Abstract
In vivo gene transfer to the ischemic heart via electroporation holds promise as a potential therapeutic approach for the treatment of heart disease. In the current study, we investigated the use of in vivo electroporation for gene transfer using 3 different penetrating electrodes and one non-penetrating electrode. The hearts of adult male swine were exposed through a sternotomy. Eight electric pulses synchronized to the rising phase of the R wave of the ECG were administered at varying pulse widths and field strengths following an injection of either a plasmid encoding luciferase or one encoding green fluorescent protein. Four sites on the anterior wall of the left ventricle were treated. Animals were euthanized 48 hours after injection and electroporation and gene expression was determined. Results were compared to sites in the heart that received plasmid injection but no electric pulses or were not treated. Gene expression was higher in all electroporated sites when compared to injection only sites demonstrating the robustness of this approach. Our results provide evidence that in vivo electroporation can be a safe and effective non-viral method for delivering genes to the heart, in vivo.
Keywords: electroporation, heart, in vivo, gene transfer, swine
Introduction
Ischemic heart disease continues to be a leading cause of mortality and disability. Despite the widespread availability of percutaneous catheter based interventions and surgical coronary artery bypass, there is a growing population of patients in which complete revascularization cannot be achieved. Gene transfer to promote neovascularization to these regions is an attractive therapeutic modality. The feasibility of delivering angiogenic factors to the myocardium has been demonstrated via cardiac catherization or direct administration into the myocardium.1–10 However, there are problems associated with its use. Adeno-viruses have been linked with tissue immune responses and non-viral mediated gene transfer, although not associated with adverse immune responses, has provided expression levels that fall below levels needed for therapeutic management of disease. Unlike viral vectors, naked DNA vectors are not associated with the immunogenicity observed with viral vectors.11–14 Therefore, an efficient gene transfer system for delivery of plasmid DNA targeted to the ischemic area of the heart at levels designed to induce increased perfusion would provide long-term support to the ischemic myocardium and reduce the possibility of recurring ischemic episodes.
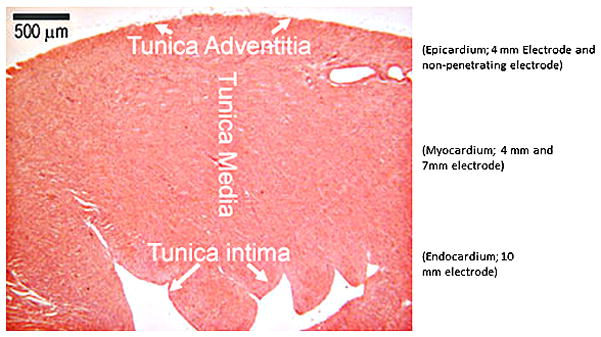
Electroporation, which is a technique involving the application of short duration, high intensity electric pulses to tissue 15,16 used in combination with plasmid DNA injection has been reported to facilitate delivery of plasmid DNA in skeletal muscle17 and cardiac tissue 18,19. Previously, we demonstrated that electroporation could be used to effectively deliver plasmid DNA directly to cardiac tissue in vivo 19. It was demonstrated that administering electric pulses that were synchronized to the rising phase of the R wave of the ECG resulted in enhanced expression of the delivered transgene without fibrillating the heart 19. In this study, the work is expanded to evaluate various electrode configurations to facilitate delivery. Utilizing these different arrays we evaluated the delivery of varying field strengths and pulse widths to the epicardium, the myocardium and to the endocardium of the heart, in vivo (Figure 1).
Figure 1.
Cartoon of the layers of the heart showing plasmid injection and electrode placement.
Results
A total of 40 animals were studied and 111 injection/electroporation sites analyzed. To enhance delivery of plasmid DNA to the heart, custom designed applicators were utilized. The basic electrode designs used were either a penetrating electrode applicator which contained 4 needle electrodes or a non-penetrating electrode applicator which contained 4 bars. Both applicators were designed to form a 5 × 5 mm square (Figure 2). For the needles arrays, the 8 pulses were divided into two sets of 4 pulses. All pulses are applied across the 5 mm gap in a 2 × 2 needle configuration. Following administration of the first 4 pulses, the next set of 4 pulses was applied in a perpendicular direction to the first (90° rotation). For all pulses, 2 needles have a positive polarity and 2 have a negative polarity. For the nonpenetrating array, the pulses are applied across two plates then an additional 4 pulses applied across the 2 perpendicular plates. Thus, the pulses are applied in two perpendicular directions as done with the needle arrays.
Figure 2.
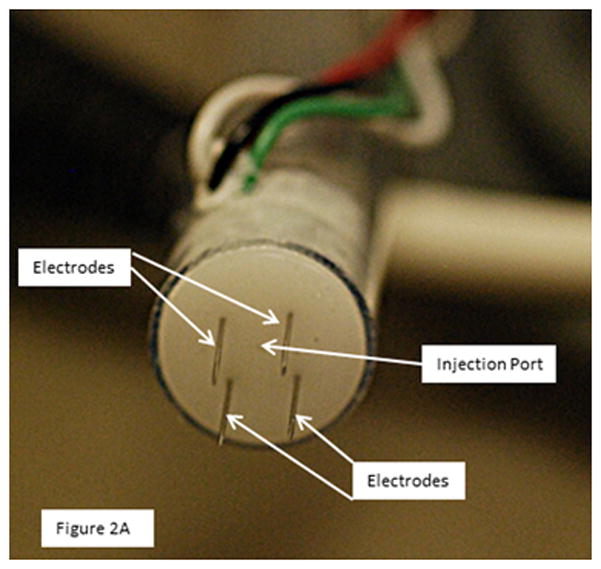
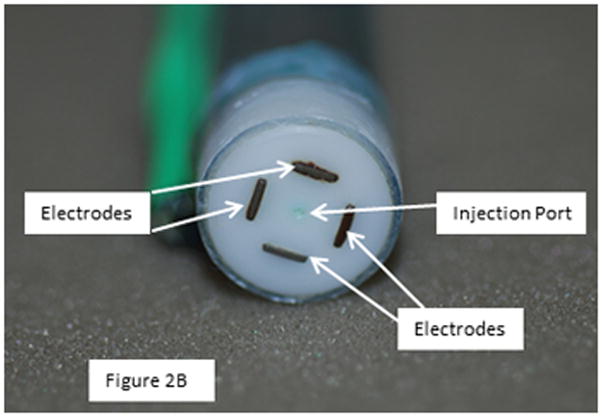
4 Needle electrode and nonpenetrating electrode
The electrode applicators contained a central injection port which allowed the injection needle to be inserted to a controlled depth. This was critical in delivering the plasmid to the desired area of the heart. A spacer was slipped over the needle to control the insertion depth. Following injection of the plasmid, the needle was drawn back into the insulated part of the applicator and was not within the applied field. To assure that the electric field was applied in the area that the plasmid was injected, three different penetrating electrode applicators were utilized which facilitated penetration to 3 different depths (4, 7 and 10 mm). For delivery to the epicardium (tunica adventitia), the injection needle was inserted to a depth of 2.5 mm and the electric pulses were administered using a penetrating applicator with 4 mm long needles. For delivery to the myocardium (tunica media) the injection needle was inserted to a depth of 3.5 mm and the electric pulses were administered using a penetrating applicator with 7 mm long needles. For delivery to the endocardium (tunica intima) the injection needle was inserted to a depth of 5.5 mm and the electric pulses were administered using a penetrating applicator with 10 mm long needles. For the nonpenetrating electrode, the injection needle was inserted to a depth of 2.5 mm.
All electroporated sites had significantly higher expression levels (p<0.001) of pLuc when compared to the plasmid injection only (no electroporation) or no treatment at all (Figure 3A–D). These results demonstrate the robustness of the approach as increased expression was obtained even with different pulsing parameters and electrode depths.
Figure 3.
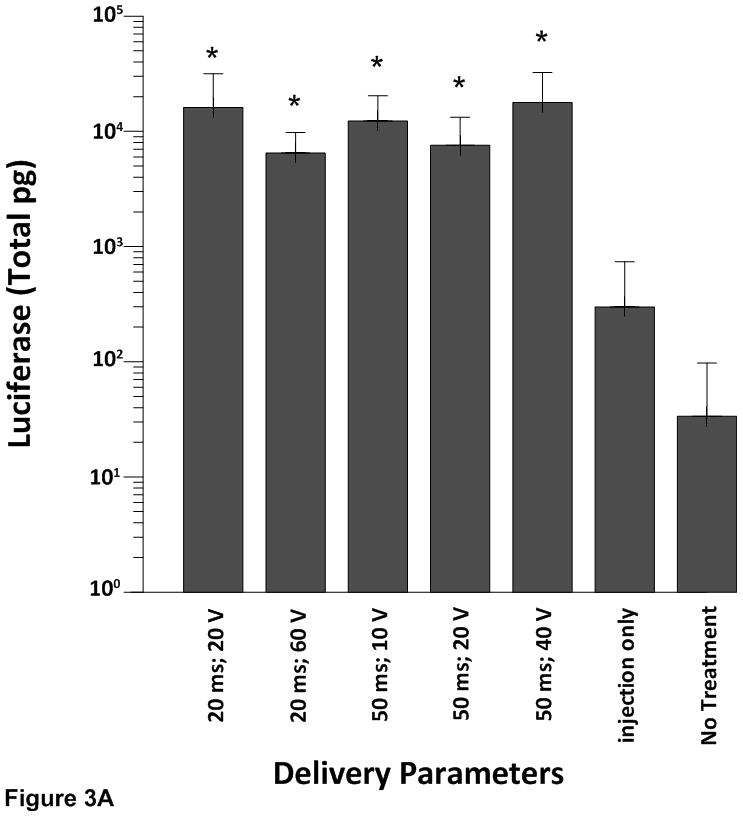
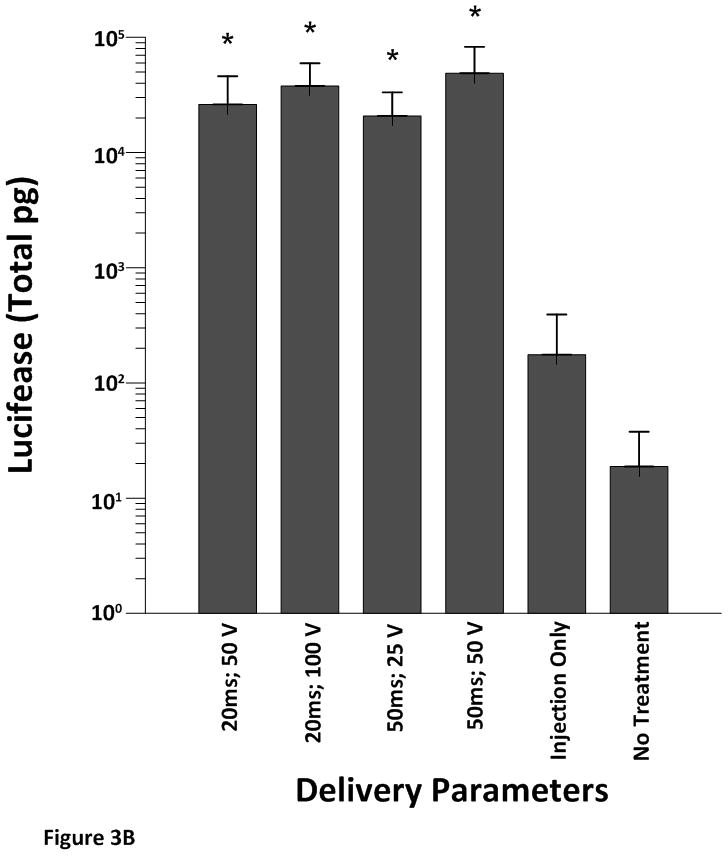
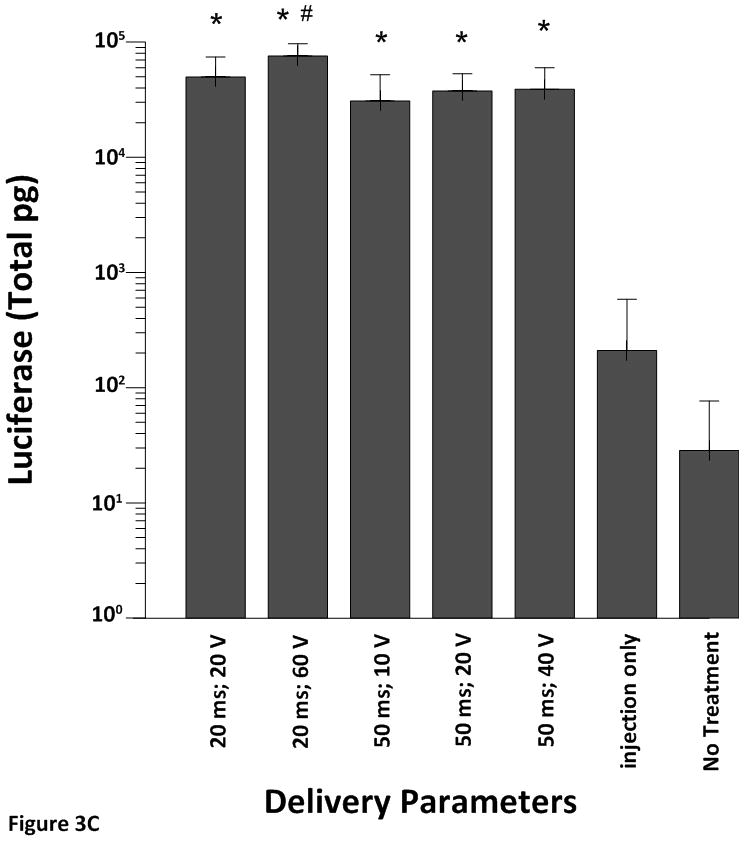
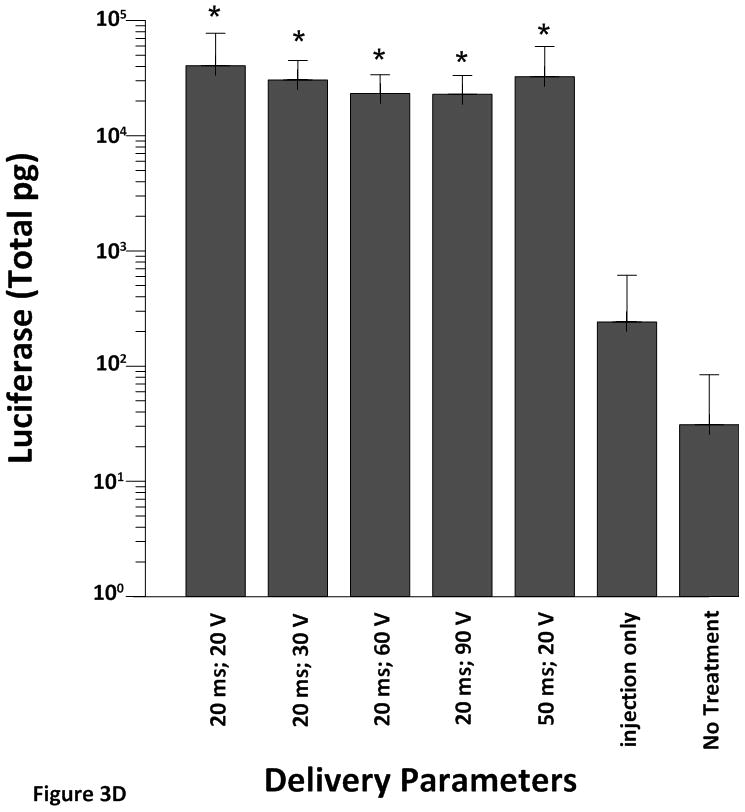
Cardiac expression of luciferase after electroporation-mediated delivery of pLuc. Expression for luciferase is given as the mean total pg ± SD in all figures.
A) Expression levels using the 4 mm penetrating electrode applicator. Injection needle was inserted to a depth of 2.5 mm. B) Expression levels using a non-penetrating electrode.Injection needle was inserted to a depth of 2.5 mm. C) Expression levels using the 7 mm penetrating electrode applicator. Injection needle was inserted to a depth of 3.5 mm. D) Expression levels using the 10 mm penetrating electrode applicator. Injection needle was inserted to a depth of 6.5 mm. Number of sites treated with each electrode delineated in Table 1. An additional 6–10 sites received an injection of pLuc without electroporation (injection only). * p<0.001; # p<0.05.
Two different electrode applicators were used to deliver plasmid DNA to the epicardium. With the 4 mm penetrating electrode applicator, when the pulse width was held constant at 20 milliseconds (ms) there was no significant difference in luciferase expression when the applied electric field was increased from 20 V to 60 V. Likewise, when pulse width was maintained at 50 ms and the applied electric field was increased, the increase in luciferase expression was not statistically significant (Figure 3A). These data suggest that plasmid injection plus electroporation increases the level of luciferase expression greater than plasmid injection alone but varying the pulse width or the electric field does not further enhance gene expression in the epicardium of the swine heart.
When we exposed the epicardium to varying field strengths and pulse widths using a non-penetrating electrode (Figure 3B) the electroporated tissue once again had significantly higher expression levels of luciferase (p< 0.001) when compared to injection without electroporation or no treatment. However, changing pulse width or the applied electric field did not cause a further increase in expression.
Although the 4 mm electrode delivered the electrical field to a small portion of the myocardium in addition to the epicardium the highest expression of pLuc was observed when the plasmid was injected into the myocardium and electroporated using the 7 mm penetrating electrode (Figure 3C). Again, all electroporation sites had significantly higher luciferase expression levels (p<0.001) when compared to the injection only (plasmid injection but no electroporation). Maintaining a constant pulse width of 20 ms and increasing the applied electric field from 20 V to 60 V resulted in no significant change in expression. When the pulse width was increased to 50 ms there was a significant decrease in luciferase expression at an electric field of 10 V and 20 V when compared to 20 ms and 60 V (p<0.05). These data suggest that a shorter pulse width and a higher electric field are more efficient at increasing luciferase expression in the myocardium.
To demonstrate the effect of plasmid injection and electroporation on luciferase expression at the level of the endocardium we used a 10 mm penetrating electrode (Figure 3D). While luciferase expression was significantly higher in the electroporation sites than in the injection only site, manipulation of the pulse width or the electric field did not result in greater expression of luciferase.
The highest luciferase expression was obtained following injection of plasmid DNA into the myocardium followed by administration of electric pulses administered with the 7 mm electrode (20 ms and 60 V). Delivery utilizing 20 ms and 20 V also yielded high levels of expression in the myocardium. As a result, we analyzed luciferase expression using the 7 mm electrode with both of these electroporation parameters following injection of pLuc at concentrations of 0.5 mg/ml, 1mg/ml or 2 mg/ml to determine if the concentration of DNA was a factor in amount of expression. When delivered with the 7 mm penetrating array, all concentrations of DNA resulted in increased luciferase expression when electroporation was used compared to injection without electroporation; however, we did not observe a dose response (Figure 4).
Figure 4.
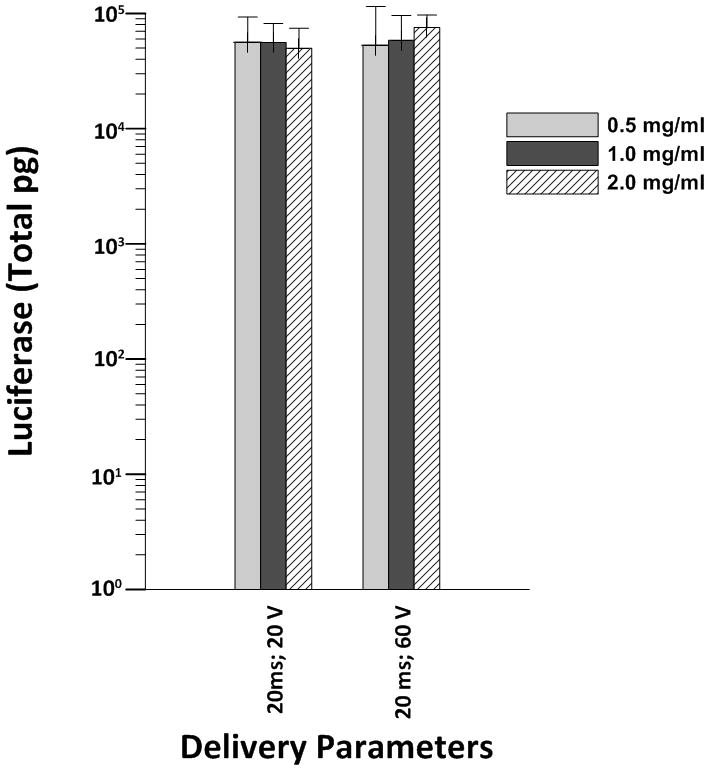
Luciferase expression response to different concentrations of plasmid after electroporation. For each plasmid concentration and electroporation parameters had between 5–6 sites.
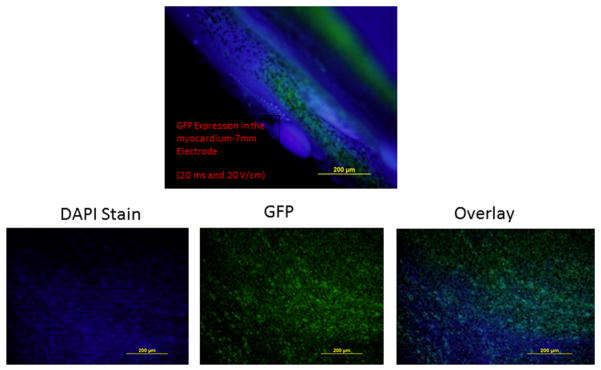
Distribution of expression within the heart was evaluated following injection of pGFP. For this experiment, the 7 mm electrode was used for electroporation. GFP was seen throughout the electroporated sites of the myocardium (Figure 5).
Figure 5.
Distribution of expression following delivering to the myocardium. Injection of pGFP following by admistration of electroporation using the 7 mm electrode. Injection needle was inserted to a depth of 3.5 mm. A total of 6 sites received injection of pGFP and electroporation and an additional 6 sites received an injection of pGFP without electroporation.
Using Ansoft Maxwell 12 software, electric fields were simulated for the different electrode geometries. Figure 6 shows a simple 2-D field simulation of the 4 mm penetrating needle electrode as well as the non-penetrating plate electrode. Both electrodes are modeled in the simulation with a 5 mm spacing and surrounded in a polyethylene sheath. The outside environment was specified to be air with the heart tissue modeled with permittivity and conductivity parameters as described previously20. The simulations used parameters that resulted in the best expressions for both electrodes (20 V for 4 mm penetrating electrode and 50 V for non-penetrating electrode). It is seen that both electrode configurations showed high localized fields at the tips of the electrodes. The injection area electric field is seen to be ~ 50 V/cm for the penetrating electrode and ~ 60 V/cm for the non-penetrating electrode. The non-penetrating electrode shows a decrease in electric field with increasing depth of tissue but still showing ~10 V/cm up to 5 mm into the tissue. The penetrating electrodes show a 50 V/cm field for the length of the electrode and ~10 V/cm up to 5 mm deeper into the tissue than the electrode length. As the needle electrode is moved to 7 and 10 mm depths, the same electric fields are also moved to these depths. With the higher voltage conditions (60 V for penetrating and 100 V non-penetrating), simulations show fields at the injection areas to be ~120 V/cm and ~110 V/cm for the penetrating and non penetrating electrodes respectively.
Figure 6.
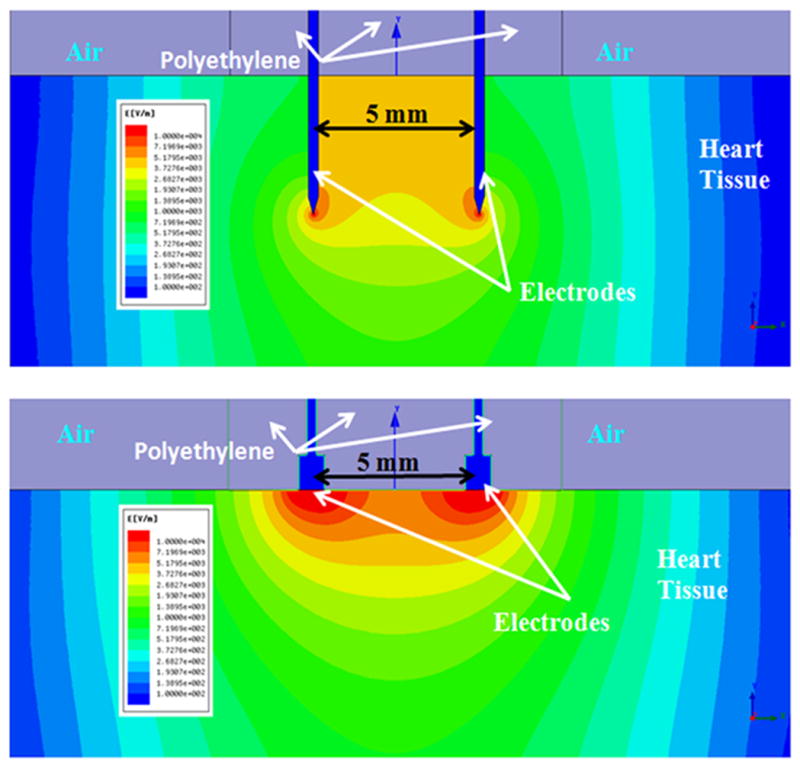
Modeling of electric field distribution. Top: 2-D field simulations for 4 mm penetrating (20 V) and bottom: non-penetrating (50 V) electrode configurations in heart tissue.
Discussion
In the present study, we confirmed that electroporation is a viable approach to deliver plasmid DNA into cells of the porcine heart, in vivo. Variables such as applied electrical field, pulse width, electrode length and DNA concentrations were tested. We analyzed 3 different electrodes with varying needle lengths (4 mm, 7 mm, 10 mm) and a non-penetrating electrode with varying pulse width and applied electric field. The electroporation parameters were selected based on several factors including size and structure of cells as well as a pulse length that could be administered during the rising phase of the R wave of the ECG and be concluded prior to the start of the t wave. Cardiac muscle is similar to skeletal muscle as both are striated. However, unlike skeletal muscle, cardiac muscle cells are mononucleated and may be branched unlike skeletal muscle cells that are typically multinucleated and linear. Skeletal muscle cells are typically centimeters in length while cardiac muscle cells are typically around 100–150 mm long. Skeletal muscle cells are also wider (100 μm diameter) than cardiac muscle cells (35 mm in diameter). Even with these differences, it was hypothesized that similar electroporation conditions that were successful to deliver to skeletal muscle could be utilized for cardiac muscle cells. It was decided to test pulse widths similar to those used by Aihara (50 ms) and Mir (20 ms) for this study21,22. These pulse widths were also short enough to be appropriately synchronized with the ECG. Interestingly, although the cardiac muscle cells are smaller, a lower applied voltage/electrode distance was needed to obtain high expression. Our findings show a statistically significant increase in luciferase expression with all electrodes, at all pulse widths and at all applied electrical fields when compared to tissue sites exposed to injection of plasmid without electroporation or non-treated tissue sites (no injection or electroporation).
The 4 mm long needle electrodes placed the applied electrical field in the epicardium and a small portion of the myocardium of the heart, in vivo. Although luciferase expression was significantly (p <0.001) greater with electroporation (for both 4 mm electrode and the non-penetrating electrode) than without it, the changes in pulse width or the applied electrical field did not cause a further increase in luciferase expression. We varied the pulse width from 20 to 50 ms and the applied electrical field from 10 V to 60 V. There was a greater tendency for changes in voltage to affect expression than changes in pulse width but the increase in expression was not statistically significant. The epicardium is a serous membrane of connective tissue covered with epithelium. The epicardium plays a morphogenetic role by emitting signals to promote and maintain cardiomyocyte proliferation. In a regenerative context, electroporation of the epicardium after injection of DNA may serve as a therapy for damaged heart and might induce a progenitor cell population that can be directed to contribute to cardiac repair.
It is clear that enhanced expression can be achieved in the epicardium by augmenting delivery with electroporation. It is interesting that higher expression in the epicardium was obtained utilizing the non-penetrating electrode compared to the 4 mm needle array. While the same injection procedure was used with both arrays, higher voltages were used with the non-penetrating array to achieve penetration of the fields. It may be possible that higher expression could be achieved with the 4 mm array if higher voltages were used, but this could also lead to tissue damage. However, it should also be noted that utilizing the non-penetrating array has a distinct advantage as only one needle (injection port) needs to penetrate the tissue. Being able to achieve appropriate expression levels without inserting multiple needles could be a benefit when attempting to deliver to the epicardium.
The most effective electrode in increasing luciferase expression was the 7 mm electrode. Delivery with this electrode yielded high reproducible expression. These studies show that luciferase expression was 133% greater when the pulse width was 20 ms and the applied electrical field was 60 V than when the pulse width was 50 ms and the applied electrical field was 10, 20 or 40 V (p<0.05). These data suggest that significant plasmid expression can be achieved when the electrode penetrates the myocardium and a voltage of at least 60 V is applied to the cardiac myocyte. The myocardium is the muscular middle layer of the wall of the heart and plays an important role in controlling the pumping function of the ventricles. It is composed of muscle fibers that contract spontaneously which allow for cardiac contraction. The function of this layer of the heart is critical to survival after ischemic episodes observed in the hibernating myocardium or infarcted heart. Thus, the myocardium is an important target for delivery of therapeutic molecules for the potential treatment of ischemia or infarcts.
We observed very few adverse responses of the heart following the application of the electric field to the heart. Fibrillation of the heart generally occurred if the animal was hypokalemic upon arrival to the facility or when the ECG tracing was not of a quality that readily allowed for recognition of the rising phase of the R wave. We corrected the hypokalemia preoperatively by administering potassium intravenously. Also, preoperatively heart rate and the ECG leads were manipulated so that there was a significant distance between the R and T waves. Applying the electric field away from the T wave in most, if not all cases prevented fibrillation of the heart even when the applied electric field was as high as 90 V.
We did not observe a dose response with either a change in pulse width or a change in the applied electrical field when we varied the concentration of injected plasmid. This is an interesting result. It is possible that because of the small area (5×5 mm) that delivery was maximized to the cells within this area and a decrease in expression may occur with a further reduction in DNA concentration. In these experiments, we utilized a constant injection volume of 100 μl. It is possible that variation of the volume may influence expression more at these concentrations. Increased volume may cause further distribution of the plasmid potentially leading to delivery to more cells and thus higher expression. This will be tested in future work. The positive message from the data presented in this report suggest that small concentrations of DNA can be used in combination with electroporation to enhance expression in the myocardium of the heart, in vivo. This is an important finding demonstrating that a large amount of DNA is not needed to get significant levels of expression. The next critical step would be to determine if these levels will be sufficient to obtain a desired effect when a therapeutic transgene is delivered.
In support of our findings of enhanced expression after electroporation we observed expression of pGFP in cells within the myocardium of the swine heart in vivo. Distribution of expression was seen in cells throughout the area of delivery. These results will form the basis for moving forward to explore therapeutic application of this approach. We previously19 demonstrated the use of electroporation for delivering plasmid DNA directly to the cardiac muscle in vivo. In the previous study, we established that the electric pulses had to be synchronized to the R wave in order to avoid fibrillation. It was also shown that a plasmid encoding VEGF165 could be effectively delivered to the heart without damage to the tissue. A recent study by Ayuni et al also demonstrated the utility of utilizing electroporation to deliver to a beating heart23. Although that study was done in a rat model it also suggests the potential for this approach. When these studies are taken together the data suggest that electroporation can be used therapeutically to increase the expression of growth factors such as VEGF in the heart in vivo19. The increased expression of VEGF could increase vessel formation and restore blood flow to ischemic regions of the heart.
A significant advance in the present study is the design of the electrode arrays. In previous studies the injection was independent of the placement of the electrode array. When performing the procedure in a small model such as a rat this is not an issue but in larger models such as the swine or when translating to eventual use in humans it will be critical to coordinate the injection with the placement of the electrodes to have a better control of delivery. Having the injection port incorporated into the electrode array assures the field will be applied where the plasmid is injected. This also enabled better control of the depth of the injection and facilitated targeting different levels within the heart. This can be a significant advantage when this approach is eventually tested in a disease model.
In summary, we analyzed a number of electroporation parameters in the heart in vivo. The 7 mm electrode gave the most reproducible enhancement of plasmid expression. With the 7 mm electrode a change in the applied electrical field was associated with a significant increase in luciferase expression. Electroporation appears to be a safe and efficient method of enhancing DNA expression in the beating heart of the swine.
Materials and Methods
Ethics Statement
Animal studies were performed at both at a PHS assured university animal research facility as well as the Naval Medical Center Portsmouth. This study was conducted in accordance with federal animal care and use guidelines and was preapproved by the Institutional Animal Care and Use Committee at both institutions.
Animals
Adult male castrated domestic Yorkshire pigs weighing 28–43 kg were purchased from Bellveue Farms, Smithville, Virginia for use in this study. All animals were singly housed in stainless steel runs with 3 animals to a room to allow socializing. The animal rooms were on a 12:12-hour light cycle. Room temperature was maintained at 15 – 17°C with a relative humidity between 40 – 65%, and 12 – 15 air changes hourly. Animals were fed twice daily a diet of Swine food (Teklad Swine Chow - #8753, Madison, WI) and provided water ad libitum via an automatic watering system (Edstrom Industries, Waterford, WI). Environmental enrichment included daily food treats, toys, and human interaction. The use of the animals in this study was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations pertaining to animals and adheres to the principles stated in the Guide for the Care and Use of Laboratory Animals.
Presurgical preparation
All animals were given the pre-anesthetic drug Ketamine (20 mg/kg) and diazepam (3–5 mg/kg IM) for sedation to facilitate catheter placement in both ear veins. Following catheter placement, the animals were anesthetized with isoflurane with a nose mask to facilitate intubation. Each animal was intubated with a #6 French endotracheal tube.
Surgical Preparation
A surgical plane of anesthesia was accomplished by allowing the animal to breath isoflurane at 2–3% with an oxygen flow rate of 2 liters per minute. The lungs were ventilated (200–300 ml tidal volume 16–18 breaths per minute) using a BonAir mechanical ventilator. Depth of anesthesia was constantly monitored and the percentage of isoflurane adjusted to maintain the animal in a surgical plane of anesthesia. Preparation for surgery included shaving the ventral thorax (chest) and initial surgical scrub. The animals were then moved to the surgical facility and placed in dorsal recumbence on a heated surgical table. ECG leads were then attached and the electrocardiogram R wave monitored for the purpose of delivering the electric field. The chest was scrubbed with 70 % alcohol and chlorhex-Q scrub.
Surgical Procedure
The chest was draped creating a sterile surgical field and the skin and soft tissues were incised using an electrosurgical unit. Once the sternum was identified, it was opened with a striker saw and the ribs spread with a rib spreader to expose the left and right ventricles of the heart. Amiodarone (300mg) was given intravenously (IV) to slow the heart rate and provide anti-arrhythmic protection to the heart. A lidocaine drip (50–75 μg/kg/min IV) was started to stabilize the heart membrane. ECG, blood pressure, and body temperature were monitored constantly. Blood gases were monitored during the surgical procedure. Four sutures were placed 2 cm apart on the anterior wall of the left ventricle to mark the location of each plasmid injection. A 5th suture was placed in the right ventricle which marked the site of injection only (no electroporation). The sternum was closed with surgical steel monofilament 18 inch surgical needles. All other incisions were closed with vicryl plus-antibacterial suture (3.5 metric). The surgical area was bandaged using sterile TelFa non-adherent pads secured by wrapping the chest with VetWrap to protect the incision from contamination. Postoperatively, all animals were allowed to remain in the operating room under constant observation until extubation. Analgesics for pain relief included Carprofen (4 mg/kg once daily for 3 days) and Buprenorphine (0.1 mg/kg) as needed.
Plasmid Injection and Electroporation
The ECG for each animal was established to clearly distinguish the rising phase of the R wave. At that time, either a plasmid encoding luciferase (pLuc) or a plasmid encoding green fluorescent protein (pGFP) was injected and 8 electric pulses administered synchronized to initiate pulse during the R wave and be complete prior to the start of the T wave. Plasmids were injected at 4 sites on the anterior wall of the left ventricle and electroporated using varying field strengths and pulse widths (Table 1). Two types of electrode designs were used a penetrating electrode applicator which contained 4 needle electrodes (4, 7 or 10 mm long) or a non-penetrating electrode applicator which contained 4 bars. Both applicators were designed to form a 5 × 5 mm square (Figure 2). Both designs facilitated the administration of 4 pulses in each of two perpendicular directions. All the electrode applicators had a central injection port which allowed the injection needle to be inserted to a controlled depth. Following injection of the plasmid the needle was drawn back into the insulated part of the applicator and was not within the applied field. The injection of plasmid was performed to three different depths. This allowed placement of the plasmid within the epicardium (tunica adventitia) using a 2.5 mm injection depth, myocardium (tunica media) using a 3.5 mm injection depth or endocardium (tunica intima) using a 5.5 mm injection depth (Figure 1). The length of the penetrating electrode was chosen to place the injected plasmid within the center of the electrode array. To accomplish this, a 4 mm long electrode was used with the 2.5 mm injection depth, a 7 mm long electrode was used for a 3.5 mm injection depth and a 10 mm long electrode was used for a 5.5 mm injection depth. The electric pulses were administered using a custom built pulse generator. The pulsing program captured the ECG signal from an Accusync 72 (Accusync Medical Research Company, Milford, CT, USA) and synchronized pulse administration with the initiation of the R wave.
Table 1.
Electroporation parameters. Four sites, each 2 cm apart on the anterior wall of the left ventricle of the porcine heart were injected with plasmid and exposed, in vivo, to the pulse widths and applied electric fields using electrodes of various lengths. Each site received 8 pulses. The injection only site which was given plasmid only (no electroporation) was placed on the anterior wall of the right ventricle.
| Electrodes | 4 mm | 7 mm | 10 mm | Non- Penetrating |
|---|---|---|---|---|
| Voltages | 50 ms; 20 V (n=3 separate sites) | 50 ms; 20 V (n=6 separate sites) | 50 ms; 20 V (n=5 separate sites) | 20 ms; 50 V (n=6 separate sites) |
| 20 ms; 60 V (n= 4 separate sites) | 20 ms; 60 V (n= 7 separate sites) | 20 ms; 60 V (n= 9 separate sites) | 50 ms; 25 V (n= 6 separate sites) | |
| 50 ms; 40 V (n=5 separate sites) | 50 ms; 40 V (n=5 separate sites) | 20 ms; 90 V (n=5 separate sites) | 20 ms; 100 V (n=6 separate sites) | |
| 50 ms; 10 V (n=5 separate sites) | 50 ms; 10 V (n=7 separate sites) | 20 ms; 30 V (n=11 separate sites) | 50 ms; 50 V (n=6 separate sites) | |
| 20 ms; 20 V (n=5 separate sites) | 20 ms; 20 V (n=6 separate sites) | 20 ms; 20 V (n=4 separate sites) |
Tissue Harvesting and Detection of Luciferase or GFP Expression
Forty eight hours after plasmid delivery, luciferase activity was quantified as previously described24. The animal was placed under a surgical plane of anesthesia the sternum re-opened and the heart tissue was harvested and frozen at −80°C until analysis. The tissue was analyzed for the presence of luciferase activity using a luciferase assay orfor the presence of GFP using florescent microscopy.
Luciferase Assay
Activity was expressed as total picograms (pg) of luciferase per tissue sample. The data was tested for normality using a Normal Quantile Plot. Statistical analysis was performed by a one-way ANOVA.
GFP Expression
Each excised sample was frozen on dry ice and embedded in tissue freeze media OCT compound (Electron Microscopy Sciences, Hatfield, PA) stored at −80°C until analysis. Several frozen sections (7 um thickness) were cut from each sample. Each section was fixed in 75% Acetone and 25% ethanol for 20 min and then washed with PBS. Each slide was placed in a dark container and allowed to dry. VECTASHIELD mounting medium with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) was placed on the tissue and the tissue examined by Olympus BX51 fluorescent microscopy for the presence of GFP.
Acknowledgments
This research was supported in part by a research grant from the National Institutes of Health R21 HL005441 and by the Frank Reidy Research Center for Bioelectrics at Old Dominion University. We thank Dr Mark Jaroszeski (University of South Florida) for construction of the electrode arrays.
Footnotes
Some of the authors of in this paper are employees of the United States Government and the United States Navy. The views expressed in this article are those of the author(s) and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense or the United States Government.
CONFLICT OF INTEREST
With respect to duality of interest and financial disclosures, Dr R Heller is an inventor on patents, which cover the technology that was used in the work reported in this paper. In addition, Dr R Heller owns stock and stock options in Inovio Pharmaceutical Corp.
COPYRIGHT INFORMATION
Some of the authors of in this paper are employees of the United States Government and the United States Navy. This work was prepared as part of our official duties. Title 17 U.S.C. 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties.
References
- 1.Folkman J. Angiogenic therapy of the human heart. Circulation. 1998;97:628–629. doi: 10.1161/01.cir.97.7.628. [DOI] [PubMed] [Google Scholar]
- 2.Unger EF, Banai S, Shou M, Lazarous DF, Jaklitsch MT, Scheinowitz M, et al. Basic fibroblast growth factor enhances myocardial collateral flow in a canine model. Am J Physiol. 1994;266:H1588–H1595. doi: 10.1152/ajpheart.1994.266.4.H1588. [DOI] [PubMed] [Google Scholar]
- 3.Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, et al. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation. 1994;89:2183–2189. doi: 10.1161/01.cir.89.5.2183. [DOI] [PubMed] [Google Scholar]
- 4.Lazarous DF, Scheinowitz M, Shou M, Hodge E, Rajanayagam S, Hunsbereger S, et al. Effect of chronic systemic administration of basic fibroblast growth factor on collateral development in the canine heart. Circulation. 1995;91:145–153. doi: 10.1161/01.cir.91.1.145. [DOI] [PubMed] [Google Scholar]
- 5.Lazarous DF, Shou M, Scheinowitz M, Hodge E, Thirumurti V, Kitsiou AN, et al. Comparative effects of basic fibroblast growth factor and vascular endo-epithelial growth factor on coronary collateral development and the arterial response to injury. Circulation. 1996;94:1074–1082. doi: 10.1161/01.cir.94.5.1074. [DOI] [PubMed] [Google Scholar]
- 6.Giordano FJ, Ping P, Mckirnan D, Nozaki S, Demaria A, Dillman WH, et al. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nat Med. 1996;2:534–539. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- 7.Guzman RJ, Lemarchand P, Crystal RG, Epstein SE, Finkel T. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ Res. 1993;73:1202–1207. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- 8.Mack CA, Patel SR, Schwartz EA, Zanzinico P, Hahn RT, Ilercil A, et al. Biologic bypass with the use of adenovirus-mediated gene transfer of the complementary deoxyribonucleic acid for VEGF-12 improves myocardial perfusion and function in the ischemic porcine heart. J ThoracCardiovasc Surg. 1998;115:168–177. doi: 10.1016/s0022-5223(98)70455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumacher B, Pecher P, von Specht BU, Stegman T. Induction of neo-angiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation. 1998;97:645–650. doi: 10.1161/01.cir.97.7.645. [DOI] [PubMed] [Google Scholar]
- 10.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, et al. Gene therapy for myocardial angio- genesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–2804. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 11.Davis HL, Demeneix BA, Quantin B, Coulombe J, Whalen RG. Plasmid DNA superior to viral vectors for direct gene transfer into adult mouse skeletal muscle. Hum Gene Ther. 1993;4:733–740. doi: 10.1089/hum.1993.4.6-733. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity of viral antigens limits E1 deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker SE, Vahlsing HL, Serfillippi LM, Franklin CL, Doh SG, Gromkowski SH, et al. Cancer gene therapy using plasmid DNA: safety evaluation in rodents and non-human primates. Hum Gene Ther. 1995;6:575–590. doi: 10.1089/hum.1995.6.5-575. [DOI] [PubMed] [Google Scholar]
- 14.Horton HM, Anderson D, Hernandez P, Barnhart KM, Norman JA, Parjer SE. A gene therapy for cancer using intramuscular injection of plasmid DNA encoding interferon α. Proc Natl Acad Sci USA. 1999;96:1553–1558. doi: 10.1073/pnas.96.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse myloma cells by electroporation in high electric fields. EMBO J. 1982;1:841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heller LC, Heller R. In Vivo Electroporation for Gene Therapy. Hum Gene Ther. 2006;17(9):890–897. doi: 10.1089/hum.2006.17.890. [DOI] [PubMed] [Google Scholar]
- 17.Hartikka J, Sukhu L, Buchner C, Hazard D, Bozoukova V, Margalith M, et al. Electroporation-Facilitated Delivery of Plasmid DNA in Skeletal Muscle: Plasmid Dependence of Muscle Damage and Effect of Poloxamer 188. Mol Ther. 2001;4:407–415. doi: 10.1006/mthe.2001.0483. [DOI] [PubMed] [Google Scholar]
- 18.Harrison RL, Byrne BJ, Leslie YI, Tung Y. Electroporation-mediated gene transfer in cardiac tissue. FEBS Letters. 1998;435:1–5. doi: 10.1016/s0014-5793(98)00987-9. [DOI] [PubMed] [Google Scholar]
- 19.Marshall WG, Jr, Boone BA, Burgos JD, Gografe SI, Baldwin MK, Danielson ML, et al. Electroporation Mediated Delivery of a Naked DNA Plasmid Expressing VEGF to the Porcine Heart Enhances Protein Expression. Gene Therapy. 2010;17:419–423. doi: 10.1038/gt.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabriel S, Lau RW, Gabriel C, et al. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys Med Biol. 1996;41:2251–2269. doi: 10.1088/0031-9155/41/11/002. [DOI] [PubMed] [Google Scholar]
- 21.Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nature Biotechnol. 1998;16:867–870. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- 22.Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud J-M, et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci USA. 1999;96:4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayuni EL, Gazdhar A, Giraud MN, Kadner A, Gugger M, Cecchini M, et al. In Vivo Electroporation Mediated Gene Delivery to the Beating Heart. PLoS ONE. doi: 10.1371/journal.pone.0014467. Research Article, published 30 Dec 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heller L, Jaroszeski MJ, Coppola D, Pottinger C, Gilbert R, Heller R. Electrically mediated plasmid DNA delivery to hepatocellular carcinomas in vivo. Gene Therapy. 2007;7:826–829. doi: 10.1038/sj.gt.3301173. [DOI] [PubMed] [Google Scholar]