Abstract
Tailoring three-dimensional (3D) biomaterial environments to provide specific cues in order to modulate function of encapsulated cells could potentially eliminate the need for addition of exogenous cues in cartilage tissue engineering. We recently developed saccharide-peptide copolymer hydrogels for cell culture and tissue engineering applications. In this study, we aim to tailor our saccharide-peptide hydrogel for encapsulating and culturing chondrocytes in 3D and examine the effects of changing single amino acid moieties differing in hydrophobicity/hydrophilicity (valine (V), cysteine (C), tyrosine (Y)) on modulation of chondrocyte function. Encapsulated chondrocytes remained viable over 21 days in vitro. Glycosaminoglycan and collagen content was significantly higher in Y-functionalized hydrogels compared to V-functionalized hydrogels. Extensive matrix accumulation and concomitant increase in mechanical properties was evident over time, particularly with the presence of Y amino acid. After 21 days in vitro, Y-functionalized hydrogels attained a modulus of 193±46 kPa, compared to 44±21 kPa for V-functionalized hydrogels. Remarkably, mechanical and biochemical properties of chondrocyte-laden hydrogels were modulated by change in a single amino acid moiety. This unique property, combined with the versatility and biocompatibility, makes our saccharide-peptide hydrogels promising candidates for further investigation of combinatorial effects of multiple functional groups on controlling chondrocyte and other cellular function and behavior.
Keywords: tissue engineering, chondrocyte, cartilage, sacchride-peptide, amino acid, functional group
1. INTRODUCTION
Articular cartilage is a connective tissue that normally provides a load-bearing, low friction, wear-resistant tissue located at the ends of long bones through its depth-varying cell and matrix properties. Upon damage due to disease, trauma, or as a consequence of aging, articular cartilage possesses limited capacity to self-repair[1, 2]. Several existing surgical repair strategies, such as microfracture[3, 4], allograft transplantation[5], mosaicplasty[6], and total knee arthroplasty[7], result in inferior tissue formation, site morbidity, and/or exhibit limited lifetime and function. Engineering cartilaginous tissues in vitro, by incorporating various cells, scaffolds or materials, as well as biophysical and biochemical cues, in combination or alone, holds promise as an alternative treatment for repair of focal cartilage defects.
A variety of biomaterials have been explored for cartilage tissue engineering applications. Chondrocytes have been encapsulated in three-dimensional (3D) matrices composed of natural materials, such as agarose[8, 9], alginate[10, 11], chitosan[12, 13], and hyaluronic acid[14], as well as synthetic materials such as poly(ethylene) glycol[15], poly(glycolic acid) or poly(lactic) acid[16, 17], thermal gelling polypeptide [18, 19], and self-assembling peptide amphiphile[20] based systems. Although the aforementioned materials have been shown to support viable chondrocytes, little is known regarding the ability of such three-dimensional (3D) matrices to modulate chondrocyte function, and resulting cell-material properties.
To date, 3D matrices have been applied in combination with growth factors and mechanical stimulation in order to modulate chondrocyte function (proliferation, matrix accumulation) as well as resulting mechanical properties of chondrocyte-laden biomaterials. Previous studies have applied a variety of growth factors (TGF-β1[21], BMP-2[22, 23], BMP-7[24], and IGF-I[25, 26]) with and without dynamic stimulation[21, 27, 28], in order to enhance matrix deposition and regulate chondrocyte growth. Application of low oxygen tension has also indicated promise in promoting cartilaginous tissue formation[29]. Although resulting tissues indicated increased matrix content, reported mechanical properties were inferior to native cartilage. Tailoring 3D biomaterial environments would provide specific biophysical and biochemical cues in order to modulate function of encapsulated cells; potentially without the need of the aforementioned exogenous cues.
Several studies have investigated the influence of microenvironment, in the form of hydrophobicity/hydrophilicity, on chondrocyte behavior; largely on two-dimensional (2D) surfaces[30–33]. Chondrocytes cultured on hydrophilic 2D surfaces[30], or, in one case, encapsulated in 3D poly(ethylene) glycol (PEG)-based hydrogels[34], exhibited greater growth than on hydrophobic surfaces. Given the potential of regulating function of mesenchymal stem cells encapsulated in a hydrogel material simply by providing cues in the form of small functional groups[35], it is likely that modulation of chondrocyte behavior is also possible.
Our laboratory has recently developed a family of saccharide-peptide hybrid copolymer-based hydrogels derived from naturally occurring saccharides and amino acids[36]. In our design, we have chosen lysine and galactaric acid as the basis for our hybrid copolymer backbone due to their biocompatibility and physiologic relevance to various glycoproteins present in vivo[37]. Our previous studies have shown that saccharide-peptide copolymers are non-toxic, non-immunogenic, and biodegradable [36, 38, 39]. Mild and quick hydrogelation, beneficial for application of 3D cell culture, was achieved by Michael addition reaction [40–45] [38] of cysteine and vinyl sulfone functionalized saccharide-peptide polymers (referred to as polymer-C and polymer-VS, respectively, in latter discussion). While this type of chemistry has been applied previously to generate PEG-peptide [41, 46], PEG-hyaluronic acid (HA)[47], or PEG-dextran gels[48], to our knowledge, our design is the first to apply Michael-type addition to form biodegradable synthetic hydrogels composed of natural building blocks without a large synthetic component such as PEG. In addition, the modular copolymer design, along with free carboxylic acid groups along the backbone, allows for convenient derivatization with small chemical functionalities, such as amino acids. In this study, our aims were to determine the utility of the VS:C hydrogel for encapsulating and culturing chondrocytes in 3D and examine the effects of changing single amino acid moieties differing in hydrophobicity/hydrophilicity[49] on modulation of chondrocyte function.
2. MATERIALS AND METHODS
2.1. Materials
Materials for cartilage explant, chondrocyte isolation, monolayer culture, hydrogel culture, biochemical procedures, and immunohistochemical procedures were obtained as described previously [50]. Mouse monoclonal anti-collagen type I antibody, and testicular hyaluronidase were from Sigma (St. Louis, MO). Mouse monoclonal anti-collagen type II antibody cocktail was from Chemicon (Temecula, CA). Non-specific mouse monoclonal IgG antibody was from Pierce (Rockford, IL).
2.2. Experimental Design
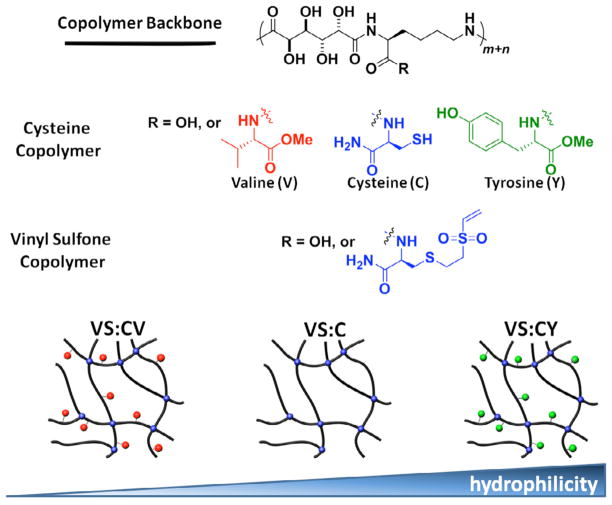
To determine the effects of cysteine, valine, and tyrosine amino acid functionalities on chondrocyte-laden hydrogel properties and matrix accumulation by encapsulated chondrocytes, cells were encapsulated in de novo synthesized saccharide-peptide hydrogels. The polymer backbone was functionalized with cysteine (designated as C), cysteine and valine (designated as V), or cysteine and tyrosine (designated Y) and used to generate VS:C, VS:CV, or VS:CY hydrogels for culture in vitro.
2.3. Polymer Synthesis and Characterization
2.3.1 Synthesis and characterizations of copolymer backbone
The galactaric acid-lysine copolymer backbone (referred to as polymer 1) was synthesized by following previously reported procedures[36].
2.3.2 Synthesis and characterizations of cysteine functionalized saccharide–peptide copolymer (here after referred to as polymer-C)
To a solution of polymer 1 (12.89 mmole of carboxylic acid, 5.16 g) and cysteine(Trt)-NH2 (0.50 eq, 6.45 mmole, 2.336 g) in 35 mL of DMF, DIPEA (19.33 mmole, 3.37 mL) and HCTU (2-(6-chloro-1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate) (8.38 mmole, 3.15 g) were added. The reaction mixture was allowed to stir at room temperature for 16 h, at which time the DMF was removed in vacuo. 150 mL of 2N HCl was added generating a white suspension in aqueous solution. The solid was filtered out and washed with 0.2N HCl to yield a white polymer. The protected polymer was further dissolved in a mixture of TFA (15.0 mL), water (4.5 mL) and triisoproylsilane (0.5 mL) solution to remove protecting groups. After stirring the reaction mixture for 3 h, the solution was diluted with water and purified by dialysis against 0.01N HCl for 3 days. Excess water was removed by lyophilization to give 3.29 g of pale brown solid polymer functionalized with cysteine (C) with a yield of 65%. The cysteine thiol groups on polymer-C was employed in latter hydrogelation via Michael-type addition with VS polymer. 1H NMR integration of the polymer’s cysteine β-protons revealed 1.27 mmole cysteine (thiol group) per gram of polymer, which is equivalent to ~50 mol% of the free carboxylate groups on the α-proton of lysine and cysteine, proton of backbone galactaric acid), 4.05–4.04 (m, 2.0H, proton of backbone galactaric acid), 3.32–3.28 (m, 2.0H, ε-proton of backbone lysine), 3.03–2.90 (m, 0.9H, β-proton of cysteine, corresponding to 0.47 cysteine unit per repeating unit), 1.97–1.86 (m, 2.0H, β-proton of backbone lysine), 1.60–1.44 (m, 4.0H, γ, σ-proton of backbone lysine).
2.3.3 Synthesis and characterizations of cysteine and tyrosine (~15 mol% to the total carboxylic acid groups) functionalized saccharide–peptide copolymer (here after referred to as polymer-CY)
To a solution of polymer 1 (3.75 mmole of carboxylic acid, 1.5 g), tyrosine(tBu)-OtBu (0.15eq, 0.56 mmole, 0.176g) and cysteine(Trt)-NH2 (0.50 eq, 1.87 mmole, 0.679 g) in 15 mL of DMF, DIPEA (7.49 mmole, 0.88 mL) and HCTU (2-(6-chloro-1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate) (3.75 mmole, 1.550 g) were added. The reaction mixture was allowed to stir at room temperature for 16 h, at which time the DMF was removed in vacuo. 150 mL of 2N HCl was added into residue to generate a white suspension in aqueous solution. The solid was filtered out and washed with 0.2N HCl to yield a white polymer. The protected polymer was further dissolved in a mixture of TFA (15.0 mL), water (4.5 mL) and triisoproylsilane (0.5 mL) solution to remove protecting groups. After stirring the reaction mixture for 3 h, the solution was diluted with water and purified by dialysis against 0.01N HCl for 3 days. Excess water was then removed by lyophilization to give 0.78 g of white solid polymer functionalized with cysteine and tyrosine (CY) with a yield of 51%. 1H NMR integration of the polymer’s cysteine β-protons reveals 1.22 mmole cysteine (thiol group) per gram of polymer, equivalent to ~48 mol% of the free carboxylic acid groups being functionalized by cysteine, comparable to the cysteine functionalization level on polymer-C. Similarly, the cysteine thiol groups on the polymer-CY will be employed in latter hydrogelation via Michael-type addition with VS polymer. 1H NMR integration of the polymer’s tyrosine β-protons indicates ~15 mol% of the free carboxylic acid groups being functionalized by Y. 1H NMR (600 MHz, D2O) δ 7.15 (s, 0.3H, aromatic proton of tyrosine), 6.86 (s, 0.3H, aromatic proton of troton o, corresponding to 0.15 tyrosine per repeating unit), 4.71–4.05 (m, 4.0H, proton of backbone galactaric acid), 3.76–3.75 (m, 0.4H), 3.43–3.28 (m, 2.0H, ε-proton of backbone lysine), 3.04–2.93 (m, 1.0H, β-proton of cysteine, corresponding to 0.5 cysteine per repeating unit), 1.94–1.74 (m, 2.0H, β-proton of backbone lysine), 1.60–1.25 (m, 4.0H, γ, σ-proton of backbone lysine).
2.3.4 Synthesis and characterizations of cysteine and valine (~15 mol%, to the total carboxylic acid groups) functionalized saccharide–peptide copolymer (here after referred to as polymer-CV)
To a solution of polymer 1 (3.75 mmole of carboxylic acid, 1.5 g), valine-OMe (0.15eq, 0.56 mmole, 0.094g) and cysteine(Trt)-NH2 (0.50 eq, 1.87 mmole, 0.679 g) in 15 mL of DMF, DIPEA (7.49 mmole, 0.88 mL) and HCTU (2-(6-chloro-1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium hexafluorophosphate) (3.75 mmole, 1.550 g) were added. The reaction mixture was allowed to stir at room temperature for 16 h, at which time the DMF was removed in vacuo. 150 mL of 2N HCl was added into residue to generate a white suspension in aqueous solution. The solid was filtered out and washed with 0.2N HCl to yield a white polymer. Then the protected polymer was further dissolved in a mixture of TFA (15.0 mL), water (4.5 mL) and triisoproylsilane (0.5 mL) solution to remove protecting groups. After stirring the reaction mixture for 3 h, the solution was diluted with water and purified by dialysis against 0.01N HCl for 3 days. Excess water was then removed by lyophilization to give 0.811 g of white solid polymer functionalized with cysteine and valine (CV) with a yield of 53%. 1H NMR integration of the polymer’s cysteine β-protons revealed 1.22 mmole cysteine (thiol group) per gram of polymer, equivalent to ~48 mol% of the free carboxylic acid groups being functionalized by cysteine, comparable to the cysteine functionalization level on polymer-C. Again, the cysteine thiol groups on the polymer-CV will be employed in latter hydrogelation via Michael-type addition with VS polymer. 1H NMR integration of the polymer’s valine β-protons revealed ~15 mol% of the free carboxylic acid groups being functionalized by V. 1H NMR (600 MHz, D2O) δ 4.70–4.33 (m, 3.8H, α-proton of cysteine and lysine, proton of backbone galactaric acid), 4.12–4.06 (m, 2.0H, proton of backbone galactaric acid), 3.89–3.80 (m, 1.0H), 3.33–3.30 (m, 2.0H, ε-proton of backbone lysine), 3.03–2.91 (m, 1.0H, β-proton of cysteine, corresponding to 0.5 cysteine per repeating unit), 2.27–2.23 (m, 0.15H, β-proton of vproto, corresponding to 0.15 valine per repeating unit), 1.93–1.87 (m, 2.0H, β-proton of backbone lysine), 1.61–1.43 (m, 4.0H, γ, σ-proton of backbone lysine), 1.00–0.97 (m, 1.0H, γ-proton of valine).
2.3.5 Synthesis and characterizations of vinyl sulfone functionalized saccharide–peptide copolymer (here after referred as polymer-VS)
To a solution of polymer-C as described in section 2.3.2 (4.90 mmole of cysteine group, 2.00g) in 100 mL PBS buffer (pH 6.0), excess divinyl sulfone (30eq, 147.0 mmole, 14.8 mL) was added. After stirring the reaction mixture at room temperature for 48 hours, the solution was diluted with water, washed with diethyl ether, and purified by dialysis against water for 3 days. Excess water was then removed by lyophilization to give 1.90 g of white solid polymer VS (here after referred to as polymer-VS). 1H NMR integration of vinyl groups on the polymer revealed 0.90 mmole vinyl sulfone group per gram of polymer. 1H NMR (600 MHz, D2O) δ 6.95–6.91 (m, 0.4H, vinyl group), 6.53–6.43 (m, 0.8H, vinyl group), 4.69–4.06 (m, 5.0H), 3.58–3.56 (m, 0.9H, α-proton of backbone lysine), 3.29–2.94 (m, 4.0H), 1.91–1.80 (m, 2.0H, β-proton of backbone lysine), 1.60–1.41 (m, 4.0H, γ, σ-proton of backbone lysine).
2.4. Preparation and Characterization of Hydrogels
All hydrogels (VS:C, VS:CV, and VS:CY) were prepared with a molar ratio of vinyl sulfone to cysteine of 1. Hydrogels were prepared by dissolving polymer-VS and one of the C functionalized copolymers at a total polymer concentration of 100 mg/ml in Dulbecco’s Modified Eagle’s Medium (DMEM) [38]. Using a pH microprobe (Fisher Scientific, Pittsburgh, PA), the copolymer solutions were adjusted separately to pH 7 by addition of the necessary amounts of HCl or NaOH prior to gelation. Polymer-VS and one of the C functionalized copolymers (polymer-C, polymer-CV, or polymer-CY) solutions were subsequently crosslinked through Michael-type conjugate addition (Figure 1), as previously reported[38], in 6.5 mm diameter Transwell® inserts (Fisher).
Figure 1.
Schematic of methods used to generate VS:CV, VS:C, VS:CY hydrogels, varying in hydrophobicity/hydrophilicity. Two types of saccharide-peptide copolymers were generated. Cysteine (C) functionalized copolymers were further functionalized with either valine (V) or tyrosine (Y) amino acids. Separately, vinyl sulfone (VS) functionalized copolymers were also generated. Hydrogelation occurred by Michael addition, when cysteine and vinyl sulfone functionalized copolymers were combined at equimolar ratio. This resulted in three types of hydrogels, varying in hydrophobicity/hydrophilicity: VS:CV, VS:C, VS:CY.
2.4.1. Swellability and Degradation of Hydrogels
Equilibrium swelling ratio (ESR), a measure of swellability of the hydrogels, was measured in DMEM at 37°C. Hydrogels were formed in triplicate and weighed wet (Wo). Dried hydrogels were weighed (Wd) after being frozen at −80°C and lyophilized. ESR was determined from the following equation:
The swollen ratio of the gels was tracked over 21 days in vitro. Gels, in triplicate, were incubated in DMEM with 10% FBS. The wet weight of the gel at time t (Wt) was normalized to the initial wet weight of the gel (Wo). Gels in Transwell® inserts were dabbed prior to weighing.
2.4.2. Gelation Kinetics by Oscillatory Rheology
Crosslinking kinetics of VS:C, VS:CV, and VS:CY hydrogels was measured by oscillatory rheology with an ARG2 rheometer (TA Instruments, Newcastle, DE). Rheological behavior during crosslinking was determined with a plate-on-plate configuration (plate diameter, 20 mm) at 25°C over 1 hour (strain = 0.1%, frequency = 1 Hz)[51]. Both components were applied to the plate and mixed by pipetting prior to the onset of the time sweep, and values for storage modulus (G′) were obtained. All analyses were done in triplicate.
2.5. Primary Culture and Formation of Hydrogel Constructs
Chondrocytes were isolated from articular cartilage slices (up to 1 mm thickness) obtained from the patellofemoral groove and femoral condyles of six immature (1–3 wk old) bovine calf knees (Research 87, Boylston, MA) and digested in medium (Dulbecco’s Modified Eagle Medium [DMEM], 10 mM HEPES, 0.1 mM nonessential amino acids, 0.4 mM L-proline, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B) with 0.2% pronase for 1 hour, and 0.02% collagenase-P for 16 hours [50].
Primary chondrocytes were encapsulated in VS:C, VS:CV, or VS:CY gels, at a cell density of 20 million cells/ml. Briefly, chondrocytes were resuspended in polymer-VS and one of the C functionalized copolymers (polymer-C, polymer-CV, or polymer-CY) solutions and then 80 μl of the cell suspension was transferred to 6.5 mm diameter Transwell® inserts (Fisher) where hydrogelation was allowed to complete[38]. Hydrogels were incubated at 37°C with 5% CO2. Media (DMEM with 10% FBS) was changed every two days and fed in slight excess of 1 ml/million cells/day for up to 21 days in vitro.
2.6. Chondrocyte-Laden Hydrogel Analyses
2.6.1. Chondrocyte Viability in Hydrogels
Cell viability was qualitatively assessed with Live/Dead staining (Invitrogen). On day 21 of culture, a quarter of the chondrocyte-laden hydrogel disk was incubated in DMEM containing 2 μM calcein AM and 4 μM ethidium homodimer probes for 20 min [38]. Portions of disks were rinsed with PBS and imaged by fluorescence microscopy (Nikon Eclipse Ti, Melville, NY).
2.6.2. Morphology of Chondrocytes Encapsulated in Hydrogels
Morphology of chondrocytes encapsulated in hydrogels was documented by phase contrast microscopy using a Nikon Eclipse Ti with Digital Sight camera (Melville, NY) on days 1, 10, and 21 of culture.
2.6.3. Biochemical Analysis
Portions of in vitro generated chondrocyte-laden hydrogels were biochemically analyzed at specified time points in culture (days 1, 10, and 21). Half of the 6.5 mm diameter hydrogel was solubilized in proteinase K overnight [52]. Portions of the digest were analyzed for DNA using PicoGreen[53], sulfated glycosaminoglycans (GAG) by 1,9 dimethylmethylene blue (DMMB) [54], and collagen by hydroxyproline. DNA content was converted to cell number using a conversion constant of 7.7 pg of DNA per cell. Hydroxyproline content was converted to collagen (COL) content by assuming a mass ratio of collagen to hydroxyproline of 7.14 [55].
2.6.4. Biomechanical Analysis
Bulk mechanical (compression) properties of chondrocyte-laden hydogels (VS:C, VS:CV, VS:CY) over time in culture (days 1, 10, and 21) were measured using an MTS Synergie 100 (MTS Systems, Eden Prairie, MN) with a 10 N load cell. [56]. Hydrogel samples were subjected to uniaxial confined compression at a constant strain rate of 1%/s from 0 to 20% strain. The compressive modulus was calculated as the slope of the linear portion of the stress-strain curve, determined at low strain regions (0–4% strain).
2.6.5. Histology and Immunohistochemistry
Portions of in vitro generated hydrogels were histologically and immunohistochemically analyzed on day 21 of culture. Samples were pre-treated in a solution of 50 mg/ml 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and lysine (K) for 30 min, in order to introduce cross-linking sites in hydrogel samples for subsequent fixation. After EDC/K treatment, samples were copiously rinsed and fixed in 10% formalin overnight. Samples were then embedded in paraffin, and sectioned vertically to 5 μm thick. Sections were deparaffinized, digested with testicular hyaluronidase (5000 U/ml), and probed with non-specific monoclonal mouse IgG, anti-collagen I, or anti-collagen II antibody cocktail using the R.T.U. Vectastain University Elite ABC kit and Vector VIP peroxidase substrate kit. Serial sections were stained with 0.5% Alcian Blue in 0.4 M MgCl2 and 0.025 M sodium acetate (pH 5.6) to stain GAG [57, 58]. Results were documented by brightfield microscopy using a Nikon Eclipse Ti with Digital Sight camera (Melville, NY).
2.7. Statistical Analysis
Effects of hydrogel type and time in culture on all endpoints were assessed by ANOVA with Tukey post-hoc tests. Data are expressed as mean±SEM.
3. RESULTS
3.1. Polymer Synthesis and Characterization
The aims of the current study were to determine the utility of the VS:C based hydrogel for encapsulating and culturing chondrocytes in 3D and examine the effects of small functional groups on modulation of chondrocyte function. For this purpose, cysteine and vinyl sulfone functionalized copolymers, polymer-C and polymer-VS respectively, were first synthesized and characterized following our reported procedure[38]. To probe the influence of small functional groups on chondrocyte behavior, copolymers containing other amino acids differing in hydrophobicity/hydrophilicity were prepared. Specifically, tyrosine or valine was co-functionalized with cysteine to the galactaric acid-lysine copolymer backbone (Figure 1). While Y or V functionalization modulated the hydrophilicity/hydrophobicity of the hydrogels, the C groups were needed in Michael-addition crosslinking during the hydrogelation. Following dialysis purification, 1H NMR integration was used to estimate the polymer’s cysteine, tyrosine and valine functionalization level. Polymers co-functionalized with cysteine/tyrosine or cysteine/valine were capable of crosslinking with polymer-VS to afford more hydrophilic or hydrophobic hydrogels compared to VS:C hydrogel, respectively. Importantly, surface functionalization was achieved without varying crosslinking density or the basic chemical structure of the hydrogels, allowing investigation of the direct influence of changes in small functional groups on chondrocyte behavior.
3.2. Preparation and Characterization of Hydrogels
3.2.1. Swellability and Degradation of Hydrogels
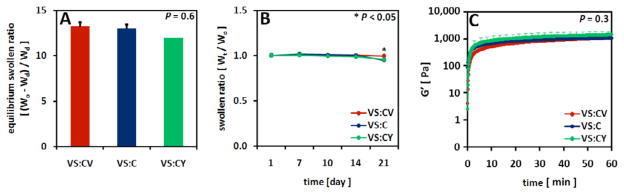
All hydrogels (VS:C, VS:CV, VS:CY) had similar swellability at the time of cross-linking (p=0.6) (Figure 2A). Similarly, the swollen ratio or extent of degradation, determined as a measure of wet weight over time (Wt), of all hydrogels over three weeks in vitro was also similar (p=0.4) for all the hydrogels used in this study (Figure 2B). Hydrogel degradation was dependent on time in culture, with gels at day 21 representing 97% of the initial hydrogel mass on day 1 (p<0.05).
Figure 2.
Effects of hydrogel type on hydrogel swelling properties, degradation, and evolution of rheological storage modulus (G′). VS:CV, VS:C, VS:CY hydrogels were analyzed for (A) equilibrium swelling ratio, (B) monitored for degradation over 21 days in vitro culture, and (C) rheologically characterized to assess evolution of G′ over time. Mean±SEM, n=3.
3.2.2. Gelation Kinetics by Oscillatory Rheology
Rheological analysis of the crosslinking of saccharide-peptide hydrogels as a function of time (Figure 2C) indicated similarity among all hydrogels (p=0.3). Gels reached an equilibrium storage modulus (G′) of 1.5 kPa.
3.3. Chondrocyte-Laden Hydrogel Analyses
3.3.1. Chondrocyte Viability in Hydrogels
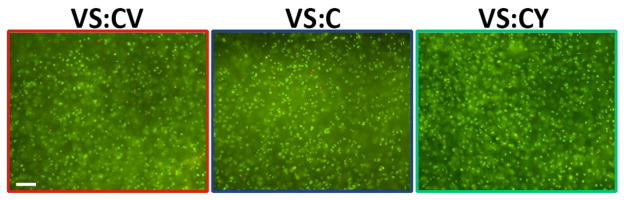
Viability of chondrocytes encapsulated in hydrogels (VS:CV, VS:C, VS:CY) was visualized qualitatively with Live/Dead staining, and appeared to be >95% after 21 days in vitro (Figure 3A–C).
Figure 3.
Viability of chondrocytes encapsulated in VS:CV, VS:C, VS:CY hydrogels, assessed by Live/Dead stain on day 21 of in vitro culture. Live cells are stained with calcein AM (green) and dead cells with ethidium homodimer-1 (red). Bar = 100 μm.
3.3.2. Morphology of Chondrocytes Encapsulated in Hydrogels
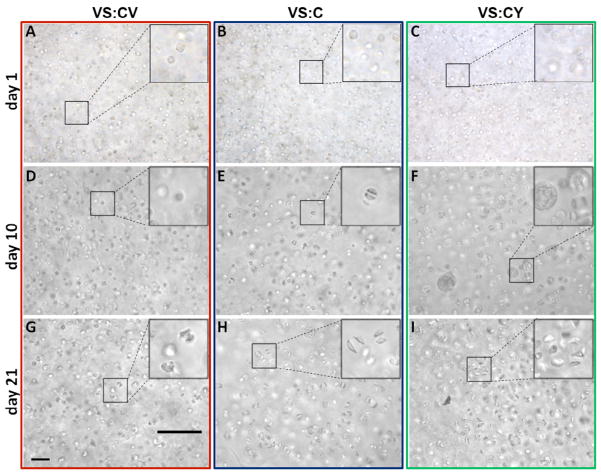
Morphology of chondrocytes encapsulated in hydrogels was observed by phase contrast microscopy during in vitro culture. After 1 day in vitro, chondrocytes encapsulated in VS:CV, VS:C, or VS:CY hydrogels were round in appearance and did not possess pericellular matrix (Figure 4A–C). By day 10 in vitro, differences in pericellular matrix accumulation were evident. Halos of pericellular matrix were evident around chondrocytes encapsulated in VS:C and VS:CY hydrogels (Figure 4EF). Chondrocytes in VS:CV hydrogels did not, qualitatively, appear to accumulate much matrix (Figure 4D). After 21 days in vitro, extensive matrix accumulation around encapsulated chondrocytes was evident in VS:CY (Figure 4I) hydrogels, followed by chondrocytes encapsulated in VS:C (Figure 4H) and VS:CV (Figure 4G) hydrogels, which appeared more similar.
Figure 4.
Matrix production and accumulation by chondrocytes encapsulated in VS:CV, VS:C, VS:CY hydrogels on (A–C) days 1, (D–F) 10, and (G–I) 21 in vitro. Matrix production and accumulation was documented by phase microscopy. Bar= 100 μm.
3.3.3. Biochemical Analysis
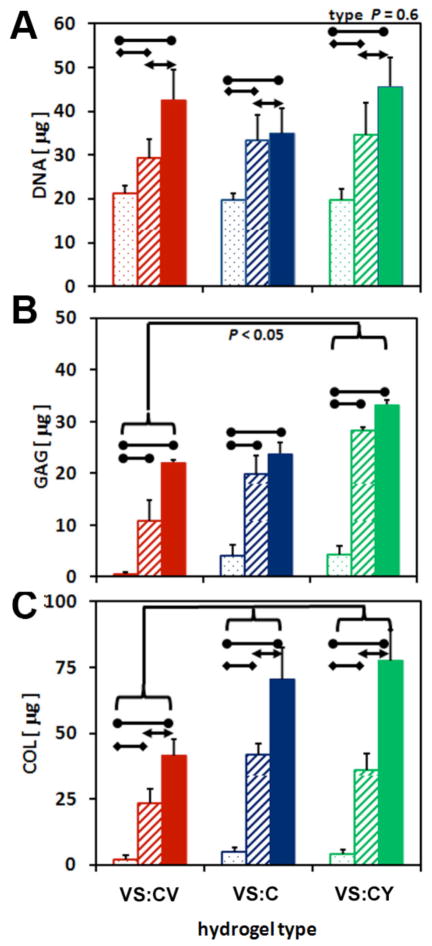
Some biochemical content varied among hydrogels based on the amino acid incorporated into the hydrogel side chain, as well as the time in culture. DNA content of hydrogels were similar regardless of the amino acid incorporated (p=0.4), but was significantly lower at day 1 compared to DNA content at day 10 (p<0.01) or 21 (p<0.001) (Figure 5A). DNA content increased 1.6-fold by day 10 and 2.0-fold by day 21 in culture. GAG content of hydrogels differed significantly based on the type of hydrogel (p<0.05) and the time in culture (p<0.001), however, interaction effects were not significant (p=0.6) (Figure 5B). GAG content of VS:CY hydrogels was 2.0-fold higher than VS:CV hydrogels (p<0.05), and tended to be significantly higher than GAG content of VS:C hydrogels (p=0.1). GAG content of VS:CV and VS:C hydrogels were similar (p=0.7). GAG content of all hydrogels increased significantly over time in culture. GAG content at day 21 and day 10 were 8.7-fold (p<0.001) and 6.5-fold (p<0.001) higher than GAG content on day 1 of culture. Total COL content also varied significantly based on the type of hydrogel (p<0.01) and time in culture (p<0.001), without significant interaction effects (p=0.4) (Figure 5C). Total COL content was 1.7-fold higher in VS:CY hydrogels compared to VS:CV hydrogels, and similar to VC:C hydrogels (p=0.9). Total COL content of VS:C hydrogels was also 1.7-fold higher than VC:CV hydrogels (p<0.05). In addition, total COL content increased over time in all hydrogels. Total COL content at day 21 and day 10 were 17.2-fold (p<0.001) and 9.2-fold (p<0.001) higher, respectively, than content at day 1 of culture.
Figure 5.
Effects of hydrogel type (VS:CV, VS:C, VS:CY) on biochemical properties of hydrogels. (A) DNA, (B) glycosaminoglycans (GAG), and (C) collagen (COL) content of hydrogels were determined on days 1 (dot), 10 (stripe), and 21 (solid)of in vitro culture. Data are mean ± SEM, n = 5–10.
3.3.4. Biomechanical Analysis
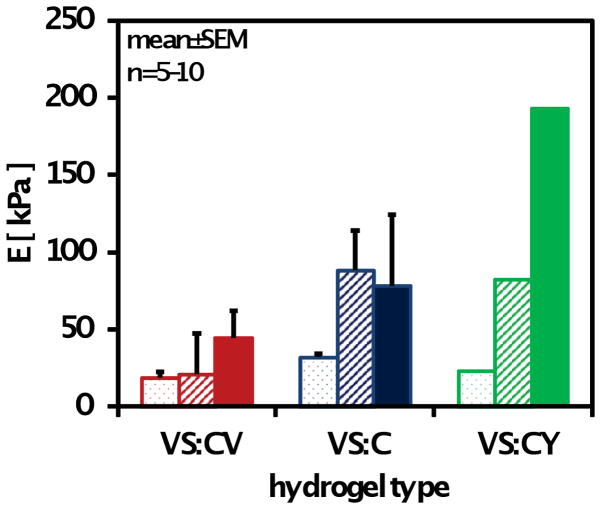
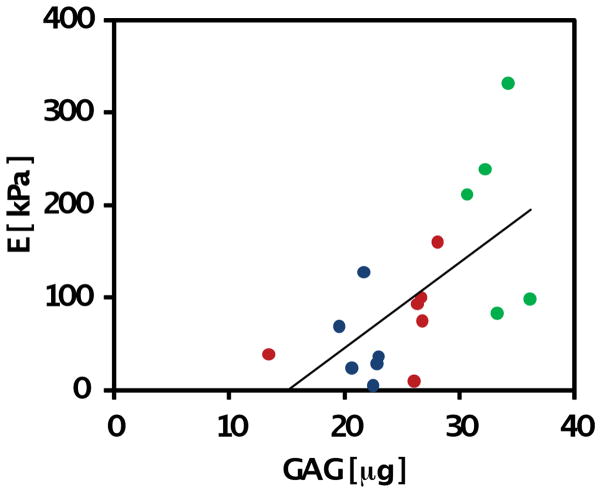
Compressive biomechanical properties of chondrocyte-laden hydrogels differed significantly based on the type of hydrogel (p<0.001), day in culture (p<0.001), and interactive effects of the two aforementioned factors (p<0.01) (Figure 6). Overall, both VS:CY (p<0.001) and VS:C (p<0.05) hydrogels were significantly stiffer than VS:CV hydrogels. Compressive properties of hydrogels significantly increased over time in culture, particularly between days 1 and 10 of culture (p<0.05). Increases in compressive modulus between day 10 and day 21 were noted (p=0.1) but were less than increases observed earlier in culture. By day 21, the compressive modulus of chondrocyte-laden VS:CY hydrogels was significantly higher than VS:C (VS:CY 2.5-fold higher than VS:C, p<0.05) and VS:CV (VS:CY 4.4-fold higher than VS:CV, p<0.05) hydrogels. VS:CY hydrogels had a modulus of 193±46 kPa, compared to 78±18 kPa for VS:C and 44±21 kPa for VS:CV hydrogels. Correlation analysis (Figure 7) indicated a significant positive correlation between GAG accumulation and compressive modulus (p<0.01).
Figure 6.
Effects of hydrogel type (VS:CV, VS:C, VS:CY) on compressive properties of hydrogels. Modulus of hydrogels were determined on days 1 (dot), 10 (stripe), and 21 (solid) of in vitro culture. Data are mean ± SEM, n = 5–10.
Figure 7.
Correlation analysis of GAG content of VS:CV, VS:C, VS:CY and compressive modulus of hydrogels on day 21 of in vitro culture. Linear regression resulted in significant correlation (P<0.01).
3.3.5. Histology and Immunohistochemistry
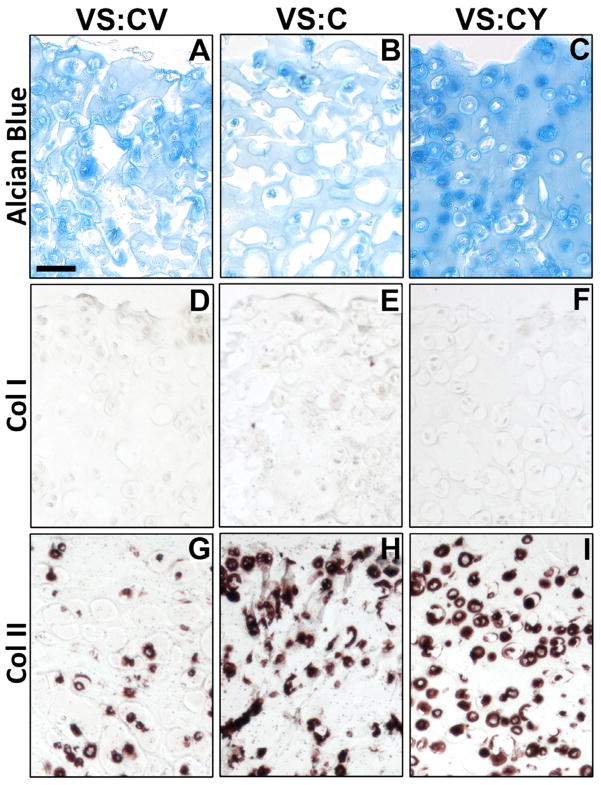
Histological and immunohistochemical analyses indicated differences in GAG and collagen staining based on hydrogel type. GAG accumulation was evident in all hydrogel types on day 21 (Figure 8ABC), although staining appeared more intense for VS:CY hydrogels (Figure 8C) compared to VS:CV (Figure 8A) or VS:C hydrogels (Figure 8B). Staining for type I collagen appeared negative for all hydrogel types (Figure 8DEF). Presence of type II collagen was evident in all hydrogel types but was increased in VS:C and VS:CY hydrogels, with intense pericellular staining (Figure 8FI) compared to VS:CV hydrogels which had comparatively little type II collagen present (Figure 8C).
Figure 8.
Effects of VS:CV, VS:C, VS:CY hydrogels on localization of glycosaminoglycans (GAG), type I collagen, and type II collagen by encapsulated chondrocytes on day 21 of in vitro culture. Sections of chondrocyte-laden hydrogels were stained with (A–C) Alcian blue, or antibodies for (D–F) type I collagen (Col I), or (G–I) type II collagen. Bar = 50 μm.
4. DISCUSSION
In this study, we have tailored our functional saccharide-peptide hydrogel material for 3D chondrocyte encapsulation. Furthermore, we have investigated the effects of amino acid moieties differing in hydrophobicity/hydrophilicity on modulation of chondrocytes three-dimensionally encapsulated in VS:C hydrogels. Cysteine or vinyl sulfone functionalized saccharide-peptide copolymers were synthesized for hydrogel preparation[38]. To investigate the effects of amino acid moieties on chondrocyte culture, the saccharide-peptide polymers were co-functionalized with either valine/cysteine (CV) or tyrosine/cysteine (CY). Whereas the cysteine moiety serves as the cross-linking site for subsequent hydrogelation, amino acids V or Y were introduced to vary the surface properties of the resulting hydrogels. We chose amino acids for our functionalization in order to maintain the biocompatibility/biodegradability of our hydrogels. While V was chosen as a representative hydrophobic amino acid, Y was selected as a hydrophilic amino acid.
When cysteine (C) and vinyl sulfone (VS) functionalized copolymers were combined in stoichiometry, hydrogelation occurred under mild conditions by Michael addition (Figure 1), generating VS:CV, VS:C, and VS:CY hydrogels, respectively. The biocompatibility of our saccharide-peptide hydrogels, combined with mild hydrogelation conditions resulted in high viability of encapsulated chondrocytes in 3D hydrogels up to 21 days in vitro (Figure 3). In contrast to many other synthetic hydrogels in which changing one property is often linked with other physical/chemical changes, all the hydrogels used in this study had identical crosslinking density, due to the same amount of cysteine and vinyl sulfone present on the polymer backbone (Figure 2). All the hydrogels had similar swelling ratios with subtle changes in amino acid moieties likely generating cellular microenvironments which differ in hydrophobicity/hydrophilicity. In addition, the synthesis procedure detailed here reproducibly produces cysteine and vinyl sulfone copolymers, resulting in reproducible gelation kinetics. This allows us to probe the effects of small functional groups on cellular behavior. Interestingly, matrix production and accumulation by chondrocytes was significantly enhanced in more hydrophilic hydrogels (VS:CY) compared to more hydrophobic hydrogels (VS:CV) (Figures 4, 5B, 8). GAG content was significantly increased in VS:CY hydrogels compared to VS:C or VS:CV hydrogels, which correlates well with increased compressive modulus of chondrocyte-laden hydrogels after 21 days of in vitro culture (Figures 6, 7). Histological differences in VS:CY hydrogels compared to VS:C or VS:CV hydrogels were evident with increased GAG and type II collagen staining in VS:CY hydrogels.
Biomaterial surface functionality and other microenvironmental cues have previously been shown to affect biological properties of cell-laden matrices. Increased collagen deposition was obtained on hydrophilic porous high density polyethylene (HDPE) matrices compared to hydrophobic HDPE matrices[59]. The presence of negatively charged, hydrophilic glycosaminoglycans, such as chondroitin sulfate, within a hydrogel matrix, were shown to support chondrocyte viability and deposition of collagen and proteoglycan production [60, 61]. Finally, encapsulation and culture of chondrocytes in a self-assembling peptide hydrogel, with alternating hydrophilic and hydrophobic moieties, indicated increased matrix deposition and stiffness over time in culture.[62] In the current study, functionalization of a single amino acid moiety, varying in side groups, to three-dimensional hydrogel matrices has resulted in significant changes in chondrocyte behavior over a relatively short period of time (21 days in vitro). In agreement with previous observations[62–65], we observed a significant increase in matrix production for VS:CY hydrogel functionalized with a hydrophilic Y moiety [49, 63–67] as compared to VS:CV hydrogel functionalized with a hydrophilic V amino acid. In addition, the mechanical properties achieved here for chondrocyte-laden VS:C hydrogels are significantly higher than those obtained previously under comparable cell culture conditions [9] or extended culture durations [68].
The mechanism underlying the observed influence of amino acid moieties on chondrocyte function warrants further investigation. Although the effect of matrix stiffness on cellular function is well known[69], in this study, the stiffness of all acellular matrices was similar, allowing us to isolate the effect of amino acid moieties on chondrocyte function. Here, the relatively low level of functionalization of amino acid moiety did not have a statistically significant effect on equilibrium swollen ratio. Presumably, a more subtle effect is present in influencing chondrocyte behavior and matrix accumulation. In native cartilage, hydrophilic proteoglycans[70] make up a significant portion of cartilage content. Hydrogels which mimic the chondrocyte microenvironment, particularly the presence of hydrophilic polysaccharides[71], have shown promise for cartilage tissue engineering. It is possible that, similarly, hydrophilic amino acids (such as Y) could have more beneficial influence on chondrocyte behavior than more hydrophobic amino acids (such as V).
In future studies, longer durations for cell culture in vitro, and individual or combinatorial exploration of other amino acids would be useful in further optimizing the hydrogel function as well as gaining mechanistic understanding of modulating chondrocyte function and related implications for cartilage repair. In addition, even though significant mechanical properties of chondrocyte-laden VS:C hydrogels were achieved here, the influence of dynamic loading during in vitro culture of these hydrogels has not been established and could potentially further enhance mechanical properties[9, 28]. Finally, the highly functional and versatile saccharide-peptide copolymer employed here could be modified with other biologically relevant components to further enhance the performance.
5. CONCLUSIONS
In this study, we have tailored a saccharide-peptide hydrogel material to impart specific microenvironmental cues, via amino acid functionalities, to encapsulated chondrocytes. Cysteine and vinyl sulfone-functionalized saccharide-peptide polymers were further functionalized with amino acid moieties differing in side groups (valine, cysteine, tyrosine). Mechanical and biochemical properties of chondrocyte-laden hydrogels were controlled by inclusion of hydrophobic/hydrophilic amino acid functional groups. Extensive matrix accumulation and concomitant increase in mechanical properties was evident over time in culture, particularly with the presence of Y amino acid. Overall, this study indicates the promise of subtly altering the cellular microenvironment with single amino acid moieties, resulting in modulation of chondrocyte-laden hydrogel properties. In the future, studies will focus on applying combinatorial effects of functional groups on cellular behavior via the VS:C hydrogel platform.
Acknowledgments
This work was supported by a grant from the NIH (R01 EB006797). We thank Dr. Pratibha Vora at Histoserv, Inc., for assistance with sample preparation and paraffin embedding and sectioning.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buckwalter JA, Mankin HJ. Articular cartilage. Part II: degeneration and osteoarthrosis, repair, regeneration, and transplantation. J Bone Joint Surg Am. 1997;79-A:612–632. [Google Scholar]
- 2.Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64-A:460–466. [PubMed] [Google Scholar]
- 3.Shapiro F, Koido S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75-A:532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Steadman JR, Rodkey WG, Singleton SB. Microfracture technique for full-thickness chondral defects: technique and clinical results. Oper Tech Orthop. 1997;7:300–304. [Google Scholar]
- 5.Bugbee WD. Fresh osteochondral allografts. J Knee Surg. 2002;15(3):191–195. [PubMed] [Google Scholar]
- 6.Lane JG, Massie JB, Ball ST, Amiel ME, Chen AC, Bae WC, et al. Follow-up of osteochondral plug transfers in a goat model: A 6-Month study. Am J Sports Med. 2004;32:1440–1450. doi: 10.1177/0363546504263945. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg VM, Caplan AI. Biological resurfacing: an alternative to total joint arthroplasty. Orthopedics. 1994;17(9):819–821. doi: 10.3928/0147-7447-19940901-25. [DOI] [PubMed] [Google Scholar]
- 8.Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745–758. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 9.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122(3):252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 10.Häuselmann HJ, Masuda K, Hunziker EB, Neidhart M, Mok SS, Michel BA, et al. Adult human chondrocytes cultured in alginate form a matrix similar to native human articular cartilage. Am J Physiol. 1996;40:C742–752. doi: 10.1152/ajpcell.1996.271.3.C742. [DOI] [PubMed] [Google Scholar]
- 11.Paige KT, Cima LG, Yaremchuk MJ, Schloo BL, Vacanti JP, Vacanti CA. De novo cartilage generation using calcium alginate-chondrocyte constructs. Plast Reconstr Surg. 1996;97:168–178. doi: 10.1097/00006534-199601000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21(24):2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 13.Hoemann CD, Sun J, Legare A, McKee MD, Buschmann MD. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthritis Cartilage. 2005;13(4):318–329. doi: 10.1016/j.joca.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Chung CE, IE, Mauck RL, Burdick JA. Differential behavior of auricular and articular chondrocytes in hyaluronic acid hydrogels. Tissue Engineering A. 2008;14(7):1121–1131. doi: 10.1089/ten.tea.2007.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59(1):63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, Rodriguez A, Vacanti M, Ibarra C, Arevalo C, Vacanti CA. Comparative study of the use of poly(glycolic acid), calcium alginate and pluronics in the engineering of autologous porcine cartilage. J Biomater Sci Polymer Ed. 1998;9:475–487. doi: 10.1163/156856298x00578. [DOI] [PubMed] [Google Scholar]
- 17.Freed LE, Marquis JC, Nohria A, Emmanual J, Mikos AG, Langer R. Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J Biomed Mater Res. 1993;27:11–23. doi: 10.1002/jbm.820270104. [DOI] [PubMed] [Google Scholar]
- 18.Choi BG, Park MH, Cho S-H, Joo MK, Oh HJ, Kim EH, et al. In situ thermal gelling polypeptide for chondrocytes 3D culture. Biomaterials. 2010;31(35):9266–9272. doi: 10.1016/j.biomaterials.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 19.Choi BG, Park MH, Cho S-H, Joo MK, Hye JO, Kim EH, et al. Thermal gelling polyalanine-poloxamine-polyalanine aqueous solution for chondrocytes 3D culture: Initial concentration effect. Soft Matter. 2011;7(2):456–462. [Google Scholar]
- 20.Shah RN, Shah NA, Del Rosario Lim MM, Hsieh C, Nuber G, Stupp SI. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc Natl Acad Sci USA. 2010;107(8):3292–3298. doi: 10.1073/pnas.0906501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003 Aug;9(4):597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 22.Grunder T, Gaissmaier C, Fritz J, Stoop R, Hortschansky P, Mollenhauer J, et al. Bone morphogenetic protein (BMP)-2 enhances the expression of type II collagen and aggrecan in chondrocytes embedded in alginate beads. Osteoarthritis and Cartilage. 2004;12(7):559–567. doi: 10.1016/j.joca.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Martin I, Suetterlin R, Baschong W, Heberer M, Vunjak-Novakovic G, Freed LE. Enhanced cartilage tissue engineering by sequential exposure of chondrocytes to FGF-2 during 2D expansion and BMP-2 during 3D cultivation. J Cell Biochem. 2001;83(1):121–128. doi: 10.1002/jcb.1203. [DOI] [PubMed] [Google Scholar]
- 24.Masuda K, Pfister BE, Sah RL, Thonar EJ-MA. Osteogenic protein-1 promotes the formation of tissue-engineered cartilage using the alginate-recovered-chondrocyte method. Osteoarthritis Cartilage. 2006;14:384–391. doi: 10.1016/j.joca.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Miller RE, Kopesky PW, Grodzinsky AJ. Growth factor delivery through self-assembling peptide scaffolds. Clin Orthop Relat Res. 2011;469:2716–2724. doi: 10.1007/s11999-011-1891-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coates E, Fisher JP. Gene expression of alginate-embedded chondrocyte subpopulations and their response to exogenous IGF-1 delivery. J Tissue Eng Regen Med. 2012;6(3):179–92. doi: 10.1002/term.411. [DOI] [PubMed] [Google Scholar]
- 27.Demarteau O, Wendt D, Braccini A, Jakob M, Schafer D, Heberer M, et al. Dynamic compression of cartilage constructs engineered from expanded human articular chondrocytes. Biochem Biophys Res Commun. 2003;310:580–588. doi: 10.1016/j.bbrc.2003.09.099. [DOI] [PubMed] [Google Scholar]
- 28.Villanueva I, Gladem SK, Kessler J, Bryant SJ. Dynamic loading stimulates chondrocyte biosynthesis when encapsulated in charged hydrogels prepared from poly(ethyleneglycol) and chondroitin sulfate. Matrix Biology. 2010;29(1):51–62. doi: 10.1016/j.matbio.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckley CT, Vinardell T, Kelly DJ. Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived MSCs and articular chondrocytes. Osteoarthritis and Cartilage. 2010;18(10):1345–1354. doi: 10.1016/j.joca.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Ke Y, Wang Y, Ren L. Surface modification of PHBV scaffolds via UV polymerization to improve hydrophilicity. J Biomater Sci Polym Ed. 2010;21(12):1589–1602. doi: 10.1163/092050609X12520505439788. [DOI] [PubMed] [Google Scholar]
- 31.Perez Olmedilla M, Garcia-Giralt N, Pradas MM, Ruiz PB, Gomez Ribelles JL, Palou EC, et al. Response of human chondrocytes to a non-uniform distribution of hydrophilic domains on poly(ethyl acrylate-co-hydroxyethyl methacrylate) copolymers. Biomaterials. 2006;27(7):1003–1012. doi: 10.1016/j.biomaterials.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 32.Hutcheon GA, Messiou C, Wyre RM, Davies MC, Downes S. Water absorption and surface properties of novel poly(ethylmethacrylate) polymer systems for use in bone and cartilage repair. Biomaterials. 2001;22(7):667–676. doi: 10.1016/s0142-9612(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 33.Ivirico JL, Martinez EC, Sanchez MS, Criado IM, Ribelles JL, Pradas MM. Structure and properties of methacrylate-endcapped caprolactone networks with modulated water uptake for biomedical applications. J Biomed Mater Res B Appl Biomater. 2007;83(1):266–275. doi: 10.1002/jbm.b.30792. [DOI] [PubMed] [Google Scholar]
- 34.Park JS, Woo DG, Sun BK, Chung HM, Im SJ, Choi YM, et al. In vitro and in vivo test of PEG/PCL-based hydrogel scaffold for cell delivery application. J Control Release. 2007;124(1–2):51–59. doi: 10.1016/j.jconrel.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 35.Benoit DSW, Schwartz MP, Durney AR, Anseth KS. Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nature Materials. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao S, Yu T, Guan Z. De novo design of saccharide-peptide hydrogels as synthetic scaffolds for tailored cell responses. J Am Chem Soc. 2009;131(48):17638–17646. doi: 10.1021/ja907097t. [DOI] [PubMed] [Google Scholar]
- 37.Herzner H, Reipen T, Schultz M, Kunz H. Synthesis of glycopeptides containing carbohydrate and peptide recognition motifs. Chem Rev. 2000;100(12):4495–4538. doi: 10.1021/cr990308c. [DOI] [PubMed] [Google Scholar]
- 38.Chawla K, Yu T, Liao SW, Guan Z. Biodegradable and biocompatible synthetic saccharide-peptide hydrogels for three-dimensional cell culture. Biomacromolecules. 2011;12(3):560–567. doi: 10.1021/bm100980w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metzke M, O’Connor N, Maiti S, Nelson E, Guan Z. Saccharide-peptide hybrid copolymers as biomaterials. Angew Chem Int Ed. 2005;44:6529–6533. doi: 10.1002/anie.200501944. [DOI] [PubMed] [Google Scholar]
- 40.Kraehenbuehl TP, Ferreira LS, Zammaretti P, Hubbell JA, Langer R. Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials. 2009;30(26):4318–4324. doi: 10.1016/j.biomaterials.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lutolf MP, Hubbell JA. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules. 2003;4(3):713–722. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 42.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003;100(9):5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21(5):513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 44.Mather BD, Viswanathan K, Miller KM, Long TE. Michael addition reactions in macromolecular design for emerging technologies. Progress in Polymer Science. 2006;31(5):487–531. [Google Scholar]
- 45.Pratt AB, Weber FE, Schmoekel HG, Muller R, Hubbell JA. Synthetic extracellular matrices for in situ tissue engineering. Biotechnol Bioeng. 2004;86(1):27–36. doi: 10.1002/bit.10897. [DOI] [PubMed] [Google Scholar]
- 46.Rizzi SC, Hubbell JA. Recombinant protein-co-PEG networks as cell-adhesive and proteolytically degradeable hydrogel matrixes. PartI: development and physicochemical characteristics. Biomacromolecules. 2005;6:1226–1238. doi: 10.1021/bm049614c. [DOI] [PubMed] [Google Scholar]
- 47.Jin R, Moreira Teixeira LS, Krouwels A, Dijkstra PJ, van Blitterswijk CA, Karperien M, et al. Synthesis and characterization of hyaluronic acid-poly(ethylene glycol) hydrogels via Michael addition: an injectable biomaterial for cartilage repair. Acta Biomaterialia. 2010;6:1968–1977. doi: 10.1016/j.actbio.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 48.Hiemstra C, van der Aa LJ, Zhong Z, Dijkstra PJ, Feijen J. Novel in situ forming, degradable dextran hydrogels by Michael addition chemistry: synthesis, rheology, and degradation. Macromolecules. 2007;40(4):1165–1173. [Google Scholar]
- 49.Wolfenden R, Andersson L, Cullis PM, Southgate CC. Affinities of amino acid side chains for solvent water. Biochemistry. 1981;20(4):849–855. doi: 10.1021/bi00507a030. [DOI] [PubMed] [Google Scholar]
- 50.Chawla K, Klein TJ, Schumacher BL, Schmidt TA, Voegtline MS, Thonar EJ-MA, et al. Tracking chondrocytes and assessing their proliferation with PKH26: effects on secretion of proteoglycan 4 (PRG4) J Orthop Res. 2006;24:1499–1508. doi: 10.1002/jor.20116. [DOI] [PubMed] [Google Scholar]
- 51.Hu BH, Su JS, Messersmith PB. Hydrogels cross-linked by native chemical ligation. Biomacromolecules. 2009;10(8):2194–2200. doi: 10.1021/bm900366e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chawla K, Klein TJ, Schumacher BL, Jadin KD, Shah BH, Nakagawa K, et al. Short-term retention of chondrocytes in stratified tissue-engineered cartilaginous constructs Implanted In Vivo in Mini-Pigs. Tissue Engineering. 2007;13(7):1525–1537. doi: 10.1089/ten.2007.0044. [DOI] [PubMed] [Google Scholar]
- 53.McGowan KB, Kurtis MS, Lottman LM, Watson D, Sah RL. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258. Osteoarthritis Cartilage. 2002;10(7):580–587. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 54.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 55.Ramachandran GN, editor. Chemistry of collagen. London: Academic Press; 1967. [Google Scholar]
- 56.Kim PD, Peyton SR, Vanstrien AJ, Putnam AJ. The influence of ascorbic acid, TGF-beta1, and cell-mediated remodeling on the bulk mechanical properties of 3-D PEG-fibrinogen constructs. Biomaterials. 2009;30(23–24):3854–3864. doi: 10.1016/j.biomaterials.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 57.Scott JE, Dorling J. Differential staining of acid glycosaminoglycans (mucopolysaccharides) by alcian blue in salt solutions. Histochemie. 1965;5:221–233. doi: 10.1007/BF00306130. [DOI] [PubMed] [Google Scholar]
- 58.Aydelotte MB, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. I. morphology and cartilage matrix production. Connect Tissue Res. 1988;18:205–222. doi: 10.3109/03008208809016808. [DOI] [PubMed] [Google Scholar]
- 59.LiVecchi AB, Tombes RM, LaBerge M. In vitro chondrocyte collagen deposition within porous HDPE: substrate microstructure and wettability effects. J Biomed Mater Res. 1994;28:839–850. doi: 10.1002/jbm.820280802. [DOI] [PubMed] [Google Scholar]
- 60.Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, et al. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6(5):385–392. doi: 10.1038/nmat1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strehin I, Nahas Z, Arora K, Nguyen T, Elisseef J. A versatile pH sensitive chondroitin sulfate-PEG tissue adhesive and hydrogel. Biomaterials. 2010;31:2788–2797. doi: 10.1016/j.biomaterials.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A. 2002;99(15):9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rose G, Geselowitz A, Lesser G, Lee R, Zehfus M. Hydrophobicity of amino acid residues in globular proteins. Science. 1985;229:834–838. doi: 10.1126/science.4023714. [DOI] [PubMed] [Google Scholar]
- 64.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 65.Betts MJ, Russell RB. Amino acid properties and consequences of substitutions. In: Barnes MR, Cray IC, editors. Bioinformatics for Geneticists. 2003. [Google Scholar]
- 66.Cornette J, Cease KB, Margalit H, Spouge JL, Berzofsky JA, DeLisi C. Hydrophobicity scales and computational techniques for detecting amphipathic structures in proteins. J Mol Biol. 1987;195:659–685. doi: 10.1016/0022-2836(87)90189-6. [DOI] [PubMed] [Google Scholar]
- 67.Janin J. Surface and inside volumes in globular proteins. Nature. 1979;277:491–492. doi: 10.1038/277491a0. [DOI] [PubMed] [Google Scholar]
- 68.Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential maturation and structure-function relationships in MSC- and chondrocyte-seeded hydrogels. Tissue Eng A. 2008;14(0) doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 70.Mow VC, Zhu W, Ratcliffe A. Structure and function of articular cartilage and meniscus. In: Mow VC, Hayes WC, editors. Basic Orthopaedic Biomechanics. New York: Raven Press; 1991. pp. 143–198. [Google Scholar]
- 71.Jin R, Moreira Teixeira LS, Dijkstra PJ, Zhong Z, von Blitterswijk CA, Karperien M, et al. Enzymatically crosslinked dextran-tyramine hydrogels as injectable scaffolds for cartilage tissue engineering. Tissue Eng Part A. 2010 doi: 10.1089/ten.TEA.2009.0764. [DOI] [PubMed] [Google Scholar]