16.1890. [DOI] [PubMed] [Google Scholar]
- 18.Tan D, Haines CJ, Limpaphayom KK, Holinka CF, Ausmanas MK. Relief of vasomotor symptoms and vaginal atrophy with three doses of conjugated estrogens and medroxyprogesterone acetate in postmenopausal Asian women from 11 countries: The Pan-Asia menopause (PAM) study. Maturitas. 2005 Sep 16;52(1):35–51. doi: 10.1016/j.maturitas.2004.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.